Abstract
Background:
Pain and sleep disturbance are common among patients with heart failure (HF) and are associated with symptom burden, disability, and poor quality of life. Little is known about the associations between specific sleep characteristics and pain in people with HF.
Objective:
Describe the relationships between nocturnal sleep characteristics, use of sleep medication, and daytime sleep characteristics and pain among people with HF.
Methods:
We conducted a cross-sectional study of stable HF participants. We administered the SF36 Bodily Pain Scale, Pittsburgh Sleep Quality Index, and Sleep Habits Questionnaire, and obtained 3 days of wrist actigraphy and one night of home unattended polysomnography. We conducted bivariate analyses and generalized linear models.
Results:
The sample included 173 participants [mean age= 60 years (SD 16.1), 65.3% (n= 113) male). Insomnia symptoms (p= .0010), sleep duration (p= .0010), poor sleep quality (p= .0153), use of sleep medications (p= .0170) and napping (p=.0029), and daytime sleepiness (p= .0094) were associated with increased pain. Patients with the longest sleep duration, who also had insomnia, had more pain (p= .0004), fatigue (p= .0028), daytime sleepiness (p= .0136), poorer sleep quality, (p< .0001) and took more sleep medications (p= .0029) than those without insomnia.
Conclusions:
Pain is associated with self-reported poor sleep quality, napping, daytime sleepiness, and use of sleep medication. The relationship between pain and sleep characteristics differs based on the presence of insomnia and sleep duration. Studies are needed to evaluate the causal relationships between sleep and pain and test interventions for these co-occurring symptoms.
Keywords: heart failure, pain, sleep, sleep initiation and maintenance disorders, actigraphy, polysomnography
Introduction
The symptoms of pain and sleep disturbance are common among patients with chronic heart failure (HF), a condition experienced by almost 6.5 million adults.1 Almost 70% of patients with stable HF report sleep disturbance and characteristics of sleep deficiency, including short sleep duration, poor sleep continuity, prolonged sleep latency, and excessive time in bed, compared with healthy controls as measured with self-report, wrist actigraphy, and abnormal sleep architecture measured with polysomnography.2,3 At least 50% of all patients with HF have sleep-disordered breathing, including Cheyne-Stokes breathing/central sleep apnea and obstructive sleep apnea. However, while approximately 50% report insomnia symptoms, self-reported sleep disturbance, and excessive daytime sleepiness, often a consequence of poor sleep, these symptoms are frequently not explained by sleep-disordered breathing.3,4 Sleep disturbance is associated with high levels of symptom burden, comorbidity, disability and poor quality of life, as well as excessive mortality and health care resource utilization.4–7
Pain is also common among patients with HF.8,9 For example, Goodlin et al. (2012) found that 80% of patients with advanced HF reported pain from multiple sources, including arthritis, ischemic heart disease, and other causes, and 29% of patients with chest pain and almost 40% of patients with pain from other sources reported that pain was severe.10 Pantilat et al. (2016) found that 48% of participants with HF experienced pain during the previous week, and the most common etiologies of pain were musculoskeletal (50%), cardiac (22%), and headache/neurological conditions (22%).11 Goebel et al. (2009) found in a sample of veterans who had HF that 55% reported pain, while 37% reported that it was moderate to severe,8 and a recent review reported that 23% to 85% of HF patients experience pain.12
Sleep deficiency is closely associated with chronic pain in numerous populations, although the causal direction of this relationship and the specific characteristics of sleep that are associated with pain are not known.13,14 Evidence suggests that self-reported insomnia symptoms, such as difficulty falling, asleep, staying asleep, waking too early in the morning, and short sleep duration contributed to pain in experimental studies.13 Brostrom et al. (2004) found that bodily pain was associated with self-reported difficulty initiating and maintaining sleep but not early morning awakening or excessive daytime sleepiness among HF patients,15 while Johansson et al. (2012) found that nighttime pain was directly associated with self-reported sleep disturbances and with self-reported difficulty initiating, maintaining, and non-restorative sleep in HF patients.16 However, few studies have systematically examined the associations between multiple objective and subjective characteristics of sleep and pain, and little is known about the co-occurrence of sleep and pain in patients with HF, a group that is at especially high risk for experiencing a high symptom burden.
In addition to nighttime sleep characteristics, pain may contribute to daytime sleep characteristics such as daytime sleepiness, fatigue, prolonged lying in bed while awake, and napping. Little research has explored the associations between these characteristics and pain. We previously found that fatigue and pain are correlated in the sample.17 However, little is known about the relationships between daytime sleepiness, snooze time (time awake in bed after awakening) and pain. Research on napping has produced conflicting findings, although other researchers found that in healthy young adults napping restores pain sensitivity to baseline in the presence of sleep restriction;18 however, daytime napping was also associated with increased pain in fibromyalgia.19
The purpose of this study, a secondary analysis of a study designed to address the contributions of sleep to functional performance among patients with stable HF conducted from 2003 to 2007,3,4,17,20–22 was to examine the relationships between specific self-reported and objective sleep characteristics and pain among patients with stable HF. We examined the relationships between 1) nocturnal sleep characteristics (sleep duration, sleep continuity, sleep efficacy, sleep onset latency) and pain; 2) use of sleep medication and pain; and 3) daytime sleep characteristics (napping, time awake in bed in the morning –snooze time, and excessive daytime sleepiness, and fatigue) and pain.
Methods
Study Design
We conducted a retrospective analysis of a cross-sectional study of 173 people with stable HF. The full details of the clinical and demographic characteristics of the sample and the study methods were reported previously.3,4,17,20–22
Sample
We recruited participants with stable HF from five HF clinics located in the northeastern United States. We included adults (ages ≥ 18) who had New York Heart Association (NYHA) class I – IV stable HF, as determined by the referring health care provider. We excluded participants who were pregnant, had previously identified sleep-disordered breathing, unstable medical or psychological disorders, end-stage renal disease, cognitive impairment, or neurological or musculoskeletal conditions affecting the non-dominant arm (due to actigraphy use).4 This study had Institutional Review Board approval, and all participants provided informed consent.
Variables and Measures
Demographic and Clinical Variables.
Demographic variables included age, race, and gender as reported by participants. Clinical variables were New York Heart Association Class, body mass index, and the Charlson Comorbidity Index,23 obtained via patient interview and medical record review. Full details of the comorbid conditions of the sample are reported previously.4
Pain.
Pain was measured using the SF-36 Bodily Pain scale and reflects about pain over the last month. The bodily pain scale is a valid measure of pain in people with chronic conditions.24,25 The bodily pain scale is a combination of pain severity and pain interference and higher numbers indicate less pain (possible range 2 – 12).
Sleep Characteristics.
We measured objective and subjective nocturnal sleep characteristics with polysomnography (PSG), actigraphy, and self-report (Pittsburgh Sleep Quality Index, Sleep Habits Questionnaire, and sleep diary). Because of our interest in the daytime response to poor sleep, we also measured excessive daytime sleepiness, fatigue, the number of naps taken (sleep diary), and snooze time (minutes elapsed between the end of the sleep period and time lights are switched on).
Polysomnography.
A single overnight unattended home polysomnography (PSG) (Safiro, Compumedics, Inc.) was conducted. Full details of the data acquisition were previously reported.3 Data were collected in 30-second epochs and were scored manually using standard methods.3 In this analysis, we used the variables of total sleep time (minutes), sleep efficiency (ratio of total sleep time to time in bed), and sleep latency (minutes to fall asleep).
Wrist Actigraphy.
Actigraphy uses human motor activity to estimate sleep parameters and provides objective sleep measures over multiple days as participants engage in normal activities of daily living.26 We measured 3 nights of actigraphy (did not overlap with the PSG night) (Actiwatch-64, Respironics Mini Mitter, Inc, Bend, OR) in 30-second epochs. We used Actiware Sleep version 5 (Respironics Mini Mitter, Inc, Bend, OR) to analyze the actigraphy data using standard methods. We used the variables of total sleep time, sleep efficiency, fragmentation index (index of restless during the sleep period), sleep onset latency, and snooze time (minutes elapsed between the end of sleep period and time lights are switched on).
Self-reported sleep characteristics.
We measured sleep quality with the sleep quality component of the Pittsburgh Sleep Quality Index (PSQI), which is a valid, reliable and sensitive measure.27 We did not use the overall PSQI score because it contains a pain question, but we computed sub-scores for sleep duration, habitual sleep efficiency, single question sleep quality component (higher score indicates worse sleep quality), and use of sleep medications in the last month. Internal consistency was 0.79 in this sample.3
We elicited insomnia symptoms with questions from the Sleep Heart Health Study28 including difficulty of initiating, maintaining sleep or awakening too early (DIMS) on a scale from “never” to “almost always” (5-item). Participants who endorsed responses of often or almost always on one or more of these questions were considered to have insomnia symptoms, and we computed the mean score based on the average response across the three questions (possible range = 0–15). The Cronbach’s α for this measure was 0.83 in this sample.4
Daytime sleepiness was measured with the Epworth Sleepiness Scale.29 Scores range from 0 to 24 and higher scores indicated more daytime sleepiness. The frequency of daily daytime naps over 4 days was elicited via self-report on a daily sleep diary. We used the Global Fatigue Index from the Multidimensional Assessment of Fatigue (MAF), a valid and reliable measure of fatigue.30,31 Scores range from 1 to 50, with higher scores indicating more severe fatigue.
Data Analysis
We performed descriptive analyses with the demographic, clinical, and sleep characteristics, and pain and computed bivariate associations between the sleep characteristics and pain with Pearson’s correlations or Spearman’s coefficients. For binary characteristics, we compared the mean of bodily pain by gender and race (white vs. non-white). Multivariate analysis was performed to identify the contributions of significant daytime/nighttime sleep characteristics to bodily pain using generalized linear models (GLM). The parsimonious model was selected with stepwise selection. We also reran the selected multivariate model separately in patients with and without insomnia. We compared the least square means of pain, fatigue, and sleep characteristics (i.e., sleep quality, sleep medication, frequency of naps, daytime sleepiness, and snooze time) between groups with insomnia and those with no insomnia across the quartiles of total sleep time (i.e., Q1: < 267.5 minutes, Q2: 267.5 – 322.5 minutes, Q3: 322.6 – 392.5 minutes, and Q4: > 392.5 minutes ) after controlling for age and comorbidity. The least square means and standard errors were obtained from the GLM with the insomnia-total sleep time interaction and the covariates. We checked the normality assumptions for the GLM with residual analysis.
Results
The sample included 173 participants with stable HF who had an average age of 60.3 years (SD 16.1), of whom 65.3% (n = 113) were male, and 64% (n = 110) were white. Table 1 reports the demographic and clinical characteristics of the participants and the descriptive statistics on the primary sleep variables. The mean bodily pain level was 8.02 (2.72) (range 2 – 12). Overall, nocturnal sleep duration ranged from 623 to 387 minutes, with the shortest duration recording by polysomnography and the longest by self-report. Overall, levels of sleep efficiency were low. Over half of the participants (n = 89; 51.45%) had insomnia symptoms.4 The majority did not report using sleeping medication, but 17.3% reported using sleep medication 3 or more times per week. The average number of naps over three days was 2.72, somewhat less than one per day. Thirty-seven (21.4%) reported taking a prescribed or over the counter medication for pain (participants could report taking more than one medication).
Table 1.
Demographic and clinical characteristics (N=173)
| Domain | Variables | Mean (SD) / N (%) |
|---|---|---|
| Demographic Characteristics | Age | 60.35 (16.07) |
| Male (%) | 113 (65.3%) | |
| White | 110 (64.6%) | |
| Clinical Characteristics | Charlson Comorbidity Index | 2.45 (1.52) |
| Body Mass Index | 30.72 (8.03) | |
| NYHA Classification | ||
| I | 5 (2.9%) | |
| II | 95 (54.9%) | |
| III | 61 (35.3%) | |
| IV | 12 (6.9%) | |
| Bodily Pain | 8.02 (2.72) | |
| Nighttime Sleep Characteristics | Sleep Duration | |
| Sleep Duration – PSQI (min) | 387.10 (100.5) | |
| Total Sleep Time – ACT (min) | 378.7 (88.6) | |
| Total Sleep Time – PSG (min) | 323.6 (96.6) | |
| Sleep Continuity | ||
| Sleep Efficiency – PSQI | 80.07 (17.70) | |
| Sleep Efficiency – ACT | 76.74 (12.86) | |
| Sleep Efficiency – PSG | 71.01 (16.25) | |
| Sleep Fragmentation – ACT | 42.24 (21.27) | |
| Sleep Latency – PSG | 20.92 (27.06) | |
| Sleep Latency – ACT | 29.36 (37.84) | |
| Sleep Quality | ||
| Sleep Quality – PSQI | 1.39 (0.85) | |
| Insomnia Symptoms Total (DIMS) | 1.73 (1.38) | |
| Frequency of Sleep Medications | ||
| None | 133 (76.9%) | |
| Less than Once per week | 5 (2.9%) | |
| Once or Twice per week | 5 (2.9%) | |
| Three or More times per week | 30 (17.3%) | |
| Pain Medications | ||
| Acetaminophen | 29 (16.8%) | |
| Non-Steroidal Anti-Inflammatory | 4 (3.31%) | |
| Gabapentin | 6 (3.47%) | |
| Daytime Sleep Characteristics | ||
| Daytime Sleepiness (ESS) | 8.31 (4.34) | |
| Fatigue | 29.80 (14.62) | |
| Frequency of Naps over 4 days | 2.72 (2.41) | |
| Snooze Time – ACT | 17.61 (18.38) |
Note. NYHA: New York Heart Association, PSQI: Pittsburgh Sleep Quality Index, ACT: Wrist Actigraphy (3 ACT nights average), PSG: Polysomnography (1 PSG night), ESS: Epworth Sleepiness Scale
Table 2 reports the correlations between the demographic and clinical characteristics of the sample, sleep variables, and pain. There were small correlations between New York Heart Association functional classification, comorbidity, and pain, with higher scores associated with more pain.17 Lower self-reported sleep efficiency, more snooze time, poorer sleep quality and more insomnia symptoms (continuous measure) were associated with pain, with moderate correlations between sleep quality, insomnia, and pain. More frequent use of sleep medication was associated with more pain. There were moderate associations between fatigue, depressive symptoms, and pain (lower values indicate more pain). There were no statistically significant associations between the actigraph and polysomnographic sleep variables and pain, except for snooze time.
Table 2.
Correlations between demographic and clinical characteristics, sleep characteristics and bodily pain
| Domain | Variables | Correlation with Bodily Pain |
|---|---|---|
| Clinical Characteristics | Charlson Comorbidity Index | −0.18 (.0179) |
| NYHA Classification | **−0.22 (.0038) | |
| Nighttime Sleep Characteristics | Sleep Duration | |
| Sleep Duration – PSQI | 0.13 (.0789) | |
| Total Sleep Time – ACT | 0.04 (.6007) | |
| Total Sleep Time – PSG | −0.11 (.1612) | |
| Sleep Continuity | ||
| Sleep Efficiency – PSQI | 0.16 (.0301) | |
| Sleep Efficiency – ACT | 0.10 (.1784) | |
| Sleep Efficiency – PSG | −0.02 (.8188) | |
| Sleep Fragmentation – ACT | −0.12 (.1142) | |
| Sleep Latency - PSG | −0.04 (.5997) | |
| Sleep Latency-ACT | −0.03 (.6620) | |
| Sleep Quality | ||
| Sleep Quality – PSQI | −0.40 (<.0001) | |
| Insomnia Symptoms (DIMS total) | −0.31 (<.0001) | |
| Frequency of Sleep Medications | **−0.29 (.0001) | |
| Daytime Sleep Characteristics | ||
| Daytime Sleepiness (ESS) | −0.20 (.0094) | |
| Global Fatigue | −0.38 (<.0001) | |
| Frequency of Naps for 4 days | −0.20 (.0070) | |
| Snooze Time-ACT | −0.17 (.0305) |
Note. NYHA: New York Heart Association, PSQI: Pittsburgh Sleep Quality Index, ACT: Wrist Actigraphy (3 ACT nights average), PSG: Polysomnography (1 PSG night), ESS: Epworth Sleepiness Scale Correlation was measured by Pearson Coefficient except NYHA Classification and Frequency of Sleep Medications (** Spearman correlation used); Bodily Pain – higher scores indicates less pain
Table 3 presents the multivariable model conducted to evaluate the contributions of daytime and nighttime sleep characteristics to pain as the outcome variable. We included variables that were associated with bodily pain that had significant levels of p < 0.10 using a stepwise approach. Insomnia symptoms (yes/no), longer total sleep time (PSG), poorer sleep quality, more frequent usage of sleep medication, and more frequent daytime naps were significantly associated with more bodily pain.
Table 3.
Multivariable model of sleep variables explaining bodily pain with stepwise selection
| Variable | Coefficient ± Std. Err | F-value | P-value |
|---|---|---|---|
| DIMS (Yes vs. No) | −1.45 ± 0.43 | 11.18 | .0010 |
| Total Sleep Time – PSG (Per 1hr) | −0.38 ± 0.12 | 11.03 | .0011 |
| Sleep Quality – PSQI | −0.63 ± 0.26 | 6.01 | .0153 |
| Frequency of Sleep Medication Use | −0.40 ± 0.17 | 5.82 | .0170 |
| Frequency of Daytime Naps | −0.23 ± 0.07 | 9.16 | .0029 |
Note. DIMS: insomnia symptoms of difficulty of initiating, maintaining sleep or awakening too early, PSG; Polysomnography, PSQI: Pittsburgh Sleep Quality Index, Bodily Pain – higher scores indicates less pain Note. R-square = 0.305
We found a positive interaction effect between insomnia and total sleep time (PSG) on pain (p = .0463) without adjusting for covariates, but the interaction was not significant when we adjusted for covariates (Figure 1). Table 4 presents the multivariable regression models separately for patients with and without insomnia. For participants with insomnia, longer total sleep time and poorer sleep quality were associated with more bodily pain, and for those without insomnia, more frequent use of sleep medication and daytime naps were associated with more bodily pain.
Figure 1.
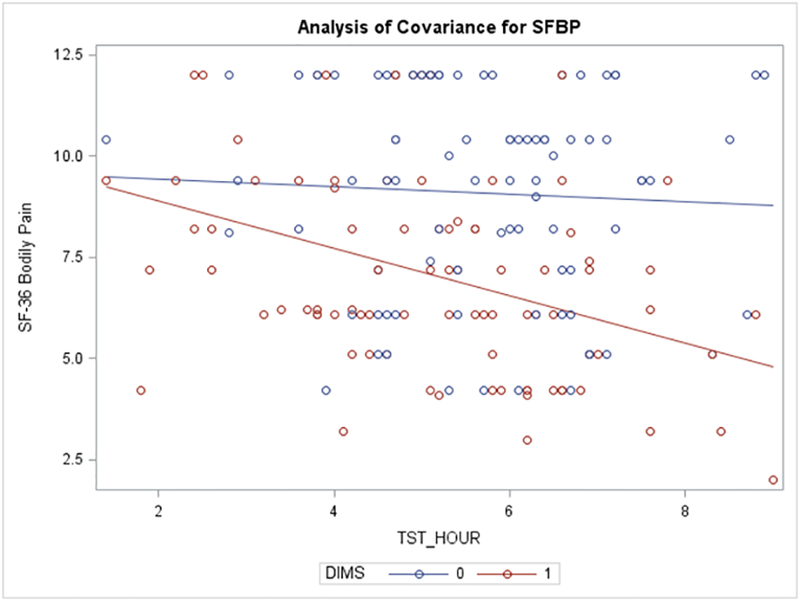
Interaction between Insomnia and Total Sleep Time (PSG) on Bodily Pain In the interaction model of insomnia and TST (PSG) without other covariates, the interaction term is significant (p=.0463). The interaction shows longer TST is associated with more pain in HF patients with insomnia only. This interaction term is not significant after adjusting for other covariates.
Table 4.
Multivariable model of sleep variables explaining bodily pain with stepwise selection in HF patients with and without insomnia
| Insomnia (N=86) | |||
|---|---|---|---|
| Variable | Coefficient ± Std. Err | F-value | P-value |
| Total Sleep Time – PSG (Per 1hr) | −0.52 ± 0.15 | 12.64 | .0006 |
| Sleep Quality – PSQI | −0.78 ± 0.32 | 5.83 | .0180 |
| Frequency of Sleep Medications | −0.22 ± 0.19 | 1.39 | .2416 |
| Frequency of Naps | −0.15 ± 0.10 | 2.06 | .1554 |
| No Insomnia (N=84) | |||
| Variable | Coefficient ± Std. Err | F-value | P-value |
| Total Sleep Time – PSG (Per 1hr) | −0.21 ± 0.19 | 1.20 | .2776 |
| Sleep Quality – PSQI | −0.32 ± 0.41 | 0.62 | .4352 |
| Frequency of Sleep Medications | −0.85 ± 0.33 | 6.65 | .0118 |
| Frequency of Naps | −0.28 ± 0.11 | 6.61 | .0120 |
Note. PSG; Polysomnography, PSQI: Pittsburgh Sleep Quality Index, Bodily Pain – higher score indicates less pain
To further understand the relationships between total sleep time and insomnia, we compared pain, daytime, and nighttime sleep characteristics by insomnia and total sleep time while adjusting for age and comorbidity (see Table 5). In people with insomnia, the participants who slept the longest (322.6 – 392.5 minutes and > 392.5 minutes) had more pain, took more sleep medications, and had more daytime sleepiness compared to those without insomnia. The shortest sleepers (< 267.5 minutes) with insomnia took fewer naps compared to those without insomnia.
Table 5.
Comparisons of pain, daytime, and nighttime sleep characteristics by insomnia and total sleep time after adjusting for age and comorbidity.
| Total Sleep Time – PSG | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| <267.5 min | 267.5 – 322.5 Min | 322.6 – 392.5 Min | > 392.5 Min | |
| N (%) | N (%) | N (%) | N (%) | |
| Insomnia | 30 (34.9%) | 19 (22.1%) | 19 (22.1%) | 18 (20.9%) |
| No Insomnia | 12 (14.3%) | 24 (28.6%) | 23 (27.4%) | 25 (29.8%) |
| Adj. Mean ± Std.Err |
Adj. Mean ± Std.Err |
Adj. Mean ± Std.Err |
Adj. Mean ± Std.Err |
|
| Bodily Pain | ||||
| Insomnia | 7.95 ± 0.45 | 8.18 ± 0.56 | 5.70 ± 0.56 | 5.91 ± 0.58 |
| No Insomnia | 9.01 ± 0.70 | 9.64 ± 0.51 | 8.88 ± 0.51 | 8.64 ± 0.49 |
| P-value | .2031 | .0549 | <.0001 | .0004 |
| Fatigue | ||||
| Insomnia | 32.11 ± 2.67 | 32.77 ± 3.33 | 35.18 ± 3.33 | 39.73 ± 3.26 |
| No Insomnia | 26.22 ± 4.14 | 22.59 ± 3.46 | 22.63 ± 3.02 | 26.50 ± 2.88 |
| P-value | .2345 | .0352 | .0059 | .0028 |
| Sleep Quality | ||||
| Insomnia | 1.77 ± 0.13 | 1.53 ± 0.16 | 2.09 ± 0.16 | 1.93 ± 0.17 |
| No Insomnia | 1.07 ± 0.21 | 0.98 ± 0.15 | 0.90 ± 0.15 | 0.84 ± 0.14 |
| P-value | .0051 | .0147 | <.0001 | <.0001 |
| Sleep Medication | ||||
| Insomnia | 0.98 ± 0.20 | 0.35 ± 0.25 | 0.86 ± 0.25 | 1.38 ± 0.26 |
| No Insomnia | 0.35 ± 0.31 | 0.20 ± 0.23 | 0.17 ± 0.23 | 0.35 ± 0.22 |
| P-value | .0913 | .6636 | .0403 | .0029 |
| Naps | ||||
| Insomnia | 2.34 ± 0.43 | 2.67 ± 0.54 | 3.61 ± 0.54 | 2.36 ± 0.56 |
| No Insomnia | 3.95 ± 0.68 | 2.86 ± 0.50 | 2.27 ± 0.49 | 2.47 ± 0.47 |
| P-value | .0472 | .7971 | .0650 | .8845 |
| Daytime Sleepiness | ||||
| Insomnia | 8.31 ± 0.78 | 8.87 ± 0.97 | 9.80 ± 0.97 | 10.05 ± 1.01 |
| No Insomnia | 8.25 ± 1.22 | 8.54 ± 0.90 | 7.02 ± 0.89 | 6.77 ± 0.85 |
| P-value | .9672 | .8040 | .0357 | .0136 |
| Snooze Time | ||||
| Insomnia | 18.16 ± 3.43 | 18.45 ± 4.37 | 19.81 ± 4.26 | 18.82 ± 4.41 |
| No Insomnia | 27.38 ± 5.60 | 11.73 ± 3.94 | 12.76 ± 3.98 | 19.09 ± 3.81 |
| P-value | .1629 | .2552 | .2266 | .9620 |
Note. Adjusted Mean and standard error were estimated from generalized linear model adjusted for age and comorbidity
Discussion
Pain is associated with nighttime sleep characteristics, including longer self-reported total sleep time and quality, insomnia symptoms, and use of sleep medication. Pain is also associated with the daytime effects of poor sleep, including longer time in the bed in the morning after awakening (snooze time), more frequent naps, fatigue, and higher levels of excessive daytime sleepiness. In the multivariable model, longer total sleep time, insomnia symptoms, use of sleep medication, and daytime naps together explained 30% of the variance in pain.
We found that in people with insomnia there was an association between longer total sleep time and pain. This contrasted with previous studies that found that pain is associated with shorter total sleep time measured with self-report and objective methods32 and that shorter total sleep time33 is a risk factor for developing chronic pain. Our finding that pain was associated with longer total sleep time was surprising because sleep deprivation can increase pain sensitivity and pain causes arousal, which may interfere with the ability to initiate and maintain sleep.14 However, none of these studies specifically included people with HF or insomnia. It is notable that the multivariate analysis revealed that the insomnia group with the longest sleep time had more severe pain scores and also took the most sleep medication. The increased use of sleep medication may partially explain the longer sleep duration. However, due to the limitations of this retrospective study, we could not determine whether they took sleep medication prior to the polysomnography study night. Additional research is needed to explore the relationships between pain and sleep in people with HF to understand the complex interplay between comorbidity, medications, and symptoms in this population, and how people with HF are using sleeping medications in relation to pain.
We found that the relationships between pain and sleep characteristics differed for people with insomnia with different total sleep times. We found that people with insomnia and longer sleep times had more symptoms of pain, fatigue, daytime sleepiness, and poor sleep quality than those without insomnia. This was in contrast to previous research that has found that fatigue and pain are associated with shorter total sleep time in other chronic conditions.34 In this study we did not measure catastrophizing; however, in adults with knee osteoarthritis, pain catastrophizing was highest in people with insomnia. Catastrophizing is maladaptive attention to specific symptoms,35 and may be associated with maladaptive behaviors such as prolonged lying in bed. Future research should measure catastrophizing and catastrophizing behaviors in this population to better understand how they may influence the relationship between total sleep time and symptoms.
The consequences of pain extended beyond the nighttime sleep period to the daytime. We found that pain was associated with the daytime symptoms of daytime sleepiness, fatigue, and increased napping. We also found that in people without insomnia, more pain was associated with taking more naps. These findings are consistent with previous reports that have found that pain is associated with increased napping and daytime sleepiness in older adults.36 Surprisingly, our findings suggest that people with very short sleep experienced few daytime symptoms including pain, fatigue, and daytime sleepiness. Other researchers have found that people with HF have less daytime sleepiness, despite having less total sleep time and reduced sleep efficiency, compared to people who do not have HF.37 This may be a result of excessive hyperarousal associated with increased sympathetic nervous system activity that may contribute to reduced daytime sleepiness in HF37,38 as well as short sleep and insomnia. However, it is also possible that objective measures, such as psychomotor vigilance testing, may be needed to determine the full extent of daytime sleepiness.39 The relationship between pain and hyperarousal is less clear; it has been proposed chronic pain may contribute to cognitive hyperarousal40 and structural changes in the brain, which lead to changes in the arousal system,41 which may lead to increased insomnia. Further research is needed to elucidate the connection between sympathetic activity, symptoms, and insomnia in HF, which may point to novel therapeutic targets for insomnia treatments for people with HF.
Previous pain and sleep research primarily focused on the relationship between total sleep time and pain. However, we also found that pain was associated with daytime sleep including increased naps, and daytime sleepiness, and increased snooze time. These findings are consistent with those in older adults, among whom frequent napping is associated with pain and excessive daytime sleepiness.36 Napping may be adaptive for people with short nocturnal sleep because naps restore pain sensitivity to baseline;18 however, daytime napping was also associated with increased pain in fibromyalgia and may be maladaptive.19 Additional research is needed to understand better the relationship between intentional and unintentional napping and the timing, frequency, and duration of napping to tease out the full implications of napping, insomnia, and pain among people with HF.
This study adds to the growing evidence that sleep and pain are associated with one another in adults with chronic conditions. Given the cross-sectional nature of this study, additional research is needed to explore the longitudinal relationships between pain and sleep in HF. A recent systematic review in the general population found that a decline in sleep quality and quantity were associated with increased risk of developing pain.42 Research is needed to better understand how these symptoms co-vary in people in HF. In addition, pain and sleep disturbance may share common biological pathways and research is needed to better understand the underlying pathways of these two symptoms. Research into inflammation42 hypothalamic-pituitary-adrenal axis dysfunction,42 or functional magnetic resonance imaging (fMRI) to measure brain activity by detecting changes related to pain location or source,43 or neurotrophic factors such as brain-derived neurotrophic factor (BDNF)44,45 are needed to explore possible common biological pathways between pain and daytime and nighttime sleep characteristics. Combining longitudinal research and biological research may indicate which type of sleep or pain interventions may be more effective at treating these co-occurring symptoms in this population to improve physical function and reduce disability in this population.
Our findings have several implications for clinical practice. Clinicians should recognize the potential relationship between pain and poor sleep and for those patients reporting pain, assess for potential co-occurring sleep disturbance and the daytime sleep characteristics related to pain. In this study, pain was also associated with increased comorbidity and New York Heart Association Class in bivariate analysis. Clinicians should be aware that pain and sleep are worse in those with comorbidity and advanced disease. Clinicians should also familiarize themselves with treatment strategies not only for pain but for sleep as well. While medications such as opioids are frequently used to treat pain, opioids may disrupt sleep quantity, quality, and sleep architecture.46 Patients with HF may prefer not to use sleeping medications such as hypnotics, due to these medications’ side effects such as feeling groggy the next day.47 An alternative approach may include the use of behavioral therapies, including cognitive behavioral therapy for insomnia (CBT-I), which combines behavioral interventions with cognitive therapies to adjust maladaptive sleep-related cognitions.48 This approach has shown promise in patients with sleep disturbance and chronic pain, improving sleep, and reducing pain interference and disability.49
This is a large well-described diverse sample with multiple self-report and objective measurements of sleep. However, due to the cross-sectional nature of the study, no conclusion about the casual or temporal relationship between pain and sleep can be drawn, and because we did not assess pain location or source, it may be that our findings may differ based on those characteristics. Additional longitudinal research is needed to better understand the relationship between multiple characteristics of sleep and pain in HF.
In conclusion, pain is associated with subjective sleep quality and daytime sleep characteristics, and the use of sleep medication but not sleep duration or continuity. In addition, the associations between pain and daytime and nighttime sleep characteristics differ based on insomnia symptoms and total sleep time. Our study highlights negative daytime sleep characteristics associated with pain including longer time in the bed in the morning after awakening (snooze time), more frequent naps, fatigue, and higher levels of excessive daytime sleepiness. Research is needed to explore the longitudinal relationship of pain and daytime and nighttime sleep characteristics in HF to ultimately develop and test interventions for these co-occurring symptoms to improve the daytime function of people with HF.
Funding:
R21NR011387, R01NR008022, T32NR008346 (PI Margaret Grey), Shelli Feder was funded by the National Clinician Scholars Program and the Department of Veterans Affairs
Footnotes
Conflicts of interest: none
Contributor Information
Samantha Conley, Yale School of Nursing Orange, CT.
Shelli L. Feder, Yale School of Medicine New Haven, CT.
Sangchoon Jeon, Yale School of Nursing Orange, CT.
Nancy S. Redeker, Yale School of Nursing Orange, CT.
Beatrice Renfield, Yale School of Nursing Orange, CT.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Redeker NS, Stein S. Characteristics of sleep in patients with stable heart failure versus a comparison group. Heart Lung. 2006;35(4):252–261. doi: 10.1016/j.hrtlng.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Redeker NS, Muench U, Zucker MJ, et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33(4):551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redeker NS, Jeon S, Muench U, Campbell D, Walsleben J, Rapoport DM. Insomnia symptoms and daytime function in stable heart failure. Sleep. 2010;33(9):1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien MY, Chen HC. Poor sleep quality is independently associated with physical disability in older adults. J Clin Sleep Med. 2015;11(3):225–232. doi: 10.5664/jcsm.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manocchia M, Keller S, Ware JE. Sleep problems, health-related quality of life, work functioning and health care utilization among the chronically ill. Qual Life Res. 2001;10(4):331–345. [DOI] [PubMed] [Google Scholar]
- 7.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 national sleep foundation sleep in america survey. J Psychosom Res. 2004;56(5):497–502. Accessed 1/3/2019 3:30:50 PM. doi: 10.1016/j.jpsychores.2004.02.010 [doi]. [DOI] [PubMed] [Google Scholar]
- 8.Goebel JR, Doering LV, Shugarman LR, et al. Heart failure: The hidden problem of pain. J Pain Symptom Manage. 2009;38(5):698–707. doi: 10.1016/j.jpainsymman.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evangelista LS, Sackett E, Dracup K. Pain and heart failure: Unrecognized and untreated. Eur J Cardiovasc Nurs. 2009;8(3):169–173. doi: 10.1016/j.ejcnurse.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodlin SJ, Wingate S, Albert NM, et al. Investigating pain in heart failure patients: The pain assessment, incidence, and nature in heart failure (PAIN-HF) study. J Card Fail. 2012;18(10):776–783. doi: 10.1016/j.cardfail.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Pantilat SZ, O’Riordan DL, Rathfon MA, Dracup KA, De Marco T. Etiology of pain and its association with quality of life among patients with heart failure. J Palliat Med. 2016;19(12):1254–1259. doi: 10.1089/jpm.2016.0095. [DOI] [PubMed] [Google Scholar]
- 12.Alemzadeh-Ansari MJ, Ansari-Ramandi MM, Naderi N. Chronic pain in chronic heart failure: A review article. J Tehran Heart Cent. 2017;12(2):49–56. [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards RR, Dworkin RH, Turk DC, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157(9):1851–1871. doi: 10.1097/j.pain.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brostrom A, Stromberg A, Dahlstrom U, Fridlund B. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19(4):234–242. [DOI] [PubMed] [Google Scholar]
- 16.Johansson P, Riegel B, Svensson E, et al. The contribution of heart failure to sleep disturbances and depressive symptoms in older adults. J Geriatr Psychiatry Neurol. 2012;25(3):179–187. doi: 10.1177/0891988712458366. [DOI] [PubMed] [Google Scholar]
- 17.Conley S, Feder S, Redeker NS. The relationship between pain, fatigue, depression and functional performance in stable heart failure. Heart Lung. 2015;44(2):107–112. doi: 10.1016/j.hrtlng.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraut B, Leger D, Medkour T, et al. Napping reverses increased pain sensitivity due to sleep restriction. PLoS One. 2015;10(2):e0117425. doi: 10.1371/journal.pone.0117425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theadom A, Cropley M, Kantermann T. Daytime napping associated with increased symptom severity in fibromyalgia syndrome. BMC Musculoskelet Disord. 2015;16:13–015–0464-y. doi: 10.1186/s12891-015-0464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon S, Redeker NS. Sleep disturbance, daytime symptoms, and functional performance in patients with stable heart failure: A mediation analysis. Nurs Res. 2016;65(4):259–267. doi: 10.1097/NNR.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritschi C, Redeker NS. Contributions of comorbid diabetes to sleep characteristics, daytime symptoms, and physical function among patients with stable heart failure. J Cardiovasc Nurs. 2015;30(5):411–419. doi: 10.1097/JCN.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redeker NS, Adams L, Berkowitz R, et al. Nocturia, sleep and daytime function in stable heart failure. J Card Fail. 2012;18(7):569–575. doi: 10.1016/j.cardfail.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 24.Singh JA, Yang S, Strand V, et al. Validation of pain and patient global scales in chronic gout: Data from two randomised controlled trials. Ann Rheum Dis. 2011;70(7):1277–1281. doi: 10.1136/ard.2010.144022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): III. tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. [DOI] [PubMed] [Google Scholar]
- 26.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. american academy of sleep medicine review paper. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ.The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the sleep heart health study. Sleep. 2001;24(1):96–105. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 30.Belza BL. Multidimensional assessment of fatigue (MAF) scale user guide. Seattle, WA: University of Washington; 1990. [Google Scholar]
- 31.Tack (Belza) B Dimensions and correlates of fatigue in older adults with rheumatoid arthritis. [PhD] San Francisco: School of Nursing, University of California; 1991. [Google Scholar]
- 32.Weingarten JA, Dubrovsky B, Basner RC, Redline S, George L, Lederer DJ. Polysomnographic measurement of sleep duration and bodily pain perception in the sleep heart health study. Sleep. 2016;39(8):1583–1589. doi: 10.5665/sleep.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Generaal E, Vogelzangs N, Penninx BW, Dekker J. Insomnia, sleep duration, depressive symptoms, and the onset of chronic multisite musculoskeletal pain. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw030. [DOI] [PubMed] [Google Scholar]
- 34.Irwin MR, Olmstead R, Carrillo C, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35(4):537–543. doi: 10.5665/sleep.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen EM, Edwards RR, Bingham CO 3rd, et al. Pain and catastrophizing in patients with rheumatoid arthritis: A prospective observational cohort study. J Clin Rheumatol. 2018. doi: 10.1097/RHU.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: Findings from the national sleep foundation ‘2003 sleep in america’ poll. Am J Geriatr Psychiatry. 2007;15(4):344–350. [DOI] [PubMed] [Google Scholar]
- 37.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716–1722. [DOI] [PubMed] [Google Scholar]
- 38.Pak VM, Strouss L, Yaggi HK, Redeker NS, Mohsenin V, Riegel B. Mechanisms of reduced sleepiness symptoms in heart failure and obstructive sleep apnea. J Sleep Res. 2018:e12778. doi: 10.1111/jsr.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riegel B, Moelter ST, Ratcliffe SJ, et al. Excessive daytime sleepiness is associated with poor medication adherence in adults with heart failure. J Card Fail. 2011;17(4):340–348. doi: 10.1016/j.cardfail.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 41.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23(1):1–13. [DOI] [PubMed] [Google Scholar]
- 42.Afolalu EF, Ramlee F, Tang NKY. Effects of sleep changes on pain-related health outcomes in the general population: A systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. 2018;39:82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krause AJ, Prather AA, Wager TD, Lindquist MA, Walker MP. The pain of sleep loss: A brain characterization in humans. J Neurosci. 2019;39(12):2291–2300. doi: 10.1523/JNEUROSCI.2408-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giese M, Unternahrer E, Huttig H, et al. BDNF: An indicator of insomnia? Mol Psychiatry. 2014;19(2):151–152. doi: 10.1038/mp.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55(1):1–10. [DOI] [PubMed] [Google Scholar]
- 46.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3(1):33–36. [PubMed] [Google Scholar]
- 47.Andrews LK, Coviello J, Hurley E, Rose L, Redeker NS. “I’d eat a bucket of nails if you told me it would help me sleep:” Perceptions of insomnia and its treatment in patients with stable heart failure. Heart Lung. 2013;42(5):339–345. doi: 10.1016/j.hrtlng.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redeker NS, Andrews L, Cline J, et al. Cognitive behavioral therapy for insomnia in stable heart failure: Feasibility, acceptability and preliminary efficacy. Sleep. 2012;34:A243. [Google Scholar]
- 49.Finan PH, Buenaver LF, Coryell VT, Smith MT. Cognitive-behavioral therapy for comorbid insomnia and chronic pain. Sleep Med Clin. 2014;9(2):261–274. doi: 10.1016/j.jsmc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


