Abstract
Background
Progression time from islet autoimmunity to clinical type 1 diabetes is highly variable and the extent that genetic factors contribute is unknown.
Methods
In 341 islet autoantibody-positive children with the human leucocyte antigen (HLA) DR3/DR4-DQ8 or the HLA DR4-DQ8/DR4-DQ8 genotype from the prospective TEDDY (The Environmental Determinants of Diabetes in the Young) study, we investigated whether a genetic risk score that had previously been shown to predict islet autoimmunity is also associated with disease progression.
Results
Islet autoantibody-positive children with a genetic risk score in the lowest quartile had a slower progression from single to multiple autoantibodies (p=0.018), from single autoantibodies to diabetes (p=0.004), and by trend from multiple islet autoantibodies to diabetes (p=0.06). In a Cox proportional hazards analysis, faster progression was associated with an increased genetic risk score independently of HLA genotype (HR for progression from multiple autoantibodies to type 1 diabetes, 1.27, 95% CI 1.02 to 1.58 per unit increase), an earlier age of islet autoantibody development (HR, 0.68, 95% CI 0.58 to 0.81 per year increase in age) and female sex (HR, 1.94, 95% CI 1.28 to 2.93).
Conclusions
Genetic risk scores may be used to identify islet autoantibody-positive children with high-risk HLA genotypes who have a slow rate of progression to subsequent stages of autoimmunity and type 1 diabetes.
Keywords: diabetes, diagnostics tests, epidemiology, immunology (including allergy)
Introduction
Type 1 diabetes begins with a preclinical phase which is defined by the presence of islet autoantibodies. This preclinical phase is variable in duration, with onset of clinical diabetes occurring months to decades after the appearance of islet autoantibodies.1 Features of autoimmunity that include autoantibody titre or specificity, age and sex have been used to stratify the rate of progression to type 1 diabetes.2–5 Genes that confer susceptibility to type 1 diabetes, in particular the human leucocyte antigen (HLA) class II genes, usually exert a stronger effect on the development of autoimmunity than on disease progression,6 and although there are reports of genes that influence the progression to clinical diabetes,5 7 8 the extent to which genetic information may be used to stratify the rate of progression to clinical diabetes in islet autoantibody-positive individuals is unknown. Here, we investigated whether a previously established genetic risk score for islet autoimmunity9 is associated with progression to clinical diabetes in the longitudinal TEDDY (The Environmental Determinants of Diabetes in the Young) study.
Research design and methods
TEDDY is an ongoing prospective cohort study that enrolled 8676 children with high-risk type 1 diabetes HLA genotypes between 2004 and 2010 in six clinical research centres located in the USA, Finland, Germany and Sweden.10–12 The families of children with risk of HLA genotypes were invited to participate in the follow-up study in which blood samples were obtained every 3 months for the first 4 years and biannually thereafter for the measurement of islet autoantibodies (glutamic acid decarboxylase antibody, insulinoma antigen-2 antibody and insulin autoantibodies) by radiobinding assays as previously described.13 14 Samples positive for islet autoantibodies were retested at the second reference laboratory for confirmation. The outcome of islet autoantibody positivity was defined as a positive result at both reference laboratories (confirmed) and by the presence of islet autoantibodies (glutamic acid decarboxylase antibody (GADA), insulinoma antigen-2 antibody (IA-2A) or insulin autantibody (IAA)) on two or more consecutive visits (persistent). The date of seroconversion to islet autoimmunity was defined as the date of drawing the first of two consecutive autoantibody-positive samples. The presence of persistent multiple islet autoantibodies was defined as the presence of at least two persistent and confirmed islet autoantibodies. The date of persistent multiple islet autoantibodies was defined as the date of drawing the first sample when the second persistent and confirmed islet autoantibody was detected. Children with positive islet autoantibodies that were due to maternal IgG transmission were not considered to be positive for that autoantibody unless the child had a negative sample before the first positive sample or the autoantibody persisted beyond 18 months of age.13 Type 1 diabetes was diagnosed according to the American Diabetes Association criteria,15 using standardised case report forms for diabetes symptoms, height and weight at diagnosis, and laboratory values such as ketones in urine and blood.
SNPs were genotyped using the Illumina ImmunoChip.16 We previously used ImmunoChip data of 4543 TEDDY participants without a family history of type 1 diabetes and with the HLA DR3/DR4-DQ8 or the HLA DR4-DQ8/DR4-DQ8 genotype to develop a genetic risk score to predict islet autoimmunity development from birth.9 The genetic score of each individual was derived from weighted values given to the HLA DR3/DR4-DQ8 or DR4-DQ8/DR4-DQ8 genotype plus a weighted value assigned to each susceptible allele of HLA class I and non-HLA SNPs (online supplementary table 1) and was applied also in this analysis. Written informed consent was obtained for all study participants from a parent or primary caretaker for genetic screening and to participate in the prospective follow-up.
jmedgenet-2018-105532supp001.docx (15.5KB, docx)
Here, we analysed the data of all 341 children who had developed islet autoantibodies during follow-up and for whom the genetic risk score could be determined. We calculated the Kaplan-Meier curves for progression from (1) any autoantibodies to multiple autoantibodies, (2) any autoantibodies to type 1 diabetes onset and (3) multiple autoantibodies to type 1 diabetes onset in children stratified by quartiles of the genetic risk score (lower quartile: <13.47; upper quartile: >14.88; the two middle quartiles were combined into one group, ie, 13.47–14.88). In order to determine the potential contribution of HLA and non-HLA SNPs and explore potential confounding by other, basically unmodifiable factors, we applied Cox proportional hazards regression analysis for the three progression times with the genetic risk score as the main predictor variable, and with HLA genotype (DR3/DR4-DQ8 or DR4-DQ8/DR4-DQ8), sex, age at onset of the previous event (eg, of any autoantibodies in the model of progression from any autoantibodies to type 1 diabetes) and country of ascertainment as confounder variables. Specifically, the HR of each outcome variable was determined as log(HR) = βGRS × genetic risk score + βHLA × HLA genotype (reference: HLA DR4-DQ8/DR4-DQ8) + βSEX × sex (reference: boys) + βAGE × age at onset (continuous variable) + βCOUNTRY × country (reference: USA). In these models, we used the genetic risk score without inclusion of the HLA class II genotypes so that the contributions of HLA class II genotype and the remainder of the genes in the risk score could be determined separately. All analyses were performed using R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria). Significance was defined by a two-sided significance level of 0.05.
Results
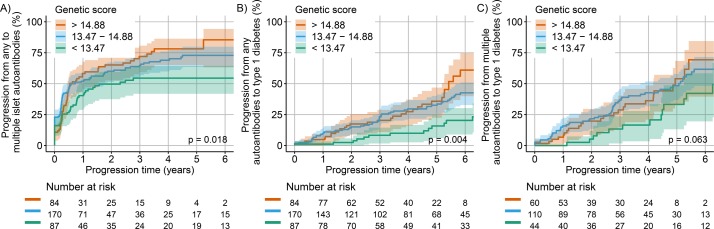
There were 341 children who developed islet autoantibodies at a median age of 2.7 (IQR, 1.5–5.0) years, of whom 141 (41.3%) were female, and 250 had the HLA DR3/DR4-DQ8 and 91 the HLA DR4-DQ8/DR4-DQ8 genotype. The subjects were followed to a median age of 7.9 (IQR, 6.2–9.5) years. During this follow-up period, 214 children (62.8%) developed multiple autoantibodies at a median age of 2.8 (IQR, 1.8–5.1) years, and 107 (31.4 %) of the children developed clinical type 1 diabetes at a median age of 5.0 (IQR, 3.0–7.1) years, with 96 children (28.2%) developing both multiple autoantibodies and type 1 diabetes. The median genetic risk score was 14.23 (IQR, 13.47–14.88) in all children and was higher in the children who developed clinical type 1 diabetes (median, 14.36; IQR, 13.73–15.03) as compared with the children who remained single islet autoantibody-positive at last visit (median, 14.01; IQR, 13.15–14.56; p=0.007 from Mann-Whitney U test). The children with a genetic risk score in the lowest quartile progressed more slowly from single to multiple islet autoantibodies (p=0.018), from single autoantibodies to diabetes (p=0.004), and by trend from multiple islet autoantibodies to type 1 diabetes (p=0.06; figure 1) than the children with genetic risk scores in the upper three quartiles. In a Cox proportional hazards analysis, an increased genetic risk score calculated without HLA genotype and an earlier age of islet autoantibody development were consistently associated with a faster progression to subsequent stages of autoimmunity and type 1 diabetes. Girls progressed faster from multiple autoantibodies to type 1 diabetes than boys. The HLA DR3/4-DQ8 genotype and country of ascertainment were not associated with the rate of progression at any stage after the appearance of islet autoantibodies (table 1).
Figure 1.
Cumulative risks of (A) development of multiple islet autoantibodies after first appearance of any autoantibodies, (B) development of type 1 diabetes after first appearance of any autoantibodies and (C) development of type 1 diabetes after first appearance of multiple autoantibodies, in children with the HLA DR3/DR4-DQ8 or the HLA DR4-DQ8/DR4-DQ8 genotype. P values were calculated using log-rank tests. The groups were defined by quartiles of the genetic risk score (green: lower quartile; blue: two medium quartiles; orange: upper quartile). HLA, human leucocyte antigen.
Table 1.
HRs and 95% CIs of development of multiple islet autoantibodies after first appearance of any autoantibodies, development of type 1 diabetes after first appearance of any autoantibodies and development of type 1 diabetes after first appearance of multiple autoantibodies in children with the HLA DR3/DR4-DQ8 or the HLA DR4-DQ8/DR4-DQ8 genotype as calculated from Cox proportional hazard models
| Progression from any to multiple autoantibodies | Progression from any autoantibodies to type 1 diabetes | Progression from multiple autoantibodies to type 1 diabetes | ||||
| HR (95% CI) | P values | HR (95% CI) | P values | HR (95% CI) | P values | |
| Genetic risk score (per unit increase)* | 1.22 (1.07 to 1.40) | 0.003 | 1.48 (1.21 to 1.80) | 0.0001 | 1.27 (1.02 to 1.58) | 0.03 |
| HLA DR3/DR4-DQ8† | 1.11 (0.81 to 1.51) | 0.52 | 1.49 (0.94 to 2.37) | 0.09 | 1.33 (0.82 to 2.18) | 0.25 |
| Female child | 1.01 (0.77 to 1.34) | 0.92 | 1.35 (0.91 to 1.98) | 0.13 | 1.94 (1.28 to 2.93) | 0.002 |
| Age at onset of previous event (per year)‡ | 0.89 (0.83 to 0.95) | 0.0003 | 0.70 (0.60 to 0.82) | <0.0001 | 0.68 (0.58 to 0.81) | <0.0001 |
| Finland§ | 0.83 (0.59 to 1.18) | 0.31 | 1.10 (0.67 to 1.81) | 0.70 | 0.95 (0.56 to 1.63) | 0.86 |
| Germany | 0.67 (0.31 to 1.46) | 0.32 | 0.40 (0.10 to 1.67) | 0.21 | 0.39 (0.09 to 1.66) | 0.20 |
| Sweden | 0.81 (0.58 to 1.12) | 0.19 | 0.98 (0.61 to 1.57) | 0.92 | 0.99 (0.60 to 1.62) | 0.96 |
*Genetic risk score is calculated without inclusion of HLA class II genotype.
†Reference is HLA DR4-DQ8/DR4-DQ8.
‡Age at onset of the previous event (ie, of any islet autoantibodies in models 1 and 2, and of multiple islet autoantibodies in model 3); the HRs for age are reported as per 1 year increase for the sake of interpretability; however, exact age (ie, not rounded) was used in the regression models.
§Country is coded as dummy variable with USA as reference.
HLA, human leucocyte antigen.
Conclusions
This study suggests that an islet autoimmunity genetic risk score is predictive of the rate of progression to clinical onset of type 1 diabetes in islet autoantibody-positive children with the HLA DR3/DR4-DQ8 or the HLA DR4-DQ8/DR4-DQ8 genotype. Importantly, this risk score was predictive also when weighting for the HLA class II genes was not included, suggesting the impact of genetic variants on progress is independent of either HLA DR3/4-DQ8 or DR4-DQ8/DR4-DQ8 genotypes in these TEDDY participants. Previous studies have indicated that protective HLA genotypes are associated with a slower progression to clinical diabetes in islet autoantibody-positive individuals,17 but there are little or no differences in the progression rate between the high-risk HLA class II genotypes.7 Our findings are consistent with previous reports of associations between the rate of progression from preclinical to clinical type 1 diabetes and individual type 1 diabetes susceptibility genes.5 7 8 Of practical relevance, a low genetic risk score may be used to identify a subset of islet autoantibody-positive children with slower progression to clinical type 1 diabetes, and therefore be an exclusion criterion for some immunotherapy prevention trials. In conclusion, our data indicate the age of islet autoantibody development and a type 1 diabetes genetic risk score may be used to stratify the rate of progression to diabetes in prevention trials.
jmedgenet-2018-105532supp002.docx (20.4KB, docx)
Footnotes
Contributors: AB (guarantor) analysed the data and wrote the first and final draft of the manuscript together with EB and A-GZ. KV, MH, CW, BIF, AKS, WAH, JPK, ÅL, MJR, J-XS, JT, BA and SSR contributed to the interpretation of the results, reviewed the manuscript and contributed to subsequent drafts.
Funding: This study was funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955 and contract no HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and the Centers for Disease Control and Prevention (CDC). This work was supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
Competing interests: A patent has been applied for (LU100334) with the title ‘Method the risk to develop type 1 diabetes’ by Helmholtz Zentrum München Deutsches Forschungszentrum für Gesundheit und Umwelt. EB, A-GZ and CW are among the inventors. The patent includes the genetic score that is examined in the manuscript.
Patient consent: Not required.
Ethics approval: The study was approved by local institutional review boards and is monitored by an external advisory board established by the US National Institutes of Health.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The program code and the data can be provided upon reasonable request.
References
- 1. Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, Eisenbarth GS. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–9. 10.1001/jama.2013.6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steck AK, Vehik K, Bonifacio E, Lernmark A, Ziegler AG, Hagopian WA, She J, Simell O, Akolkar B, Krischer J, Schatz D, Rewers MJ. TEDDY Study Group. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: the environmental determinants of diabetes in the young (TEDDY). Diabetes Care 2015;38:808–13. 10.2337/dc14-2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Köhler M, Beyerlein A, Vehik K, Greven S, Umlauf N, Lernmark Å, Hagopian WA, Rewers M, She JX, Toppari J, Akolkar B, Krischer JP, Bonifacio E, Ziegler AG. TEDDY study group. Joint modeling of longitudinal autoantibody patterns and progression to type 1 diabetes: results from the TEDDY study. Acta Diabetol 2017;54:1009–17. 10.1007/s00592-017-1033-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steck AK, Dong F, Frohnert BI, Waugh K, Hoffman M, Norris JM, Rewers MJ. Predicting progression to diabetes in islet autoantibody positive children. J Autoimmun 2018;90:59–63. 10.1016/j.jaut.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonifacio E, Warncke K, Winkler C, Wallner M, Ziegler AG. Cesarean section and interferon-induced helicase gene polymorphisms combine to increase childhood type 1 diabetes risk. Diabetes 2011;60:3300–6. 10.2337/db11-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity 2010;32:468–78. 10.1016/j.immuni.2010.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krischer JP, Liu X, Lernmark Å, Hagopian WA, Rewers MJ, She JX, Toppari J, Ziegler AG, Akolkar B. TEDDY Study Group. The influence of Type 1 diabetes genetic susceptibility regions, age, sex, and family history on the progression from multiple autoantibodies to Type 1 diabetes: a TEDDY study report. Diabetes 2017;66:3122–9. 10.2337/db17-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pöllänen PM, Lempainen J, Laine AP, Toppari J, Veijola R, Vähäsalo P, Ilonen J, Siljander H, Knip M. Characterisation of rapid progressors to type 1 diabetes among children with HLA-conferred disease susceptibility. Diabetologia 2017;60:1284–93. 10.1007/s00125-017-4258-7 [DOI] [PubMed] [Google Scholar]
- 9. Bonifacio E, Beyerlein A, Hippich M, Winkler C, Vehik K, Weedon MN, Laimighofer M, Hattersley AT, Krumsiek J, Frohnert BI, Steck AK, Hagopian WA, Krischer JP, Lernmark Å, Rewers MJ, She JX, Toppari J, Akolkar B, Oram RA, Rich SS, Ziegler AG. TEDDY Study Group. Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: A prospective study in children. PLoS Med 2018;15:e1002548 10.1371/journal.pmed.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagopian WA, Erlich H, Lernmark A, Rewers M, Ziegler AG, Simell O, Akolkar B, Vogt R, Blair A, Ilonen J, Krischer J, She J. TEDDY Study Group. The environmental determinants of diabetes in the young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011;12:733–43. 10.1111/j.1399-5448.2011.00774.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. TEDDY Study Group. The environmental determinants of diabetes in the young (TEDDY) Study. Ann N Y Acad Sci 2008;1150:1–13. 10.1196/annals.1447.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. TEDDY Study Group. The environmental determinants of diabetes in the young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–98. 10.1111/j.1399-5448.2007.00269.x [DOI] [PubMed] [Google Scholar]
- 13. Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark Å, Hagopian WA, Rewers MJ, She JX, Simell OG, Toppari J, Ziegler AG, Akolkar B, Bonifacio E. TEDDY Study Group. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–7. 10.1007/s00125-015-3514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM, Adler K, Ziegler AG, Mueller PW, Schatz DA, Krischer JP, Steffes MW, Akolkar B. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–7. 10.1210/jc.2010-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care 2014;37:S14–80. 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 16. Törn C, Hadley D, Lee HS, Hagopian W, Lernmark Å, Simell O, Rewers M, Ziegler A, Schatz D, Akolkar B, Onengut-Gumuscu S, Chen WM, Toppari J, Mykkänen J, Ilonen J, Rich SS, She JX, Steck AK, Krischer J; TEDDY Study Group. Role of Type 1 Diabetes-Associated SNPs on risk of autoantibody positivity in the TEDDY Study. Diabetes 2015;64:1818–29. 10.2337/db14-1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pugliese A, Boulware D, Yu L, Babu S, Steck AK, Becker D, Rodriguez H, DiMeglio L, Evans-Molina C, Harrison LC, Schatz D, Palmer JP, Greenbaum C, Eisenbarth GS, Sosenko JM. Type 1 Diabetes TrialNet Study Group. HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 Haplotype protects autoantibody-positive relatives from type 1 diabetes throughout the stages of disease progression. Diabetes 2016;65:1109–19. 10.2337/db15-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jmedgenet-2018-105532supp001.docx (15.5KB, docx)
jmedgenet-2018-105532supp002.docx (20.4KB, docx)