Abstract
Psychosis is linked to aberrant salience or viewing neutral stimuli as self-relevant, suggesting a possible impairment in self-relevance processing. Psychosis is also associated with increased dopamine in the dorsal striatum, especially the anterior caudate (Kegeles et al., 2010). Critically, the anterior caudate is especially connected to (a) the cortical default mode network (DMN), centrally involved in self-relevance processing, and (b) to a lesser extent, the cortical frontoparietal network (FPN; Choi, Yeo, & Buckner, 2012). However, no previous study has directly examined striatal-cortical DMN connectivity in psychosis risk. In Study 1, we examined resting-state functional connectivity in psychosis risk (n = 18) and control (n = 19) groups between (a) striatal DMN and FPN subregions and (b) cortical DMN and FPN. The psychosis risk group exhibited decreased connectivity between striatal subregions with the cortical DMN. In contrast, the psychosis risk group exhibited intact connectivity between striatal subregions with the cortical FPN. Additionally, recent distress was also associated with decreased striatal-DMN connectivity. In Study 2, to determine if decreased striatal-cortical DMN connectivity was specific to psychosis risk or related to recent distress more generally, we examined the relationship between connectivity and distress in individuals diagnosed with a non-psychotic emotional distress disorder (N = 25). In contrast to Study 1, here we found distress was associated with evidence of increased striatal-cortical DMN connectivity. Overall, the current results suggest that decreased striatal-cortical DMN connectivity is associated with psychosis risk and could contribute to aberrant salience.
Keywords: dorsal caudate, corticostriatal loops, attenuated psychotic symptoms, positive schizotypy, temporal lobe
There is a long line of evidence that the striatum plays an important role in psychosis, with psychosis associated with increased dopamine in the striatum (Howes et al., 2012). Increased dopamine in the dorsal striatum, especially in the anterior dorsal caudate (Kegeles et al., 2010), has been found both in people with psychotic disorders (Howes et al., 2012; Jauhar et al., 2017) and in people at clinical high risk for psychosis (e.g., Fusar-Poli et al., 2011). Given that the striatum is the main input layer of the basal ganglia (Haber, 2016), an important aspect of striatal functioning is its connectivity with cortical regions, with most areas of the cortex projecting to the striatum and with striatal projections eventually returning to the cortex (i.e., cortico-striatal-thalamic loops; Haber, 2014; Nelson & Kreitzer, 2014). There is evidence that psychosis and psychosis risk are associated with decreased striatal resting-state connectivity (Dandash et al., 2014; Fornito et al., 2013; Sarpal et al., 2015), with the possibility that increased dopamine and striatal connectivity are related (Horga et al., 2016). Importantly, distinct striatal subregions have been found to be preferentially associated with distinct cortical networks (Choi et al., 2012). Hence, an important way of assessing striatal connectivity is to measure the connectivity of striatal subregions with distinct cortical networks. For the anterior dorsal caudate, it has been found to be especially connected with the cortical default mode network and, to a lesser extent, with the frontoparietal network. However, to our knowledge no previous study has directly examined resting-state functional connectivity between distinct dorsal caudate striatal subregions and these cortical networks in psychosis risk.
Alterations in functional connectivity in individuals with schizophrenia have often been found in the default mode network and the frontoparietal network (Northoff & Duncan, 2016). The default mode network is a group of functionally connected brain regions that has been found to directly support internal mental activity, such as remembering the past, thinking about the future, and imagining alternative scenarios to the present (Menon, 2011). It is also critical for self-relevance and self-referential processing (Raichle, 2015). Furthermore in individuals with schizophrenia, decreased default mode network connectivity with the insula has been found to be associated with attentional deficits, with some researchers positing that misallocation of attention leads to aberrant salience (Sheffield & Barch, 2016). In contrast to the default mode network, the frontoparietal network is thought to play an integral role in executive cognitive processes, such as attention, conflict monitoring, and goal directed behavior (Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). In addition, the frontoparietal network includes a substantial part of the dorsolateral prefrontal cortex, which has long been thought to be important for psychotic disorders (e.g., Barch & Ceaser, 2012; Kerns, Nuechterlein, Braver, & Barch, 2008; Lesh, Niendam, Minzenberg, & Carter, 2011) and has been linked to control of cognition and emotion (e.g., Barbey, Koenigs, & Grafman, 2013; Buhle et al., 2014; Duncan, 2013) and hence could play a role in regulating aberrant salience experiences. It is also thought that striatal dopamine might play an important role in either updating or gating prefrontal cortex representations (e.g., Chatham & Badre, 2015; Cools & D’Esposito, 2011; Frank, Loughry, & O’Reilly, 2001), and hence an updating/gating impairment could contribute to aberrant updating of dorsolateral prefrontal cortex representations, potentially producing aberrant salience.
It is possible that decreased connectivity between the striatum with the default mode network, and perhaps also with the frontoparietal network, could contribute to psychosis risk by contributing to aberrant salience. The aberrant salience hypothesis of psychosis posits that striatal hyperdopaminergia causes inappropriate assignment of salience to external and internal stimuli and leads to the development of hallucinations and delusions (Howes & Nour, 2016; Kapur, 2003). Given the default mode network’s involvement in self-relevance processing (Buckner, Andrews-Hanna, & Schacter, 2008) and the frontoparietal network’s involvement in cognitive control (Duncan, 2013), this suggests that decreased connectivity between the striatum and these cortical networks could contribute to psychosis risk. However, again, it is unclear whether resting-state functional connectivity between dorsal caudate striatal subregions and these cortical networks is impaired in psychosis risk, and research examining connectivity with distinct striatal subregions could aid in detection of psychosis risk or the identification of targets for treatment.
The current research consisted of two studies. Study 1 examined resting-state functional connectivity between distinct subregions of the dorsal caudate with the cortical default mode network and with the cortical frontoparietal network in individuals at risk for psychosis. We hypothesized that there would be decreased resting-state functional connectivity between the striatum and these two cortical networks. Additionally, psychosis risk has been found to be associated with higher levels of emotional distress (e.g., Karcher, Martin, & Kerns, 2015; Kerns, 2006). This suggests the possibility that any abnormalities in resting-state functional connectivity in psychosis risk could be related to emotional distress and not specific to psychosis risk. However, although previous research suggests psychosis risk could be related to decreased connectivity between the striatum and the default mode and frontoparietal cortical networks, in contrast emotional distress disorders have been found to be associated with increased connectivity between the dorsal caudate and regions of the default mode network (Furman, Hamilton, & Gotlib, 2011; Hwang et al., 2016; but see Bluhm et al., 2009) and regions of the frontoparietal network (Kerestes et al., 2015). Hence, Study 2 examined whether decreased resting-state functional connectivity between these striatal subregions and cortical networks was specific to psychosis risk or whether it would also be found in people with a non-psychotic emotional distress disorder. We hypothesized that decreased connectivity between the striatum and these cortical networks would be specific to psychosis risk and that individuals with emotional distress disorders would have increased connectivity between the striatum and these cortical networks.
Study 1
Method
Participants.
Participants were right-handed undergraduate students at the University of Missouri. We followed the approach of Chapman et al. (1994) and used a combined questionnaire psychometric high-risk approach with a semi-structured interview of attenuated psychotic symptoms. Given the results of Chapman et al. and the possibility that it is specifically the combination of both extremely elevated positive schizotypy and attenuated psychotic symptoms together that predict increased risk of psychotic disorder in general population samples, participants in the psychosis risk group had to have both elevated positive schizotypy and attenuated psychotic symptoms (see Table 1 for group descriptives). Consistent with Chapman et al. (1994), we included participants in the psychosis risk group who had longstanding (i.e., more than one year) and stable attenuated psychotic symptoms (i.e., indicative of trait-like vulnerability rather than imminent risk). Participants in the psychosis risk group (n = 19) (a) scored 1.96 sex-normed standard deviations above the mean on the Perceptual Aberration Scale (PerAb; Chapman, Chapman, & Raulin, 1978) or Magical Ideation Scale (MagicId; Eckblad & Chapman, 1983) or scored a combined three sex-normed standard deviations (SDs) above the mean on PerAb and MagicId; and (b) endorsed experiencing attenuated psychotic symptoms on a weekly basis in the past month on the Structured Interview for Prodromal Syndromes Scale of Prodromal Symptoms (SIPS SOPS; Miller et al., 2003; i.e., a SIPS SOPS score ≥ 3, with 3 = “Moderate” symptom severity, on either Unusual Thought Content/Delusional Ideation, Suspiciousness/Persecutory Ideas, or Perceptual Abnormalities/Hallucinations domains; using only these three SIPS domains following Chapman et al., 1994). Previous research has found that people with both elevated positive schizotypy plus attenuated psychotic symptoms have a 14% rate of psychotic disorders at 10-year follow-up (Chapman et al., 1994), with an estimated lifetime risk of psychotic disorders greater than 20% (based on Pedersen et al., 2014), a lifetime risk of psychotic disorder that is at least as high as first-degree relatives of people with a psychotic disorder (Faridi, Pawliuk, King, Joober, & Malla, 2009). One psychosis risk participant was excluded from analyses because of a permanent retainer that produced large imaging artifacts, resulting in a final group of 18 participants. Participants in the psychosis risk group had a mean age of 18.33 years (SD = 0.59) and were 66.67% female, 66.67% Caucasian, 27.78% African-American, and 5.56% Asian-American.
Table 1.
Descriptives and Group Comparisons of Self-Report and Interview Measures
| Psychosis Risk | Control | Group Comparison |
|
|---|---|---|---|
| Positive Schizotypy | Mean (SD) | Mean (SD) | d [95% CI] |
| Perceptual Aberration | 17.06 (6.86) | 4.16 (1.38) | 2.57 [1.67, 3.48]*** |
| Magical Ideation | 20.06 (3.65) | 7.79 (1.62) | 4.38 [3.15, 5.61]*** |
| SIPS SOPS | Scored 3-5 (%) | Scored 3-5 (%) | |
| Unusual Thought Content / Delusional Ideasa | 88.9% | 0.0% | 3.84 [2.71, 4.96]*** |
| Suspiciousness / Persecutory Ideas | 55.6%b | 0.0% | 1.57 [0.81, 2.34]*** |
| Perceptual Abnormalities / Hallucinations | 94.4% | 0.0% | 5.59 [4.11, 7.07]*** |
| Grandiose Ideas | 22.2%b | 0.0% | 0.73 [0.04, 1.42]* |
| Disorganized Communication | 33.3%c | 0.0% | 0.99 [0.28, 1.69]** |
| Distress | Mean (SD) | Mean (SD) | |
| DASS-21 | 58.56 (25.75) | 26.95 (12.60) | 1.55 [0.79, 2.31]*** |
| IPIP Neuroticism | 28.83 (6.99) | 20.21 (6.44) | 1.28 [0.55, 2.01]*** |
Note. SIPS SOPS = Structured Interview for Prodromal Syndromes Scale of Prodromal Symptoms; DASS-21 = Depression Anxiety and Stress Scales-21; IPIP = International Personality Item Pool.
p < .05.
p < .01.
p < .001.
Unusual Thought Content / Delusional Ideas scores reflect both persecutory and non-persecutory ideation.
Domain not assessed due to time constraints for one participant.
Domain not assessed due to time constraints for two participants.
The control group (n = 19) consisted of participants who scored between −0.6 to 0.6 sex-normed SDs around the mean on PerAb and MagicId. Hence, the control group’s mean scores were close to population averages and were not an extreme scoring group. Additionally, control participants did not endorse attenuated psychotic symptoms (i.e., SIPS SOPS score < 3). Participants in the control group had a mean age of 18.37 years (SD = 0.60) and were 73.68% female, 89.47% Caucasian, 5.26% Asian-American, and 5.26% biracial.
There were no significant between-group differences on demographic variables age (χ2 [2, N = 37] = .08, p = .959), effect size V = .05, 95% CI [−.28, .37], sex (χ2 [1, N = 37] = .22, p = .641, V = .08 [−.25, .39]), or ethnicity (χ2 [3, N = 37] = 6.84, p = .077, V = .43 [.12, .66]). Both groups were antipsychotic medication naïve. Groups also did not significantly differ in proportion of psychotropic medication usage, χ2 [1, N = 37] = .78, p = .380, V = .15 [−.18, .45] (in psychosis risk group: n = 5 taking a medication, with n = 4 taking antidepressants, n = 3 taking anxiolytics, n = 3 taking stimulants; in control group: n = 3 taking a medication, n = 2 taking antidepressants, n = 1 taking anxiolytics, n = 1 taking stimulants). Medication use was not significantly correlated with resting-state functional connectivity, and we found a similar pattern of results if we excluded people using medication. Previous striatum-related behavioral (Karcher, Martin, & Kerns, 2015) and EEG (Karcher, Bartholow, Martin, & Kerns, 2017) studies comparing psychosis risk and control groups have found large effect size differences (ds = 1.36 and 1.14, respectively; power to detect these effect sizes in the current study .98 and .92; current study with .80 power to detect effect size = 0.95; power analyses conducted using G*Power 3.1 [Faul, Erdfelder, Buchner, & Lang, 2009]). The samples in the current study were not shared with previous studies (Karcher et al., 2015; 2017).
Materials.
Positive schizotypy measures.
Positive schizotypy, which is thought to reflect phenotypic manifestations of risk for positive psychotic symptoms (e.g., delusions) in schizophrenia-spectrum disorders (Kwapil & Barrantes-Vidal, 2015; Lenzenweger, 2010), was assessed with the PerAb (α = .93 in the current study; Chapman et al., 1978) and MagicId (α = .87; Eckblad & Chapman, 1983) scales. PerAb is a 35-item true/false scale that detects psychotic-like experiences involving distorted perceptions of one’s own body. MagicId is a 30-item true/false scale that detects unconventional beliefs.
Structured Interview for Prodromal Syndromes Scale of Prodromal Symptoms (SIPS SOPS).
Attenuated psychotic symptoms were assessed with the SIPS SOPS (Miller et al., 2003). SIPS SOPS attenuated psychotic symptoms have been used to identify people at clinical high risk for psychosis and have been found to predict future conversion to psychotic disorder (e.g., Cannon et al., 2016). SIPS SOPS items are rated on a 7-point Likert scale (0 = “No Symptoms” to 6 = “Severe and Psychotic”) based on severity of symptoms. In the current study and in line with previous research (Karcher et al., 2015), participants who scored three or higher on either of two core SIPS SOPS domains, Perceptual Abnormalities/Hallucinations and Unusual Thought Content/Delusional Ideation, were considered to have current attenuated psychotic symptoms. All interviews were videotaped and conducted by an advanced graduate student, NRK, who was extensively trained and had considerable experience in SIPS SOPS administration and scoring. NRK was blind to group membership and participants’ questionnaire scores.
Trait and recent distress.
Participants completed negative affect measures to assess whether negative affect was related to resting-state functional connectivity. Trait neuroticism or disposition to experience negative affect was assessed with the neuroticism 10-item subscale of the International Personality Item Pool (IPIP; α = .83; Goldberg, 1999). Participants rated each item on a 5-point Likert scale indicating how well each statement applied to them (1= “Strongly Disagree” to 5 = “Strongly Agree”). The total score for IPIP neuroticism was used in analyses. Recent distress was assessed with the Depression Anxiety Stress Scales (DASS-21; α = .90; Henry & Crawford, 2005), a 21-item self-report scale assessing experiences of depression, anxiety, and emotional stress. Participants rated each item on a 4-point Likert scale based on the severity of their experience over the past week (0 = “Did not apply to me at all” to 3 = “Applied to me very much or most of the time”). As recommended by the scale developers, the total scale score was multiplied by two to make the score comparable to the original DASS.
Procedure.
All questionnaires and computer tasks in Study 1 were administered on computers using E-Prime 2.0 (Psychology Software Tools Inc., 2012). In this study, participants first completed positive schizotypy scales and other questionnaires as well as the SIPS interview assessing attenuated psychotic symptoms in an earlier behavioral session. Eligible psychosis risk and control participants were then invited to complete a brain imaging session (raw data for this study: Hua et al., 2018). During the brain imaging session, in addition to the resting-state scan, participants were scanned while completing behavioral tasks not reported here (e.g., Karcher, Hua, & Kerns, in press). This study was approved by the University of Missouri’s Institutional Review Board.
Image acquisition and preprocessing.
Scanning took place on a Siemens Trio 3T scanner equipped with an 8-channel head coil. During resting-state fMRI scanning, consistent with previous studies and recommendations (e.g., Patriat et al., 2013; Smith et al., 2013; Van Dijk et al., 2010), participants were instructed to relax and keep their eyes open as they viewed a blank screen with a cross-hair fixation point. Throughout the entirety of the resting-state scan, NRK continuously monitored every scan in real-time to ensure that the participants were aware of the resting-state scan instructions and also to ascertain the wellbeing and wakefulness of the participants. High-resolution structural T1 weighted images were acquired for anatomical localization: MPRAGE, repetition time (TR) = 1920 ms, echo time (TE) = 2.92 ms, flip angle = 9˚, field of view (FOV) = 256 × 256, matrix size = 256 × 256, slice thickness = 1 mm. Functional T2* echoplanar weighted images were acquired with 32 contiguous interleaved slices: TR = 2000 ms, TE = 30 ms, flip angle = 90˚, FOV = 256 × 256 mm, matrix size = 64 × 64, slice thickness = 4 mm. Following previous research (Alexander & Brown, 2010; Deichmann, Gottfried, Hutton, & Turner, 2003), scanned acquisition was tilted 30˚ towards the coronal plane from the AC-PC line to improve scanning of the striatum and to minimize imaging artifacts.
Data preprocessing was carried out using SPM8 (Wellcome Trust Centre for Neuroimaging, 2009). Structural and functional images were reoriented to align with the AC-PC line. Images were corrected for slice acquisition timing, and remaining images were realigned to each participant’s mean image. Using the realigned images, these images were then coregistered with the participant’s T1 image in order to better normalize each participant’s data to the MNI template. Each participant’s T1 image was then segmented into cerebrospinal fluid (CSF), white matter, and grey matter. Using the segmentation file that was produced, each participant’s functional data were normalized by warping the data into standard MNI template space. A 6-mm spatial smoothing filter was then applied to the images. To account for artifacts in the BOLD response, nuisance signals (CSF, white matter, and grey matter [i.e., Global Signal Regression; Satterthwaite et al., in press]) and six head-motion parameters (three translation and three rotation) plus their temporal derivatives were entered into the model as covariates and regressed out from each ROI’s time series (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012; Van Dijk, Sabuncu, & Buckner, 2012). Note that time series for CSF, white matter, and grey matter time series were extracted using the MarsBar toolbox for SPM8. A temporal bandpass filter (.01 < f < .08 Hz) was also applied to the data. Images were then scrubbed for motion and intensity, and time points exceeding two standard deviations from the participant’s mean for any motion or intensity parameters were identified and removed (range of time points removed across participants = 4 to 14 of 187 in time series; no group difference in number of time points removed, p = .865). These preprocessed images were used in the rest of the analyses.
Image Analyses.
Regions of interest (ROIs).
All ROIs were taken from Choi et al. (2012) and from Yeo et al. (2011) and consisted of (a) the subregions of the striatum most associated with either the default mode network or the frontoparietal network and (b) the regions of the cortex associated with either the default mode network or the frontoparietal network. These ROIs were initially identified in a sample of n = 500 and were replicated in an additional sample of n = 500 (Choi et al., 2012; Yeo et al., 2011). In the current research, we used bilateral ROIs (results were similar when using unilateral ROIs). To see the exact ROIs that were used in the study, for the striatum see Choi et al. (2012) Figure 7; and for cortical networks see Yeo et al (2011) Figure 11 (note that in both of these figures that default mode network = red; frontoparietal network = orange; for specific striatal subregion color patches, see Supplemental Figure 1; for specific cortical network color patches, see Supplemental Figure 2).
Resting-state functional connectivity ROI analyses.
Resting-state time series were estimated for each participant by sampling and averaging voxel time series for each ROI in MATLAB (The Mathworks Inc., 2010). Time series for each striatal subregion and each cortical network were correlated with each other, and correlations were then z-transformed using Fisher’s r-to-z transformation to allow for comparison between groups. We followed up group differences in striatal-cortical default mode network connectivity by examining striatal connectivity with five distinct cortical default mode subnetworks and their spatially distinct cortical regions (additional details regarding default mode subnetworks/distinct cortical regions and analyses in Supplement).
Striatal seed to whole-brain resting-state functional connectivity analyses.
In addition to examining striatal connectivity with default mode and frontoparietal networks as ROIs, we also used striatal subregions as seeds to examine connectivity in a whole-brain analysis using SPM8. In ROI analyses, the signal across the entire ROI is averaged, thus, it is possible that between-group differences in connectivity of the striatum with particular brain regions within cortical networks might be missed. In the first-level within-subject analysis, contrast images were created for each participant by estimating the regression coefficient between each seed’s time series and all voxels in the brain. In the second-level between-group analysis, psychosis risk and control groups were compared using a two-sample t-test for each of the striatal subregion seeds. Between-group seed to whole-brain functional connectivity maps were thresholded at an uncorrected voxel-wise threshold of p < .001 and at a family-wise error corrected cluster-level threshold of p < .05 and 20 voxels.
Default mode network integrity analyses.
We also examined whether significant group differences in striatal-cortical default mode network connectivity were related to the degree of connectivity within the cortical default mode network itself. To do this, we first divided the cortical default mode network into its distinct subnetworks (Yeo et al., 2011) and then divided each subnetwork into spatially distinct cortical regions. We correlated the time series for each distinct default mode network cortical region with the time series of all other default mode network cortical regions; each correlation was then z-transformed using Fisher’s r-to-z transformation. We then correlated the average of these correlations with striatal-cortical default mode network connectivity.
Resting-state functional connectivity with distress analyses.
To examine whether results were specific to psychosis risk or were related to psychological distress in general, we examined correlations between resting-state functional connectivity with negative affect measures.
Results and Discussion
Group differences in connectivity between striatal subregions and cortical networks.
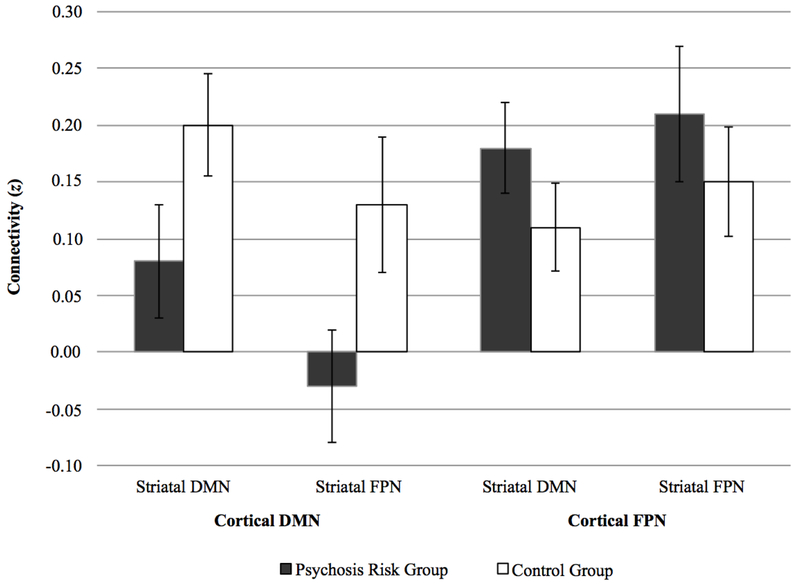
Data were analyzed using a 2 (striatal subregion: striatal default mode network [DMN] vs. striatal frontoparietal network [FPN]) by 2 (cortical network: cortical DMN vs. cortical FPN) by 2 (group: psychosis risk vs. control). Hence, as can be seen in Table 2, there were four striatal-cortical connectivity value dependent variables (i.e., Fischer’s r-to-z transformed correlations): 1) striatal DMN with cortical DMN; 2) striatal DMN with cortical FPN; 3) striatal FPN with cortical DMN; and 4) striatal FPN with cortical FPN.
Table 2.
Striatal-Cortical Connectivity Means (SDs) and 95% Confidence Intervals between Striatal Subregions with Cortical Networks
| Psychosis Risk |
Control |
|||
|---|---|---|---|---|
| Striatal-Cortical Connectivity | M (SD) | 95% CI | M (SD) | 95% CI |
| 1. Striatal DMN with Cortical DMN | 0.08 (0.23) | [−0.04, 0.19] | 0.20 (0.20) | [0.11, 0.30] |
| 2. Striatal FPN with Cortical DMN | −0.03 (0.20) | [−0.13, 0.07] | 0.13 (0.26) | [0.01, 0.26] |
| 3. Striatal DMN with Cortical FPN | 0.18 (0.18) | [0.09, 0.26] | 0.11 (0.17) | [0.03, 0.20] |
| 4. Striatal FPN with Cortical FPN | 0.21 (0.24) | [0.09, 0.33] | 0.15 (0.21) | [0.05, 0.25] |
Note. DMN = Default Mode Network; FPN = Frontoparietal Network.
As expected (Choi et al., 2012), there was a significant Striatal Subregion by Cortical Network interaction, F(1,35) = 20.66, p < .001, d = 1.50, 95% CI [0.74, 2.25], such that, as can be seen in Table 2, resting-state functional connectivity correlations were higher within network (i.e., striatal DMN with cortical DMN; and striatal FPN with cortical FPN) than between networks (i.e., striatal FPN with cortical DMN; and striatal DMN with cortical FPN). However, as can also be seen in Table 2, there was also significant connectivity between each striatal subregion with the other cortical network (with one exception: for psychosis risk, striatal FPN subregion with cortical DMN connectivity). This makes sense as these striatal subregions were identified based on what cortical networks they were most connected with and not on what cortical networks they were exclusively connected with. In addition, we also examined across all subjects Spearman correlations between the four striatal-cortical connectivity values dependent variables (e.g., to what extent striatal DMN with cortical DMN connectivity is correlated with striatal FPN with cortical DMN connectivity), which as can be seen in Table 3 results in six different correlations among the four dependent variables. And, as can be seen in Table 3, overall connectivity between the two striatal subregions with the cortical DMN was very highly correlated; conversely, connectivity between the two striatal subregions with the cortical FPN was also very highly correlated.
Table 3.
Study 1 Zero-Order Spearman Correlations [95% Confidence Intervals] for Connectivity Values between Striatal Subregions with Cortical Networks Across All Subjects
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Striatal-Cortical Connectivity | ||||
| 1. Striatal DMN with Cortical DMN | — | |||
| 2. Striatal FPN with Cortical DMN | .80** [.64, .89] | — | ||
| 3. Striatal DMN with Cortical FPN | .15 [−.18, .45] | .23 [−.10, .52] | — | |
| 4. Striatal FPN with Cortical FPN | .11 [−.22, .42] | .29 [−.03, .56] | .78** [.61, .88] | — |
Note. DMN = Default Mode Network; FPN = Frontoparietal Network.
p < .01.
Most importantly, there was also a significant Group by Cortical Network interaction, F(1,35) = 7.33, p = .010, d = 0.89, 95% CI [0.19, 1.59]. As seen in Table 2 and Figure 1, this interaction reflected that, compared to the control group, the psychosis risk group showed a greater difference in striatal connectivity to the cortical DMN versus striatal connectivity to the cortical FPN. In addition, the psychosis risk group also exhibited decreased resting-state functional connectivity with the cortical DMN than the control group, F(1,35) = 4.30, p = .046, d = 0.68 [0.005, 1.37] (note that this is no longer significant if we use a sequential Bonferroni correction), with this decrease being found for striatal FPN with cortical DMN connectivity, t(35) = 2.14, p = .039, d = 0.70 [0.02, 1.39 (note: striatal DMN with cortical DMN connectivity, t(35) = 1.79, p = .082, d = 0.59 [−0.09, 1.27]). On the other hand, there were no significant between-group differences for correlations between striatal subregions and the cortical FPN, with if anything these correlations being numerically larger in the psychosis risk group, F(1,35) = 0.99, p = .326, d = 0.33 [−0.34, 1.00]; for striatal FPN with cortical FPN, t(35) = −0.81, p = .423, d = −0.27 [−0.94, 0.40; for striatal DMN with cortical FPN, t(35) = −1.08, p = .289, d = −0.36 [−1.02, 0.31]. Note that within the psychosis risk group attenuated psychotic symptoms were not related to striatal connectivity measures (all ps > .562). Hence, overall, the psychosis risk group exhibited decreased connectivity between striatal subregions with the cortical DMN.
Figure 1.
Study 1 resting-state functional connectivity correlations (r-to-z transformed values) between striatal subregions with cortical networks in psychosis risk and control groups. Error bars represent standard errors. DMN = Default Mode Network; FPN = Frontoparietal Network.
Seed to whole-brain resting-state functional connectivity.
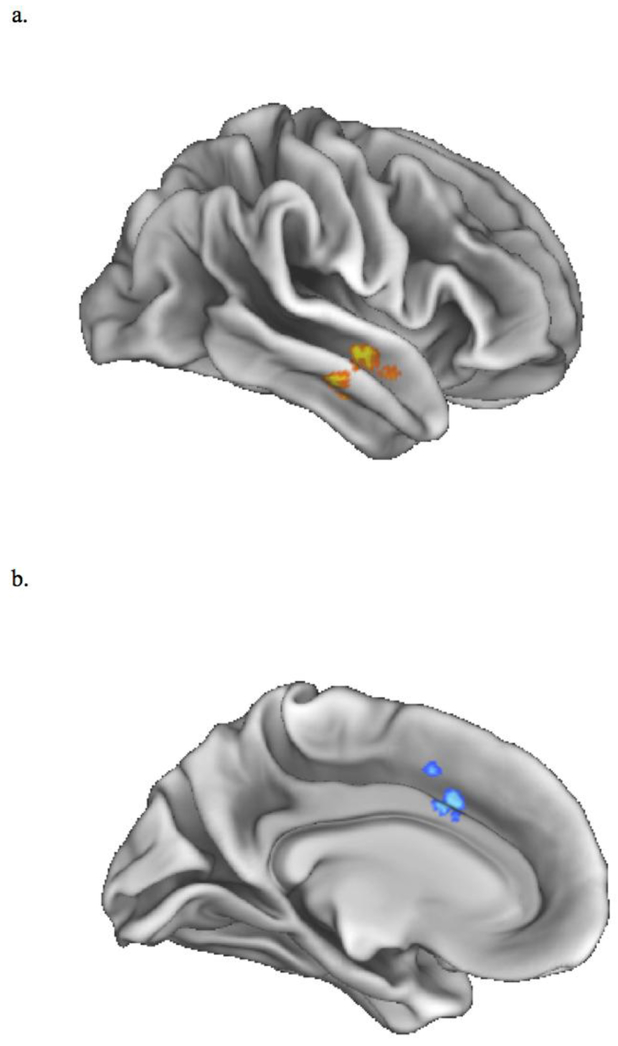
Thus far, we have been reporting results based on cortical network ROIs. Next, we compared the psychosis and control groups using whole-brain analysis to examine connectivity with striatal subregions. As can be seen in Table 4 and Figure 2, the psychosis risk group exhibited significantly decreased connectivity between the striatal FPN subregion and a right anterior middle and superior temporal gyrus region. Note that this right temporal lobe region was located predominantly within DMN Subnetwork 16 (Yeo et al., 2011), a subnetwork consisting of this right temporal lobe region as well as medial prefrontal cortex and posterior cingulate cortex regions. In contrast, the psychosis risk group exhibited greater connectivity than the control group between the striatal FPN and part of the anterior cingulate cortex (ACC), with this part of the ACC within the ventral attention network (Yeo et al., 2011).
Table 4.
Brain Regions Exhibiting Greater Activation in Group Comparisons for Striatal Seeds
| Seed | Cluster-Level | Peak-Level | MNI | |||||
|---|---|---|---|---|---|---|---|---|
| pFWE-corr | kE | T | puncorr | Anatomical Peak Regions | mm | mm | mm | |
| Control Group > Psychosis Risk Group | ||||||||
| Striatal FPN | < .05 | 132 | 5.31 | < .001 | Middle Temporal Gyrus | 54 | −8 | −18 |
| 4.49 | < .001 | Superior Temporal Gyrus | 58 | 0 | −14 | |||
| Psychosis Risk Group > Control Group | ||||||||
| Striatal FPN | < .05 | 156 | −4.91 | < .001 | Cingulate Gyrus | 2 | 16 | 38 |
| −3.57 | < .001 | Paracingulate Gyrus | −6 | 8 | 46 | |||
| −3.53 | < .001 | Cingulate Gyrus | −6 | 16 | 30 | |||
Note. FPN = Frontoparietal Network; FWE-corr = Family-Wise Error corrected; kE = volume of voxels (mm3); uncorr = uncorrected; MNI = Montreal Neurological Institute.
Figure 2.
Whole-brain connectivity results when using the striatal subregion most connected to cortical frontoparietal network as seed: (a) psychosis risk group exhibited decreased connectivity between the striatal subregion and a region in the right middle and superior temporal gyrus; (b) psychosis risk group exhibited greater connectivity between the striatal subregion and a region in the cingulate gyrus
Correlations with DMN integrity.
Given that psychosis risk was associated with decreased connectivity between the striatum and the cortical DMN, we next examined whether this relationship could be related to integrity of the cortical DMN itself. Across participants, we correlated each participant’s striatal-cortical DMN connectivity scores with their within cortical DMN connectivity scores. Overall, these correlations were very close to zero (striatal DMN, rs = −.007, 95% CI [−.33, .32], p = .961; striatal FPN, rs = −.001 [−0.32, 0.32], p = .993). Hence, it did not appear that decreased striatal-cortical DMN connectivity in psychosis risk was related to connectivity within the cortical DMN itself.
Associations between resting-state functional connectivity and distress measures.
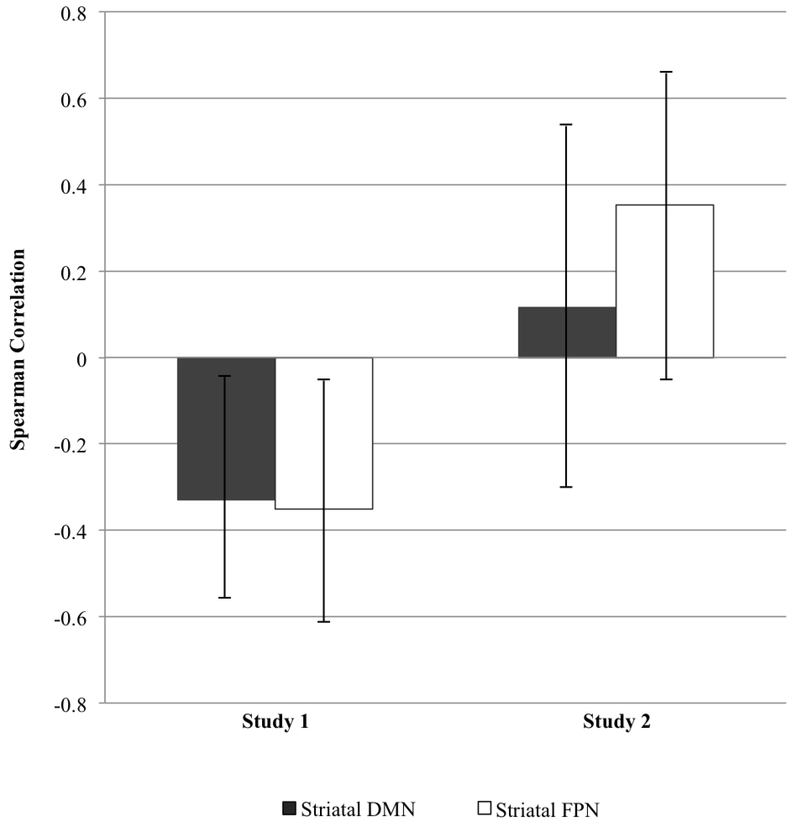
Thus far, we have reported decreased connectivity between striatal subregions with the cortical DMN in psychosis risk. However, as can been seen in Table 1, given that the psychosis risk group reported more recent and trait distress than controls, with between-group effect sizes being large, we next examined whether decreased connectivity between striatal subregions with the cortical DMN was related to measures of distress. There were no significant associations between trait neuroticism with resting-state functional connectivity between striatal subregions with the cortical DMN. However, within the full sample we did find that self-reported recent distress on the DASS-21 (i.e., depression, anxiety, and stress) was associated with decreased connectivity between striatal subregions with the cortical DMN: for striatal DMN, Spearman rs = −.33, 95% CI [−.59, −.007], p = .045; for striatal FPN, rs = −.35 [−.61, −.03], p = .034. When examining groups separately, these associations were not significant: within just the psychosis group, rs = −.21 [−.50, .12] and rs = −.28 [−.55, .05]; within just the control group, these same associations were close to zero and numerically positive, rs = .004 [−.32, 0.33] and .07 [−.26, .39] (see Supplemental Figure 3 for scatterplots of these associations). This suggested the possibility that the decreased connectivity between striatal subregions with the cortical DMN in psychosis risk could be related to recent distress in people at risk for psychosis. We examined this issue further in Study 2.
Study 2
In Study 1, the psychosis risk group exhibited significantly decreased connectivity between striatal subregions with the cortical DMN compared to the control group. However, decreased connectivity was also associated with increased recent emotional distress. This suggests that decreased connectivity in Study 1 might have been related to increased emotional distress and not specifically to psychosis risk. We were able to examine this further in Study 2 in a sample of people with a non-psychotic emotional distress disorder who experienced (a) a comparable average level of emotional distress to the psychosis risk group assessed with the same measure of distress and (b) were scanned using procedures identical to Study 1. If decreased connectivity between striatal subregions and the cortical DMN is specifically related to psychosis risk, then it might be expected that the correlation between connectivity and distress might be different in emotional distress disorder than in psychosis risk. Consistent with the latter, at least one previous study has reported increased striatal-cortical DMN connectivity in emotional distress (Hwang et al., 2016). Hence, it is possible that instead of a negative correlation as in Study 1, in Study 2 with emotional distress disorders we might find that striatal connectivity with the cortical DMN might be, if anything, positively correlated with recent distress.
Method
Participants.
Participants were 29 right-handed women, with recent distress data not completed by four participants, resulting in a final group of 25 participants. Participants were currently receiving mental health treatment and met Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; American Psychiatric Association, 2013) criteria for at least one of the following emotional distress disorders: mood disorder (Major Depressive Disorder, Bipolar I Disorder, or Bipolar II Disorder); one of several anxiety disorders (specifically either Generalized Anxiety Disorder, Post-Traumatic Stress Disorder, or Social Anxiety Disorder); or Borderline Personality Disorder. Participants with a history of psychosis were excluded. Participants were interviewed with the Mini-International Neuropsychiatric Interview (M.I.N.I. 7.0 for DSM-5; Sheehan et al., 1998) and the Structured Interview for DSM-IV Personality (SIDP-IV; Pfohl, Blum, & Zimmerman, 1997) completed by trained graduate students. Note that in contrast to Study 1, the primary goals of Study 2 did not involve the striatum and were related to connectivity between the amygdala and the medial orbitofrontal cortex (main results for this study will be published elsewhere). Thus, given potential heterogeneity across multiple diagnostic groups and evidence that sex moderates the relationship between amygdala-medial orbitofrontal cortex connectivity and emotional distress symptoms (Burghy et al., 2012), to attempt to decrease heterogeneity the study was limited to women. Twenty-three participants were taking psychiatric medication (82.6% anti-depressant, 26.1% mood stabilizer, and 30.4% other anxiety medication). Participants had a mean age of 25.28 years (SD = 6.05) and were 84% Caucasian, 8% African American, 4% Latino/Latina, and 4% Native American.
Materials.
Recent distress.
As in Study 1, recent distress was assessed with the DASS-21 (α = .92). The range of scores on the DASS-21 in the Study 2 sample was comparable to those in Study 1. In Study 2, the emotional distress disorder group had a mean = 53.68, SD = 25.73, 95% CI [43.06, 64.30], range = 14–124. In Study 1, the entire sample had a mean = 42.32, SD = 25.48, [33.83, 50.82], range = 0–110. Specifically, the Study 1 psychosis risk group had a mean = 58.56, SD = 25.75, [45.75, 71.36], range = 26–110; alternatively, the Study 1 control group had a mean = 26.95, SD = 12.60, [20.87, 33.02], range = 0–48 (and if the one control participant who had a score of 0 was dropped, then the next lowest control participant score was equal to 10).
Imaging procedures and analyses.
Protocols for acquiring and analyzing resting-state fMRI data were identical to Study 1.
Procedure.
In Study 2, after an initial interview and questionnaire session, eligible participants were then invited to complete the brain imaging portion of the study. Most participants completed the DASS-21 in the first session before the scanning session; however, due to variability in diagnostic interview duration, eight participants completed the DASS-21 one week after the scanning session (note that there was no significant difference in distress between participants that completed the DASS-21 before versus after the scanning session). This study was approved by the University of Missouri’s Institutional Review Board.
Results and Discussion
Associations between resting-state functional connectivity and negative affect measures.
In this study, we found that increased self-reported recent experiences of distress on the DASS-21 (i.e., depression, anxiety and stress) were positively but nonsignificantly associated with increased connectivity between striatal subregions with the cortical DMN: striatal DMN, rs = .12, p = .580, 95% CI [−.29, .48]; striatal FPN, rs = .35, p = .084, [−.05, .66]. Hence as can be seen in Figure 3, in contrast to Study 1, we found that the associations between recent distress and striatal connectivity with the cortical DMN were quite different in Study 2, with if anything these associations being positive in individuals with non-psychotic emotional distress disorders (whereas they were significantly negative in individuals at risk for psychosis). Using Fisher’s r-to-z transformation, we directly compared striatal connectivity with the cortical DMN for Study 1 and Study 2. The comparison between the two studies was significant for striatal FPN subregion z = 2.68, p = .007 (note: striatal DMN subregion z = 1.68, p = .093). It should be noted that there are systematic differences between the samples in Study 1 and Study 2 (e.g., Study 2 participants included individuals who had graduated from college and included only females; although note that correlations with distress among only females in Study 1 were generally similar to the correlations among the full Study 1 sample).
Figure 3.
Spearman correlations between recent distress with resting-state functional connectivity of striatal subregions and cortical DMN in Study 1 (N = 37) and in Study 2 (N = 25). Bars represent 95% confidence intervals. DMN = Default Mode Network; FPN = Frontoparietal Network.
General Discussion
To our knowledge, the current study is the first to directly examine resting-state functional connectivity between striatal subregions with the cortical default mode and frontoparietal networks in psychosis risk. We found that psychosis risk was associated with decreased striatal connectivity with the default mode network but intact connectivity with the frontoparietal network. Thus, the current results seem generally consistent with the aberrant salience view that decreased connectivity with the default mode network might affect the processing of self-relevance in individuals experiencing trait-like vulnerability for psychosis. As the psychosis risk sample in the current sample was not recruited based on multiple factors that predict imminent risk (e.g., Fusar-Poli et al., 2016), this suggests that impairment in connectivity between the striatum and the cortical default mode network can be best generalized to individuals in the general population who endorse trait-like vulnerability for psychosis.
In the current study, we examined connectivity between two dorsal anterior caudate striatal subregions with the cortical default mode network and the frontoparietal network. Consistent with previous research (Choi et al., 2012), we also found that these striatal subregions were more strongly associated with their own cortical network than with the other cortical network. Nevertheless, we also found that each striatal subregion was also associated with the other cortical network. This makes sense given that these striatal subdivisions were based on which cortical network the subregion was more strongly associated with and was not based on each subregion being exclusively correlated with just one or the other cortical network (Choi et al., 2012). Furthermore, we found that the extent of connectivity between the two striatal subregions with the same cortical network was very strongly correlated.
Using these striatal subregions, the current study provided novel evidence that psychosis risk was associated with altered striatal-cortical connectivity. Compared to the control group, in psychosis risk there was relatively less striatal-default mode network connectivity than striatal-frontoparietal network connectivity. Further, in whole-brain analyses we also found decreased striatal connectivity in psychosis risk specifically with a right middle and superior temporal lobe region that overlaps with a default mode subnetwork (i.e., Subnetwork 16) that is comprised of three regions: medial prefrontal cortex, posterior cingulate cortex, and right temporal lobe (Yeo et al., 2011). Although no other study has directly examined connectivity between striatal subregions and the cortical default mode network, the current results are generally consistent with some previous research finding decreased striatal connectivity with cortical default mode network regions in both individuals at risk for psychosis risk and individuals with schizophrenia (Dandash et al., 2014; Fornito et al., 2013; Sarpal et al., 2015; Wang, Ettinger, Meindl, & Chan, 2018). Furthermore, we found that decreased connectivity between the striatum and cortical default mode network was unrelated to connectivity within the cortical default mode network itself. In fact, previous research has found that, if anything, the cortical default mode network might exhibit increased within-network connectivity in psychosis risk and psychotic disorders (Shim et al., 2010; Whitfield-Gabrieli & Ford, 2012). Hence, the current research provides further evidence that psychosis risk might be associated with altered striatal-cortical connectivity, especially with the cortical default mode network.
Previous research has found that the default mode network is strongly related to processing self-relevant information and autobiographical memory (Nelson et al., 2009). Hence our results suggest that psychosis risk is associated with decreased striatal connectivity with regions centrally involved in self-relevant information processing and autobiographical memory processing. This seems generally consistent with other evidence that this subnetwork is related to decreased insight in people at risk for psychosis, and that psychosis risk is associated with decreased self-concept clarity (Cicero, Becker, Martin, Docherty, & Kerns, 2013) and other indicators of altered self-processing (Nelson et al., 2009; Sass & Parnas, 2003). This also seems generally consistent with the aberrant salience view of psychosis (Kapur, 2003). Potentially, decreased striatal connectivity with the default mode network would detrimentally affect the ability to accurately process self-relevant information and would contribute to aberrant salience.
In the current research, striatal-cortical default mode network connectivity in psychosis risk was also associated with increased recent distress. Thus in Study 2, we examined the association between striatal-cortical default mode network connectivity and recent distress in people with non-psychotic emotional distress disorders. Comparing the two studies, we found that distress was significantly more positively associated with striatal-cortical default mode network connectivity in Study 2 than in Study 1. Our results in Study 2 appear generally consistent with one previous study reporting increased striatal-cortical default mode network connectivity in emotional distress (Hwang et al., 2016; but see Bluhm et al., 2009). Hence, overall, it appears that the association between distress and decreased striatal-cortical default mode network connectivity might be specific to psychosis risk and not shared by people with non-psychotic emotional distress disorders. However, it is possible that within psychosis risk that emotional distress might impact striatal connectivity, with previous evidence finding that stress can contribute to psychosis (Walker, Mittal, & Tessner, 2008) and might increase striatal dopamine (Egerton et al., 2016). This suggests that it may be the case that distress is implicated in decreased striatal connectivity in psychosis risk, with distress having a different effect in psychosis risk than it does in non-psychotic emotional distress disorders. Thus, since associations were in opposite directions and significantly different between Study 1 and Study 2, this might be an interesting question to further examine in future research.
In contrast to finding decreased striatal-cortical default mode network connectivity in psychosis risk, the current research did not find evidence of decreased striatal-cortical frontoparietal network connectivity. However, there is other evidence of decreased integrity within the frontoparietal network itself in psychotic disorders (Baker et al., 2014). Furthermore, there are some findings that appear consistent with decreased striatal-cortical frontoparietal network connectivity in psychosis (e.g., Dandash et al., 2014; Fornito et al., 2013). Given the marked heterogeneity within psychotic disorders (Tandon, Keshavan, & Nasrallah, 2008), a potentially important issue is what aspect of psychotic disorders might be associated with frontoparietal network dysfunction. Our results suggest that the presence of attenuated psychotic symptoms and risk for full-blown positive psychotic symptoms may not be specifically related to decreased connectivity between the striatum and the frontoparietal network. Future research could continue to examine what specific aspects of psychosis risk and psychotic disorders are associated with altered striatal-cortical frontoparietal network connectivity.
Limitations of the study were the small sample size for each group and that participants in Study 1 were undergraduate students. Another possible limitation was that some participants in both Study 1 and 2 were taking psychiatric medication, which could have affected imaging results, although importantly both samples were antipsychotic medication-naïve. However, there was no evidence in Study 1 that medication use was related to striatal functional connectivity or altered the pattern of group differences. Furthermore, Study 1 participants were at risk for a psychiatric disorder, whereas Study 2 participants were all being treated for a psychiatric disorder, and it is possible that distress might be related to striatal connectivity differently in at-risk versus disorder groups. In addition, Study 1 did not formally assess for the presence of comorbid psychiatric diagnoses, and Study 2 did not assess for attenuated psychotic symptoms.
Future research should examine the self-relevance processing correlates of decreased striatal-cortical default mode network connectivity. For instance, potentially decreased connectivity is related to behavioral indices of aberrant salience (Roiser et al., 2009). Another issue for future research is to examine the relationship between level of attenuated psychotic symptoms and striatal connectivity. Note that in the current study within just the psychosis risk group level of attenuated psychotic symptoms was not associated with striatal connectivity. It is possible that this reflects truncated range, as all people in the psychosis risk group had current attenuated psychotic symptoms. However, it also suggests the possibility that striatal connectivity is a marker of psychosis risk and is not specifically related to level of attenuated psychotic symptoms. Thus it might also be relevant to examine whether decreased striatal-cortical default mode network connectivity has some value in psychosis risk detection or prediction, or as a treatment target. In addition, decreased activation in core regions of the salience network have been associated with impaired salience related to delusions and hallucinations (Menon, 2011). There is also evidence that atypical interactions among the salience network with the default mode and frontoparietal networks are associated with auditory hallucinations (Alderson-Day et al., 2016). Hence, future research should further examine the relationship of the salience network in psychosis risk. Furthermore, researchers have varied in whether they measured resting-state connectivity with eyes closed or eyes open. To our knowledge, we are not aware of any study examining the effect of these methodological differences on FPN connectivity. Thus, future research should examine the impact of eyes open versus eyes closed on FPN connectivity.
Overall, the current study found novel evidence of decreased resting-state functional connectivity between the striatum and the cortical default mode network in psychosis risk. This adds to other research implicating the dorsal caudate as a region where decreased connectivity to cortical regions is associated with psychosis (e.g., Horga et al., 2016). The current findings also appear to be generally consistent with the aberrant salience view of psychosis (Kapur, 2003) and with evidence of self-relevance processing disturbances in psychosis risk and psychotic disorders (Nelson et al., 2009).
Supplementary Material
Acknowledgements
We would like to thank Mason H. Price, Jeffrey D. Johnson, and Shawn E. Christ for their coding and technical expertise regarding the neuroimaging analyses. This research was supported by NIMH grant T32 MH014677 (NRK); NIMH grant R21 MH100359 (JGK & TJT); and University of Missouri research funds (JGK).
This work was supported by the National Institute of Mental Health (N.R.K., grant number T32 MH014677), (J.G.K. and T.J.T., grant number R21 MH100359); and University of Missouri research funds (J.G.K).
References
- Alderson-Day B, Dierderen K, Fernyhough C, Ford JM, Horga G, Marguiles DS, … Jardri R (2016). Auditory hallucinations and the brain’s resting-state networks: Findings and methodological obsevations. Schizophenia Bulletin, 42(5), 1110–1123. 10.1093/schbul/sbw078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, & Brown JW (2010). Competition between learned reward and error outcome predictions in anterior cingulate cortex. NeuroImage, 49(4), 3210–3218. 10.1016/j.neuroimage.2009.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington DC. [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BTT, Krienen F, Buckner RL, & Öngür D (2014). Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry, 71(2), 109–118. 10.1001/jamapsychiatry.2013.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, & Grafman J (2013). Dorsolateral prefrontal contributions to human working memory. Cortex, 49(5), 1195–1205. 10.1016/j,cortex.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, & Ceaser A (2012). Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Science, 16(1). 10.1016/j.tics.2011.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Tháberge J, Densmore M, Bartha R, … Osuch E (2009). Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: Decreased connectivity with caudate nucleus. Psychiatry and Clinical Neurosciences, 63(6), 754–761. 10.1111/j.1440-1819.2009.02030.x [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network. Annals of the New York Academy of Sciences, 1124(1), 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Oshsner KN (2014). Cognitive reappriasal of emotion: A meta-analysis of human neuorimaging studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, … Birn RM, (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–1741. https://doi.org/10.1038nn.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, … Kattan MW (2016). An individualized risk calculator for research in prodromal psychosis. American Journal of Psychiatry, 173(10), 980–988. 10.1176/appi.ajp.2016.15070890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, & Zinser M (1994). Putatively psychosis-prone subjects 10 years later. Journal of Abnormal Psychology, 103(2), 171–183. 10.1037/0021-843X.103.2.171 [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, & Raulin ML (1978). Body-image aberration in schizophrenia. Journal of Abnormal Psychology, 87(4), 399–407. 10.1037/0021-843X.87.4.399 [DOI] [PubMed] [Google Scholar]
- Chatham CH, & Badre D (2015). Multiple gates on working memory. Current Opinion in Behavioral Sciences, 1, 23–31. 10.1016/j.cobeha.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, & Buckner RL (2012). The organization of the human striatum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 108(8), 2242–2263. 10.1152/jn.00270.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero DC, Becker TM, Martin EA, Docherty AR, & Kerns JG (2013). The role of aberrant salience and self-concept clarity in psychotic-like experiences. Personality Disorders, 4(1), 33–42. 10.1037/a0027361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SV, Mittal VA, Bernard JA, Ahmadi A, King TZ, & Turner JA (in press). Stronger default mode network connectivity is associated with poorer clinical insight in youth at ultra high-risk for psychotic disorders. Schizophrenia Research. 10.1016/j.schres.2017.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, & D’Esposito M (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry, 69(12), e113–e125. 10.1016/j.biopsych.2011.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O, Fornito A, Lee J, Keefe RSE, Chee MWL, Adcock RA, … Harrison BJ (2014). Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophrenia Bulletin, 40(4), 904–913. 10.1093/schbul/sbt093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, & Turner R (2003). Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage, 19(2), 430–441. [DOI] [PubMed] [Google Scholar]
- Duncan J (2013). The structure of cognition: Attentional episodes in mind and brain. Neuron, 80(1), 35–50. 10.1016/j.neuron.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad M, & Chapman LJ (1983). Magical ideation as an indicator of schizotypy. Journal of Consulting and Clinical Psychology, 51(2), 215–225. 10.1037/0022-006X.51.2.215 [DOI] [PubMed] [Google Scholar]
- Egerton A, Valmaggia LR, Howes OD, Day F, Chaddock CA, Allen P, … McGuire P (2016). Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophrenia Research, 176(2–3), 171–176. 10.1016/j.schres.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi K, Pawliuk N, King S, Joober R, & Malla AK (2009). Prevalence of psychotic and non-psychotic disorders in relatives of patients with a first episode psychosis. Schizophrenia Research, 114(1–3), 57–63. 10.1016/j.schres.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang AG (2009). Statistical power analyses using G*Power 3.1: Tests for correlatoin and regression analyses. Behavior Research Methods, 41(4), 1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, … Bullmore ET (2013). Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry, 70(11), 1143–1151. 10.1001/jamapsychiatry.2013.1976 [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, & O’Reilly RC (2001). Interactions between frontal cortex and basal ganglia in working memory: A computational model. 10.3758/CABN.1.2.137 [DOI] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, & Gotlib IH (2011). Frontostriatal functional connectivity in major depressive disorder. Biology of Mood & Anxiety Disorders, 1(1), 11 10.1186/2045-5380-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Borgwardt S, Woods SW, Addington J, Nelson B, … McGuire PK (2016). Heterogeneity of psychosis risk within individuals at clinical high risk: A meta-analytical stratification. JAMA Psychiatry, 73(2), 113–120. 10.1001/jamapsychiatry.2015.2324 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin M-C, … McGuire P (2011). Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Molecular Psychiatry, 16(1), 67–75. 10.1038/mp.2009.108 [DOI] [PubMed] [Google Scholar]
- Goldberg LR (1999). A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models In Mervielde II, Deary F, Fruyt D, & Ostendorf F (Eds.), Personality Psychology in Europe (pp. 7–28). Tilburg: Tilbur University Press. [Google Scholar]
- Haber SN (2014). The place of dopamine in the cortico-basal ganglia circuit. Neuroscience, 282, 248–257. 10.1016/j.neuroscience.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18(1), 7–21. 10.1007/978-1-4614-6434-1_135-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2005). The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 44(2), 227–239. 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- Horga G, Cassidy CM, Xu X, Moore H, Slifstein M, Van Snellenberg JX, & Abi-Dargham A (2016). Dopamine-related disruption of functional topography of striatal connections in unmedicated patients with schizophrenia. JAMA Psychiatry, 73(8), 862–870. 10.1001/jamapsychiatry.2016.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, & Kapur S (2012). The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of General Psychiatry, 69(8), 776–786. 10.1001/archgenpsychiatry.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, & Nour MM (2016). Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry, 15(1), 3–4. 10.1002/wps.20276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua JPY, Karcher NR, Merrill AM, O’Brien KJ, Straub KT, Trull TJ & Kerns JG (2018, December 21). Data for: Psychosis risk is associated with decreased resting-state functional connectivity between the striatum and the default mode network. Retrieved from osf.io/gs8bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Xin SC, Ou YM, Zhang WY, Liang YL, Chen J, … Kong J (2016). Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. Journal of Psychiatric Research, 76, 111–120. 10.1016/j.jpsychires.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhar S, Nour MM, Veronese M, Rogdaki M, Bonoldi I, Azis M, … Howes OD (2017). A test of the transdiagnostic dopamine hypothesis of psychosis using Positron Emission Tomographic imaging in bipolar affective disorder and schizophrenia. JAMA Psychiatry, 74(12), 1206–1213. 10.1001/jamapsychiatry.2017.2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S (2003). Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry, 160(1), 13–23. 10.1176/appi.ajp.160.1.13 [DOI] [PubMed] [Google Scholar]
- Karcher NR, Hua JPY, & Kerns JG (in press). Probabilistic category learning and striatal functional activation in psychosis risk. Schizophrenia Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher NR, Martin EA, & Kerns JG (2015). Examining associations between psychosis risk, social anhedonia, and performance of striatum-related behavioral tasks. Journal of Abnormal Psychology, 124(3), 507–518. 10.1037/abn0000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher NR, Bartholow BD, Martin EA, & Kerns JG (2017). Associations between electrophysiological evidence of reward and punishment based-learning and psychotic experiences and social anhedonia in at-risk groups. Neuropsychpharmacology, 42, 925–932. 10.1038/npp.2016.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, … Laruelle M (2010). Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archivers of General Psychiatry, 67(3), 231–239. 10.1001/archgenpsychiatry.2010.10 [DOI] [PubMed] [Google Scholar]
- Kerestes R, Harrison BJ, Dandash O, Stephanou K, Whittle S, Pujol J, & Davey CG (2015). Specific functional connectivity alteraions of the dorsal striatum in young people with depression. NeuroImage: Clinical, 7, 266–272. 10.1016/j.nicl.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG (2006). Schizotpy facets, cognitive control, and emotion. Journal of Abnormal Psychology, 115(3), 418–427. 10.1037/0021-843X.115.3.418 [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, & Barch DM (2008). Executive functioning component mechanisms and schizophrenia. Biological Psychiatry, 64(1),26–33. 10.1016/j.biopsych.2008.04.027 [DOI] [PubMed] [Google Scholar]
- Kwapil TR, & Barrantes-Vidal N (2015). Schizotypy: Looking back and moving forward. Schizophrenia Bulletin, 41(Suppl 2), S366–S373. 10.1093/schbul/sbu186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF (2010). Schizotypy and Schizophrenia: The View from Experimental Psychopathology. New York: The Guilford Press. [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, & Carter CS (2011). Cognitive control deficits in schizophrenia: Mechanisms and meaning. Neuropsychopharmacology, 36(1), 316–38. 10.1038/npp.2010.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, … Woods SW (2003). Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin, 29(4), 703–715. 10.1093/oxfordjournals.schbul.a007040 [DOI] [PubMed] [Google Scholar]
- Nelson AB, & Kreitzer AC (2014). Reassessing models of basal ganglia function and dysfunction. Annual Review of Neuroscience, 37(1), 117–135. 10.1146/annurev-neuro-071013-013916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Fornito A, Harrison BJ, Yücel M, Sass LA, Yung AR, … McGorry PD (2009). A disturbed sense of self in the psychosis prodrome: Linking phenomenology and neurobiology. Schizophrenia Bulletin, 33(6), 807–817. 10.1016/j.neubiorev.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Northoff G, & Duncan NW (2016). How do abnormalitis in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Progress in Neurobiology, 145–146, 26–45. 10.1016/j.pneurobio.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Patriat R, Molloy EK, Meier TB, Kirk GR, Nair VA, Meyerand ME, … Birn RM (2013). The effect of resting condition on resting-state fMRI reliability and consistency: A comparison between resting with eyes open, closed, and fixated. Neuroimage, 78, 463–473. 10.1016/j.neuroimage.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, McGrath JJ, … Eaton WW (2014). A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry, 71(5), 573–581. 10.1001/jamapsychiatry.2014.16 [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum NS, & Zimmerman M (1997). Structured interview for DSM-IV personality: SIDP-IV. Washington DC: American Psychiatric Press. [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology Software Tools Inc. (2012). E-Prime 2.0. [Google Scholar]
- Raichle ME (2015). The brain’s default mode network. Annual Review of Neuroscience, 38(1), 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Roiser JP, Stephan KE, den Ouden HEM, Barnes TRE, Friston KJ, & Joyce EM (2009). Do patients with schizophrenia exhibit aberrant salience? Psychological Medicine, 39(2), 199–209. 10.1017/S0033291708003863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, … Malhotra AK (2015). Antipsychotic treatment and functional connectivity of the striatum: A prospective controlled study in first-episode schizophrenia. JAMA Psychiatry, 72(1), 5–13. 10.1001/jamapsychiatry.2014.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass LA, & Parnas J (2003). Schizophrenia, consciousness, and the self. Schizophrenia Bulletin, 29(3), 427–444. 10.1093/oxfordjournals.schbul.a007017 [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Ciric R, Roalf DR, Davatzikos C, Bassett DS, & Wolf DH (in press). Motion artifact in studies of functional connectivity: Characteristics and mitigation strategies. Human Brain Mapping. 10.1002/hbm.23665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl 2), 22–33. [PubMed] [Google Scholar]
- Sheffield JM, & Barch DM (2016). Cognition and resting-state functional connectivity in schizophrenia. Neuroscience and Biobehavioral Reviews, 61, 108–120. 10.1016/j.neubiorev.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim G, Oh JS, Jung WH, Jang JH, Choi C-H, Kim E, … Kwon JS (2010). Altered resting-state connectivity in subjects at ultra-high risk for psychosis: An fMRI study. Behavioral and Brain Functions, 6, 58 10.1186/1744-9081-6-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Beckman CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, … WU-Minn HCP Consortium. (2013). Resting-state fMRI in the Human Connectome Project. Neuroimage, 80, 144–168. 10.1016/j.neuroimage.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, & Schacter DL (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience, 25(1), 74–86. 10.1162/jocn_a_00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Keshavan MS, & Nasrallah HA (2008). Schizophrenia, “Just the Facts”: What we know in 2008 Part 1: Overview. Schizophrenia Research, 100(1–3), 4–19. 10.1016/j.schres.2008.01.022 [DOI] [PubMed] [Google Scholar]
- The Mathworks Inc. (2010). MATLAB. Natick, MA. [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, & Buckner RL (2010). Intrinsice functional connectivity as a tool for human connectomics: Theory, properties, and optimization. Neurophysiology, 103(1), 297–321. 10.1152/jn.00783.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, & Buckner RL (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, & Buckner RL (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342. 10.1152/jh.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Mittal V, & Tessner K (2008). Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual Review of Clinical Psychology, 4(1), 189–216. 10.1146/annurev.clinpsy.4.022007.141248 [DOI] [PubMed] [Google Scholar]
- Wang Y, Ettinger U, Meindl T, & Chan RCK (2018). Association of schizotypy with striatocortical functional connectivity and its asymmetry in healthy adults. Human Brain Mapping, 39(1), 288–299. 10.1002/hbm.23842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust Centre for Neuroimaging. (2009). SPM8. [Google Scholar]
- Whitfield-Gabrieli S, & Ford JM (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8(1), 49–76. 10.1146/annurev-clinpsy-032511-143049 [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.