Abstract
Association of plasma membrane BKCa channel with auxiliary BK-β (1–4) subunits profoundly affects regulatory mechanisms and physiological processes in which this channel participate. However, functional association of mitochondrial BK (mitoBKCa) with regulatory subunits is unknown. We report that mitoBKCa functionally associates with its regulatory subunit BK-β1 in adult rodent cardiomyocytes. Cardiac mitoBKCa is a calcium and voltage activated channel, sensitive to paxilline with a large conductance for K+ of 300 pS. Additionally, mitoBKCa displays a high open probability (Po) and voltage half of activation (V1/2 = −55 mV, n=7) that resembles that of plasma membrane BKCa when associated with its regulatory BK-β1 subunit. Immunochemistry assays demonstrated an interaction between mitochondrial BKCa-α and its BK-β1 subunit. Mitochondria from the BK-β1 KO mice showed sparse mitoBKCa currents (5 patches with mitoBKCa activity out of 28 total patches from n=5 different hearts), displaying a depolarized V1/2 activation (+47 mV in 12 µM matrix Ca2+). The reduced activity of mitoBKCa was accompanied with a high expression of BKCa transcript in the BK-β1 KO, suggesting less abundance of mitoBKCa channels in this genotype. Accordingly, BK-β1subunit increased two-fold the localization of BKDEC (the splice variant of BKCa that specifically targets mitochondria) into mitochondria. Importantly, both paxilline treated and BK-β1 KO mitochondria displayed a more rapid Ca2+ overload, featuring an early opening of the mitochondrial transition pore (mPTP). We provide strong evidence that mitoBKCa associates with its regulatory BK-β1 subunit in cardiac mitochondria, ensuring proper targeting and activation of the mitoBKCa channel helps to maintain mitochondrial Ca2+ homeostasis.
Keywords: cardiomyocytes, mitochondria, potassium channel, Paxilline, mitochondrial calcium retention capacity
Introduction
Large conductance for K+, voltage and Ca2+-activated channels (Slo1, MaxiK, BKCa) are expressed at the plasma membrane of a wide variety of cell types and tissues (Latorre & Brauchi, 2006; Salkoff et al., 2006; Cui et al., 2009a; Berkefeld et al., 2010; Latorre et al., 2010; Lee & Cui, 2010a; Contreras et al., 2013; Hoshi et al., 2013). BKCa activity governs cellular excitability, Ca2+ homeostasis, signaling cascades and neurotransmitter release (Lancaster & Nicoll, 1987; Meredith et al., 2006; Cui et al., 2009b; Lee & Cui, 2010b). In the cardiovascular system, the activity of BKCa channels regulates vascular tone and blood pressure (Brayden & Nelson, 1992; Ledoux et al., 2006). In adult cardiomyocytes, BKCa channels are targeted almost exclusively to the inner mitochondrial membrane (Singh et al., 2013; Balderas et al., 2015) where their activation exerts a cardioprotective effect (Xu et al., 2002; Sato et al., 2005; Singh et al., 2013; Soltysinska et al., 2014). While the biophysical properties of plasma membrane BKCa (i.e. voltage dependence, Ca2+-sensitivity and gating) depend on their interactions with auxiliary β and γ subunits (Latorre et al., 2017), the expression of the auxiliary BK-β1 subunit in cardiac mitochondria has been demonstrated by two independent groups (Ohya et al., 2005; Bautista et al., 2009). However, the functional association of BKCa channels with its regulatory BK-β1 subunits in cardiac mitochondria has yet to be determined.
An initial characterization of mitoBKCa channels by the O’Rourke laboratory revealed a K+ conductance of ~300 pS in cardiac mitoplast-attached patches; this channel was also found to be sensitive to changes in mitochondrial Ca2+ and inhibited by Charybdotoxin (CTx) when applied into the patch pipette (Xu et al., 2002). These results, together with Siemen’s pioneering work in a glioma cell line LN229, established that mitoBKCa shares key properties, such as large conductance for K+ and sensitivity to voltage and Ca2+ (Siemen et al., 1999), with the plasma membrane BKCa (Barrett et al., 1981; Latorre et al., 1982; Latorre et al., 1989). The conductance of mitoBKCa varies among different tissues and cell types, however several laboratories have reported a conductance between 200 and 550 pS for mitoBKCa as recently reviewed by our group (Singh et al., 2012; Balderas et al., 2015). A conductance of 145 pS for cardiac mitoBKCa, slightly smaller to the commonly reported conductance for mitoBKCa and the one reported in the present work was recently reported (Frankenreiter et al., 2017) (see discussion). Current findings indicate rodent cardiac mitoBKCa is a splice variant of plasma membrane BKCa (Kcnma1) containing an extra 50 amino acids at the end of the C-terminus (the “DEC” sequence) essential to target BKCa to the mitochondria in adult cardiomyocytes (Singh et al., 2013). Hearts exposed to BKCa openers NS1619 (Xu et al., 2002; Stowe et al., 2006; Singh et al., 2013), NS11021 (Bentzen et al., 2010), and Naringenin (Testai et al., 2013) reduced infarct size in ischemia-reperfusion assays, whereas mice hearts lacking BKCa (Kcnma1−/−) were not protected (Singh et al., 2013; Soltysinska et al., 2014; Frankenreiter et al., 2017). The cardioprotective effect of opening mitoBKCa has been linked to the capacity of mitochondria to tolerate Ca2+ loading (Sato et al., 2005; Stowe et al., 2006; Singh et al., 2013).
Despite of the growing evidence for a role of mitoBKCa channels in mitochondrial and heart physiology, their voltage-dependence and association with regulatory BK-β subunits are unknown. We identify a population of mitoBKCa channels in adult rodent cardiomyocytes with high voltage sensitivity. Molecular and immunochemistry analyses indicate that such a characteristic arises from the association of mitoBKCa with its regulatory β1 subunit. In addition a second population of mitoBKCa channels with a more depolarized voltage dependency of activation (V1/2= +40 mV at 12 µM matrix Ca2+, n=7) was identified, suggesting a different subunit composition.
Materials and Methods
Ethical approval
The animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC). At UCLA, animal care and use program is guided by federal and state laws, regulations, and guidelines, and by additional institutional policies implemented to systematically address federal requirements and as the result of a voluntary accreditation process. The Public Health Service Policy on Humane Care and Use of Animals (PHS Policy) and USDA Animal Welfare Regulations (AWRs) provide the primary regulatory basis for the existence and function of the IACUC.
The authors understand, and the work conforms to, the principles and regulations of The Journal of Physiology (Grundy, 2015).
Animals.
Sprague-Dawley male rats (3 months old), and three-month-old wild type (C57BL/6NCrL, Charles River laboratories) and β1 KO (β1−/−) male mice were raised in the animal facilities at UCLA. Animals were housed in a 12h light-dark cycle with ad libitum access to food and water. Animals were euthanized by an overdose of pentobarbital sodium.
The BK-β1 KO (kcnmb1−/−) line was generated in the laboratory as described in a previous publication (Li et al., 2013).
Antibodies.
Polyclonal antibody against BKCa (Rabbit Anti-KCNMA1 Cat. APC-021 and APC-151), and polyclonal antibody for BK-β1 subunit (Rabbit Anti-KCNMB1, Cat. APC-036) were from Alomone labs. Monoclonal antibody against GRIM19 (Mouse Ab110240) was from Abcam. Polyclonal antibody against SERCA (Rabbit, Anti-ATP2A2/SERCA2 cat. 4388) was from Cell Signaling. Alexa Fluor 555® anti-goat and anti-rabbit (A21428), and anti-donkey and anti-mouse (A28180) were from Invitrogen. Anti-HA monoclonal antibody (Ab. H3663), and Anti-Flag polyclonal antibody (Ab. F7425) were from Sigma.
Isolation of left ventricle cardiomyocytes (LVC)
Adult male Sprague Dawley rats (3 months old) were injected intraperitonealy (i. p.) with heparin (200 IU/kg). After 20 min, animals were euthanized with single dose of pentobarbital sodium (60 mg/kg) (i. p.) followed by cervical dislocation. The heart was surgically removed and arrested immediately on ice-cold PBS solution (in mM: KCl 2, KH2PO4 1.5, NaCl 138, and Na2HPO4 8.1), the surrounding vasculature tissue was removed followed by quick aortic cannulation on a modified Langendorff apparatus. Hearts were perfused for 5 min with Tyrode’s solution (in mM: NaCl 130, KCl 5.4, MgCl2 1, Na2HPO4 0.6, glucose 10, taurine 5, 2, 3-butanedione monoxime 10, and HEPES 10, pH 7.4, oxygenated with 95% (v/v) O2, 5% (v/v) CO2, 37°C) at a constant pressure of 80 cm H2O. Once all the internal blood was rinsed out, the hearts were digested by enzyme perfusion with Tyrode’s solution supplemented with 372 U/ml Collagenase Type-2 and 1.0 U/ml Protease Type-XIV. After 15–20 min, the enzymatic solution was substituted with oxygenated Krebs buffer (KB) (in mM: KCl 25, KH2PO4 10, MgSO4 2, Glucose 20, Taurine 20, Creatinine 5, K-Glutamate 100, Aspartic acid 10, EGTA 0.5, HEPES 5, and 1% (w/v) BSA, pH 7.2). After 10 min hearts were dismounted and washed on ice cold KB solution to remove the right ventricle and the vascular tissue. The left ventricle was manually dissociated and cardiomyocytes were released into the KB solution. Isolated cardiomyocytes were filtered through a 100 µm mesh and centrifuged at 1000 xg for 60 s in KB buffer.
All the experiments were performed according to NIH guidelines and received ethical approval from the UCLA Office of Animal Research Oversight (OARO).
Labeling cardiomyocytes with Mitotracker red
Freshly dissociated cardiomyocytes were incubated with 200 nM Mitotracker red (Invitrogen) for 15 min at 37°C. The excess of Mitotracker red was removed with KB buffer.
Isolation of mitochondria from LVC
Cardiomyocytes were suspended in isolation buffer “A” (in mM: 230 Mannitol, 70 sucrose, 10 HEPES, 2 EDTA, pH 7.2 supplemented with 1 mg/ml fatty acid-free BSA), loaded into a cold glass potter and gently homogenized on ice (up to 10 strokes). The homogenate was recovered and centrifuged at low speed (1,300 xg 4°C) for 5 min. The supernatant was collected and centrifuged at high speed (10,000 xg 4°C) for 10 min. Isolated mitochondria were maintained on ice.
Mitoplast preparation
Isolated mitochondria were resuspended in 2 ml hypotonic buffer “B” (in mM: 5 HEPES, 2 EDTA, pH 7.2) and shaken on ice for 12 min. After this time, samples were centrifuged for 5 min at 12,500 rpm 4°C. The pellet (mitoplasts: mitochondrial internal membrane stripped of outer membrane, was dissolved in isotonic buffer “C” (in mM: 150 KCl, 5 HEPES, pH 7.2.) and stored at 4°C for further usage.
Electrophysiology and analysis
Mitoplasts were placed into a recording chamber filled with bath solution (in mM: 140 KMeSO3, 10 KCl, 5 HEPES, pH 7.4. Only those mitoplasts that showed mitotracker red signal were used for patch-clamp. The concentration of free Ca2+ was calculated with MaxChelator (Stanford University), [Ca2+] was controlled with EGTA or HEDTA and measured with a calcium electrode (World Precision Instruments). Borosilicate glass microelectrodes (~25 MΩ) filled with pipette solution (in mM: 140 KMeSO3, 10 KCl, 5 HEPES, pH 7.4) were approached to the mitoplast until a high resistance seal (>5 GΩ) was formed. Recordings were performed within ~48 h after preparation of mitoplasts using the inside-out patch configuration. The membrane potential was clamped with an Axopatch 200A amplifier (Molecular Devices, Sunny Valley CA) controlled with a custom made software (G-patch). Single channel recordings were acquired at 5 kHz, filtered at 1 kHz and digitized through an analog/digital interface connected to a PC. Data analysis was performed with QuB software (SUNY Buffalo). NPo was determined from total point amplitude histograms by measuring the fraction of time (Pk) spent at each open level (k) using a half amplitude criteria and summing the contributions of each channel Data points of NPo were normalized to the NPo max at +80 mV in 12 µM Ca2+ and plotted as a function of voltage (V). The half activation potential (V1/2) was estimated by fitting NPo data with a Boltzmann equation
where V is the command pulse (mV), V1/2 is the half voltage of activation and z the slope value, k the Boltzman constant, T the absolute temperature. Bars represent SEM with a minimum of 3 different experiments and p values were obtained by performing two tailed Student’s t-test.
Co-immunoprecipitation
Isolated cardiac mitochondria were harvested and solubilized with ice-cold RIPA buffer supplemented with protease and phosphatase inhibitors (4,000 and 6,000 µg/ml, respectively) (100x, Cell Signaling cat # 5872). Protein G magnetic beads (100 µl, SureBeads™, Biorad) were washed and saturated with specific polyclonal antibody (4 µg BKCa Ab-Alomone, APC-021) and pre-incubated with 500 µl (2 mg protein) of precleared lysate for 1 h at room temperature. Beads were washed 3 times with PBS-T (PBS-0.1% Tween 20) and eluted in 40 µl of 1x Laemmli buffer (Biorad) by incubating for 10 minutes at 70°C. Total and mitochondrial fraction lysates, and immunoprecipitated proteins were analyzed on a 4–20% SDS-polyacrylamide gel and transferred onto 0.2 µm PVDF membrane. Western blots were probed with primary antibodies anti-BKCa (Alomone APC-151, 1:100), and anti-BK-β1 subunit (Alomone APC-036, 1:200). For control blots the membranes were striped and re-blotted with anti-SERCA (Cell Signaling 4388, 1:1000) and anti-GRIM19 (Abcam ab110240, 1:1000), and revealed by immunofluorescence using either goat anti-rabbit Alexa Fluo 555® (Invitrogen A21426, 1:5,000) or donkey anti-mouse AlexaFluo 555® (Invitrogen, 1:10,000) and a Chemidoc system (Biorad). Signal of the BK-β1 subunit in the whole lysate was detected with primary antibody against BK-β1 (APC-036, Alomone) 1:200, at 4°C on a rocker. Secondary antibody: goat anti rabbit-HRP (Jackson, 111-035-144) 1:10000, incubated for 1 h at room temperature on a rocker. Detection was performed with chemiluminescent (ECL) substrate Super Signal™ West Femto Maximum Sensitivity (Thermo, 34095), and imaged with Chemidoc system (Biorad).
Real-time quantitative PCR
Total RNA was purified from isolated adult mice (C57BL/6NCrL) cardiomyocytes lysed with TRIzol (Ambion, 15596018). RNA samples were digested for 10 min at room temperature with RNase-free DNase and cleaned-up with RNeasy mini kit (Qiagen). cDNA was synthesized using 0.5 μg of cleaned RNA following the provided instructions of the iScript cDNA Synthesis Kit (Bio-Rad, 170–8891). qPCR was performed using SsoFast EvaGreen Supermix (Bio-Rad, 172–5201) on a BioRad C1000 thermocycle. Primers used are detailed in Table S1. PCR was performed as follows: 98°C for 2min; [98°C for 5 sec; 60°C 5 sec] 60 cycles; for real time Melting curve [65°C for 5 sec increasing by half a degree each cycle until 95°C]. As control we used primers of glyceraldehyde 3-phosphate dehydrogenase (GAPDH, see Table 1). All samples are from duplicates and were normalized using the comparative Delta Delta Ct (ΔΔCt) method.
Table 1.
Quantitative PCR primers
| Accession number | Primers | Sequence |
|---|---|---|
| MaxiK U40603 (alpha subunit) | Forward Reverse |
5’ -TACTTCAATGACAATATCCTCACCCT-3’ 5’ -ACCATAACAACCACCATCCCCTAAG-3’ |
| MaxiK AF020712 (β1 subunit) | Forward Reverse |
5’ -GTATCACACAGAAGACACTCGGGA-3’ 5’ -AAGAAGGAGAAGAGGAGGATTTGGG-3’ |
| GAPDH | Forward Reverse |
5’-AGGTCGGTGTGAACGGATTTG-3’ 5’-TGTAGACCATGTAGTTGAGGTCA-3’ |
Transient transfection of HeLa cells
HeLa cells were seeded on 25 mm circle coverslips (0.13 mm thick, Fisher Scientific, Pittsburg, PA) coated with 0.1% (v/v) laminin (Sigma) in PBS and cultured in DMEM medium supplemented with 10% (v/v) FBS and antibiotics. When a confluence of >60% was reached cells were transfected. Transfection was performed with Lipofectamine 2000 (Invitrogen) following the protocol provided by the manufacturer. Briefly, cells incubated in presence of cDNA-Lipofectamine 2000 complexes (in 1:3 ratios) in Opti-MEM media (Invitrogen). One group of cells was transfected with the clone of full length human α subunit of BK-DEC channel alone, this construct includes the splice insert DEC sequence (EKKWFTDEPDNAYPRNIQIKPMSTHMANQINQYKSTSSLIPPIREVEDEC) at the C-terminus. A second group of cells was co-transfected with both HA tagged-BKCa DEC and Flag-human β1 subunit in a 1:3 cDNA ratio. Cells transfected with empty vector were used as negative control (MOCK). Transfection was stopped after 6 h. Cells were incubated for 72 h at 37°C.
Immunolabeling
After 72 h of transfection, slides were loaded with 200 nM Mitotracker red CMXRos incubated for 15 min at 37°C followed by three washes with PBS. Cells were fixed with 4% paraformaldehyde (15 min at room temperature) followed by three washes with PBS. Samples were permeabilized with 0.5% (v/v) Triton-X 100 in PBS (10 min at RT). Nonspecific binding of the antibodies was prevented by incubation of cells in 5% (v/v) Normal Goat Serum (NGS, Jackson Immuno Research) in 2% BSA (Sigma) diluted in PBS 0.5% (v/v) Triton-X 100 for 30 min at room temperature. After this time, cells were incubated overnight at 4°C with specific primary antibodies AntiHA mAb, AntiFlag pAb diluted in PBS containing 0.5% Triton-X 100, 0.5% NGS, and 0.2% BSA. After washing the cells three times with PBS 0.5% Triton X-100 the slides were incubated for 60 min at RT with conjugated secondary antibodies Atto-647N (1 µg/ml anti mouse) and Alexa-488 (1 µg/ml) diluted in PBS containing 0.5% Triton-X 100, 0.5% NGS, and 0.2% BSA. Slides were mounted with ProLong Gold (Invitrogen). Confocal images (Fig. 5) were acquired with a Nikon Confocal microscope using a 60X oil immersion objective with 1.42 NA (Plan Apo) at scanning resolution of 0.27 µm/pixel. Sequential confocal images of HeLa cells were acquired from the same field. Images were median filtered (median intensities of 32 X 32-pixel squares centered at the target pixel were subtracted from the target pixel) and analyzed using a custom built software to determine the Cross correlation index (Cci) (Li et al., 2010; Wu et al., 2010).
Figure 5. Paxilline reversibly blocks mouse cardiac mitoBKCa channel.

Single channel current in absence (A) and in presence (B) of paxilline at the indicated voltage and matrix Ca2+. C) Current amplitude histogram of the 6 pA channel recorded in A, paxilline reduced the Po from 0.7±0.1 to 0.2±0.09 (n=3) (D). E) Single channel current recorded after the patch was perfused again with 12 µM matrix Ca2+. F) Current amplitude histogram that shows the reappearance of the 6 pA current (Po=0.4) after washing out paxilline.
Mitochondrial Calcium Retention Capacity (mCRC)
Freshly isolated mice cardiac mitochondria was gently dissolved in ice-cold buffer “C” (in mM, Sucrose 150, KCl 50, KH2PO4 2, Succinic Acid 5, Tris 20, pH 7.4 HCl). Basal fluorescence was measured in buffer “C” without mitochondria using a Fluorolog-3 (HORIBA LTD). Calcium green-5N (500nm excitation, 530nm emission) reports the Ca2+ content of the extra mitochondrial media. When mitochondria were added to the cuvette a sudden drop in fluorescence was recorded due to a lower transmittance of the solution and mitochondrial Ca2+ uptake. The signal stabilized in ~90 s. After this time, normoxic mitochondria were challenged with pulses of 10 nmol CaCl2 applied every 60 s. CRC was defined as the total amount of Ca2+ added until the mitochondrial permeability transition pore (mPTP) opened. Recordings were normalized to the maximum fluorescence recorded before the addition of mitochondria (F/Fmax). Values of CRC were expressed as nmol of Ca2+/mg mitochondrial protein.
RESULTS
Developing a method to purify intact mitoplasts for patch-clamp recording.
Mitochondria represent more than 30% of cell volume in adult cardiomyocytes. We purified mitochondria from rat left ventricle cardiomyocytes (Fig. 1A). The outer mitochondrial membrane was disrupted by hypotonic shock and the mitoplasts were recovered. Micrographs of fixed mitoplasts (2% polyethylene glycol) show the integrity of the purified mitoplasts. This method yielded a highly enriched mitoplast preparation (Fig. 1B). Only mitoplasts displaying Mitotracker red signal (Fig. 1C) were used for voltage-clamp (arrow in Fig. 1C, D). Only excised inside-out mitochondrial patches were used in this study. Inside-out configuration was achieved by pulling out the patch pipette after Giga seal (>10 GΩ) formation. Inside-out configuration was interpreted as the matrix side of the mitochondria exposed to the bath solution (Fig. 1E). In those patches were the inside-out configuration was not achieved the activity of mitoBKCa channel was not observed even at high Ca2+ (>10 µM) in the bath solution.
Figure 1. Preparation of mitoplast from left ventricle cardiomyocytes.

A) Healthy rat cardiomyocytes isolated with a Langendorff perfusion apparatus. B) Electron microscopy image of mitoplasts (inner mitochondrial membrane devoid of outer membrane), red arrow. A low percentage of intact swollen mitochondria remained (top-left panel B). C) Mitoplasts labeled with Mitotracker Red visualized with differential interference contrast (DIC) Nomarski microscopy through a 60X objective. The arrow in C indicate a selected mitoplast for patch-clamp. D) DIC image of panel C show a borosilicate patch pipette next to a mitoplast. E) Schematic representation of the preparation of mitoplasts. In an excised patch (inside-out configuration), the bath solution represents the mitochondrial matrix.
Cardiac mitochondrial inner membrane expresses a large conductance, voltage- and Ca2+-dependent channel with a relatively high Po: mitoBKCa.
We characterized the biophysical properties of mitoBKCa in rat cardiac mitoplasts by exposing excised inside-out patches to symmetric bath and pipette solutions (150 KMeSO3 and 5 µM Ca2+). Transitions from closed (c) to open state (o) are the upward and downward deflections of the current recordings at positive and negative membrane potentials, respectively (Fig. 2A–C). MitoBKCa Po increased with membrane depolarization: the normalized NPo versus membrane potential plot shows a half activation membrane potential (V1/2) ~−55 mV at 12 µM matrix Ca2+ (Po = 0.8 ± 0.05, n=5, Fig. 2A, D). The Ca2+-dependence of mitoBKCa channel, was evident from the progressive shift of the voltage dependent activation curve towards more depolarized potentials when matrix Ca2+ was reduced to 5 and 0.02 µM (V1/2= +42 mV, and +70 mV, respectively, Fig. 2D). The apparent mitoBKCa Ca2+ affinity was estimated by fitting the average Po (n=4) at 3 different matrix Ca2+ and constant voltage (+20 mV) to a Hill function, yielding a K1/2 = 8 µM and a Hill coefficient nH = 2. MitoBKCa displays a unitary conductance of 308 ± 19 pS (n=11) (Fig. 2E).
Figure 2. Voltage-dependence of MitoBKCa channel.

In an excised inside-out patch, the bath solution represents the matrix side of the mitoplast. Channel activity was recorded in symmetric 150 KMeSO4. A) Single channel currents from a patch that contains at least three channels obtained at 12 µM matrix Ca2+ (c=closed state; o=open state). B) The same patch as in A exposed to 5 µM matrix Ca2+. Recordings in B show the activity of only one channel at positive membrane potentials. C) Same patch as in A and B exposed to 0.02 µM matrix Ca2+, single channel current was observed only at +80 mV. D) Plot of normalized NPo vs V. Data points were fit to a Boltzmann distribution (continuous lines) with a V1/2=−55, +42 and +70 mV at 12 (n=8), 5 (n=4), and 0.02 µM Ca2+ (n=3), respectively. Z values of 2.2, 1.4 and 1.6 were calculated for 12, 5 and 0.02 µM matrix Ca2+, respectively. E) Plots of I vs. V of mitoBKCa channels with a slope conductance of 309±19 pS (n=11). Bars represent mean and SEM here and throughout.
Paxilline inhibits the cardiac mitoBKCa channel by stabilizing its closed state.
To confirm the identity of this large K+ conductance as BKCa channel, we used paxilline, a specific BKCa blocker (Sanchez & McManus, 1996); 100 nM paxilline applied to the matrix side of inside-out patches exposed to 5 µM matrix Ca2+ decreased the Po of the channel from 0.7 ± 0.1 to 0.2 ± 0.1 in <5 minutes (n=3) (Fig. 3A–D). The mean open time of the channel (7.2 ms ± 2.4 ms, control) did not change significantly in presence of paxilline (4.6 ms ± 1.2 ms) p>0.05, n=3, whereas the mean closed time augmented significantly from 3.8 ms ± 0.5 ms to 27 ms ± 0.4 ms (Fig. 3E–F, n=3, p<0.05). Thus, paxilline reduced the Po of the channel mainly by stabilizing its closed state, a kinetic effect that has been previously reported for the plasma membrane BKCa channel (Zhou & Lingle, 2014). Later, we used paxilline as a pharmacological tool to evaluate the physiological role of mitoBKCa in mitochondrial Ca+2-retention capacity.
Figure 3. Paxilline reduces the open probability of mitoBKCa channels.
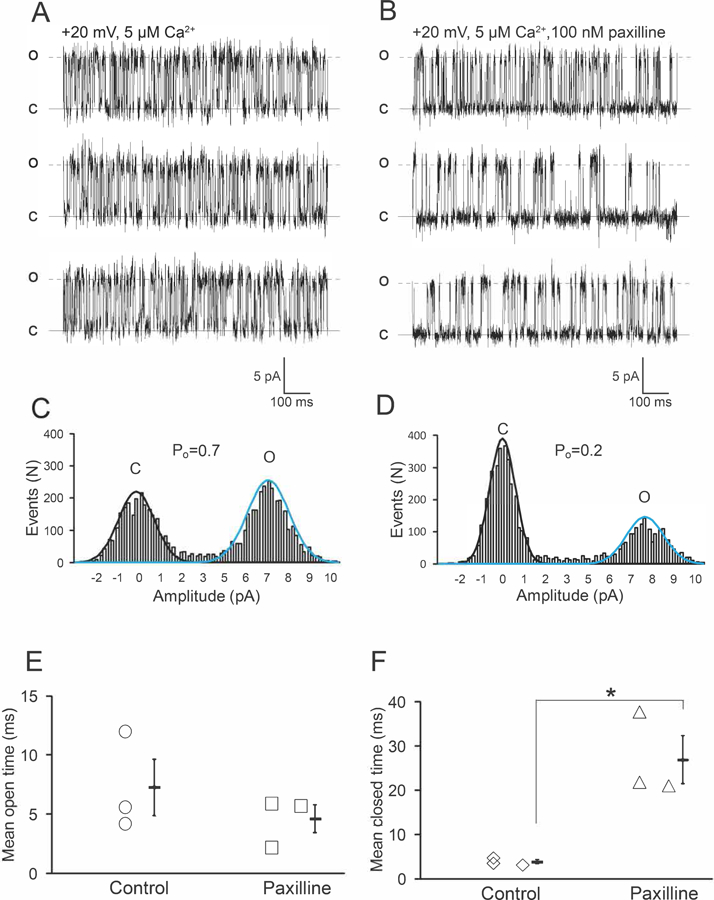
Single-channel currents of mitoBKCa recorded from the same mitoplast in absence (A) and in presence of paxilline (B) at the indicated matrix Ca2+ and membrane potential. Paxilline decreased the Po of the 7 pA channel from 0.77±0.1 to 0.41±0.1 (n=3) as indicated in the current amplitude histograms (C and D). Mean open time (E) and mean closed time (F) observed in 3 different patches. Paxilline augmented significantly the mean closed time of the channel (*p<0.05 Student’s t-test, n=3 hearts).
MitoBKCa functionally interacts with its auxiliary BK-β1 subunit; the activity of mitoBKCa channel was negligible in the BK-β1 KO mice.
Figure 4A shows the immunoprecipitation of BKCa alpha and the successful co-immunoprecipitation of BK-β1 subunit from cardiac mitochondrial lysate with a specific antibody against BKCa alpha (see methods). Both subunits were also detected in the pre-immune mitochondrial fraction (input) as well as in the whole heart lysate. While the mitochondrial protein GRIM19 was also detected in both whole lysate and in the input, we found no signal in the IP product, indicating specificity of the association between BK-β1 subunit and BKCa. SERCA was used as a negative control and to discard cross-contamination of our mitochondrial fraction with membranes from the sarcoplasmic reticulum (SR). These results indicate that BKCa channel interacts with its regulatory β1-subunit in cardiac mitochondria. To test how the interaction of mitoBKCa with its regulatory β1-subunit affects the biophysical properties of mitoBKCa we characterize this channel in cardiac Wt mitochondria and in mitochondria from the β1 KO mice.
Figure 4. MitoBKCa alpha and auxiliary β1 subunit association in cardiac mitochondria.
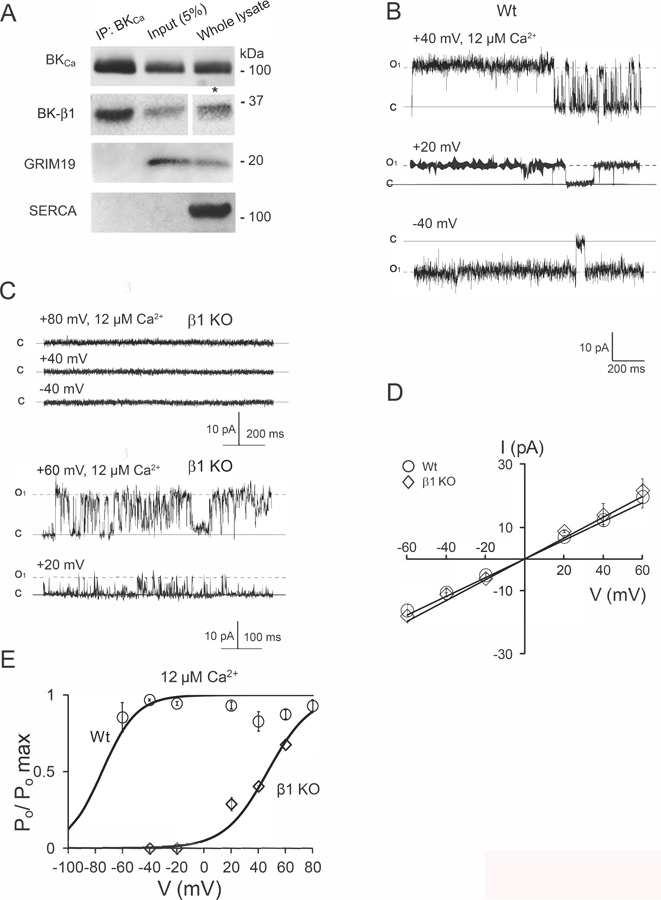
A) Immunoprecipitation (IP) of BKCa alpha and BK-β1 subunit with an antibody against BKCa alpha (see methods). Western blots revealed the presence of GRIM 19 and SERCA as markers of mitochondrial and sarcoplasmic reticulum membranes (4A, lower panels). *The signal of BK-β1 in the whole lysate was detected with chemiluminescent (ECL) substrate Supersignal™ West Femto Maximun sensitivity (see methods). Taken together, these results indicates an association between mitoBKCa and its auxiliary β1 subunit. Having demonstrated this, we characterized the biophysical properties of mitoBKCa in Wt and β1-KO cardiac mitoplasts. B) Single-channel currents of mitoBKCa recorded from Wt mitoplasts. The Po of the channel was 0.9 ± 0.08, n=4, in average at +80 mV and the indicated matrix Ca2+. C) Representative traces of a mitoplast from the β1 KO, were no single-channel current was detected at the indicated voltages and matrix Ca2+ (upper traces). Few mitoplasts (5 out of 28, mitoplasts, n=5 hearts) showed mitoBKCa channel activity with a Po of 0.4 ± 0.004, n=3 at +40 mV in the indicated matrix Ca2+. D) Plots of I vs. V at 12 µM matrix Ca2+ from Wt and β1 KO mitoplasts (data from 6 and 3 patches, respectively). A slope conductance of 302±26 (n=4) and 335 ±15 pS (n=3) was calculated for Wt and β1 KO, respectively. E) Plots of Po vs. V for mitoBKCa channels, data points represent the average of 4 and 3 mitoplasts for Wt and BK-β1 KO, respectively. Bars SEM.
MitoBKCa from Wt hearts displays a high Po in 100% of the patches at all the membrane potentials tested (Fig. 4B–E). Interestingly, the activity of mitoBKCa channel was negligible in mitochondria from the BK-β1 KO mice, where the channel was observed in only 5 patches from a total of 28 experiments (Fig. 4C). The remaining mitoBKCa observed in the BK-β1 KO displayed the same slope conductance (~300 pS, Fig. 4D) as that of Wt mitoBKCa, but with a more depolarized voltage-dependence of activation (V1/2= +47 mV at 12 µM matrix Ca2+, n=4) (Fig. 4E). MitoBKCa from the Wt displayed a Ca2+-dependency similar to that observed in rat cardiac mitochondria (Po~0.6, at +60 mV in 5 µM matrix Ca2+, n=3, to Po ~0.9, n=3 in 12 µM matrix Ca2+) (Fig. 3). In addition, murine mitoBKCa showed also sensitivity to paxilline, that reduced the Po of the channel from 0.6 (n=3) (Fig. 5A and C) to 0.1 (n=3) (Fig. 5B and D) in 5 µM matrix Ca2+ at +20 mV. The blocking effect of paxilline was partially reduced by washing the patches with bath solution (Po=0.4, n=3) (Fig. 5E and F).
Co-expression of regulatory BK-β1 subunit increases targeting of BKDEC to the mitochondria in HeLa cells.
The low Po and the rare frequency in which mitoBKCa channel was observed in the BK-β1 KO (Fig. 4B) suggests a reduction in the number of channels expressed and or properly targeted to the mitochondria in this genotype. Interestingly, the level of BKCa transcript was significantly elevated in the BK-β1 KO, compared to Wt mice (GADPH served as a control of expression, see Methods). To test whether auxiliary BK-β1 subunits are required to target BKCa into the inner mitochondrial membrane, we co-transfected HeLa cells with both BKDEC (Singh et al., 2013) and auxiliary β1 subunit. Figure 6A–D show confocal images from mitochondria of HeLa cells co-expressing BKDEC and BK-β1 subunit. Panel A shows Mitotracker red signal (DAPI is in blue), panel B the BKDEC (green), panel C represents the signal of BK-β1 (blue). Panel D represents the merge of the four signals, white color indicates colocalization of BKDEC with BK-β1 and mitotracker red. E) Quantification of the co-localization index (Cci) relative to mitotracker red signal revealed that Cci was significantly higher in cells co-expressing BKDEC and BK-β1 subunit than those cells expressing only BKDEC.
Figure 6. Localization of BKDEC and β1 subunit in HeLa cells.

A) Cultured cells co-transfected with BKDEC alpha+β1subunit, loaded with mitotracker red, and DAPI B) β1-flag labeled with an anti-flag C) BKDEC labeled with anti-HA. D) Merge of mitotracker red, BKDEC and β1. E) Cross-correlation index (CCi), relative to mitotracker red signal, calculated from cells overexpressing BKDEC alone (Cci=0.53±0.05) and co-expressed with β1 subunit (Cci=0.77±0.01, n=11 cells from 3 different preparations).*p<0.05.
Inhibition of mitoBKCa reduces mitochondrial Ca2+ retention capacity.
Following the characterization of the biophysical properties of the large conductance BKCa channel in mitochondria from adult rodent cardiomyocytes, we began to address its physiological role in mitochondria. Recent observations indicate that pharmacological opening of mitoBKCa enhanced mitochondrial Ca2+ retention capacity (mCRC) (Sato et al., 2005; Stowe et al., 2006; Singh et al., 2013). We measured mCRC in presence of paxilline as a way to investigate the effect that mitoBKCa inhibition has on mitochondrial Ca2+ handling. Figures 7A and B show representative mCRC experiments, where the upward deflections in the fluorescence (Ca2+ green) recordings report Ca2+ elevations, caused by Ca2+ addition to the extra mitochondrial media (upward arrows) and by the release of Ca2+ from mitochondria; downward deflection represents mitochondrial Ca2+ uptake. Freshly isolated cardiac mitochondria were challenged with pulses of 10 nmol CaCl2 (Fig. 7A, arrows). Wt mitochondria were able to handle ~22 Ca2+ pulses before opening of the mitochondrial transition pore (mPTP) (Bopassa et al., 2010; Singh et al., 2013), due to mitochondrial Ca2+ overload (Fig. 7A, green line). Pre-incubation of mitochondria with 10 µM paxilline caused a significant reduction of mCRC, where ~15 pulses of CaCl2 were sufficient to trigger the opening of mPTP (Fig. 7A, red line). These data indicate that a reduction in the Po of mitoBKCa channel with paxilline reduced mCRC (711 ± 30 for control and 494 ± 26 nmol/µg mitochondrial protein for mitochondria treated with paxilline, n=7) (Fig. 7C). Notably, mCRC was also reduced in the BK-β1 KO (553 ± 58 nmol/µg protein nmol Ca2+, Fig. 7B blue line, n=5) compared to the Wt (Fig. 7C). Together, this data indicates that opening of mitoBKCa channel and expression of its regulatory BK-β1 subunit are necessary to maintain mitochondrial Ca2+ homeostasis in cardiac cells.
Figure 7. Mitochondrial calcium uptake is impaired by Paxilline and by genetic ablation of β1-subunit.
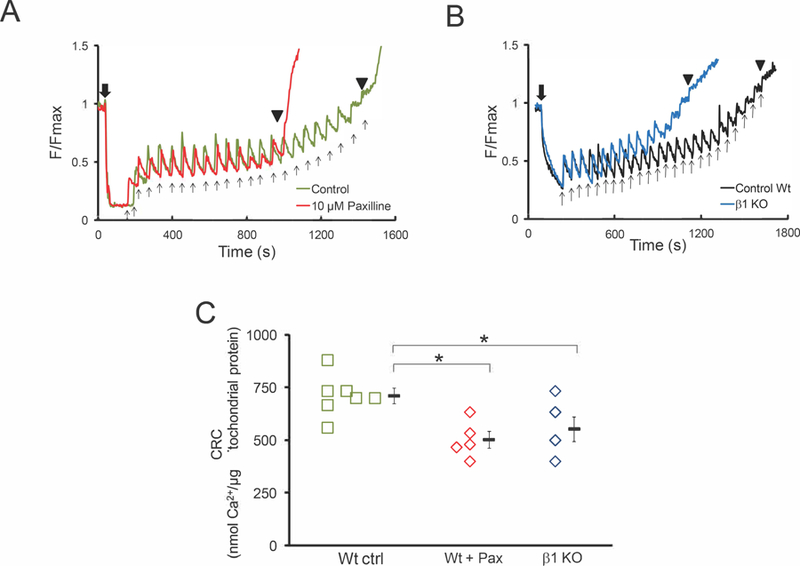
A) Paxilline reduces the amount of mitochondrial Ca2+ necessary to induce formation and opening of mPTP. B) Mitochondria from the β1 KO required less Ca2+ than mitochondria from the Wt to trigger the opening of mPTP. C) Calcium retention capacity values obtained in A for paxilline and in B for the β1-KO mitochondria. Bars SEM, (*p<0.05 Student’s t-test). Black downward arrow, addition of mitochondria. Black upward arrows, 10 nmol CaCl2 pulses. (▼) opening of mPTP.
A second population of mitoBKCa channels with low Po in rat cardiac mitochondria.
This population of channels was present in about 40% of the inside-out patches (7 out of 18 membrane patches). These channels also display a large conductance for K+ (284 ± 6 pS, n=7 patches from 5 hearts) (Fig. 8A, D) and a V1/2 = +42 mV in 12 µM matrix Ca2+ (n=4, Fig. 8C). The Po of this channel was reduced when matrix [Ca2+] was decreased (n=3) (Fig. 8A–C). A full characterization of the biophysical properties of this second population of mitoBKCa channel is required to elucidate its association with regulatory subunits.
Figure 8. A different population of mitoBKCa in cardiac mitochondria.
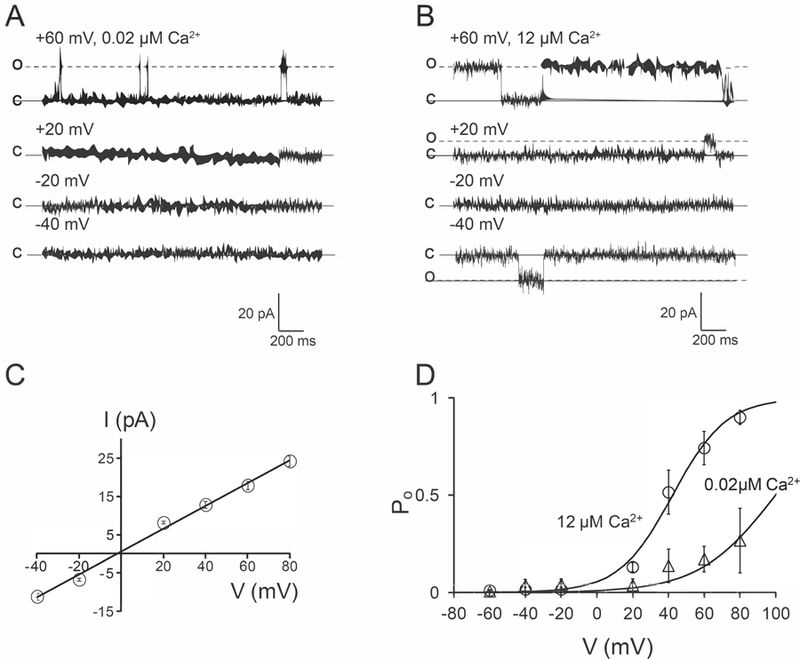
A) Single channel currents recorded in 0.02 µM matrix Ca2+ at the indicated voltages. B) Same patch as in A exposed to 12 µM matrix Ca2+. C) Plot of I vs. V, a slope conductance of 284 ± 6 pS (n=7) was calculated. D) Plots of Po vs. V from the currents observed in A and B, data points represent an average of ≥4 different patches at the indicated matrix Ca2+.
Discussion
MitoBKCa is a large conductance K+ channel.
Single channel conductance of mitoBKCa varies between different tissues and cell types (Singh et al., 2012; Balderas et al., 2015). Cardiac mitoBKCa channel has a conductance ranging between 190–300 pS (Xu et al., 2002; Ohya et al., 2005; Soltysinska et al., 2014). Likewise, we reported that cardiac mitoBKCa is a Ca2+ dependent, voltage and paxilline sensitive channel, with a conductance for K+ of 300 pS. A recent report has shown a slightly smaller conductance (145 pS) for cardiac mitoBKCa (Frankenreiter et al., 2017). Although a detailed analysis is necessary to explain the differences observed between different groups, including the present study, it is clear that heterogeneous conductances exist in cardiac mitochondrial inner membrane as recently demonstrated (Soltysinska et al., 2014), where a conductance of 190 pS was reported for mitoBKCa, among other conductances ranging from 60 to 370 pS. We observed that substitution of Cl− ions in the recording solutions, as well as the use of low concentrations of paxilline (100 nM) helped to dissect the biophysical properties of cardiac mitoBKCa channel as well as its pharmacological profile.
Activation of mitoBKCa channel depends on its regulatory β1 subunit.
Cell function and survival rely on adequate mitochondrial function. Oxidative phosphorylation (OXPHOS), ATP synthesis and the formation and opening of the mitochondrial PTP, among others are physiological processes regulated by mitochondrial Ca2+. Since the discovery of BKCa channel at the inner mitochondrial membrane (Siemen et al., 1999), a considerable effort has been made to establish the biophysical properties as well as the molecular composition of BKCa channel in mitochondria and its role in cardioprotection (Singh et al., 2013; Balderas et al., 2015). Despite significant advances, the physiological conditions under which mitoBKCa channel might be active in respiring mitochondria remains unknown. The opening of a large K+ conductance such as mitoBKCa channel, would rapidly depolarize the mitochondrial membrane potential (ΔΨm), reducing the driving force for Ca2+, thus modulating mitochondrial Ca2+ uptake. The relative depolarized V1/2 of activation of mitoBKCa (V1/2=−55 mV at ~10 µM matrix Ca2+) with respect to mitochondrial membrane potential made the Po of this channel presumably negligible under mitochondrial steady-state conditions (∆ψm=−180 mV; [Ca2+]mit<200 nM; pH=7.2; etc.) The remarkable high Po displayed by mitoBKCa at both negative and positive membrane potentials is similar to that previously reported for mitoBKCa from various tissues and cell types (Siemen et al., 1999; Xu et al., 2002; Fahanik-Babaei et al., 2011). In addition, its relative hyperpolarized V1/2 (Fig. 2) compared to that of the BKCa-α subunit expressed alone (Meera et al., 1996; Tanaka et al., 1997; Nimigean & Magleby, 1999; Brenner et al., 2000; Bao & Cox, 2005; Sweet & Cox, 2009), together with the expression of regulatory BK-β1 subunits in cardiac mitochondria (Fig. 4A) (Ohya et al., 2005; Bautista et al., 2009), supports the hypothesis of a functional association between cardiac mitoBKCa and its auxiliary BK-β1 subunit. It is clear that a combination of physiological regulatory elements, rise in matrix [Ca2+], are required to increase mitoBKCa channel Po in steady-state respiring mitochondria.
The activity of plasma membrane BKCa is independently regulated by the co-assembly of different β (1, 2) subunits together with γ (1) subunits (Yan & Aldrich, 2010, 2012) (Gonzalez-Perez et al., 2015), thus interactions of cardiac mitoBKCa channels with different regulatory subunits, β and or γ, are likely to occur. The most recent subproteomal analysis developed by our group revealed the interaction of mitoBKCa channel with ~150 different mitochondrial proteins (Zhang et al., 2017), including outer membrane import proteins, TOM 22 and TOM 70; as well as proteins of the respiratory complex (Zhang et al., 2017). The mitochondrial subproteome also revealed association of mitoBKCa with the ADP/ATP carrier, ANT (Zhang et al., 2017), a regulator of the mitochondrial PTP (Kokoszka et al., 2016). Therefore, interactions of mitoBKCa channels with mitochondrial proteins and complexes may confer novel biophysical properties within the same population of mitochondria.
Regulatory BK-β1 subunit targets and activates mitochondrial BKCa channel.
Biophysical characterization demonstrated that the large conductance for K+, voltage dependent, Ca2+ and paxilline sensitive mitoBKCa channel is present in adult rodent cardiomyocytes (Figs. 2–6). We also demonstrate a molecular interaction of mitoBKCa channel with its regulatory β1 subunit in adult cardiomyocytes (Fig. 4). The low Po and the scarcity of mitoBKCa–containing membrane patches in the BK-β1 KO (5 patches with active channels, out of 28 patches) indicates that BK-β1 subunit is necessary to effectively target BKCa to the mitochondria, as has been seen in other cell systems, where targeting of plasma membrane BKCa depends on the level of expression of BK-β1 subunit (Toro et al., 2006). We observed that co-expression of BKDEC with its regulatory β1 subunit targets more channels to the mitochondria (Fig. 6). We recently reported that BKCa channel is delivered into mitochondria through its interaction with the TOM22 import system (Zhang et al., 2017). Despite this novel information, the molecular details and how the regulatory BK-β1 subunit contributes to target BKCa channel to the mitochondria remains to be established.
With regard to the physiological role of mitoBKCa, it has been hypothesized that activation of this large conductance helps to modulate mitochondrial Ca2+ handling, the details of this mechanism remains unclear. Current models suggest that when open mitoBKCa would rapidly depolarize the ΔΨm (Testai et al., 2013), hence reducing the mitochondrial driving force for Ca2+, preventing mitochondrial Ca2+ overload (Wang et al., 2004; Ohya et al., 2005; Aon et al., 2010). Under this scenario, activation of mitoBKCa contributes to mitochondrial Ca2+ handling, controlling indirectly the formation and opening of mPTP, an essential step for cell survival (Halestrap & Richardson, 2015). In agreement with this hypothesis, reduced mitoBKCa channel activity caused by pharmacological inhibition with paxilline or by lack of its regulatory β1 subunit, derivate in loss of mitochondrial Ca2+ handling (Fig. 7). This evidence support the hypothesis that association of mitoBKCa channel with its auxiliary BK-β1 subunit is essential to maintain mitochondrial Ca2+ homeostasis in cardiac cells.
It is worth to consider that mitoBKCa might be associated with regulatory subunits other than β1, as suggested by the second population of mitoBKCa found in this work (Fig. 8), displaying a depolarized voltage-sensitivity (V1/2 = +47 mV at 12 µM matrix Ca2+) (Meera et al., 1996; Brenner et al., 2000). Heterogeneous populations of mitoBKCa and association with different auxiliary subunits would increase the modes of regulation and the therapeutic targets that modulate this channel in cardiac mitochondria.
Supplementary Material
Key Points.
Association of plasma membrane BKCa channels with BK-β subunits shape their biophysical properties and physiological roles; however, functional modulation of mitochondrial BKCa channel (mitoBKCa) by BK-β subunits is not established.
MitoBKCa-α, and regulatory BK-β1 subunit associate in mouse cardiac mitochondria.
A large fraction of mitoBKCa displayed properties similar to that of plasma membrane BKCa when associated with BK-β1 (left-shifted voltage dependence of activation, V1/2=−55mV, 12 µM matrix Ca2+).
In the BK-β1 KO mice, cardiac mitoBKCa displayed low Po and depolarized V1/2 of activation (+47mV at 12 µM matrix Ca2+)
Co-expression of BKCa with BK-β1 subunit in HeLa cells doubled the density of BKCa in mitochondria.
This work supports the view that cardiac mitoBKCa channel is functionally modulated by the BK-β1 subunit; proper targeting and activation of mitoBKCa shapes mitochondrial Ca2+ handling.
Acknowledgements
We thank the members of the Olcese laboratory for many insightful discussions and the Microscopy Techniques Core at UCLA members Marianne Cilluffo for assistance with electron microscopy. We thank MBA. Hlne Zammarchi and BS. Anthony Balynas for their valuable comments on this paper.
Funding
This work was supported by NIH HL107418 (LT, ES, RO), 1R01HL134346 (RO), HL124070 and HL141353 (DC), UC MEXUS-CONACyT Postdoctoral Fellowship FE-13–248 (EB) and AHA-WSA 15POST22490015 (EB).
Abbreviations:
- mitoBKCa
mitochondrial large conductance for K+, voltage- and Ca2+-activated channel
- Po
open probability
- CRC
calcium retention capacity
Footnotes
Competing interests
The authors declare no competing financial interests.
References
- Aon MA, Cortassa S, Wei AC, Grunnet M & O’Rourke B. (2010). Energetic performance is improved by specific activation of K+ fluxes through K(Ca) channels in heart mitochondria. Biochim Biophys Acta 1797, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderas E, Zhang J, Stefani E & Toro L. (2015). Mitochondrial BKCa channel. Front Physiol 6, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L & Cox DH. (2005). Gating and ionic currents reveal how the BKCa channel’s Ca2+ sensitivity is enhanced by its beta1 subunit. J Gen Physiol 126, 393–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JN, Barrett EF & Dribin LB. (1981). Calcium-dependent slow potassium conductance in rat skeletal myotubes In press 82. [DOI] [PubMed] [Google Scholar]
- Bautista L, Castro MJ, Lopez-Barneo J & Castellano A. (2009). Hypoxia inducible factor-2alpha stabilization and maxi-K+ channel beta1-subunit gene repression by hypoxia in cardiac myocytes: role in preconditioning. Circ Res 104, 1364–1372. [DOI] [PubMed] [Google Scholar]
- Bentzen BH, Andersen RW, Olesen SP, Grunnet M & Nardi A. (2010). Synthesis and characterisation of NS13558: a new important tool for addressing KCa1.1 channel function ex vivo. Naunyn Schmiedebergs Arch Pharmacol 381, 271–283. [DOI] [PubMed] [Google Scholar]
- Berkefeld H, Fakler B & Schulte U. (2010). Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90, 1437–1459. [DOI] [PubMed] [Google Scholar]
- Bopassa JC, Eghbali M, Toro L & Stefani E. (2010). A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 298, H16–H23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden JE & Nelson MT. (1992). Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256, 532–535. [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y & Aldrich RW. (2000). Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275, 6453–6461. [DOI] [PubMed] [Google Scholar]
- Contreras GF, Castillo K, Enrique N, Carrasquel-Ursulaez W, Castillo JP, Milesi V, Neely A, Alvarez O, Ferreira G, Gonzalez C & Latorre R. (2013). A BK (Slo1) channel journey from molecule to physiology. Channels (Austin ) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Yang H & Lee US. (2009a). Molecular mechanisms of BK channel activation. Cell Mol Life Sci 66, 852–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Yang H & Lee US. (2009b). Molecular mechanisms of BK channel activation. Cell Mol Life Sci 66, 852–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahanik-Babaei J, Eliassi A & Saghiri R. (2011). How many types of large conductance Ca(+)(2)-activated potassium channels exist in brain mitochondrial inner membrane: evidence for a new mitochondrial large conductance Ca(2)(+)-activated potassium channel in brain mitochondria. Neuroscience 199, 125–132. [DOI] [PubMed] [Google Scholar]
- Frankenreiter S, Bednarczyk P, Kniess A, Bork NI, Straubinger J, Koprowski P, Wrzosek A, Mohr E, Logan A, Murphy MP, Gawaz M, Krieg T, Szewczyk A, Nikolaev VO, Ruth P & Lukowski R. (2017). cGMP-Elevating Compounds and Ischemic Conditioning Provide Cardioprotection Against Ischemia and Reperfusion Injury via Cardiomyocyte-Specific BK Channels. Circulation 136, 2337–2355. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez V, Xia XM & Lingle CJ. (2015). Two classes of regulatory subunits coassemble in the same BK channel and independently regulate gating. Nat Commun 6, 8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D. (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP & Richardson AP. (2015). The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury. J Mol Cell Cardiol 78, 129–141. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Pantazis A & Olcese R. (2013). Transduction of voltage and Ca2+ signals by Slo1 BK channels. Physiology (Bethesda ) 28, 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Flierl A, Sweeney KM, Angelin A, MacGregor GR & Wallace DC. (2016). Deficiency in the mouse mitochondrial adenine nucleotide translocator isoform 2 gene is associated with cardiac noncompaction. Biochim Biophys Acta 1857, 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B & Nicoll RA. (1987). Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol 389, 187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R & Brauchi S. (2006). Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res 39, 385–401. [DOI] [PubMed] [Google Scholar]
- Latorre R, Castillo K, Carrasquel-Ursulaez W, Sepulveda RV, Gonzalez-Nilo F, Gonzalez C & Alvarez O. (2017). Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol Rev 97, 39–87. [DOI] [PubMed] [Google Scholar]
- Latorre R, Morera FJ & Zaelzer C. (2010). Allosteric interactions and the modular nature of the voltage- and Ca2+-activated (BK) channel. J Physiol 588, 3141–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P & Alvarez O. (1989). Varieties of calcium-activated potassium channels. Annu Rev Physiol 51, 385–399. [DOI] [PubMed] [Google Scholar]
- Latorre R, Vergara C & Hidalgo C. (1982). Reconstitution in planar lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc Natl Acad Sci U S A 79, 805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE & Nelson MT. (2006). Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda ) 21, 69–78. [DOI] [PubMed] [Google Scholar]
- Lee US & Cui J. (2010a). BK channel activation: structural and functional insights. Trends Neurosci [DOI] [PMC free article] [PubMed]
- Lee US & Cui J. (2010b). BK channel activation: structural and functional insights. Trends Neurosci 33, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Tanaka Y, Alioua A, Wu Y, Lu R, Kundu P, Sanchez-Pastor E, Marijic J, Stefani E & Toro L. (2010). Thromboxane A2 receptor and MaxiK-channel intimate interaction supports channel trans-inhibition independent of G-protein activation. Proc Natl Acad Sci U S A 107, 19096–19101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang Z, Koh H, Lu R, Jiang Z, Alioua A, Garcia-Valdes J, Stefani E & Toro L. (2013). The beta1-subunit of the MaxiK channel associates with the thromboxane A2 receptor and reduces thromboxane A2 functional effects. J Biol Chem 288, 3668–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Jiang Z & Toro L. (1996). A calcium switch for the functional coupling between alpha (hslo) and beta subunits (Kv,cabeta) of maxi K channels. FEBS Lett 385, 127–128. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF & Aldrich RW. (2006). BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci 9, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean CM & Magleby KL. (1999). The beta subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J Gen Physiol 113, 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S, Kuwata Y, Sakamoto K, Muraki K & Imaizumi Y. (2005). Cardioprotective effects of estradiol include the activation of large-conductance Ca(2+)-activated K(+) channels in cardiac mitochondria. Am J Physiol Heart Circ Physiol 289, H1635–1642. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C & Wei A. (2006). High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7, 921–931. [DOI] [PubMed] [Google Scholar]
- Sanchez M & McManus OB. (1996). Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35, 963–968. [DOI] [PubMed] [Google Scholar]
- Sato T, Saito T, Saegusa N & Nakaya H. (2005). Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation 111, 198–203. [DOI] [PubMed] [Google Scholar]
- Siemen D, Loupatatzis C, Borecky J, Gulbins E & Lang F. (1999). Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun 257, 549–554. [DOI] [PubMed] [Google Scholar]
- Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E & Toro L. (2013). MitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci U S A 110, 10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Stefani E & Toro L. (2012). Intracellular BK(Ca) (iBK(Ca)) channels. J Physiol 590, 5937–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltysinska E, Bentzen BH, Barthmes M, Hattel H, Thrush AB, Harper ME, Qvortrup K, Larsen FJ, Schiffer TA, Losa-Reyna J, Straubinger J, Kniess A, Thomsen MB, Bruggemann A, Fenske S, Biel M, Ruth P, Wahl-Schott C, Boushel RC, Olesen SP & Lukowski R. (2014). KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury. PLoS One 9, e103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG & Jiang MT. (2006). Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol 290, H434–H440. [DOI] [PubMed] [Google Scholar]
- Sweet TB & Cox DH. (2009). Measuring the influence of the BKCa {beta}1 subunit on Ca2+ binding to the BKCa channel. J Gen Physiol 133, 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Meera P, Song M, Knaus H-G & Toro L. (1997). Molecular constituents of maxi KCa channels in human coronary smooth muscle. Predominant α + β subunit complexes. J Physiol 502, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testai L, Martelli A, Marino A, D’Antongiovanni V, Ciregia F, Giusti L, Lucacchini A, Chericoni S, Breschi MC & Calderone V. (2013). The activation of mitochondrial BK potassium channels contributes to the protective effects of naringenin against myocardial ischemia/reperfusion injury. Biochem Pharmacol 85, 1634–1643. [DOI] [PubMed] [Google Scholar]
- Toro B, Cox N, Wilson RJ, Garrido-Sanabria E, Stefani E, Toro L & Zarei MM. (2006). KCNMB1 regulates surface expression of a voltage and Ca2+-activated K+ channel via endocytic trafficking signals. Neuroscience 142, 661–669. [DOI] [PubMed] [Google Scholar]
- Wang X, Yin C, Xi L & Kukreja RC. (2004). Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol Heart Circ Physiol 287, H2070–2077. [DOI] [PubMed] [Google Scholar]
- Wu Y, Eghbali M, Ou J, Lu R, Toro L & Stefani E. (2010). Quantitative determination of spatial protein-protein correlations in fluorescence confocal microscopy. Biophys J 98, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A & O’Rourke B. (2002). Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science 298, 1029–1033. [DOI] [PubMed] [Google Scholar]
- Yan J & Aldrich RW. (2010). LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466, 513–516. [DOI] [PubMed] [Google Scholar]
- Yan J & Aldrich RW. (2012). BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc Natl Acad Sci U S A 109, 7917–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li M, Zhang Z, Zhu R, Olcese R, Stefani E & Toro L. (2017). The mitochondrial BKCa channel cardiac interactome reveals BKCa association with the mitochondrial import receptor subunit Tom22, and the adenine nucleotide translocator. Mitochondrion 33, 84–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y & Lingle CJ. (2014). Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J Gen Physiol 144, 415–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


