Abstract
Survivors of blast-induced traumatic brain injury (bTBI) have increased susceptibility to Parkinson’s disease (PD), characterized by α-synuclein aggregation and the progressive degeneration of nigrostriatal dopaminergic neurons. Using an established bTBI rat model, we evaluated the changes of α-synuclein and tyrosine hydroxylase (TH), known hallmarks of PD, and acrolein, a reactive aldehyde and marker of oxidative stress, with the aim of revealing key pathways leading to PD post-bTBI. Indicated in both animal models of PD and TBI, acrolein is likely a point of pathogenic convergence. Here we show that after a single mild bTBI, acrolein is elevated up to a week, systemically in urine, and in whole brain tissue, specifically the substantia nigra and striatum. Acrolein elevation is accompanied by heightened α- synuclein oligomerization, dopaminergic dysregulation, and acrolein/α-synuclein interaction in the same brain regions. We further show that acrolein can directly modify and oligomerize α- synuclein in vitro. Taken together, our data suggests acrolein likely plays an important role in inducing PD pathology following bTBI by encouraging α-synuclein aggregation. These results are expected to advance our understanding of the long-term post-bTBI pathological changes leading to the development of PD, and suggest intervention targets to curtail such pathology.
Keywords: oxidative stress, Parkinson’s disease, neuroinflammation, acrolein, neurodegeneration, neurotrauma
INTRODUCTION
In the general civilian population, traumatic brain injury (TBI) has been independently identified as a risk factor for Parkinson’s disease (PD), suggesting TBI increases PD susceptibility (Bower et al., 2003; Goldman et al., 2012; Goldman et al., 2006). Athletes, boxers, and veterans, all of which are at-risk sub-populations for TBI, have a higher chance of developing Parkinson’s disease (PD) symptoms later in life (Acosta et al., 2015). Veterans, specifically, have been reported to be three times as likely to develop PD when compared to the general populous (Bell et al., 2009; Jafari et al., 2013; Johnson et al., 2012; Laino, 2005; Lee et al., 2012; Van Den Eeden et al., 2003). The majority of combat-related TBI results from explosive blasts (DePalma et al., 2005; Galarneau et al., 2008), which are most commonly mild in nature (Carlson et al., 2010; Hoge et al., 2008; Shively and Perl, 2012; Terrio et al., 2009). Mild brain injuries often go undiagnosed or unreported, fostering growing concern that clinically ‘silent’ injuries may later precipitate PD, among other consequences (Elder and Cristian, 2009). Unfortunately, despite mounting clinical evidence associating TBI and PD, mechanisms by which TBI-related injurious processes increase PD susceptibility are poorly understood.
During TBI, including those from blast exposure, the brain immediately undergoes primary physical damage to neurons, glial cells, and microvasculature. Primary injury induces secondary cellular and biochemical processes including oxidative stress, lipid peroxidation, abnormal protein aggregation, inflammation, and neuronal death (Acosta et al., 2015; Garcia-Gonzalez et al., 2018; Glover et al., 2012; Walls et al., 2016; Werner and Engelhard, 2007; Yu et al., 2009). Secondary injuries initiate on the order of minutes after injury and, even after mild injuries, can continue for months (Cernak et al., 2011; DePalma et al., 2005; Desmoulin and Dionne, 2009; Miller, 2012; Rosenfeld et al., 2013; Walls et al., 2016). In contrast, idiopathic PD pathophysiological processes occur over the course of many years. In humans, the TBI-PD relationship is thus difficult to mechanistically study as a result of the separation between TBI occurrence (usually during younger, more active years) and PD onset (average age: 60), which would require involved, expensive longitudinal studies. As such, animal models are not only suitable, but necessary for such investigation. Despite the temporal disconnect in humans, it has been demonstrated that TBI secondary injury processes overlap with the degenerative processes observed in PD (Acosta et al., 2015; Saing et al., 2012; Xiong et al., 2013), giving invaluable guidance for investigation using animal models.
PD is classified as a synucleinopathy due to its hallmark pathological finding, Lewy body inclusions, which are primarily composed of insoluble, abnormally aggregated α-synuclein (α-syn) protein fibrils (Spillantini et al., 1997; Stefanis, 2012). α-syn is thought to play an essential role in PD pathogenesis, with its aggregation leading to dopaminergic cell death in the substantia nigra (SN) and depletion of the brain’s supply of dopamine in the striatum (STR) (Castellani et al., 1996; Lo Bianco et al., 2004; Spillantini et al., 1997; Stefanis, 2012). In conjunction to dopamine depletion, the overall activity and protein levels of tyrosine hydroxylase, a rate-limiting enzyme for catecholamine synthesis (including dopamine), are decreased in the SN of PD patients (Mogi et al., 1988; Nakashima et al., 2013). Consequently, due to the critical role of the dopaminergic nigrostriatal pathway in motor coordination, the cardinal clinical signs of PD emerge: tremor, rigidity, slowness of movement, and postural instability (Bernheimer et al., 1973; Kish et al., 1988; Morrish et al., 1995). Interestingly, α-syn protein is highly vulnerable to attack by reactive oxygen species and reactive aldehydes, which are suspected to promote its oligomerization and further induce oxidative stress (Shamoto-Nagai et al., 2007), perhaps leading to elevated oxidative stress markers in PD patients (Castellani et al., 1996; Owen et al., 1997; Yan et al., 2013). As previously mentioned, oxidative stress is a known consequence of TBI, suggesting a possible link and pathological convergence between TBI and PD.
We have previously documented typical TBI primary and secondary injuries in a mild blast induced-traumatic brain injury (mb-TBI). Specifically, we have noted conspicuous microvascular damage, neuroinflammation, and increased oxidative stress in the hours and days following injury (Walls et al., 2016). Furthermore, we have discovered a significant elevation of acrolein, an α,β-unsaturated aldehyde that is both a product and catalyst of lipid peroxidation and stimulator of oxidative stress and inflammation (Garcia-Gonzalez et al., 2018; Walls et al., 2016). Finally, we have demonstrated that acrolein plays a key role in the pathogenesis of PD in a 6-OHDA injection model, highlighting the need for additional mechanistic investigations (Ambaw et al., 2018).
The present study extends our previous findings in the context of PD-relevant mechanisms after mb-TBI, delving deeper into overlapping neurodegenerative processes shared by mb-TBI and PD in the same animal preparation. Specifically, we investigated α-syn aggregation, dysregulation of tyrosine hydroxylase, and lipid peroxidation in the whole brain, striatum and substantia nigra regions post-blast injury. We demonstrate that acrolein can directly modify α-syn and lead to its aberrant expression in vitro and likely in vivo as well, in addition to the dysregulation of tyrosine hydroxylase. To our knowledge, this is the first direct in vivo evidence implicating the post-TBI role of acrolein, or any other TBI secondary injury-related molecule, in promoting PD-like abnormal expression of α-syn and dysregulation of tyrosine hydroxylase in the brains of rats exposed to mild blast-induced traumatic brain injury.
MATERIALS AND METHODS
Mild-blast traumatic brain injury rat model
All live animal procedures were conducted under animal use protocols approved by the Purdue University Animal Care and Use Committee. The mild blast TBI (mb-TBI) model was carried out as previously described in Walls et. al. 2016 (Walls et al., 2016). In brief, 300 gram adult male Sprague Dawley rats were anesthetized with 80 mg/kg ketamine and 20 mg/kg xylazine cocktail. After verifying the absence of toe-withdrawal reflex, the animals were secured in an open-ended shock tube style blast apparatus and a body shield was placed over the animals for protection during injury allowing for the study of mild TBI without systemic confounders. Mild bTBI was produced by a blast wave generator, which delivered a global blast pressure wave in a laboratory setting. Blast generation was achieved when pressure built up in a reservoir until it exceeded the burst strength of the diaphragm. The blast wave was directed downward at a distance of 50 mm from the nozzle of the blast generator to the head of the animal, with a peak pressure of 150 kPa. Sham (Control) animals were anesthetized accordingly and place in the same room of the blast set-up but outside the blast wave range.
3-hydroxypropyl mercapturic acid (3-HPMA) quantification in urine
Acrolein metabolite, 3-HPMA levels in the urine were measured utilizing LC-MS-MS as described in Zheng et al. 2013 (Zheng et al., 2013). Urine samples were collected in standard metabolic collection cages before mb-TBI and 1, 2, 5, and 7 days post-injury. ENV+ cartridges (Biotage, Charlotte, NC, USA) were used to prepare solid phase extraction before LC-MS-MS analysis. Each cartridge was conditioned with 1 mL of methanol, water, and 0.1% fromic acid diluted in water. A volume of 500 μL of urine was spiked with 200 ng of deuterated 3-HPMA (d3-3-HPMA) (Toronto Research Chemicals Inc., New York, Ontario) and mixed with 500 μL of 50 mM ammonium formate and 10 μL of undiluted formic acid. Subsequently, this urine mixture was added to the cartridge and washed twice with 1 mL of 0.1% formic acid, then followed by 1 mL of 10% methanol, followed by 1 mL solution of 10% methanol/ 90% 0.1% fromic acid. The cartridges were dried with nitrogen gas and eluted with 600 μL methanol plus 2% fromic acid three times. The combined eluates were dried with an evaporation centrifuge and reconstituted in 100 μL of 0.1% formic acid before LC-MS-MS analysis. An Agilent 1200 Rapid Resolution liquid chromatography (LC) system coupled to an Agilent 6460 series QQQ mass spectrometer (MS) (Santa Clara, CA, USA) was used to analyze 3-HPMA in each sample (Zheng et al., 2013).
Levels of 3-HPMA were normalized to urine creatinine levels. Creatinine measurements were performed using creatinine (urinary) assay kit (Cayman Chemical Company, MI, USA). In brief, creatinine standards, 12x, and 24x diluted urine samples were prepared in a 96-well plate and incubated with an alkaline picrate solution for approximately 20 min at room temperature. Initial reading was detected using standard spectrophotometry at the 490-500 nm absorbance. Next, 5 μL of an acid solution was added to each of the samples and the plate was incubated on a shaker for 20 min at room temperature. Similarly, final measurement was acquired at 490-500 nm absorbance using standard spectroscopy. The difference between the initial and final reading were calculated, and the creatinine standard curve, generated according to the assay protocol, were used for quantitative analysis for each of the sample. These values were then used to normalize the 3-HPMA measurements.
Western blot
Control (sham) and injured rats were sacrificed for Western blot analysis at 2, or 7 days post treatment. After deeply anesthetized with 80 mg/kg ketamine and 20 mg/kg xylazine cocktail, animals were transcardially perfused with Krebs solution and decapitated. Whole brains were quickly removed, frozen on dry ice, and stored at −80°C until processing. Tissue from the striatal regions (STR: B5 (anterior), C5 (posterior)) and substantia nigral region (SN: E8) was dissected out according to Paxinos & Watson’s The Rat Brain Atlas as a reference guide for the regional assessment (Paxinos and Watson, 1986). Brain tissues were sonicated in 1x RIPA buffer (Sigma, #R0278) with protease inhibitor cocktail at 1:100 final concentration (Sigma, P8340). Samples were centrifuged at 15,000 g for 40 min at 4°C and only the supernatant was used for the Western blot study. Protein concentrations were measured using the Bicinochoninic Acid protein assay kit (Pierce, Rockford, IL, USA) and SPECTRAmax (Molecular Devices, Sunnyvale, CA). Sixty micrograms of protein with 20% SDS, β-mercaptoethanol, and 2x Laemmli buffer were loaded to a 15% Tris-HCL gels and electrophoresed at 80 volts for 2-3 hours. Proteins were then transferred to a nitrocellulose membrane by electro blotting in 70 volts for 1-2 hours depending on the protein size at 4°C in 1x transfer buffer with 20% methanol (Tris-Glycine buffer from BioRad, Hercules). The membrane was blocked in 1x casein (Vector, #SP-5020) at room temperature for 1 hr, and immunolabeled with one of the following primary antibodies overnight at 4°C : anti-ACR (Abcam, #37110); anti- α-synuclein (α-syn) (BD Transduction, #610786); anti-TH (Cell Signaling, #2792S); anti-THpSer31 (Cell Signaling, #3370S); anti-THpSer40 (Novus, #NB300-173); and anti-actin (Sigma, #A2066). The experiments were run from the same samples, accordingly from the whole brain, STR (B5, C5), and SN (E8) lysates; and the antibodies were run in parallel. The membranes were further incubated with either biotinylated anti-mouse or anti-rabbit secondary antibody (Vector, #BA-2000, #BA-1000) at room temperature for 1 hr. The DuoLux substrate (Vector, #SK-6605) immunodetection kit was used for chemiluminescent signal acquisition and the Azure c300 Western blot imaging system (Azure Biosystems, Dublin, CA) was used to image the membrane. The AlphaView software (Protein Simple, San Jose, CA) was used to quantify the relative signal for each band. Data are normalized with actin, pTH-Ser31 and Ser 40 are normalized with unp-TH, and expressed as percent control.
Detection of α-syn protein modifications by acrolein
Purified wild-type α-syn protein (a generous gift from Dr. Jean-Christophe Rochet, Purdue University) (2.5 μM in the final concentration) was incubated in the presence of 0.125, 0.25, 0.5, 1, 5, and 10 mM of ACR at 37°C for 20 h. Then equal volume of the loading buffer (Laemmli sample buffer + β-Mercaptoethanol) was added to the reaction mixture and heated at 95°C for 5 min. The samples were subjected to Western blot analysis, by loading on a 15% SDS-page gel, blotted on a nitrocellulose membrane, and probed with either anti- α-syn or anti-ACR antibody. The sample with only α-syn protein was used as a control and normalization factor for quantification analysis.
Immunoprecipitation assay α-syn in samples from rat brains post-mild bTBI
Immunoprecipitation of the whole brain lysates from sham (control), 2-days, and 7-days post-injury was carried out using Pierce Classic IP Kit (ThermoFisher, #26146). In short, 2mg/mL protein lysate from each sample group was pre-cleared using the control agarose resin. The lysate (2 mg protein) was incubated with either 5 μL of anti-α-syn or anti-ACR primary antibody at 4°C overnight to form an immune complex. Twenty μL of Pierce A/G Agarose was added into the mixture to capture the immune complex and incubated at 4°C overnight. The elution of the immune complex was carried out using the 2x non-reducing lane marker sample buffer and DTT. The collection tubes were incubated at 100°C for 5 min and eluates were applied to 15% SDS-PAGE for immunoblotting with anti- α-syn and ACR was imaged using Azure c300 Western blot imaging system (Azure Biosystems, Dublin, CA).
Immunofluorescence staining
Animals were anesthetized and perfused with 4% paraformaldehyde in Krebs solution. Whole brains were extracted and placed in 15% sucrose solution until the tissue sinks, followed by a subsequent addition of 30% sucrose solution again until the tissue sinks. When ready, tissue was frozen in OCT and sectioned coronally at 25μm in a cryostat (Leica). Sections were mounted on glass slides immediately after cutting and stored at −20°C. Representative sections of the striatum and substantia nigra (Bregmas approximately 0.7mm and −6.04mm) were selected for staining. Sections were first hydrated in 1xPBS then permeabilized with 0.1% Triton X-100 followed by 3% Triton. Blocking was done with 10% immunobuffer and primary antibodies anti-ACR (Abcam, #37110) and anti- α-synuclein (α-syn) (BD Transduction, #610786), and were mixed in 10% immunobuffer and left to costain overnight at 4°C. Sections were incubated for 2hours at room temperature in secondary antibodies conjugated to fluorophores (Jackson Immuno, Alexa 488 and 594). Cell nuclei were labeled with 4′, 6-diamidino-2-phenylindole. Imaging was done with an Olympus IX51 fluorescence microscope. Figures were constructed using ImageJ with the FigureJ plugin.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). One way ANOVA with Tukey or Fisher post hoc and Student’s t test were used for statistical assessment. The statistical significance was set at p < 0.05.
RESULTS
Elevation of acrolein metabolite, 3-HPMA, post-mild blast traumatic brain injury
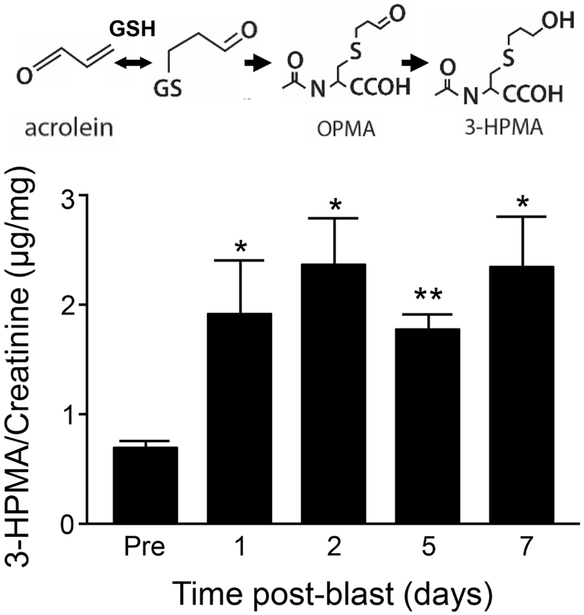
As indicated in Fig. 1, the endogenous acrolein metabolite, 3-HPMA (N-acetyl-S-(3hydroxypropyl) cysteine), was significantly increased at 1-day (1.93 ± 0.46 μg/mg, n=5), 2- day (2.37 ± 0.40 μg/mg, n=5), 5-days (1.78 ± 0.15 μg/mg, n=4), and 7-day (2.35 ±0.44 μg/mg, n=5) post-injury, when compared to their pre-injury baseline (1.13 ± 0.22 μg/mg, n=5, p < 0.05 or p < 0.01).
Figure 1.
Elevation of 3-HPMA in urine after mb-TBI. Upper panel: schematic of the acrolein reaction with glutathione and production of the metabolites OPMA and 3-HPMA. Lower panel: Urine 3-HPMA detected by LC-MS/MS normalized to urine creatinine content in the blast rats. Urine was collected before injury (baseline), 1, 2, 5 and 7 days post-injury. Repeated measures ANOVA indicate significant effect of day [F(4,2)=5.20, p=0.005]. Pairwise comparison of post-injury 3-HPMA levels to baseline show significant elevation of 3-HPMA that persists to at least the seventh day post-injury. Data presented as mean ± SEM. Fisher’s LSD post-hoc test. n=5 *p<0.05, **p<0.01.
Increased levels of acrolein-lysine protein adducts in the whole brain, STR and SN regions
Acrolein levels after mb-TBI were measured in the whole, STR (anterior and posterior) and substantia nigral brain region by Western blotting (Fig. 2). The data shows a significant increase of the acrolein-lysine protein adducts after injury compared to the control (uninjured) group in all cases. Specifically, a significant increase of acrolein-lysine adducts in the whole brain were detected in 2 day (22.96 ± 4.97 %, n=8) and 7 day (22.53 ± 8.01 %, n=8) post-injury groups compared to the control (100 ± 4.28 %, n=9; p<0.05) (Fig 2A,B).
Figure 2.
Acrolein-lysine adducts increase in whole brain preparation, striatum, and substantia nigra after mild bTBI. (A) Western Blot image of acrolein-lysine modified proteins (FDP-Lys) and β-actin for each group at control, 2-day and 7-day post-blast. All bands on the acrolein-lysine blot were used for analysis for each group. (B) Quantification demonstrated significant difference between control, 2-day post-injury, and 7-day post-injury [F(2,22)=5.20, p=0.014]. (C) Western Blot image of acrolein-lysine modified proteins (FDP-Lys) and β -actin for each group at control, 2-day and 7-day post-blast of the B5 (anterior) striatal region. All bands on the acrolein-lysine blot were used for analysis for each group. (D) Quantification demonstrated significant difference between control, 2-day post-injury, and 7-day post-injury [F(2,9)=6.58, p=0.017]. (E) Western Blot image of acrolein-lysine modified proteins (FDP-Lys) and β-actin for each group at control, 2-day and 7-day post-blast of the C5 (posterior) striatal region. (F) Quantification demonstrated significant difference between control, 2-day post-injury, and 7-day post-injury [F(2,10)=6.22, p=0.018]. . Acrolein-lysine adducts increase in substantia nigra region after mild bTBI. (G) Western Blot image of acrolein-lysine modified proteins (FDP-Lys) and β -actin for each group at control, 2-day and 7-day post-blast. All bands on the acrolein-lysine blot were used for analysis for each group. (H) Quantification demonstrated significant differences between control, 2-day post-injury, and 7-day post-injury [F(2,10)=5.61, p=0.023]. Data are relative, normalized as percent control and presented as mean ± SEM. Tukey’s post-hoc test. n=8-9 (whole brain) and n=4-5 (striatum and substantia nigra). *p<0.05, **p<0.01.
In addition, an elevation of acrolein-lysine adducts was detected in the anterior region of striatum in 2 day (93.18 ± 24.68 %, n = 4), and 7 day (60.89 ± 10.41 %, n = 4) post-injury when compared to control (100 ± 17.37 %, n=4; p < 0.01 for 2 days and p < 0.05 for 7 days post-injury) (Fig 2C,D). Similarly, elevation was detected in posterior striatal region in 2 day (98.44 ± 24.84 %, n=5), and 7 day (72.80 ± 20.49 %, n=4) postinjury when compared to control (100 ± 12.62 %, n=4; p < 0.01 for 2 days and p < 0.05 for 7 days post-injury comparison) as indicated in Fig 2E,F. In the substantia nigral region, there was also a significant increase of acrolein-lysine adducts in 2 day (81.66 ± 23.64 %, n=5), and 7 day (117.57 ± 29.07 %, n=5) post-injury compared to control (100 ± 19.8 %, n=4; p<0.05 or p<0.01 for 2 day or 7 day comparison) (Fig 2G,H).
Overall aberrant increased of α-syn levels post-mild bTBI
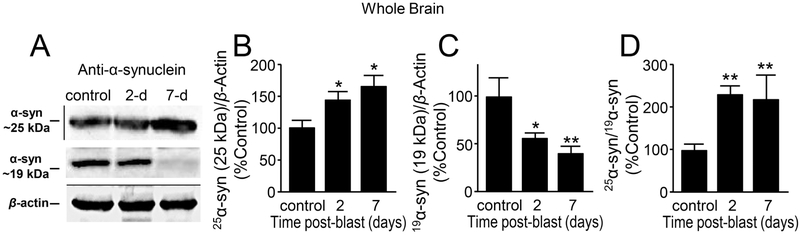
α-syn is the main protein present in the Lewy bodies (LB) of the surviving dopaminergic neurons in PD patients. It is a small, soluble protein at 14 kDa that is highly localized in the presynaptic terminals and can be essential for normal brain function (Galvin et al., 2001; Kim et al., 2000; Souza et al., 2000; Takeda et al., 1998). We measured the α-syn protein levels using Western blotting in the whole, striatal, and substantia nigral brain region lysates (Fig. 3, 4, 5). We observed two consistent bands of the α-syn protein at 25 kDa and 19 kDa (19 kDa is the predicted size of the antibody used, per mfr. BD Transduction). These bands were confirmed to be species of α-syn by blocking the antibody with α-syn purified protein before incubating the blots (Supplementary Fig. 1).
Figure 3.
Aberrant expression of α-synuclein in whole brain preparation after mb-TBI. (A) Western Blot image of α-syn at approximately 25kDA, α-syn at 19kDA, and β-actin at control, day 2 and day 7 post-blast. There are significant differences between control and 2-day post-injury, and 7-day post-injury in both (B) 25 kDa α-syn form [F(2,21)=5.77 p=0.01] and (C) 19 kDa α-syn form [F(2,24)=6.56, p=0.005]. (D) The normalized ratio of the 25 kDa and 19 kDa α-syn at control, 2 and 7 days post-blast indicates an increase in the proportion of 25 kDa α-syn [F(2,20)=5.83, p=0.01). Data in B-D are relative, normalized as percent control, and presented as mean ± SEM. Tukey’s post-hoc test. n=6-9 *p<0.05, **p<0.01.
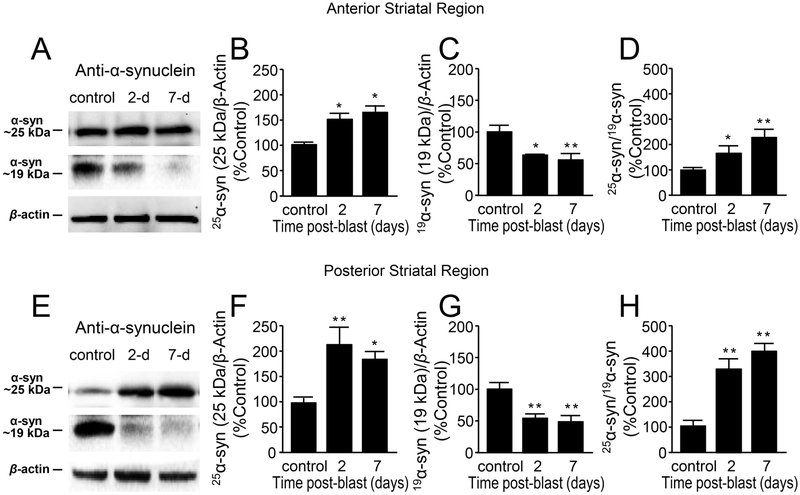
Figure 4.
Aberrant expression of α-synuclein in striatal region after mild bTBI. (A) B5 (anterior) striatal region Western blot image of α-syn at approximately 25kDA, α-syn at 19kDA and β-actin for control, day 2 and day 7 post-blast. There were significant differences between control, 2-day post-injury, and 7-day post-injury in both (B) 25 kDa [F(2,9)=5.05 p=0.034] and (C) 19 kDa [F(2,9)=6.24, P=0.020] forms of α-syn. (D) The normalized ratio of the 25 kDa and 19 kDa forms of α-syn at control, 2 and 7 days postblast indicates an increase in the proportion of 25kDa form of α-syn [F(2,9)=6.46, p=0.018]. (E) C5 (posterior) striatal region Western blot image of α-syn at approximately 25kDA, α-syn at 19kDA and β-actin for control, day 2 and day 7 post-blast. There were significant differences between control, 2-day post-injury, and 7-day post-injury in both (F) 25 kDa [F(2,10)=7.97 p=0.008] and (G) 19 kDa [F(2,10)=10.27, P=0.004] forms of α-syn. (H) The normalized ratio of the 25 kDa and 19 kDa forms of α-syn for control, 2 and 7 days post-blast indicates an increase in the proportion of 25kDa form of α-syn [F(2,10)=23.74, p=0.000]. Data in B-D and F-G are relative, normalized as percent control, and presented as mean ± SEM. Fisher’s LSD post-hoc test. n=4-5 *p<0.05, **p<0.01.
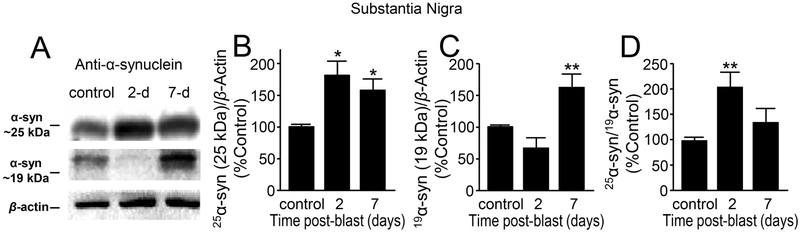
Figure 5.
Aberrant expression of α-synuclein in substantia nigra region after mb-TBI. (A) Western Blot image of α-syn at approximately 25kDA, α-syn at 19kDA and β -actin at control, day 2 and day 7 post-blast. There were significant differences between control, 2-day post-injury, and 7-day post-injury in both (B) 25 kDa [F(2,10)=5.15 p=0.02] and (C) 19 kDa forms of α-syn [F(2,10)=12.9, P=0.002]. (D) The normalized ratio of the 25 kDa and 19 kDa forms of a α-syn at control, 2 and 7 days post-blast indicates an increase in the proportion of 25 kDa α-syn [F(2,10)=5.08, p=0.03]. Data in B-D are relative, normalized as percent control, and presented as mean ± SEM. Fisher’s LSD post-hoc test. n=4-5 *p<0.05, **p<0.01.
In the whole brain analysis, α-syn at 25 kDa demonstrates a significant increase in 2 day (47.21 ± 11.10 %, n=9), 7-day (54.81 ± 15.61 %, n=7) post-injury compared to control (100 ± 10.39 %, n=8; p<0.05) (Fig. 3A,B). However, α-syn at 19 kDa was significantly decreased in at 2 day (44.54 ± 5.62 %, n=9), and 7 day (59.12 ± 5.78 %, n=7) post-injury (when compared to control, 100 ± 18.8 %, n=8) (p < 0.05 and p < 0.01 for 2 days and 7 days post-injury comparison respectively) (Fig. 3A,C). The ratio of 25 kDa and 19 kDa α-syn showed a significant increase in 2 day (132.80 ± 17.62 %, n=9) and 7 day (118.72 ± 55.75 %, n=6) post-injury compared to the control (100 ± 12.57 %, n=8; p<0.01) (Fig. 3D).
In the anterior striatal regions (Fig. 4A,B), the 25 kDa α-syn protein levels were significantly elevated at 2 day (49.50 ± 17.04 %, n = 4), and 7 day (63.07 ± 18.28 %, n=4) post-injury compared to control (100 ± 5.47 %, n = 4; p < 0.05). In the posterior striatal region, a similar increase of 25 kDa α-syn in 2 day (111.36 ± 37.08 %, n=4), and 7 day (84.87 ± 10.64 %, n=4) post-injury compared to the control (100 ± 8.89 %, n=5; p < 0.05) (Fig. 4E,F) was observed. However, as seen in the whole brain preparation (Fig. 3A,C), α-syn protein levels at 19 kDa show a significant decrease in both anterior and posterior striatal regions (p < 0.05 or p < 0.01, Fig. 4C,G). In addition, the ratio of 25 kDa and 19 kDa α-syn showed a significant increase in 2 day and 7 day post-injury in both anterior (Fig. 4D) and posterior (Fig. 4H) striatal regions compared to the control group (p<0.05 or p<0.01).
Finally, the α-syn at 25 kDa in the substantia nigral region also showed a significant increase in 2 day (42.08 ± 15.73 %, n=4), and 7 day (58.75 ± 19.61 %, n=4) post-injury compared to the control (100 ± 5.18 %, n=5; p < 0.05) (Fig. 5A,B). The α-syn at 19 kDa, however, had a decreased trend at the 2 day time point (28.23 ± 12.64 %, n=4), and a significant increase in 7-day (64.82 ± 20.39 %, n=4) post-injury compared to the control (100 ± 2.81 %, n=5, p<0.01) (Fig. 5A,C). The ratio of the 25 kDa and 19 kDa α-syn shows a significant increase at 2 day (105.11 ± 20.69 %, n=4), and a trend of increase at 7-day (19.77 ± 25.4, n=4) post-injury compared to the control group (100 ± 5.24, n=5; p < 0.01) (Fig. 5D).
Acute alterations in the tyrosine hydroxylase protein following mild bTBI
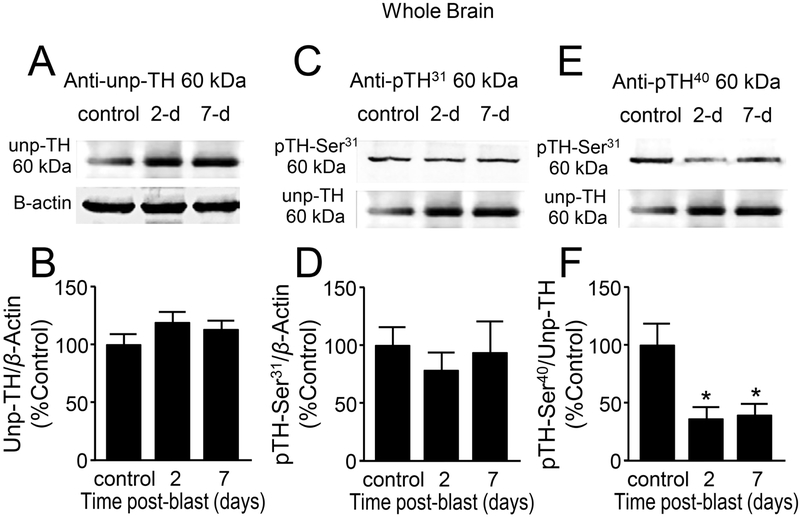
Tyrosine hydroxylase (TH) is the rate limiting enzyme in the production of dopamine from the amino acid tyrosine (Khan et al., 2012). In PD patients the TH activity is decreased in the nigro-striatal area resulting in decreased dopamine levels (Mogi et al., 1988; Nagatsu et al., 1964). We measured the protein levels of TH, un-phosphorylated form (Unp-TH), those with phosphorylation at either serine 31 or at serine 40, in the whole brain (Fig. 6), striatal (Fig. 7) and susbstantia nigral (Fig. 8) regions. The Unp-TH were normalized using β-actin and the phosphorylated TH, pSer31 and pSer40, were normalized with the Unp-TH, and values were expressed as percent control. In the whole brain lysates (Fig. 6A,C,E), there were no significant changes of Unp-TH protein levels (Fig. 6B, p > 0.05) and TH pSer31 (Fig. 6D, p > 0.05) when compared to control in both 2 and 7 days post-injury. However, TH pSer40 was significantly decreased at day 2 (62.70 ± 9.09%, n=8) and day 7 (60.10 ± 9.74%, n=7) post-injury compared to control (100 ± 21.74%, n = 9; p < 0.05) (Fig. 6F).
Figure 6.
Decreased phosphorylation of tyrosine hydroxylase (TH) at Ser40 suggests reduced TH activity after mb-TBI in whole brain preparation. (A,C,E) Western Blot images of un-phosphorylated TH (A), phosphorylated TH at Ser31(B), phosphorylated TH at Ser40(C), and β -actin for each group at control, 2 and 7 days post-blast. (B,D,F) Comparisons were made between control, 2-day post-injury, and 7-day post-injury in all three forms of TH. No changes were observed in (B) un-phosphorylated or (D) Ser31-phosphorylated TH. (F) Phosphorylation at Ser40, TH’s primary activation site, demonstrated significant reductions at both 2 and 7 days post-injury [F(2,21)=5.24, p=0.014]. Data in B,D,F are relative, normalized as percent control, and presented as mean ± SEM. Tukey’s post-hoc test. n=7-9 *p<0.05.
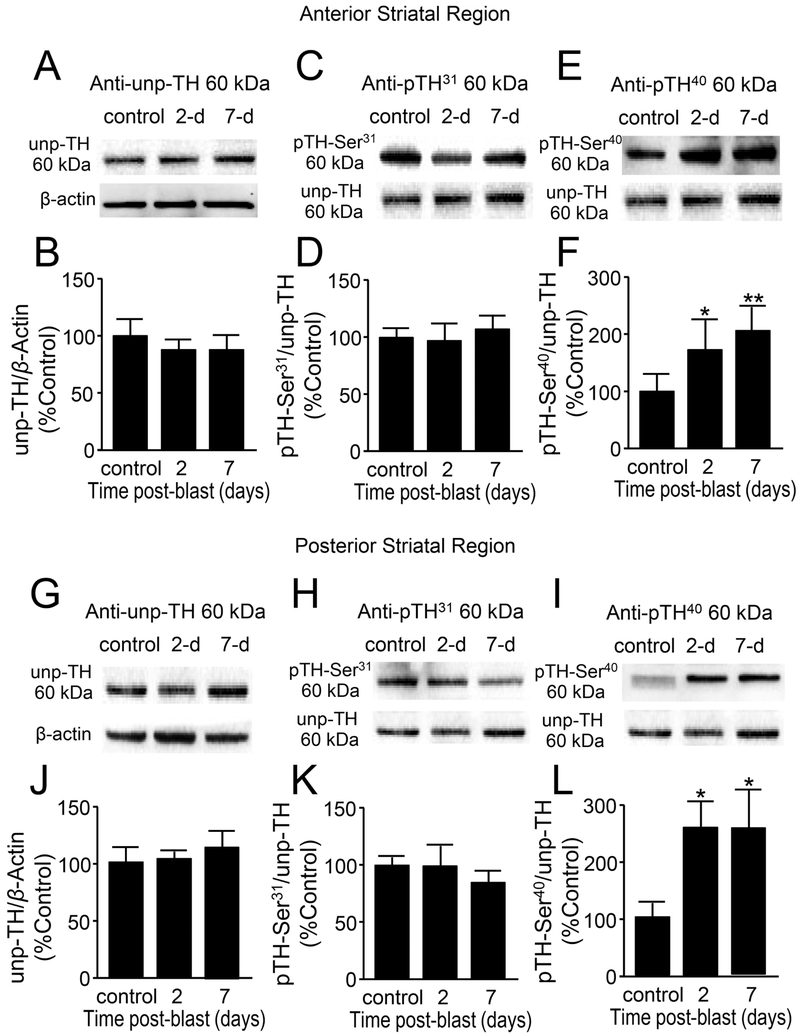
Figure 7.
Increased phosphorylation of tyrosine hydroxylase (TH) at Ser40 suggests increased TH activity in the striatum after mb-TBI. (A,C,E) B5 (anterior) striatal region Western Blot images of (A) un-phosphorylated TH, (C) phosphorylated TH at Ser31, (E) phosphorylated TH at Ser40, and β-actin for each group at control, 2 and 7 days postblast. (B,D,F) Comparisons were made between control and 2-day post-injury, as well as 7-day post-injury in al three forms of TH. (B) There were no significant changes in the un-phosphorylated TH at 2 and 7 days post-blast [F(2,10)=0.216, p=0.81]. (D) There were no significant changes in the Ser31-phosphorylated TH [F(2,10)=1.04, p=0.38] and (F) There is a significant increase in the Ser40-phosphorylated TH [F(2,10)=6.24, p=0.02]. (G,H,I) C5 (posterior) striatal region Western Blot images of (G) un-phosphorylated TH, (H) phosphorylated TH at Ser31, (I) phosphorylated TH at Ser40, and β-actin for each group at control, 2 and 7 days post-blast. (J,K,L) Comparisons were made between control and 2-day post-injury, as well as 7-day post-injury in all three forms of TH. (J) There were no significant changes in the un-phosphorylated TH at 2 and 7 days post-blast [F(2,10)=0.665, p=0.53]. (K) There were no significant changes in the Ser31-phosphorylated TH [F(2,10)=0.524, p=0.60] and (L) There is a significant increase in the Ser40-phosphorylated TH [F(2,10)=6.41, p=0.016]. Data in B,D,F and J,K,L are relative, normalized as percent control, and presented as mean ± SEM. Fisher’s LSD post-hoc test. n=4-5 *p<0.05, **p<0.01.
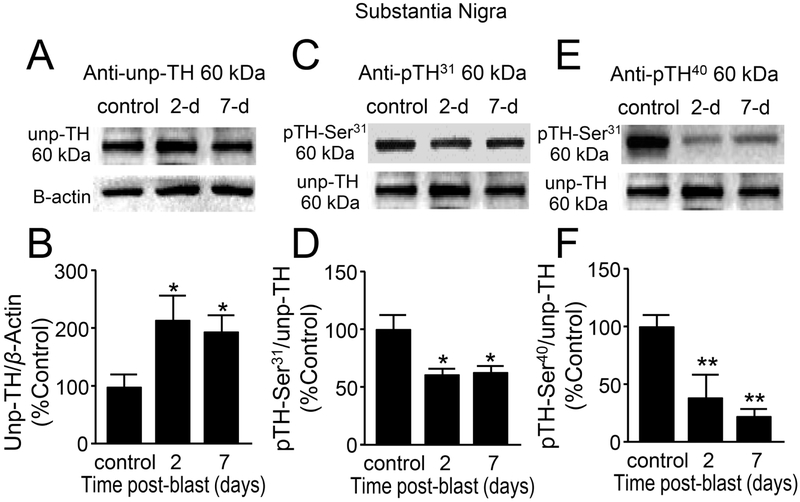
Figure 8.
Decreased phosphorylation of tyrosine hydroxylase (TH) at Ser40 and Ser31 suggest reduced TH activity in the substantia nigra region after mb-TBI. (A,C,E) Western Blot images of (A) un-phosphorylated TH, (C) phosphorylated TH at Ser31, (E) phosphorylated TH at Ser40, and β-actin for each group at control, 2 and 7 days postblast. (B,D,F) Comparisons were made between control and 2-day post-injury, as well as 7-day post-injury in all three forms of TH. (B) Un-phosphorylated TH was significantly increased at 2 and 7 days post-blast [F(2,10)=5.61, p=0.033]. (D) Ser31-phosphorylated TH [F(2,10)=5.96, p=0.02] and (F) Ser40-phosphorylated TH [F(2,10)= 12.81, p=0.002], TH’s two most common activation sites, both demonstrated significant reductions at 2 and 7 days post-injury. Data in B,D,F are relative, normalized as percent control, and presented as mean ± SEM. Fisher’s LSD post-hoc test, n-4-5 *p<0.05, **p<0.01.
In the striatal brain region, there were no significant changes of Unp-TH levels in both anterior, (Fig. 7A,B) and posterior striatal regions (Fig 7G,J) (compared to control, p > 0.05). Similarly, TH pSer31 expression showed no significant changes in anterior (Fig. 7C,D) or posterior striatal regions (Fig. 7H,K), when compared to control in both days 2 and 7 post-injury (p > 0.05). However, there was a significant increase of TH pSer40 in both anterior (Fig. 7E,F) and posterior regions (Fig. 7I, L) in days 2(n=4-5, p < 0.05 or p < 0.01 when compared to control) and 7 post injury (n=4-5, p<0.05 when compared to control).
In the substantia nigral region, three forms of TH were also measured (Fig. 8A-F) using Western blotting. Unlike in the whole brain or striatum, TH protein (Unp-TH) levels were significantly increased at 2 days (114.84 ± 40.08%, n=4) and 7 days (96.47 ± 26.29%, n=5) post-injury compared to the control group (100 ± 20.20%, n=4) (Fig. 8B, p < 0.05). However, TH pSer31 showed a significant decrease at 2 days (38.17 ± 3.62%, n=4) and 7 days (37.28 ± 5.04%, n=5) post-injury compared to control (100 ± 12.66 %, n=4; p<0.05) (Fig. 8D). Similarly, TH pSer40 showed a significant decrease at 2 days (60.87 ± 18.33%, n=4) and 7 days (77.98 ± 6.81%, n=5) post-injury compared to control (100 ± 8.43 %, n=4; p<0.01) (Fig. 8F).
Acrolein induces α-syn protein oligomerization and aggregation in vitro
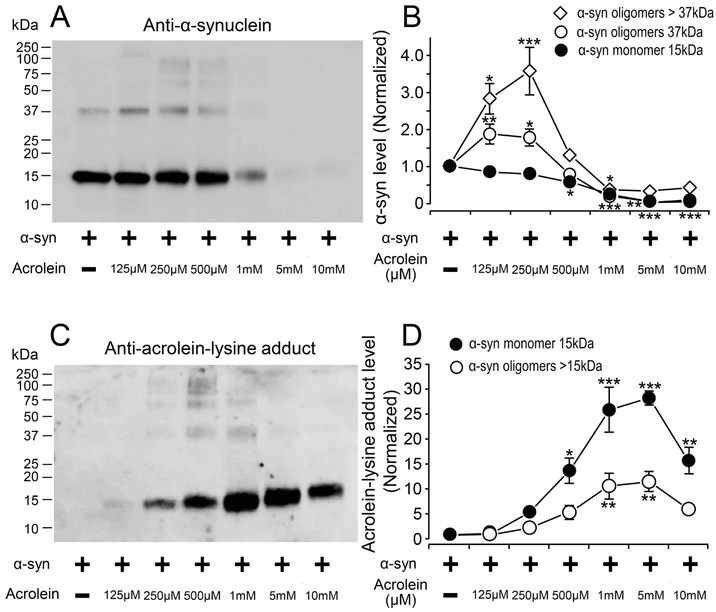
The effects of acrolein on the α-syn protein were studied in vitro using a protein. Purified α-syn protein samples were treated with acrolein for 20 hrs. All sample groups were run in triplicates. Western blot detection using anti-α-syn (Fig. 9A) indicates that α-syn at 15 kDa remained relatively constant with increasing acrolein concentrations up to 500 μM. At 1 mM of acrolein, this band started to fade as the acrolein concentration increased. However, quantification of the band at 37 kDa, which is a species of α-syn protein, shows a broader band with increasing acrolein concentrations starting at 125 μM. At 1 mM acrolein, this band starts to fade as the acrolein concentration increased, similar to that observed with 15 kDa band (Fig. 9A,B). In addition, speculated oligomerized and aggregated forms α-syn (bands above 37 kDa) were observed at 125 μM up to1 mM of acrolein and faded with higher concentrations of acrolein (Fig. 9A). Quantification of these observed bands is shown in Fig. 9B.
Figure 9.
Acrolein induces α-synuclein oligomerization and aggregation in vitro, α-synuclein (2.5 μM) was incubated alone and in the presence of ACR at different concentrations (0.125, 0.25, 0.5, 1, 5, and 10 mM) at 37°C for 20h. All samples were run in triplicates. Samples were separated by SDS-PAGE and blotted using either α-syn (A,B) or acrolein-lysine antibodies (C,D). (A) Immunoblotting image using anti- α-syn. (B) Quantification of the image in (A). Comparison of immunoreactivity for bands at 15 kDa [F(6,20)=32.16; p<0.001], 37 kDa [F(6,20)=31; p<0.001], and >37kDa [F(6,20)=19.83, p<0.001] showed significant effect across different acrolein concentrations. Compared to isolated α-syn incubation, there was a significant increase in α-syn oligomers when incubated with 125μM and 250μM acrolein (≥ 37kD), as well as a decrease in oligomers and monomers for all acrolein concentrations ≥500μM. (C) Immunoblotting images using anti-acrolein-lysine. (D) Quantification graph of the image on (C). Analysis of the bands at 15 kDa [F(6,20)=23.7; p<0.001] and >15 kDa [F(6,20)=8.8; p<0.001] showed significant effects. Compared to isolated α-syn incubation, there was a significant change in acrolein-modified α-syn levels with the addition of acrolein. Significant increases in acrolein-modified α-syn monomers were observed at all concentrations ≥500μM acrolein, while acrolein-modified α-syn oligomers significantly increased at 1 and 5 mM acrolein. Data in B,D are relative, normalized as percent control, and presented as mean ± SEM. Tukey’s post-hoc test. *p<0.05, **p<0.01, ***p<0.001.
Western blot detection using anti-acrolein-lys adduct antibody (Fig. 9C) demonstrates that the acrolein-modified α-syn protein bands at 15 kDa becomes broader with increasing acrolein concentrations and started to decrease at 10 mM of acrolein. Quantification of these bands is shown in Fig. 9D. Acrolein-modified proteins at higher molecular weights (> 15kDa) were also observed starting at 250 μM and up to 1 mM of acrolein (Fig. 9C). Quantification of these bands are shown in Fig. 9D. These data suggest that the α-syn aggregation and oligomerization is dose-dependent to acrolein exposure; and the formation of acrolein adducts is also proportional to the level of acrolein. Furthermore, these data suggest that the native α-syn protein likely degrades at the higher concentrations of acrolein, or it is modified so extensively by acrolein at its high concentrations that the antibody binding sites are blocked.
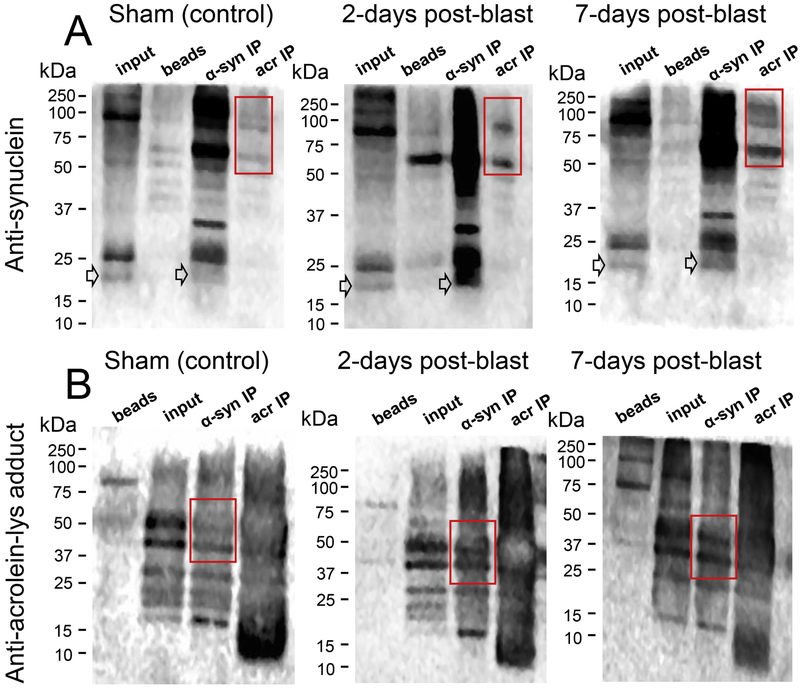
Increased interaction and co-localization between α-syn and acrolein in post-mild bTBI rats
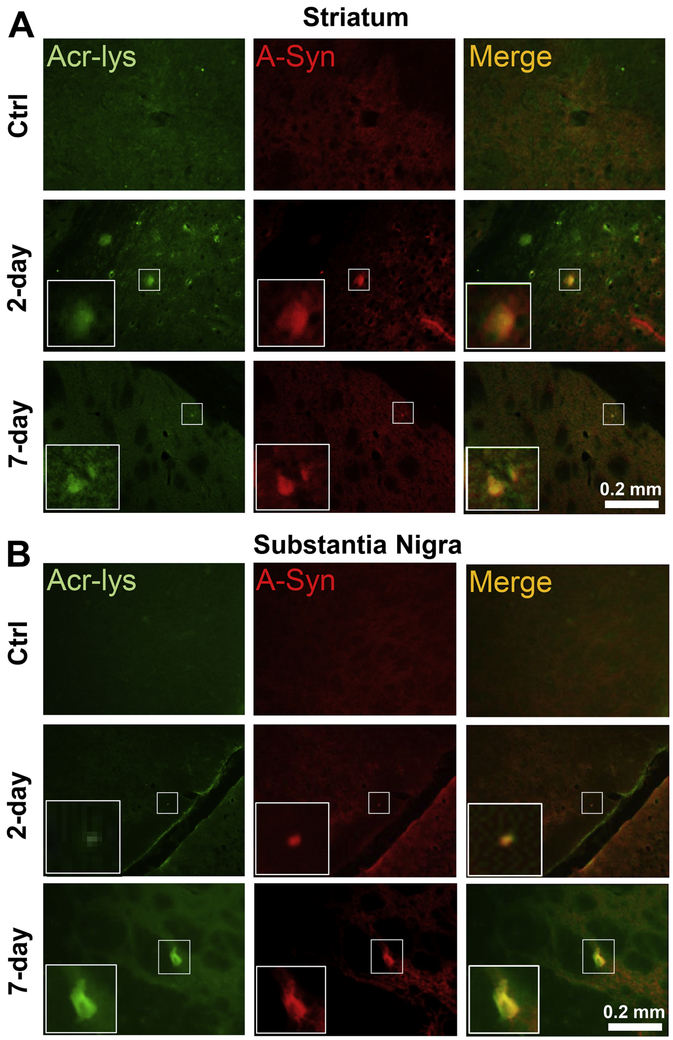
Coimmunoprecipitations (co-IPs) were conducted on whole brain lysates of the control, 2-day, and 7-day post-injury groups, with antibodies against α-syn and acrolein-lysine adducts. Consequently, immunoblots (IBs) using the same antibodies were performed to detect co-localization of these two molecules in each sample group. In the acrolein-lys-precipitated (Acr-IP) row for each sample group, α-syn species were detected in the α-syn IB (Fig. 10A), particularly distinct bands (red box) were observed and these bands are intensified in the 2-day and 7-day post-injury groups. Similarly, in the Acr-Lys adducts IB (Fig. 10B), bands in the α-syn IP row (red box) were detected and the intensity is increased in the 2-day and 7-day post-injured groups compared to the control group. Furthermore, immunofluorescence staining show increased co-localization of α-syn protein and acrolein-lysine adducts in the striatum (Fig 11A) and substantia nigra (Fig 11B) in both the 2-day and 7-day post-injury groups. These data suggest increasing levels of α-syn protein and acrolein-lysine adducts, and provide direct visual confirmation of their co-localization after blast injury, supporting the in vitro (Fig. 9) and in vivo (Fig. 10A,B) protein analyses and suggesting such a relationship may be possible.
Figure 10.
Acrolein modifies α-synuclein in vivo after mb-TBI. Whole brain lysates from control, 2 and 7 day post-blast rats were homogenized and immunoprecipitated with either α-syn or acrolein-lysine antibodies. Then, samples were subjected to SDS-PAGE and immunoblotting detection using anti-α-syn (A) or anti-acrolein-lysine (B) to complete the co-immunoprecipitation procedure. (A) Immunoblotting using α-syn antibody from each group’s brain lysate. Red boxes indicate bands of interest that are acrolein-modified α-syn proteins and show increased expression in injured rats. Arrows denote monomeric α-syn that can be observed at 19kDa in the α-syn IP and Input lanes. (B) Immunoblotting using acrolein-lysine antibody from each group’s brain lysate. Red boxes indicate acrolein-modified α-syn proteins and show increased expression after blast injury, reinforcing the result from (A).
Figure 11.
Post-injury elevation of co-localization of FDP-lysine and α-syn immunoreactivities. A) Representative immunofluorescence staining in the striatum shows sham Control (“Ctrl”) 2-day and 7-day post injury. Acrolein shown in green and α-syn in red, with areas of co-localization (“Merged”) appearing yellow. (B) Corresponding images for the substantia nigra region. White zoom boxes represent areas of increased immunoreactive staining intensity.
DISCUSSION
In the present investigation, we report the first direct evidence indicating the ability of acutely upregulated post-TBI oxidative stress / lipid peroxidation-related reactive aldehydes, namely acrolein, to modify α-syn, both in vitro and in vivo. We also demonstrate dysregulation of the primary dopamine synthesis pathway via alterations in TH phosphorylation sites in the whole brain and more specifically the nigrostriatal pathway. We assert that early post-injury oxidative stress / lipid peroxidation products after TBI play an important role in initiating and perpetuating neurodegenerative processes mimicking those observed in idiopathic PD, perhaps accelerating onset or heightening the susceptibility to Parkinsonian pathologies in TBI patients. Together, these data provide insight into putative biochemical mechanism/s which may link the acute and subacute pathophysiology of TBI to known PD-relevant neurodegenerative processes.
Oxidative stress and lipid peroxidation, as observed via increased acrolein, play a key role in secondary injury progression after TBI
We observed significant increases of 3-HPMA, an acrolein metabolite (Fig. 1), in the urine 1-7 days post injury, and acrolein-lysine adducts in post-mortem whole brain, STR and SN regions at 2 and 7 days post-injury (Fig. 2). These data support and expand the results of our previous study where we established a consistent animal mb-TBI model mimicking the human condition and observed brain elevation of acrolein-lysine adducts at 1 and 5 days post-injury alongside urine 3-HPMA elevation at 1 and 2 days post-injury(Walls et al., 2016). The examination of long-term elevation of acrolein following mb-TBI is ongoing, and is expected to be the topic of a separate, future publication. Our speculation is that the elevation of acrolein post-mb-TBI beyond one week is likely, as we have consistently observed in spinal cord injury, another CNS trauma (Due et al., 2014; Park et al., 2014, 2015). We reason that, even when acrolein elevation cannot be detected through systemic (urine) or brain tissue (Western blot), it is still possible that using more sensitive techniques (immunohistochemistry/microscopy), we could reveal the elevation of acrolein in certain brain or cellular regions. The elevation of acrolein and other reactive aldehydes in TBI has long been associated with the mortality rate in TBI, suggesting this class of molecules as putative surrogate biomarkers for the injury (Butterfield and Reed, 2016; Lorente et al., 2015; Santos et al., 2005; Shao et al., 2006; Wu et al., 2014).
In the central nervous system, the majority of oxidative stress is manifested by lipid peroxidation, which leads to the generation of reactive aldehydes such as acrolein, 4-hydroxy-2-nonenal (HNE), and malionaldehyde (MDA) (Butterfield et al., 2002; Pugazhenthi et al., 2006; Uchida, 2003). Acrolein is the most reactive of the α,β-unsaturated aldehydes produced endogenously, has a longer half-life in comparison to reactive oxidative stress molecules (Burcham et al., 2002; Luo and Shi, 2005), and has been independently associated with PD (Ambaw et al., 2018; Shamoto-Nagai et al., 2007), Alzheimer’s disease (Butterfield et al., 2001; Lovell et al., 2001), multiple sclerosis (MS) (Leung et al., 2011; Tully et al., 2018), and spinal cord injury (SCI) pathophysiology (Luo and Shi, 2004).
On proteins, specifically, acrolein preferentially binds to three functional groups with high affinity: the sulfhydryl group of cysteine, the amino group of lysine, and imidazole group of histidine, forming covalent adducts (Esterbauer et al., 1991; Uchida et al., 1998a; Uchida et al., 1998b) which can subsequently affect protein expression and function. TBI has been documented to alter the proteome, namely via post-translational modifications (PTMs) of key proteins such as microtubule-associated proteins (MAP2A/2B), hexokinase, and ubiquitin carboxy-terminal hydrolase L1 protein (UCHL-1) (Guingab-Cagmat et al., 2013; Kobeissy et al., 2006; Lazarus et al., 2015). These PTMs can lead to the dysfunction of the proteins and increase the risk of diseases (Butterfield et al., 2002; Butterfield and Reed, 2016). Our data suggest PTMs by acrolein and subsequent dysregulation of PD-related proteins (α-syn and TH, discussed in more detail below) in the nigrostriatal regions after TBI as a possible point of convergence between brain trauma and PD. More broadly, our findings implicate oxidative stress as a common post-TBI phenomenon capable of triggering a biochemical cascade altering protein structure and function which, left unmitigated, could lead to long-term post-TBI consequences including neurodegeneration.
Acrolein affects expression and promotes oligomerization of α-syn: A PD-like post-TBI neuropathology
α-Syn is the main protein present in the Lewy Bodies found in the surviving dopaminergic neurons of PD patients. It is a small, soluble protein that is highly localized in presynaptic terminals (Khan et al., 2012; Maroteaux et al., 1988), and can be essential to normal brain function (Galvin et al., 2001; Kim et al., 2000; Souza et al., 2000; Takeda et al., 1998). While its exact function(s) are not completely understood, α-syn has been reported to facilitate membrane trafficking, dopamine regulation, and synaptic plasticity (Rokad et al., 2016). Overexpression of α-syn has been observed in animal models of TBI (Acosta et al., 2015; Shahaduzzaman et al., 2013; Surgucheva et al., 2014; Uryu et al., 2003), but the mechanisms by which PD-like neuropathologies are exacerbated by TBI remain ambiguous.
In this study we observed, in the whole brain, STR and the SN areas, two species of α-syn protein: 25 kDa and predicted form at 19 kDa. Confirmatory experiments have shown that both of these bands are α-syn (Supplement Fig. 1). In the whole brain (Fig. 3B), STR (Fig. 4B,F), and SN (Fig. 5B) regions the expression of the 25 kDa α-syn was increased post-injury. Similarly the ratio of the 25 kDa to 19 kDa forms (Fig. 3D, 4D,H, 5D) was elevated. These data indicate an increase in the proportion of overall α-syn accounted for by the 25 kDa form of α-syn in the whole brain, STR and SN regions 2 and 7 days post-mb-TBI. These results are in accordance with preclinical and clinical studies where TBI promoted the overexpression of α-syn (Acosta et al., 2015; Bower et al., 2003; Goldman et al., 2012; Goldman et al., 2006; Shahaduzzaman et al., 2013; Surgucheva et al., 2014). In contrast, we observed a gradual significant decrease in levels of 19 kDa predicted form of α-syn in the whole brain (Fig. 3C) and the STR (Fig. 4C,G) post-injury. Interestingly, in the SN-area there was a trend of decrease in 19 kDa α-syn two days after blast and a significant increase seven days post injury (Fig. 5C). We suspect that the 19 kDa predicted form α-syn is the “native/normal” state of the protein and our data suggest that this is decreasing post-injury. Consequently, we speculate the 25 kDa to be a post-translationally modified (PTM) form of α-syn and increase post-injury. However, the nature of this species of α-syn needs to be further investigated. In addition, the elevation of 19 kDa α-syn may represent an up regulation of native α-syn in the SN-area which remains to be investigated. Together, these results suggest that a spatiotemporally dynamic, aberrant expression pattern of α-syn occurs after mb-TBI.
Currently, little is known about the mechanism leading to post-TBI aggregation of α-syn. However, one study suggests PTMs and protein dimerization can be a rate-limiting step of its aggregation en route to LB formation (Pivato et al., 2012). The structure of α-syn may be one reason that this protein is particularly vulnerable to PTMs by reactive aldehyde species, namely acrolein. The protein structure of α-syn contains seven imperfect repeats of 11 amino acids, which form N-terminal helices, a central hydrophobic domain, and acidic rich C-terminus. The amino acid lysine makes up 13.39% of the total amino acids of the α-syn proteome. These lysine-rich regions are ubiquitination sites, a common PTM event for α-syn, and contribute to the structural stability of the protein (Ulmer and Bax, 2005; Ulmer et al., 2005). We speculate that the disruption of these lysine residues, one of the aforementioned high-affinity targets for acrolein-protein binding, are potentially interrupting the structural stability of the protein leading to PTM and oligomerization as observed in our mb-TBI rats. In support of this notion, we build upon previous work with A53T mutant α-syn by demonstrating that acrolein induces in vitro oligomerization not only of an oligomerization prone variant, but also of wild-type α-syn, (Fig. 9) and that acrolein modifies/co-localizes with α-syn in vivo post-blast TBI (Fig. 10,11) (Ambaw et al., 2018). These results resemble the familial mutants of α-syn that promote its toxic oligomerization and aggregation, a critical step in Lewy body (LB) formation (Chung et al., 2001; Winner et al., 2011).
To our knowledge, these findings document the first evidence of acrolein co-localization with α-syn after TBI. Interestingly, however, acrolein-α-syn co-localization has previously been observed in LBs within the SN of PD patients (Shamoto-Nagai et al., 2007) and injection of acrolein into the SN induces α-syn aggregation and cell death (Wang et al., 2017), further suggesting acrolein is a capable and likely causal factor promoting α-syn aggregation. We thus emphasize that the elevation of acrolein may be a critical secondary injury process in promoting PD-like degenerative processes after TBI, specifically via α-syn oligomerization/aggregation.
Disruption of dopaminergic synthesis via dysregulation of tyrosine hydroxylase
Tyrosine hydroxylase (TH) is the key enzyme for dopamine (DA) synthesis in the dopaminergic neurons and its terminals. TH function is regulated via its phosphorylation sites, mainly at serine 31 and serine 40 (Nagatsu et al., 1964; Zhu et al., 2012). In PD patients, TH activity is decreased in the nigro-striatal area, which results in the reduction of dopamine levels leading to the cardinal symptoms of PD (Bademci et al., 2010). In addition, decreased TH is an indirect indication of dopaminergic neuron damage/death in the SN in TBI rodent models (Acosta et al., 2015; Shahaduzzaman et al., 2013).
In our study, whole brain levels of TH pSer40 (Fig. 6F), were significantly decreased after mb-TBI while total TH (Unp-TH) and TH pSer31 (Fig. 6B, 6D) were not affected. In the SN-area, both TH pSer31 and pSer40 sites (Fig. 8D,F) levels were decreased and the total TH protein (Fig. 8b) showed significant increase. The decreased levels of TH pSer40, the most studied and directly implicated phosphorylation / activation site of DA synthesis, suggest decreased activity of TH after injury in the whole brain and SN. Furthermore, the changes observed in the TH protein and TH pSer31 in the SN (Fig. 8B,D) is perhaps due to the specific function of these neurons. The dopaminergic neurons of the SN mainly regulate motor function via the nigrostriatal projections into the striatum (Maxwell and Li, 2005). Interestingly, in the STR region TH pSer40 levels were significantly increased post injury (Fig. 7F,L). There have been conflicting results regarding striatal TH activity after TBI (Lindgren et al., 2000; Shin et al., 2011). The increase of striatal TH activity is thought to be a compensatory action of the striatum (Shin et al., 2011). The different levels of TH and its phosphorylation sites we observed post-injury may be due to the complexity of the dopaminergic neurotransmission, specifically in the SN and STR. The TH protein is found to be expressed much greater in the terminal fields compared to the somatodendritic compartments of the nigrostriatal pathways (Salvatore et al., 2016). Furthermore, dopaminergic neurons in the SN are known to be more susceptible to oxidative processes than dopaminergic neurons found elsewhere in the brain (Burns et al., 1983; German et al., 1988; Hung and Lee, 1998; Wang and Michaelis, 2010; Waters et al., 1987). Differential mechanical injury profiles between brain regions could also contribute, wherein the SN experiences more injurious loading conditions than the STR, as our lab’s computational biomechanics work in this mb-TBI model has suggested (Garcia-Gonzalez et al., 2018). Regardless of the underlying mechanism, these data suggest differential localization and regulation of the TH protein between different brain loci, in a similar fashion to the α-syn protein, after injury. Our data suggest a putative mechanism for disruption of the brain’s supply of dopamine, but the source of the disruption remains unclear. To our knowledge, this is the first time that the expression of TH protein levels and its phosphorylation sites have been quantified in the whole brain, STR, and SN-area after a single mild blast TBI. This study provides further evidence that the dopaminergic system is acutely altered by TBI.
The decrease of TH activity as measured by the protein levels specifically on its pSer40 and pSer31 phosphorylation sites in the whole brain and SN-area after mb-TBI may occur through several mechanisms. Possibly the most important regulatory protein of TH activity is α-syn, which is also directly implicated in PD pathology as discussed above. The abnormal expression of α-syn has been demonstrated to inhibit TH expression and activity in both in vitro and in vivo studies (Khan et al., 2012). TH function is hindered in both transgenic mice overexpressing α-syn and in dopaminergic cells transfected with α-syn (Beal, 2003; Zhang et al., 2003). Additionally, transfection of α-syn in MES 23.5 dopaminergic cell line induces cell injury and decreases TH gene expression and protein levels (Daadi et al., 2006). Furthermore, α-syn has similar protein homology to adapter protein 14-3-3, which promotes TH activation and is thought to function as a chaperone protein (Fujita et al., 2006; Kim et al., 2002; Kim et al., 2000; Ostrerova et al., 1999; Souza et al., 2000). The 14-3-3 chaperone protein enhances the major kinases’ function to activate TH at pSer40, thereby increasing DA synthesis (Itagaki et al., 1999; Tofaris et al., 2003; Toska et al., 2002). We speculate that the overall increased of aberrant α-syn levels post mb-TBI may affect TH regulation as observed in our study.
It is also possible that acrolein can modulate the structure and function of TH directly, as we observed for α-syn (Ambaw et al., 2018), or indirectly by altering entangled signaling pathways or molecules involved in dopamine synthesis. For the indirect option, TH is an enzyme in which its activity is regulated by many kinases and phosphatases. The main activating kinases that regulate TH phosphorylation at Ser40 are the cAMP-dependent protein kinase A (PKA) and the mitogen-activated protein kinase-activated protein kinase 2 (MAPKAP-K2) (Khan et al., 2012; Sutherland et al., 1993; Thomas et al., 1997). In contrast, protein kinase C (PKC), which is highly expressed in SN dopaminergic neurons and co-localizes with TH (Khan et al., 2012; Polanski et al., 2011), has been shown to decrease TH activity and DA synthesis. Many of acrolein’s documented effects on biological systems are attributed to activation of kinases similar to those mentioned above (Ranganna et al., 2002; Tanel and Averill-Bates, 2007), however the exact mechanisms remain unknown (Randall et al., 2013; Ranganna et al., 2002; Tanel and Averill-Bates, 2007). Such pathways would be logical targets to investigate how acrolein may impact TH activity-modifying molecules.
The potential of oxidative stress, specifically mediated by acrolein, to modulate any of the aforementioned proteins may be critical for PD progression and/or induction of PD-like pathologies resulting from TBI. We speculate that the aberrations of α-syn after mb-TBI due to acrolein elevation play a role in the dysregulation of TH in the whole brain and SN-area. In relation to the interplay between the kinases and phosphatases mentioned above, TH and α-syn alterations in the presence of acrolein need to be further validated and investigated in depth to fully understand how TBI pathophysiology can potentiate PD-like degenerative processes.
CONCLUSIONS
Increased acrolein levels in our mb-TBI rat model indicate ongoing acute to subacute oxidative stress and lipid peroxidation cascades in the brain post-TBI consistent with prior reports, suggesting these pathways may be critical initiators of later stage TBI pathophysiology including inflammatory and degenerative processes. As demonstrated herein, acrolein may serve as a pathological link between TBI and PD, opening new avenues for therapeutic targets to mitigate the long-term consequences in TBI patients. These findings warrant further investigation into the clinical relevance of acrolein and related aldehydes, as well as its direct role in and mechanisms for modifying α-syn post-injury. We hope this study sparks new avenues for mechanistic and translational research, particularly for preventative therapeutic interventions targeting oxidative stress and/or lipid peroxidation after TBI.
Supplementary Material
Supplemental Figure 1. Western Blot confirming the 25 kDa and 19 kDa forms α-synuclein. Whole brain protein lysates from rats were used at the same concentration for samples 1-3. Blots were run simultaneously. (A) Western blot image of samples 1-3 incubated with the α-syn antibody. (B) Western blot image of samples 1-3 incubated with the blocked α-syn antibody. The antibody was blocked with purified α -syn protein for 2 hours at 4°C prior to blot incubation. The absence of distinct bands at 25 kDa and 19 kDa confirmed that these were α -syn protein species.
Highlights:
Acrolein is elevated systemically in urine, and in whole brain tissue, specifically the substantia nigra and striatum, following a mild blast-induced traumatic brain injury (bTBI).
Heightened α- synuclein oligomerization, dopaminergic dysregulation, and acrolein/α-synuclein interaction cab be detected in substantia nigra and striatum post-bTBI.
Acrolein can directly modify and oligomerize α- synuclein in vitro.
Acrolein likely plays an important role in inducing Parkinson’s diseases pathology following bTBI by encouraging α-synuclein aggregation.
ACKNOWLEDGMENTS:
This work was supported by the Indiana State Department of Health [Grant # 204200 to RS], National Institutes of Health [Grant # NS073636 to RS]. Glen Acosta was partially supported by Eli Lilly Stark Neurosciences Pre-Doctoral Research Fellowship. The authors thank Dr. Rob Schuster from the Laboratory of Dr. Chris Rochet at Department of Medicinal Chemistry and Molecular Pharmacology of Purdue University for providing the purified α-syn protein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
Riyi Shi is the co-founder of Neuro Vigor, a start-up company with business interests of developing effective therapies for CNS neurodegenerative diseases and trauma.
REFERENCES
- Acosta SA, Tajiri N, de la Pena I, Bastawrous M, Sanberg PR, Kaneko Y, Borlongan CV, 2015. Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson's disease. J Cell Physiol 230, 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambaw A, Zheng L, Tambe MA, Strathearn KE, Acosta G, Hubers SA, Liu F, Herr SA, Tang J, Truong A, Walls E, Pond A, Rochet JC, Shi R, 2018. Acrolein-mediated neuronal cell death and alpha-synuclein aggregation: Implications for Parkinson's disease. Mol Cell Neurosci 88, 70–82. [DOI] [PubMed] [Google Scholar]
- Bademci G, Edwards TL, Torres AL, Scott WK, Zuchner S, Martin ER, Vance JM, Wang L, 2010. A rare novel deletion of the tyrosine hydroxylase gene in Parkinson disease. Hum Mutat 31, E1767–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, 2003. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann N Y Acad Sci 991, 120–131. [DOI] [PubMed] [Google Scholar]
- Bell RS, Vo AH, Neal CJ, Tigno J, Roberts R, Mossop C, Dunne JR, Armonda RA, 2009. Military traumatic brain and spinal column injury: a 5-year study of the impact blast and other military grade weaponry on the central nervous system. J Trauma 66, S104–111. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F, 1973. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20, 415–455. [DOI] [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA, 2003. Head trauma preceding PD: a case-control study. Neurology 60, 1610–1615. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, Pyke SM, 2002. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology 181-182, 229–236. [DOI] [PubMed] [Google Scholar]
- Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ, 1983. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A 80, 4546–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J, 2002. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging 23, 655–664. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A, 2001. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med 7, 548–554. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed TT, 2016. Lipid peroxidation and tyrosine nitration in traumatic brain injury: Insights into secondary injury from redox proteomics. Proteomics Clin Appl 10, 1191–1204. [DOI] [PubMed] [Google Scholar]
- Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, Sayer NA, 2010. Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. J Trauma Stress 23, 17–24. [DOI] [PubMed] [Google Scholar]
- Castellani R, Smith MA, Richey PL, Perry G, 1996. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res 737, 195–200. [DOI] [PubMed] [Google Scholar]
- Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, Xu L, Slack N, Windle D, Ahmed FA, 2011. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis 41, 538–551. [DOI] [PubMed] [Google Scholar]
- Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM, 2001. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 7, 1144–1150. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Pivirotto P, Bringas J, Cunningham J, Forsayeth J, Eberling J, Bankiewicz KS, 2006. Distribution of AAV2-hAADC-transduced cells after 3 years in Parkinsonian monkeys. Neuroreport 17, 201–204. [DOI] [PubMed] [Google Scholar]
- DePalma RG, Burris DG, Champion HR, Hodgson MJ, 2005. Blast injuries. The New England journal of medicine 352, 1335–1342. [DOI] [PubMed] [Google Scholar]
- Desmoulin GT, Dionne JP, 2009. Blast-induced neurotrauma: surrogate use, loading mechanisms, and cellular responses. J Trauma 67, 1113–1122. [DOI] [PubMed] [Google Scholar]
- Due M, Park J, Zheng L, Walls M, Allette Y, White F Shi R, 2014. Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat. J Neurochem 128, 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Cristian A, 2009. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt Sinai J Med 76, 111–118. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H, 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine 11, 81–128. [DOI] [PubMed] [Google Scholar]
- Fujita M, Wei J, Nakai M, Masliah E, Hashimoto M, 2006. Chaperone and anti-chaperone: two-faced synuclein as stimulator of synaptic evolution. Neuropathology 26, 383–392. [DOI] [PubMed] [Google Scholar]
- Galarneau MR, Woodruff SI, Dye JL, Mohrle CR, Wade AL, 2008. Traumatic brain injury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg 108, 950–957. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Lee VM, Trojanowski JQ, 2001. Synucleinopathies: clinical and pathological implications. Arch Neurol 58, 186–190. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalez D, Race NS, Voets NL, Jenkins DR, Sotiropoulos SN, Acosta G, Cruz-Haces M, Tang J, Shi R, Jerusalem A, 2018. Cognition based bTBI mechanistic criteria; a tool for preventive and therapeutic innovations. Sci Rep 8, 10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Dubach M, Askari S, Speciale SG, Bowden DM, 1988. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience 24, 161–174. [DOI] [PubMed] [Google Scholar]
- Glover LE, Tajiri N, Lau T, Kaneko Y, van Loveren H, Borlongan CV, 2012. Immediate, but not delayed, microsurgical skull reconstruction exacerbates brain damage in experimental traumatic brain injury model. PLoS One 7, e33646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SM, Kamel F, Ross GW, Jewell SA, Bhudhikanok GS, Umbach D, Marras C, Hauser RA, Jankovic J, Factor SA, Bressman S, Lyons KE, Meng C, Korell M, Roucoux DF, Hoppin JA, Sandler DP, Langston JW, Tanner CM, 2012. Head injury, alpha-synuclein Rep1, and Parkinson's disease. Annals of neurology 71, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW, 2006. Head injury and Parkinson's disease risk in twins. Annals of neurology 60, 65–72. [DOI] [PubMed] [Google Scholar]
- Guingab-Cagmat JD, Cagmat EB, Hayes RL, Anagli J, 2013. Integration of proteomics, bioinformatics, and systems biology in traumatic brain injury biomarker discovery. Front Neurol 4, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA, 2008. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. The New England journal of medicine 358, 453–463. [DOI] [PubMed] [Google Scholar]
- Hung HC, Lee EH, 1998. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med 24, 76–84. [DOI] [PubMed] [Google Scholar]
- Itagaki C, Isobe T, Taoka M, Natsume T, Nomura N, Horigome T, Omata S, Ichinose H, Nagatsu T, Greene LA, Ichimura T, 1999. Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry 38, 15673–15680. [DOI] [PubMed] [Google Scholar]
- Jafari S, Etminan M, Aminzadeh F, Samii A, 2013. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Movement disorders : official journal of the Movement Disorder Society 28, 1222–1229. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2012. Axonal pathology in traumatic brain injury. Experimental neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W, Priyadarshini M, Zakai HA, Kamal MA, Alam Q, 2012. A brief overview of tyrosine hydroxylase and alpha-synuclein in the Parkinsonian brain. CNS Neurol Disord Drug Targets 11, 456–462. [DOI] [PubMed] [Google Scholar]
- Kim TD, Paik SR, Yang CH, 2002. Structural and functional implications of C-terminal regions of alpha-synuclein. Biochemistry 41, 13782–13790. [DOI] [PubMed] [Google Scholar]
- Kim TD, Paik SR, Yang CH, Kim J, 2000. Structural changes in alpha-synuclein affect its chaperone-like activity in vitro. Protein Sci 9, 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O, 1988. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. The New England journal of medicine 318, 876–880. [DOI] [PubMed] [Google Scholar]
- Kobeissy FH, Ottens AK, Zhang Z, Liu MC, Denslow ND, Dave JR, Tortella FC, Hayes RL, Wang KK, 2006. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol Cell Proteomics 5, 1887–1898. [DOI] [PubMed] [Google Scholar]
- Laino C, 2005. Military deployment may raise risk of Parkinson disease. Neurology Today 5, 48. [Google Scholar]
- Lazarus RC, Buonora JE, Jacobowitz DM, Mueller GP, 2015. Protein carbonylation after traumatic brain injury: cell specificity, regional susceptibility, and gender differences. Free Radic Biol Med 78, 89–100. [DOI] [PubMed] [Google Scholar]
- Lee PC, Bordelon Y, Bronstein J, Ritz B, 2012. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology 79, 2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G, Sun W, Zheng L, Brookes S, Tully M, Shi R, 2011. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse. Neuroscience 173, 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Lindskog M, Herrera-Marschitz M, Goiny M, Haycock J, Goldstein M, Hokfelt T, Fisone G, 2000. Regulation of tyrosine hydroxylase activity and phosphorylation at Ser(19) and Ser(40) via activation of glutamate NMDA receptors in rat striatum. J Neurochem 74, 2470–2477. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P, 2004. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc Natl Acad Sci U S A 101, 17510–17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente L, Martin MM, Abreu-Gonzalez P, Ramos L, Argueso M, Caceres JJ, Sole-Violan J, Lorenzo JM, Molina I, Jimenez A, 2015. Association between serum malondialdehyde levels and mortality in patients with severe brain trauma injury. J Neurotrauma 32, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR, 2001. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiology of Aging 22, 187–194. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R, 2004. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem Int 44, 475–486. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R, 2005. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int 46, 243–252. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH, 1988. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8, 2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SL, Li M, 2005. Midbrain dopaminergic development in vivo and in vitro from embryonic stem cells. J Anat 207, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, 2012. Neuropathology. Blast injuries linked to neurodegeneration in veterans. Science 336, 790–791. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kiuchi K, Kojima K, Kondo T, Narabayashi H, Rausch D, Riederer P, Jellinger K, Nagatsu T, 1988. Homospecific activity (activity per enzyme protein) of tyrosine hydroxylase increases in parkinsonian brain. J Neural Transm 72, 77–82. [DOI] [PubMed] [Google Scholar]
- Morrish PK, Sawle GV, Brooks DJ, 1995. Clinical and [18F] dopa PET findings in early Parkinson's disease. J Neurol Neurosurg Psychiatry 59, 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S, 1964. Tyrosine Hydroxylase. The Initial Step in Norepinephrine Biosynthesis. J Biol Chem 239, 2910–2917. [PubMed] [Google Scholar]
- Nakashima A, Ota A, Kaneko YS, Mori K, Nagasaki H, Nagatsu T, 2013. A possible pathophysiological role of tyrosine hydroxylase in Parkinson's disease suggested by postmortem brain biochemistry: a contribution for the special 70th birthday symposium in honor of Prof. Peter Riederer. J Neural Transm (Vienna) 120, 49–54. [DOI] [PubMed] [Google Scholar]
- Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B, 1999. alpha-synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci 19, 5782–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AD, Schapira AH, Jenner P, Marsden CD, 1997. Indices of oxidative stress in Parkinson's disease, Alzheimer's disease and dementia with Lewy bodies. J Neural Transm Suppl 51, 167–173. [DOI] [PubMed] [Google Scholar]
- Park J, Zheng L, Acosta G, Vega-Alvarez S, Chen Z, Muratori B, Cao P, Shi R., 2015. Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury. J Neurochem 135, 987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zheng L, Marquis A, Walls M, Duerstock B, Pond A, Vega-Alvarez S, Wang H, Ouyang Z Shi R, 2014. Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J Neurochem 129, 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1986. The rat brain in stereotaxic coordinates 3rd ed. Academic Press, Sydney. [DOI] [PubMed] [Google Scholar]
- Pivato M, De Franceschi G, Tosatto L, Frare E, Kumar D, Aioanei D, Brucale M, Tessari I, Bisaglia M, Samori B, de Laureto PP, Bubacco L, 2012. Covalent alpha-synuclein dimers: chemico-physical and aggregation properties. PLoS One 7, e50027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanski W, Reichmann H, Gille G, 2011. Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-beta-carboline: a new anti-Parkinson drug? Expert Rev Neurother 11, 845–860. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Phansalkar K, Audesirk G, West A, Cabell L, 2006. Differential regulation of c-jun and CREB by acrolein and 4-hydroxynonenal. Free Radic Biol Med 40, 21–34. [DOI] [PubMed] [Google Scholar]
- Randall MJ, Spiess PC, Hristova M, Hondal RJ, van der Vliet A, 2013. Acrolein-induced activation of mitogen-activated protein kinase signaling is mediated by alkylation of thioredoxin reductase and thioredoxin 1. Redox Biol 1, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganna K, Yousefipour Z, Nasif R, Yatsu FM, Milton SG, Hayes BE, 2002. Acrolein activates mitogen-activated protein kinase signal transduction pathways in rat vascular smooth muscle cells. Mol Cell Biochem 240, 83–98. [DOI] [PubMed] [Google Scholar]
- Rokad D, Ghaisas S, Harischandra DS, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG, 2016. Role of neurotoxicants and traumatic brain injury in alpha-synuclein protein misfolding and aggregation. Brain Res Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS, 2013. Blast-related traumatic brain injury. Lancet Neurol 12, 882–893. [DOI] [PubMed] [Google Scholar]
- Saing T, Dick M, Nelson PT, Kim RC, Cribbs DH, Head E, 2012. Frontal cortex neuropathology in dementia pugilistica. J Neurotrauma 29, 1054–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Calipari ES, Jones SR, 2016. Regulation of Tyrosine Hydroxylase Expression and Phosphorylation in Dopamine Transporter-Deficient Mice. ACS Chem Neurosci 7, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A, Borges N, Cerejo A, Sarmento A, Azevedo I, 2005. Catalase activity and thiobarbituric acid reactive substances (TBARS) production in a rat model of diffuse axonal injury. Effect of gadolinium and amiloride. Neurochem Res 30, 625–631. [DOI] [PubMed] [Google Scholar]
- Shahaduzzaman M, Acosta S, Bickford PC, Borlongan CV, 2013. alpha-synuclein is a pathological link and therapeutic target for Parkinson's disease and traumatic brain injury. Med Hypotheses 81, 675–680. [DOI] [PubMed] [Google Scholar]
- Shamoto-Nagai M, Maruyama W, Hashizume Y, Yoshida M, Osawa T, Riederer P, Naoi M, 2007. In parkinsonian substantia nigra, alpha-synuclein is modified by acrolein, a lipid-peroxidation product, and accumulates in the dopamine neurons with inhibition of proteasome activity. J Neural Transm (Vienna) 114, 1559–1567. [DOI] [PubMed] [Google Scholar]
- Shao C, Roberts KN, Markesbery WR, Scheff SW, Lovell MA, 2006. Oxidative stress in head trauma in aging. Free Radic Biol Med 41, 77–85. [DOI] [PubMed] [Google Scholar]
- Shin SS, Bray ER, Zhang CQ, Dixon CE, 2011. Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res 1369, 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively SB, Perl DP, 2012. Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military--past, present, and future. J Head Trauma Rehabil 27, 234–239. [DOI] [PubMed] [Google Scholar]
- Souza JM, Giasson BI, Lee VM, Ischiropoulos H, 2000. Chaperone-like activity of synucleins. FEBS Lett 474, 116–119. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M, 1997. Alpha-synuclein in Lewy bodies. Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- Stefanis L, 2012. alpha-synuclein in Parkinson's disease. Cold Spring Harb Perspect Med 2, a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgucheva I, He S, Rich MC, Sharma R, Ninkina NN, Stahel PF, Surguchov A, 2014. Role of synucleins in traumatic brain injury - an experimental in vitro and in vivo study in mice. Mol Cell Neurosci 63, 114–123. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Alterio J, Campbell DG, Le Bourdelles B, Mallet J, Haavik J, Cohen P, 1993. Phosphorylation and activation of human tyrosine hydroxylase in vitro by mitogen-activated protein (MAP) kinase and MAP-kinase-activated kinases 1 and 2. Eur J Biochem 217, 715–722. [DOI] [PubMed] [Google Scholar]
- Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Sisk A, Masliah E, 1998. Abnormal distribution of the non-Abeta component of Alzheimer's disease amyloid precursor/alpha-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. Lab Invest 78, 1169–1177. [PubMed] [Google Scholar]
- Tanel A, Averill-Bates DA, 2007. P38 and ERK mitogen-activated protein kinases mediate acrolein-induced apoptosis in Chinese hamster ovary cells. Cell Signal 19, 968–977. [DOI] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D, 2009. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil 24, 14–23. [DOI] [PubMed] [Google Scholar]
- Thomas G, Haavik J, Cohen P, 1997. Participation of a stress-activated protein kinase cascade in the activation of tyrosine hydroxylase in chromaffin cells. Eur J Biochem 247, 1180–1189. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG, 2003. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem 278, 44405–44411. [DOI] [PubMed] [Google Scholar]
- Toska K, Kleppe R, Armstrong CG, Morrice NA, Cohen P, Haavik J, 2002. Regulation of tyrosine hydroxylase by stress-activated protein kinases. J Neurochem 83, 775–783. [DOI] [PubMed] [Google Scholar]
- Tully M, Tang J, Zheng L, Acosta G, Tian R, Hayward L, Race N, Mattson D, Shi R, 2018. Systemic Acrolein Elevations in Mice With Experimental Autoimmune Encephalomyelitis and Patients With Multiple Sclerosis. Front Neurol 9, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, 2003. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 42, 318–343. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E, 1998a. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem 273, 16058–16066. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T, 1998b. Protein-bound acrolein: potential markers for oxidative stress. Proceedings of the National Academy of Sciences of the United States of America 95, 4882–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer TS, Bax A, 2005. Comparison of structure and dynamics of micelle-bound human alpha-synuclein and Parkinson disease variants. J Biol Chem 280, 43179–43187. [DOI] [PubMed] [Google Scholar]
- Ulmer TS, Bax A, Cole NB, Nussbaum RL, 2005. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem 280, 9595–9603. [DOI] [PubMed] [Google Scholar]
- Uryu K, Giasson BI, Longhi L, Martinez D, Murray I, Conte V, Nakamura M, Saatman K, Talbot K, Horiguchi T, McIntosh T, Lee VM, Trojanowski JQ, 2003. Age-dependent synuclein pathology following traumatic brain injury in mice. Experimental neurology 184, 214–224. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM, 2003. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. American journal of epidemiology 157, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Walls MK, Race N, Zheng L, Vega-Alvarez SM, Acosta G, Park J, Shi R, 2016. Structural and biochemical abnormalities in the absence of acute deficits in mild primary blast-induced head trauma. J Neurosurg 124, 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK, 2010. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Lin HC, Zhao WZ, Huang HJ, Lo YL, Wang HT, Lin AM, 2017. Acrolein acts as a neurotoxin in the nigrostriatal dopaminergic system of rat: involvement of alpha-synuclein aggregation and programmed cell death. Sci Rep 7, 45741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Hunt SP, Jenner P, Marsden CD, 1987. Localization of neurotensin receptors in the forebrain of the common marmoset and the effects of treatment with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res 412, 244–253. [DOI] [PubMed] [Google Scholar]
- Werner C, Engelhard K, 2007. Pathophysiology of traumatic brain injury. Br J Anaesth 99, 4–9. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R, 2011. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A 108, 4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F, 2014. Dietary strategy to repair plasma membrane after brain trauma: implications for plasticity and cognition. Neurorehabil Neural Repair 28, 75–84. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2013. Animal models of traumatic brain injury. Nat Rev Neurosci 14, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan MH, Wang X, Zhu X, 2013. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 62, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Kaneko Y, Bae E, Stahl CE, Wang Y, van Loveren H, Sanberg PR, Borlongan CV, 2009. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res 1287, 157–163. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Calon F, Zhu C, Boado RJ, Pardridge WM, 2003. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum Gene Ther 14, 1–12. [DOI] [PubMed] [Google Scholar]
- Zheng L, Park J, Walls M, Tully M, Jannasch A, Cooper B, Shi R, 2013. Determination of urine 3-HPMA, a stable acrolein metabolite in a rat model of spinal cord injury. J Neurotrauma 30, 1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang J, Zeng Y, 2012. Overview of tyrosine hydroxylase in Parkinson's disease. CNS Neurol Disord Drug Targets 11, 350–358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Western Blot confirming the 25 kDa and 19 kDa forms α-synuclein. Whole brain protein lysates from rats were used at the same concentration for samples 1-3. Blots were run simultaneously. (A) Western blot image of samples 1-3 incubated with the α-syn antibody. (B) Western blot image of samples 1-3 incubated with the blocked α-syn antibody. The antibody was blocked with purified α -syn protein for 2 hours at 4°C prior to blot incubation. The absence of distinct bands at 25 kDa and 19 kDa confirmed that these were α -syn protein species.