Abstract
Antifreeze proteins (AFPs) confer the ability to survive at subzero temperatures and are found in many different organisms, including fish, plants, and insects. They prevent the formation of ice crystals by non-colligative adsorption to the ice surface and are essential for the survival of organisms in cold environments. These proteins are also widely used for cryopreservation, food technology, and metabolic genetic engineering over a range of sources and recipient cell types. This review summarizes successful applications of AFPs in the cryopreservation of animals, insects, and plants, and discusses challenges encountered in cryopreservation. Applications in metabolic genetic engineering are also described, specifically with the overexpression of AFP genes derived from different organisms to provide freeze protection to sensitive crops seasonally exposed to subzero temperatures. This review will provide information about potential applications of AFPs in the cryopreservation of animals and plants as well as in plant metabolic genetic engineering in hopes of furthering the development of cold-tolerant organisms.
Keywords: Antifreeze proteins, Cold-tolerant organisms, Cryopreservation, Ice crystals, Metabolic genetic engineering
Introduction
Antifreeze proteins and discovery
Antifreeze proteins (AFPs) are biological antifreeze materials found in many organisms that live in extreme cold environments. These proteins bind to ice in such a way that inhibits the growth of the ice crystals, allowing the organisms to survive these harsh conditions. Scholander et al. (1957) first discovered antifreeze proteins during an investigation into why Arctic fish can survive in water colder than the freezing point of their blood. Similarly, DeVries and Wohlschlag (1969) isolated an antifreeze protein during an investigation of Antarctic fish. The presence of AFP in insects was discovered by Husby and Zachariassen (1980), and their existence in plants, fungi, and bacteria was uncovered by Griffith et al. (1992) and Duman and Olsen (1993). The naming of AFPs ranges from antifreeze proteins to ice structuring or binding proteins or thermal hysteresis proteins. For the purposes of this review, all such proteins will be referred to as AFPs.
Source of antifreeze proteins
Antifreeze proteins were first detected in Arctic fish (Scholander et al. 1957) and later grouped into types I, II, III, and IV based on their sequences and structures (Fig. 1). All these proteins share an ability to alter the freezing point of solutions. Duman and Olsen (1993) first discovered AFPs in microorganisms such as bacteria and fungi, and discoveries of other AFPs in bacteria and fungi followed (Gilbert et al. 2005; Hoshino et al. 2003; Kawahara et al. 2007; Muryoi et al. 2004; Newsted et al. 1994; Singh et al. 2014). Similarly, plant AFPs have been observed in 60 plant species, and among them, 11 of these proteins have been purified and characterized (Gupta and Deswal 2014). AFPs observed in plants, such as winter rye (Griffith et al. 1997), carrot (Meyer et al. 1999; Zhang et al. 2004), grass (Sidebottom et al. 2000), winter cereals (Yeh et al. 2000), peach (Wisniewski et al. 1999), and Japanese radish (Kawahara et al. 2009), demonstrate high sequence homology.
Fig. 1.
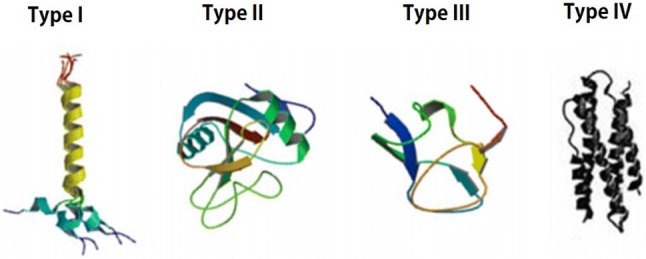
Structural differences among types of fish AFPs (I, II, III, and IV)
AFPs have also been discovered in insects, such as milkweed bugs (Patterson et al. 1981), budworm moths (Hew et al. 1983), snow scorpionflies (Husby and Zachariassen 1980), stoneflies (Gehrken and Somme 1987), the beetle Dendroides canadensis (Wu et al. 1991), Alaskan insects and spiders (Duman et al. 2004), and wood cockroaches (Duman 1979). AFPs allow these insects to survive subzero winter temperatures by decreasing the freezing points of their bodily fluids and inhibiting recrystallization.
Antifreeze proteins have been isolated from many different organs, such as the liver, stomach, heart, seeds, stems, bark, leaves, and flowers (reviewed in Cheung et al. 2017). Although their structures and amino acid sequences vary, all bind to different faces of the ice crystal (Jia and Davies 2002).
Mechanism of action and role of antifreeze proteins
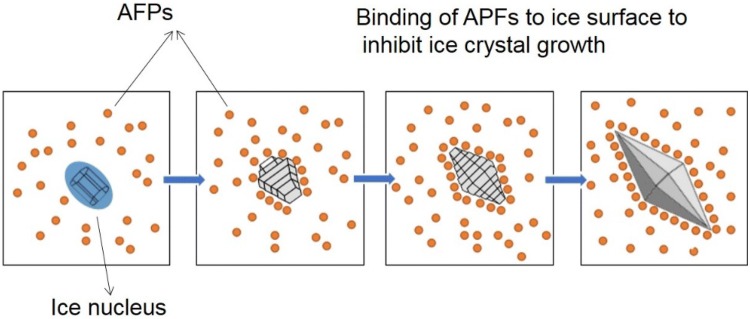
AFPs allow organisms to survive in harsh environments by lowering the freezing point of water through binding with ice nuclei and inhibiting recrystallization (Figs. 2, 3). Recently, Liu et al. (2018) discovered that AFPs from the fungus Pichia pastoris lowered freezing temperatures, controlled ice crystal sizes, and reduced damage from the freezing of hydrated gluten. Similar activities by plant AFPs in protecting plant cells from freezing damage have been reported, as well. AFPs from winter-hardy coniferous species have been shown to inhibit ice crystal formation (Jarzabek et al. 2009). The capacity for insect AFPs to reduce solution freezing points has also been well documented (Duman and Serianni 2002; Duman 2002; Olsen and Duman 1997; Graham et al. 1997; Tomczak et al. 2003). Interestingly, AFP structures in these organisms, such as the ocean pout, winter flounder, beetle, moth, and snow flea, are distinct from one another (Fig. 4, PDB 101).
Fig. 2.
Binding of antifreeze proteins (AFPs) to the ice nucleus to prevent the formation of large ice crystals
Fig. 3.
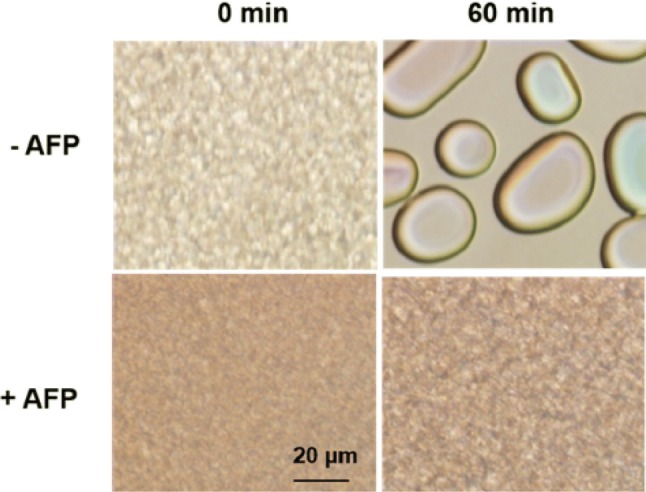
Comparison of the status of ice recrystallization in solutions with (+) or without (–) AFP at – 6 °C for 60 min
Fig. 4.
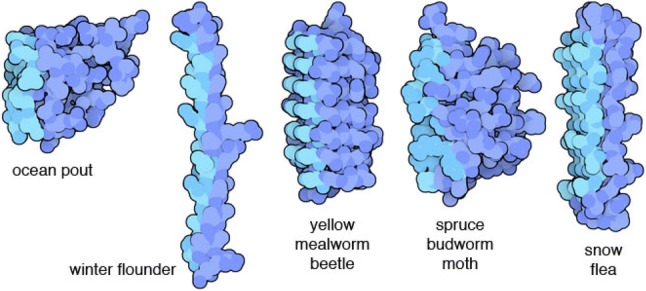
Summary of structural differences among antifreeze proteins in different taxa (PDB 101)
Classification of antifreeze proteins based on activity
AFPs can be classified as moderately active or hyperactive based on their ice binding positions. Moderately active AFPs bind to the prism and pyramidal planes of the ice crystal and generate a hexagonal bipyramidal ice crystal shape, whereas hyperactive AFPs bind to these planes and the basal plane of the ice crystal (Fig. 5), resulting in a circular disk-like ice crystal morphology (Knight et al. 1991; Drori et al. 2014; Park et al. 2012). The binding of hyperactive AFPs to the basal plane may result in a greater inhibitory effect on ice growth from whole ice surfaces than that of moderately active AFPs, with much higher thermal hysteresis (TH) activity than that of their moderately active counterparts (Kong et al. 2016; Pertaya et al. 2008; Drori et al. 2014).
Fig. 5.
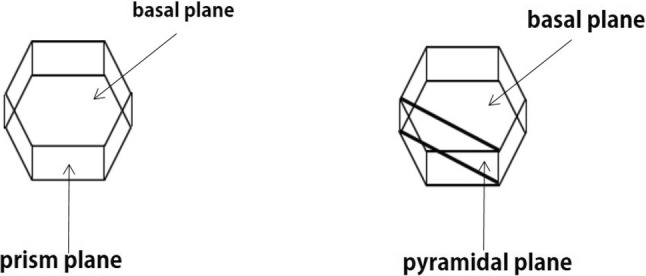
Different ice crystal-binding sites for hyperactive versus moderately active antifreeze proteins
Classification of antifreeze proteins based on TH values
The changing of melting and freezing points conferred by AFPs is known as thermal hysteresis (TH). AFPs can also be classified based on TH values to indicate their level of antifreeze activity. AFPs with high TH values, such as insect AFPs (TH values 5−10 °C), are considered hyperactive (Lin et al. 2010), while those from plants and fish (TH values, 0.2−0.6 °C and 1−2 °C) are classified as moderately active (Sicheri and Yang 1995; Griffith and Yaish 2004). However, hyperactive AFPs do not necessarily ensure better cryopreservation than moderately active proteins; for example, moderately active AFPs have been shown to protect mouse ovarian tissue ten times more effectively than hyperactive proteins (Kim et al. 2015; Lee et al. 2015a, b).
Application of antifreeze proteins
The reduction of freezing points by AFPs is non-colligative and does not significantly alter melting points regardless of concentration (Raymond et al. 2007; Lee et al. 2010; Tomczak et al. 2003). Effective inhibition of ice recrystallization by low concentrations of AFPs has been reported in previous studies (Knight et al. 1984, 1988), and differs from common antifreeze agents like methanol, glycerol, or ethylene glycol that lower freezing points in proportion to their concentrations. AFPs are highly sought after for use in cryopreservation, biotechnology, and the food industry (Christner 2010) owing to their unique abilities. The addition of AFPs to cells, organs, and tissues of plants and animals has been shown to improve cryopreservation efficiency (Jeon et al. 2015; Seo et al. 2018). With regards to food, AFPs improve the texture of ice cream (Regand and Goff 2006) and the quality of preserved meat (Griffith and Ewart 1995). The expression of AFPs in transgenic plants increases ice growth therein (Griffith et al. 1997; Hoshino et al. 1999; Maunsbach et al. 2001; Holmberg et al. 2001). This review discusses the applications of diverse AFPs in plant and animal biotechnology in detail.
Application of AFPs in cryopreservation
Cell, tissue, and organ cryopreservation methods are well established for plants and animals through the use of cryoprotectants like dimethyl sulfoxide (DMSO), glycerol, and polyvinylpyrrolidone (PVP). However, as cell and organ membranes are extremely sensitive to freezing and thawing cycles, high concentrations of these compounds are necessary to dehydrate the cytosol and minimize the formation of intracellular ice crystals during freeze and thaw cycles (Taylor and Fletcher 1998, 1999). These high concentrations can also cause cytotoxicity by altering the epigenetic regulation of cells (Adler et al. 2006; Thaler et al. 2012) and has led to a demand for alternative cryoprotectants with less toxicity. As described above, AFPs can inhibit the growth of ice during freezing and thawing without significantly affecting the melting point. In addition, as these proteins lower the freezing point non-colligatively, they are considered less toxic than existing cryoprotectants. Relatively low concentrations of AFPs can inhibit the recrystallization of ice compared to other cryoprotectants. Many researchers have focused on the use of AFPs in the cryopreservation of cells, tissues, and organs of plants and animals, owing to their unique properties. According to a review by Kim et al. (2017), type III AFPs are most commonly used for cryopreservation, followed by type I AFPs. Type II AFPs are rarely used as cryoprotectants (Fig. 6).
Fig. 6.
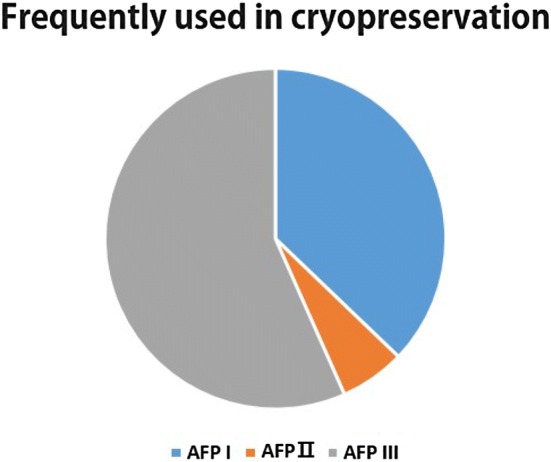
Use of different types of AFPs in cryopreservation research
Application of AFPs to the cryopreservation of animal cells, tissues, and organs
Different types of fish AFPs, especially types I and III, have been used to improve the cryopreservation of animal cells, tissues, and organs, such as oyster oocytes (Naidenko 1997), bovine and porcine oocytes (Rubinsky et al. 1991, 1992), vertebrate and invertebrate cell lines (Koushafar and Rubinsky 1997), intact livers (Lee et al. 1992; Rubinsky et al. 1994), and bull sperm (Prathalingam et al. 2006). Improved motility and reduced enzyme leakage have been observed in sperm cryopreserved with AFPs compared to those without (Uperti et al. 1996). The presence of AFPs also improves the quality of cryopreserved sheep embryos (Baguisi et al. 1997). Rubinsky et al. (1994) reported that hearts preserved with AFPs remained viable, as evidenced by electron microscopy, while those preserved without AFP did not. AFPs have been shown to protect the heart from freezing damage and improve viability during cryopreservation in other studies, as well (Amir et al. 2004, 2005; Soltys et al. 2001). Embryos from Sparus aurata injected with AFP exhibited improved tolerance to chilling at 0 and – 10 °C with a hatching rate of approximately 100% (Robles et al. 2006). Jo et al. (2011) found that the addition of fish AFP (500 ng/mL) to vitrification solution improved the survival rate of immature mouse oocytes, while Lee et al. (2015a) reported that a diverse array of proteins from yeast, bacteria, and fish improved murine oocyte quality and embryonic development. Zilli et al. (2014) reported that the addition of type III fish AFP to the cryopreservation medium protected sperm from freezing and improved their viability as compared to other treatments (control, DMSO, and DMSO + type I fish AFP). The cryoprotective effect of type III fish AFP has also been observed in rabbit embryos (Nishijima et al. 2014). Ideta et al. (2014) reported that bovine embryos stored in medium containing 10 mg/mL AFP survived for 10 days at 4 °C. Fish embryos incubated in a solution containing type I AFP exhibit a significantly increased survival rate upon exposure to 4 or 10 °C (Martínez-Páramo et al. 2008a, b, 2009). Type I AFPs block potassium and calcium ion channels, reducing ion leakage from lipid membranes at 4 °C (Rubinsky et al. 1992; Baguisi et al. 1997), thereby helping to maintain the transmembrane ionic gradient and improve cryopreservation of cells and tissues (Arav et al. 1993). Similarly, type III AFPs stabilize the plasma membrane by interacting with lipids (Wang and Huang 1996). Unlike type I and III AFPs, type II AFPs have been associated with cytotoxicity in cells, tissues, and organs during cryopreservation (Naidenko 1997; Pham et al. 1999; Wang et al. 1999) and use thereof is infrequent in cryopreservation (Fig. 6). Higher concentrations of AFPs can cause the formation of destructive, needle-like ice and lead to a decrease in the post-thaw survival of cryopreserved cells (Lee et al. 2015a, b; Hansen et al. 1993; Carpenter and Hansen 1992). Low concentrations of AFPs are thus preferred in cryopreservation studies. However, the optimal concentration for cryopreservation differs depending on cell type and AFP source, and the utilization of AFPs in cryopreservation requires fine-tuning depending on these different parameters.
Application of AFPs to the cryopreservation of plant cells, tissues, and organs
The cryopreservation of plant cells, tissues, and organs has also been attempted for long-term species conservation (Engelmann 2011; Jeon et al. 2015; Seo et al. 2018). However, since commonly used explants, such as the callus or shoot tips, contain high amounts of cellular water, freezing injuries are likely via the crystallization of this water into ice during freezing and thawing, leading to low survival rates. In addition, as mentioned above, commonly used cryoprotectants, such as glycerol, sugars, and DMSO, are toxic to certain plant tissues. In 1989, Cutler et al. (1989) investigated the effects of AFP vacuum infiltration into the leaves of potato (Solanum tuberosum L.), canola (Brassica napus), and Arabidopsis thaliana (L.) plants, and found that AFPs lowered their freezing temperatures significantly compared to that of water-infiltrated controls, with the amount of freezable water reduced across a range of low temperatures. In canola, the freezing temperature was decreased by an average of 1.8 °C, indicating that AFP infiltration could depress its freezing point to a level that would substantially improve crop survival in typical agricultural environments. Wang et al. (2001) further reported that the utilization of AFPs improves cryopreservation efficiency in rice embryogenic cells. Jeon et al. (2015) also observed that the addition of type I fish AFP to vitrification solution significantly increased cryopreservation efficiency in the chrysanthemum. Seo et al. (2018) found that the inclusion of type III fish AFPs in cryoprotection solutions improved the cryopreservation efficiency of potato shoot tips. Pe et al. (2019) observed involvement of AFPs in regulation of cold-responsive genes in Hosta capitate under low-temperature condition. Currently, however, AFPs are utilized less frequently in plant cryopreservation than in that for animals and animal tissues.
Application of AFPs in metabolic genetic engineering
The introduction of genes encoding AFPs via metabolic genetic engineering is another promising strategy to improve freeze tolerance in transgenic plants and animals. Many studies have attempted to generate freeze-tolerant plants and animals via the overexpression of AFP genes in otherwise cold-sensitive organisms. Success in this area has recently increased substantially.
The production of transgenic plants overexpressing fish AFPs has been reported for many species, including Arabidopsis, tobacco, tomatoes, and potatoes (Hightower et al. 1991; Kenward et al. 1993; Wallis et al. 1997; Worrall et al. 1998). Ice recrystallization has been successfully inhibited in fish AFP-overexpressing transgenic tomato leaf extracts (Hightower et al. 1991), and Balamurugan et al. (2018) claimed that the overexpression of the AFP gene from rye grass (Lolium perenne) in tomato plants resulted in significantly higher freeze tolerance than in wild-type plants through a threefold increase in relative water content and 2.6-fold reduction in the electrolyte leakage index. Wallis et al. (1997) observed that transgenic potatoes overexpressing AFP genes exhibited significantly less electrolyte leakage than control plants after freezing at – 2 °C. The overexpression of insect AFP in Arabidopsis resulted in significant antifreeze activity and improved frost resistance (Meyer et al. 1999), and transgenic Arabidopsis plants overexpressing insect AFP showed increased cold tolerance through a decrease in their freezing temperature (Huang et al. 2002). Lin et al. (2011) confirmed that the integration of insect AFP in transgenic Arabidopsis reduced its freezing temperature of by 2−3 °C as compared to wild-type plants. Similarly, improved freeze tolerance was observed in transgenic Arabidopsis overexpressing an insect AFP from a spruce bud worm; the transgenic lines had less ion leakage and malondialdehyde than wild-type lines in temperatures as low as – 20 °C for 30 min and 4 °C overnight (Zhu et al. 2010). Similarly, the introduction of insect AFPs and type I fish AFPs into tobacco inhibits ice recrystallization (Holmberg et al. 2001; Kenward et al. 1993) in these plants. Deng et al. (2014) claimed that the heterologous expression of AnAFP in tobacco resulted in less wilting and less change in relative electrical conductivity under cold stress (– 3 °C) compared to wild-type plants after a 16 h freeze and 1 h thaw. Conversely, the overexpression of type II fish AFPs in tobacco does not confer cold tolerance (Kenward et al. 1999). Wang et al. (2008) also observed that transgenic tobacco overexpressing AFP from the insect Microdera punctipennis demonstrated improved freeze tolerance over wild-type plants through reductions in ion leakage and malondialdehyde levels. Fan et al. (2002) confirmed that transgenic expression of the carrot AFP gene could enhance the tolerance of tobacco plants to cold through a significantly greater reduction in ion leakage (1−30%) than that of the wild type (1−80%). Transgenic wheat overexpressing AFP showed significant freeze tolerance at – 7 °C, with high levels of antifreeze activity (Khanna and Daggard 2006). The most effective antifreeze proteins for different plants, however, depend on AFP expression level, localization, and stability.
The introduction of type I fish AFP to salmon fish eggs resulted in the generation of cold-tolerant transgenic salmon fishes (Hew et al. 1992). Hew et al. (1999) went on to report that the integration of fish AFP into salmon resulted in inherited expression of the AFP gene in the F3 generation. Similarly, the microinjection of ocean pout type III AFP into goldfish oocytes resulted in transgenic goldfish with improved resistance to low temperatures (Wang et al. 1995), and Hobbs and Fletcher (2008) also transferred the AFP gene to salmon to improve freeze resistance.
The overexpression of type III fish AFP has been shown to improve antifreeze activity and protect against freezing damage during the cryopreservation of transgenic mouse ovaries (Bagis et al. 2006, 2008). Uhlig et al. (2011) also reported that the heterologous expression of AFPs in Escherichia coli yielded antifreeze activity and caused crystal deformation, recrystallization inhibition, and TH. Similarly, the expression of fish AFP in Drosophila increased antifreeze activity, and the transgenic flies were able to survive significantly longer in near freezing temperatures than controls through the prevention of apoptosis (Nicodemus et al. 2006; Neelakanta et al. 2012).
Conclusion
AFPs from diverse taxa, including fish, bacteria, fungi, insects, and plants, allow organisms to survive at subzero temperatures by reducing the freezing point for ice growth therein. AFPs derived from different species have been used successfully for the cryopreservation of plant and animal cells and organs, although their ability to reduce the freezing temperature is dependent on the species and cell type from which the AFP is derived, AFP type and concentration, and cryopreservation protocol. Extensive research has focused on the development of AFP-overexpressing transgenic animals or plants to improve their survival in extreme cold conditions. However, the cytotoxicity of certain AFPs somewhat limits their cryopreservation applications. As such, the identification of novel AFPs better suited to cryopreservation would have important practical implications. The development of a technique for direct delivery of the AFPs into cells to control crystal growth without damaging the cells is also necessary for improving the applicability of AFP in future cryopreservation techniques.
Future perspectives
Successful application of AFPs in cryopreservation of animals and plants has been reported in several different studies. However, due to low yield and high cost, future applications for AFPs remain uncertain. Increasing production yields with different molecular biological techniques is needed, and lowering the cost of AFP use in cryopreservation and the food industry will increase future usage. The overexpression of AFP genes derived from different organisms has been shown to confer freeze protection to sensitive crops exposed to seasonally subzero temperatures, and costs associated with transgenic plants have been decreasing rapidly. We expect the production of AFP-expressing transgenic plants via metabolic genetic engineering to increase, especially to improve frost tolerance in garden plants and ornamental cut flowers, supporting further development in the global horticultural industry.
Authors’ contributions
AHN collected the literature and wrote the manuscript; CKK advised and assisted with the writing of the manuscript.
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bio industry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant# 315002-5)
Compliance with ethical standards
Conflict of interest statement
The authors report no conflicts of interest.
References
- Adler S, Pellizer C, Paparella M, Hartung T, Bremer S. The effects of solvents on embryonic stem cell differentiation. Toxicol Vitro. 2006;20:265–271. doi: 10.1016/j.tiv.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Amir G, Rubinsky B, Horovitz L, Yousif BS, Leor J, Smolinsky AK, Lavee J. Improved viability and reduced apoptosis in sub-zero 21 h preservation of transplanted rat hearts using antifreeze proteins. J Heart Lung Transpl. 2004;23:171–172. [Google Scholar]
- Amir G, Rubinsky B, Basheer SY, Horowitz L, Jonathan L, Feinberg MS, Smolinsky AM, Lavee J. Improved viability and reduced apoptosis in sub-zero 21 h preservation of transplanted rat hearts using anti-freeze proteins. J Heart Lung Transpl. 2005;24:1915–1929. doi: 10.1016/j.healun.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Arav A, Rubinsky B, Fletcher G, Seren E. Cryogenic protection of oocytes with antifreeze proteins. Mol Reprod Dev. 1993;36:488–493. doi: 10.1002/mrd.1080360413. [DOI] [PubMed] [Google Scholar]
- Bagis H, Aktoprakligil D, Mercan HO, Yurdusev N, Turgut G, Sekmen S, Arat S, Cetin S. Stable transmission and transcription of newfoundland ocean pout type III fish antifreeze protein (AFP) gene in transgenic mice and hypothermic storage of transgenic ovary and testis. Mol Reprod Dev. 2006;73:1404–1411. doi: 10.1002/mrd.20601. [DOI] [PubMed] [Google Scholar]
- Bagis H, Akkoc T, Tass A, Aktoprakligil D. Cryogenic effect of antifreeze protein on transgenic mouse ovaries and the production of live offspring by orthotopic transplantation of cryopreserved mouse ovaries. Mol Reprod Dev. 2008;75:608–613. doi: 10.1002/mrd.20799. [DOI] [PubMed] [Google Scholar]
- Baguisi A, Arav A, Crosby TF, Roche JF, Boland MP. Hypothermic storage of sheep embryos with antifreeze proteins: development in vitro and in vivo. Theriogenology. 1997;48:1017–1024. doi: 10.1016/s0093-691x(97)00328-2. [DOI] [PubMed] [Google Scholar]
- Balamurugan S, Ann JS, Varghese IP, Murugan SB, Harish MC, Kumar SR, Sathishkumar R. Heterologous expression of Lolium perenne antifreeze protein confers chilling tolerance in tomato. J Integr Agric. 2018;17:1128–1136. [Google Scholar]
- Carpenter JF, Hansen TN. Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth. Proc Natl Acad Sci USA. 1992;89:8953–8957. doi: 10.1073/pnas.89.19.8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung RC, Ng TB, Wong JH. Antifreeze proteins from diverse organisms and their applications: an overview. Curr Protein Pept Sci. 2017;18:262–283. doi: 10.2174/1389203717666161013095027. [DOI] [PubMed] [Google Scholar]
- Christner B. Bioprospecting for microbial products that affect ice crystal formation and growth. Appl Microbiol Biotechnol. 2010;85:481–489. doi: 10.1007/s00253-009-2291-2. [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Saleem M, Kendall E, Gusta LV, Georges F, Fletcher GL. Winter flounder antifreeze protein improves the cold hardiness of plant tissues. J Plant Physiol. 1989;135:351–354. [Google Scholar]
- Deng LQ, Yu HQ, Liu YP, Jiao PP, Zhou SF, Zhang SZ, Li WC, Fu FL. Heterologous expression of antifreeze protein gene AnAFP from Ammopiptanthus nanus enhances cold tolerance in Escherichia coli and tobacco. Gene. 2014;539:132–140. doi: 10.1016/j.gene.2014.01.013. [DOI] [PubMed] [Google Scholar]
- DeVries AL, Wohlschlag DE. Freezing resistance in some Antarctic fishes. Science. 1969;163:1073–1075. doi: 10.1126/science.163.3871.1073. [DOI] [PubMed] [Google Scholar]
- Drori R, Celik Y, Davies PL, Braslavsky I. Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. J R Soc Interface. 2014;11:20140526. doi: 10.1098/rsif.2014.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman JG. Thermal-hysteresis-factors in overwintering insects. J Insect Physiol. 1979;25(10):805–810. [Google Scholar]
- Duman JG. The inhibition of ice nucleators by insect antifreeze proteins is enhanced by glycerol and citrate. J Comp Physiol. 2002;172:163–168. doi: 10.1007/s00360-001-0239-7. [DOI] [PubMed] [Google Scholar]
- Duman JG, Olsen TM. Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. Cryobiology. 1993;30:322–328. [Google Scholar]
- Duman JG, Serianni AS. The role of endogenous antifreeze protein enhancers in the hemolymph thermal hysteresis activity of the beetle Dendroides canadensis. J Insect Physiol. 2002;48:103–111. doi: 10.1016/s0022-1910(01)00150-0. [DOI] [PubMed] [Google Scholar]
- Duman JG, Bennett T, Sformo T, Hochstrasser R, Barnes BM. Antifreeze proteins in Alaskan insects and spiders. J Insect Physiol. 2004;50:259–266. doi: 10.1016/j.jinsphys.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Engelmann F. Cryopreservation of embryos: an overview. In: Thorpe TA, Yeung EC, editors. In plant embryo culture. Methods in molecular biology. New York: Humana Press; 2011. pp. 155–184. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu B, Wang H, Wang S, Wang J. Cloning of an antifreeze protein gene from carrot and its influence on cold tolerance in transgenic tobacco plants. Plant Cell Rep. 2002;21:296–301. [Google Scholar]
- Gehrken U, Somme L. Increased cold hardiness in eggs of Arcynopteryx compacta (Plecoptera) by dehydration. J Insect Physiol. 1987;33(12):987–991. [Google Scholar]
- Gilbert JA, Davies PL, Laybourn-Parry J. A hyperactive, Ca2 + -dependent antifreeze protein in an Antarctic bacterium. FEMS Microbiol Lett. 2005;245(1):67–72. doi: 10.1016/j.femsle.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Graham LA, Liou YC, Walker VK, Davies PL. Hyperactive antifreeze protein from beetles. Nature. 1997;388:727–728. doi: 10.1038/41908. [DOI] [PubMed] [Google Scholar]
- Griffith M, Ewart KV. Antifreeze proteins and their potential use in frozen foods. Biotechnol Adv. 1995;13:375–402. doi: 10.1016/0734-9750(95)02001-j. [DOI] [PubMed] [Google Scholar]
- Griffith M, Yaish MW. Antifreeze proteins in overwintering plants: a tale of two activities. Trends Plant Sci. 2004;9:399–405. doi: 10.1016/j.tplants.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Griffith M, Ala P, Yang DS, Hon WC, Moffatt BA. Antifreeze protein produced endogenously in winter rye leaves. Plant Physiol. 1992;100:593–596. doi: 10.1104/pp.100.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M, Antikainen M, Hon WC, Pihakaski-Maunsbach K, Yu XM, Chun JU, Yang DSC. Antifreeze proteins in winter rye. Physiol Plantarum. 1997;100:327–332. [Google Scholar]
- Gupta R, Deswal R. Antifreeze proteins enable plants to survive in freezing conditions. J Biosci. 2014;39(5):931–944. doi: 10.1007/s12038-014-9468-2. [DOI] [PubMed] [Google Scholar]
- Hansen TN, Smith KM, Brockbank KG. Type I antifreeze protein attenuates cell recoveries following cryopreservation. Transpl Proc. 1993;25:3182–3184. [PubMed] [Google Scholar]
- Hew CL, Kao MH, So Y-P, Lim K-P. Presence of cysteine containing antifreeze proteins in the spruce bud worm, Choristoneura fumiferana. Can J Zool. 1983;61(10):2324–2328. [Google Scholar]
- Hew CL, Davies PL, Fletcher G. Antifreeze protein gene transfer in Atlantic salmon. Mol Mar Biol Biotechnol. 1992;1:309–317. [PubMed] [Google Scholar]
- Hew C, Poon R, Xiong F, Gauthier S, Shears M, King M, Davies P, Fletcher G. Liver-specific and seasonal expression of transgenic Atlantic salmon harboring the winter flounder antifreeze protein gene. Transgenic Res. 1999;8:405–414. doi: 10.1023/a:1008900812864. [DOI] [PubMed] [Google Scholar]
- Hightower R, Baden C, Penzes E, Lund P, Dunsmuir P. Expression of antifreeze proteins in transgenic plants. Plant Mol Biol. 1991;17:1013–1021. doi: 10.1007/BF00037141. [DOI] [PubMed] [Google Scholar]
- Hobbs RS, Fletcher GL. Tissue specific expression of antifreeze protein and growth hormone transgenes driven by the ocean pout (Macrozoarces americanus) antifreeze protein OP5a gene promoter in Atlantic salmon (Salmo salar) Transgenic Res. 2008;17:33–45. doi: 10.1007/s11248-007-9128-5. [DOI] [PubMed] [Google Scholar]
- Holmberg N, Farr´es J, Bailey JE, Kallio PT. Targeted expression of a synthetic codon optimized gene, encoding the spruce budworm antifreeze protein, leads to accumulation of antifreeze activity in the apoplasts of transgenic tobacco. Gene. 2001;275:115–124. doi: 10.1016/s0378-1119(01)00635-7. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Odaira M, Yoshida M, Tsuda S. Physiological and biochemical significance of antifreeze substances in plants. J Plant Res. 1999;112:255–261. [Google Scholar]
- Hoshino T, Kiriaki M, Ohgiya S, Fujiwara M, Kondo H, Nishimiya Y, Yumoto I, Tsuda S. Antifreeze proteins from snow mold fungi. Can J Bot Rev Can Bot. 2003;81:1175–1181. [Google Scholar]
- Huang T, Nicodemus J, Zarka DG, Thomashow MF, Wisniewski M, Duman JG. Expression of an insect (Dendroides canadensis) antifreeze protein in Arabidopsis thaliana results in a decrease in plant freezing temperature. Plant Mol Biol. 2002;50:333–344. doi: 10.1023/a:1019875922535. [DOI] [PubMed] [Google Scholar]
- Husby JA, Zachariassen KE. Antifreeze agents in the body fluid of winter active insects and spiders. Experientia. 1980;36(8):963–964. [Google Scholar]
- Ideta A, Aoyagi Y, Tsuchiya K, Nakamura Y, Hayama K, Shirasawa A, Sakaguchi K, Tominaga N, Nishimiya Y, Tsuda S. Prolonging hypothermic storage (4 °C) of bovine embryos with fish antifreeze protein. J Reprod Dev. 2014;61:1–6. doi: 10.1262/jrd.2014-073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzabek M, Pukacki PM, Nuk K. Cold-regulated proteins with potent antifreeze and cryoprotective activities in spruces (Picea spp.) Cryobiology. 2009;58:268–274. doi: 10.1016/j.cryobiol.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Naing AH, Park KI, Kim CK. The effect of antifreeze protein on the cryopreservation of chrysanthemums. Plant Cell Tissue Organ Cult. 2015;123:665–671. [Google Scholar]
- Jia Z, Davies PL. Antifreeze proteins: an unusual receptor-ligand interaction. Trends Biochem Sci. 2002;27(2):101–106. doi: 10.1016/s0968-0004(01)02028-x. [DOI] [PubMed] [Google Scholar]
- Jo JW, Jee BC, Lee JR, Suh CS. Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertil Steril. 2011;96:1239–1245. doi: 10.1016/j.fertnstert.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Iwanaka Y, Higa S, Muryoi N, Sato M, Honda M, Omura H, Obata H. A novel, intracellular antifreeze protein in an antarctic bacterium, Flavobacterium xanthum. Cryo Lett. 2007;28:39–49. [PubMed] [Google Scholar]
- Kawahara H, Fujii A, Inoue M, Kitao S, Fukuoka J, Obata H. Antifreeze activity of cold acclimated Japanese radish and purification of antifreeze peptide. Cryo Lett. 2009;30:119–131. [PubMed] [Google Scholar]
- Kenward KD, Altschuler M, Hilderbrand D, Davies PL. Accumulation of type I fish antifreeze protein in transgenic tobacco in cold specific. Plant Mol Biol. 1993;23:377–385. doi: 10.1007/BF00029012. [DOI] [PubMed] [Google Scholar]
- Kenward KD, Brandle J, McPherson J, Davies PL. Type II fish antifreeze protein accumulation in transgenic tobacco does not confer frost resistance. Transgenic Res. 1999;8:105–117. doi: 10.1023/a:1008886629825. [DOI] [PubMed] [Google Scholar]
- Khanna HK, Daggard GE. Targeted expression of redesigned and codon optimized synthetic gene leads to recrystallisation inhibition and reduced electrolyte leakage in spring wheat at sub-zero temperatures. Plant Cell Rep. 2006;25:1336–1346. doi: 10.1007/s00299-006-0191-9. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Shim HE, Lee JH, Kang Y-C, Hur YB. Ice-binding protein derived from Glaciozyma can improve the viability of cryopreserved mammalian cells. J Microbiol Biotechnol. 2015;25:1989–1996. doi: 10.4014/jmb.1507.07041. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Hur YB, Lee CW, Park SH, Koo BW. Marine antifreeze proteins: structure, function, and application to cryopreservation as a potential cryoprotectant. Mar Drugs. 2017;15:27. doi: 10.3390/md15020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CA, DeVries AL, Oolman LD. Fish antifreeze protein and the freezing and recrystallization of ice. Nature. 1984;308:295–296. doi: 10.1038/308295a0. [DOI] [PubMed] [Google Scholar]
- Knight CA, Hallett J, DeVries AL. Solute effects on ice recrystallization: an assessment technique. Cryobiology. 1988;25:55–60. doi: 10.1016/0011-2240(88)90020-x. [DOI] [PubMed] [Google Scholar]
- Knight CA, Cheng CC, DeVries AL. Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes. Biophys J. 1991;59:409–418. doi: 10.1016/S0006-3495(91)82234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong CH, Hamid N, Liu T, Sarojini V. Effect of antifreeze peptide pretreatment on ice crystal size, drip loss, texture, and volatile compounds of frozen carrots. J Agric Food Chem. 2016;64:4327–4335. doi: 10.1021/acs.jafc.6b00046. [DOI] [PubMed] [Google Scholar]
- Koushafar H, Rubinsky B. Effect of antifreeze proteins on frozen primary prostatic adenocarcinoma cells. Urology. 1997;49:421–425. doi: 10.1016/S0090-4295(96)00572-9. [DOI] [PubMed] [Google Scholar]
- Lee CY, Rubinsky B, Fletcher GL. Hypothermic preservation of whole mammalian organs with antifreeze proteins. Cryo Lett. 1992;13:59–66. [Google Scholar]
- Lee JK, Park KS, Park S, Park H, Song YH, Kang SH, Kim HJ. An extracellular ice-binding glycoprotein from an Arctic psychrophilic yeast. Cryobiology. 2010;60:222–228. doi: 10.1016/j.cryobiol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Lee HH, Lee HJ, Kim HJ, Lee JH, Ko Y, Kim SM, Lee JR, Suh CS, Kim SH. Effects of antifreeze proteins on the vitrification of mouse oocytes: comparison of three different antifreeze proteins. Hum Reprod. 2015;30:2110–2119. doi: 10.1093/humrep/dev170. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim SK, Youm HW, Kim HJ, Lee JR, Suh CS, Kim SH. Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLoS One. 2015;10:e0126252. doi: 10.1371/journal.pone.0126252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, O'Tousa JE, Duman JG. Expression of two selfenhancing antifreeze proteins from the beetle Dendroides Canadensis in Drosophila melanogaster. J Insect Physiol. 2010;56(4):341–349. doi: 10.1016/j.jinsphys.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Lin X, Wisniewski M, Duman JG. Expression of two self-enhancing antifreeze proteins from the beetle Dendroides canadensis in Arabidopsis thaliana. Plant Mol Biol Rep. 2011;29:802–813. [Google Scholar]
- Liu M, Liang Y, Wange Y, Zhang H, Wu G, Wang L, Qian H, Qi X. Effects of recombinant carrot antifreeze protein from Pichia pastoris GS115 on the physicochemical properties of hydrated gluten during freeze−thawed cycles. J Cereal Sci. 2018;83:245–251. [Google Scholar]
- Martínez-Páramo S, Perez-Cerezales S, Barbosa V, Robles V, Herraez MP. Advances on fish embryo cryopreservation using antifreeze proteins. Biol Reprod. 2008;78:152. [Google Scholar]
- Martínez-Páramo S, Pérez-Cerezales S, Robles V, Anel L, Herraez MP. Incorporation of antifreeze proteins into zebrafish embryos by a non-invasive method. Cryobiology. 2008;56:216–222. doi: 10.1016/j.cryobiol.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Martínez-Páramo S, Barbosa V, Pérez-Cerezales S, Robles V, Herraez MP. Cryoprotective effects of antifreeze proteins delivered into zebrafish embryos. Cryobiology. 2009;58:128–133. doi: 10.1016/j.cryobiol.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Maunsbach PK, Moffatt B, Testillano P, Risueno M, Yeh S, Griffith M, Maunsbach AB. Genes encoding chitinase antifreeze proteins are regulated by cold and expressed by all cell types in winter rye shoots. Physiol Plantarum. 2001;112:359–371. doi: 10.1034/j.1399-3054.2001.1120309.x. [DOI] [PubMed] [Google Scholar]
- Meyer K, Keil M, Naldrett MJ. A leucine-rich repeat protein of carrot that exhibits antifreeze activity. FEBS Lett. 1999;447:171–178. doi: 10.1016/s0014-5793(99)00280-x. [DOI] [PubMed] [Google Scholar]
- Muryoi N, Sato M, Kaneko S, Kawahara H, Obata H, Yaish MW, Griffith M, Glick BR. Cloning and expression of afpA, a gene encoding an antifreeze protein from the arctic plant growth promoting rhizobacterium Pseudomonas putida GR12-2. J Bacteriol. 2004;186(17):5661–5671. doi: 10.1128/JB.186.17.5661-5671.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidenko T. Cryopreservative of Crassostrea gigas oocytes embryo and larvae using antioxidants echinochromes A and antifreeze protein AFP–I. Cryo Lett. 1997;18:375–382. [Google Scholar]
- Neelakanta G, Hudson AM, Sultana H, Cooley L, Fikrig E. Expression of Ixodes scapularis antifreeze glycoprotein enhances cold tolerance in Drosophila melanogaster. PLoS One. 2012;7:e33447. doi: 10.1371/journal.pone.0033447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsted WJ, Polvi S, Papish B, Kendall E, Saleem M, Koch M, Hussain A, Cutler AJ, Georges F. A low molecular weight peptide from snow mold with epitopic homology to the winter flounder antifreeze protein. Biochem Cell Biol. 1994;72(3−4):152–156. doi: 10.1139/o94-022. [DOI] [PubMed] [Google Scholar]
- Nicodemus J, O’Tousa JE, Duman JG. Expression of a beetle, Dendroides canadensis, antifreeze protein in Drosophila melanogaster. J Insect Physiol. 2006;52:888–896. doi: 10.1016/j.jinsphys.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Nishijima K, Tanaka M, Sakai Y, Koshimoto C, Morimoto M, Watanabe T, Fan J, Kitajima S. Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit. Cryobiology. 2014;69:22–25. doi: 10.1016/j.cryobiol.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Olsen TM, Duman JG. Maintenance of the supercooled state in the gut of overwintering pyrochroid beetle larvae, Dendroides canadensis: role of gut ice nucleators and antifreeze proteins. J Comp Physiol B. 1997;167:114–122. [Google Scholar]
- Park KS, Do H, Lee JH, Park SI, Kim EJ, Kim SJ, Kang SH, Kim HJ. Characterization of the ice-binding protein from Arctic yeast Leucosporidium sp. AY30. Cryobiology. 2012;64:286–296. doi: 10.1016/j.cryobiol.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Patterson JL, Kelly TJ, Duman JG. Purification and composition of a thermal hysteresis producing protein from the milkweed bug, Oncopeltus fasciatus. J Comp Physiol. 1981;142(4):539–542. [Google Scholar]
- Pe PPW, Naing AH, Chung MY, Park KI, Kim CK (2019) The role of antifreeze proteins (AFPs) in the regulation of genes involved in response of Hosta capitate to cold. 3 Biotech. 10.1007/s13205-019-1859-5 [DOI] [PMC free article] [PubMed]
- Pertaya N, Marshall CB, Celik Y, Davies PL, Braslavsky I. Direct visualization of spruce budworm antifreeze protein interacting with ice crystals: basal plane affinity confers hyperactivity. Biophys J. 2008;95:333–341. doi: 10.1529/biophysj.107.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham L, Dahiya R, Rubinsky B. An in vivo study of antifreeze protein adjuvant cryosurgery. Cryobiology. 1999;38:169–175. doi: 10.1006/cryo.1999.2158. [DOI] [PubMed] [Google Scholar]
- Prathalingam NS, Holt WV, Revell SG, Mirczuk S, Fleck RA, Watson PF. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze−thaw. Theriogenology. 2006;66:1894–1900. doi: 10.1016/j.theriogenology.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Raymond JA, Fritsen C, Shen K. An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol Ecol. 2007;61:214–221. doi: 10.1111/j.1574-6941.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- Regand A, Goff HD. Ice recrystallization inhibition in ice cream as affected by ice structuring proteins from winter wheat grass. J Dairy Sci. 2006;89:49–57. doi: 10.3168/jds.S0022-0302(06)72068-9. [DOI] [PubMed] [Google Scholar]
- Robles V, Cabrita E, Anelb L, Herraez MP. Microinjection of the antifreeze protein type III (AFPIII) in turbot (Scophthalmus maximus) embryos: toxicity and protein distribution. Aquaculture. 2006;261:1299–1306. [Google Scholar]
- Rubinsky B, Devries A, Arav A (1994) Interaction of thermal hysteresis protein with cells and cell membranes and associated applications. US Patent 5358931
- Rubinsky B, Arav A, Fletcher GL. Hypothermic protection—a fundamental property of “antifreeze” proteins. Biochem Biophys Res Comm. 1991;180:566–571. doi: 10.1016/s0006-291x(05)81102-7. [DOI] [PubMed] [Google Scholar]
- Rubinsky B, Mattioli M, Arav A, Barboni B, Fletcher GL. Inhibition of Ca++ and K+ currents by “antifreeze” proteins. Am J Physiol. 1992;262:542–545. doi: 10.1152/ajpregu.1992.262.3.R542. [DOI] [PubMed] [Google Scholar]
- Rubinsky B, Arav A, Hong JS, Lee CY. Freezing of mammalian livers with glycerol and antifreeze proteins. Biochem Biophys Res Commun. 1994;200:732–741. doi: 10.1006/bbrc.1994.1512. [DOI] [PubMed] [Google Scholar]
- Scholander PF, van Dam L, Kanwisher JW, Hammel HT, Gordon MS. Supercooling and osmoregulation in Arctic fish. J Cell Comp Physiol. 1957;49:5–24. [Google Scholar]
- Seo JH, Naing AH, Jeon SM, Kim CK. Anti-freezing-protein type III strongly influences the expression of relevant genes in cryopreserved potato shoot tips. Plant Mol Biol. 2018;97:347–355. doi: 10.1007/s11103-018-0743-8. [DOI] [PubMed] [Google Scholar]
- Sicheri F, Yang DS. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995;375:427–431. doi: 10.1038/375427a0. [DOI] [PubMed] [Google Scholar]
- Sidebottom C, Buckley S, Pudney P, Twigg S, Jarman C, Holt C, Telford J, McArthur A, Worrall D, Hubbard R, Lillford P. Heat-stable antifreeze protein from grass. Nature. 2000;406:256. doi: 10.1038/35018639. [DOI] [PubMed] [Google Scholar]
- Singh P, Hanada Y, Singh SM, Tsuda S. Antifreeze protein activity in Arctic cryoconite bacteria. FEMS Microbiol Lett. 2014;351(1):14–22. doi: 10.1111/1574-6968.12345. [DOI] [PubMed] [Google Scholar]
- Soltys KA, Batta AK, Koneru B. Successful nonfreezing, sub-zero preservation of rat liver with 2, 3-butanediol and type I antifreeze protein. J Surg Res. 2001;96:30–34. doi: 10.1006/jsre.2000.6053. [DOI] [PubMed] [Google Scholar]
- Taylor R, Fletcher RL. Cryopreservation of eukaryotic algae—a review of methodologies. J Appl Phycol. 1998;10:481–501. [Google Scholar]
- Taylor R, Fletcher RL. A simple method for the freeze-preservation of zoospores of the green macroalga Enteromorpha intestinalis. J Appl Phycol. 1999;11:257–262. [Google Scholar]
- Thaler R, Spitzer S, Karlic H, Klaushofer K, Varga F. DMSO is a strong inducer of DNA hydroxymethylation in pre-osteoblastic MC3T3-E1 cells. Epigenetics. 2012;7:635–651. doi: 10.4161/epi.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczak MM, Marshall CB, Gilbert JA, Davies PL. A facile method for determining ice recrystallization inhibition by antifreeze proteins. Biochem Biophys Res Commun. 2003;311:1041–1046. doi: 10.1016/j.bbrc.2003.10.106. [DOI] [PubMed] [Google Scholar]
- Uhlig C, Kabisch J, Palm GJ, Valentin K, Schweder T, Krell A. Heterologous expression, refolding and functional characterization of two antifreeze proteins from Fragilariopsis cylindrus (Bacillariophyceae) Cryobiology. 2011;63:220–228. doi: 10.1016/j.cryobiol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Uperti GC, Payen SR, Duganzich DM, Oliver JE, Smith JF. Enzyme leakage during cryopreservative of ram spermatozoa. Anim Reprod. Sci. 1996;41:27–36. [Google Scholar]
- Wallis JG, Wang H, Guerra DJ. Expression of a synthetic antifreeze protein in potato reduces electrolyte release at freezing temperatures. Plant Mol Biol. 1997;35:323–330. doi: 10.1023/a:1005886210159. [DOI] [PubMed] [Google Scholar]
- Wang JH, Huang CN. Antifreeze proteins: in hypothermic and cryogenic preservation. Chinese J Cell Biol. 1996;18:107–111. [Google Scholar]
- Wang R, Zhang P, Gong Z, Hew CL. Expression of the antifreeze protein gene in transgenic goldfish (Carassius auratus) and its implication in cold adaptation. Mol Mar Biol Biotechnol. 1995;4:20–26. [PubMed] [Google Scholar]
- Wang JH, Bian HW, Huang CN, Ge JG. Studies on the application of antifreeze proteins in cryopreservation of rice embryogenic suspension cells. Acta Biol Exp Sinica. 1999;32:271–276. [PubMed] [Google Scholar]
- Wang JH, Bian HW, Zhang YX, Cheng HP. The dual effect of antifreeze protein on cryopreservation of rice (Oryza sativa L.) embryogenic suspension cells. Cryo Lett. 2001;22:175–182. [PubMed] [Google Scholar]
- Wang Y, Qiu L, Dai C, Wang J, Luo J, Zhang F, Ma J. Expression of insect (Microdera puntipennis dzungarica) antifreeze protein MpAFP149 confers the cold tolerance to transgenic tobacco. Plant Cell Rep. 2008;27:1349–1358. doi: 10.1007/s00299-008-0562-5. [DOI] [PubMed] [Google Scholar]
- Wisniewski M, Webb R, Balsamo R, Close TJ, Yu XM, Griffith M. Purification, immunolocalization, cryoprotective, and antifreeze activity of PCA60: a dehydrin from peach (Prunus persica) Physiol Plant. 1999;105:600–608. [Google Scholar]
- Worrall D, Elias L, Ashford D, Smallwood M, Sidebottom C, Lillford P, Telford J, Holt C, Bowles D. A carrot leucine-rich-repeat protein that inhibits ice recrystallization. Science. 1998;282:115–117. doi: 10.1126/science.282.5386.115. [DOI] [PubMed] [Google Scholar]
- Wu DW, Duman JG, Cheng CHC, Castellino FJ. Purification and characterization of antifreeze proteins from larvae of the beetle Dendroides canadensis. J Comp Physiol B. 1991;161:271–278. [Google Scholar]
- Yeh S, Moffatt BA, Griffith M, Xiong F, Yang DSC, Wiseman SB, Sarhan F, Danyluk J, Xue YQ, Hew CL, Doherty-Kirby A, Lajoie G. Chitinase genes responsive to cold encode antifreeze proteins in winter cereals. Plant Physiol. 2000;124:1251–1263. doi: 10.1104/pp.124.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu B, Feng D, He Y, Wang J. Expression, purification, and antifreeze activity of carrot antifreeze protein and its mutants. Protein Expres Purif. 2004;35:257–263. doi: 10.1016/j.pep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Zhu B, Xiong AS, Peng RH, Xu J, Jin XF, Meng XR, Yao QH. Overexpression of ThpI from Choristoneura fumiferana enhances tolerance to cold in Arabidopsis. Mol Biol Rep. 2010;37:961–966. doi: 10.1007/s11033-009-9759-0. [DOI] [PubMed] [Google Scholar]
- Zilli L, Beirao J, Schiavone R, Herraez MP, Gnoni A, Vilella S. Comparative proteome analysis of cryopreserved flagella and head plasma membrane proteins from sea bream spermatozoa: effect of antifreeze proteins. PLoS One. 2014;9:e99992. doi: 10.1371/journal.pone.0099992. [DOI] [PMC free article] [PubMed] [Google Scholar]