Abstract
Background
The value of alpha-fetoprotein (AFP) as a prognostic indicator in patients with hepatocellular carcinoma (HCC) has been proposed in recent studies, but the evidence so far is still contradictory. This analysis aims to evaluate the prognostic value of preoperative AFP level in patients undergoing curative resection.
Methods
This retrospective study reviewed the prospectively collected data of all patients who underwent initial liver resection for HCC at Queen Mary Hospital during the period from March 1999 to March 2013. Patients with palliative resection, positive margin after pathological examination or distant metastasis were excluded from the study. Survival of patients with AFP level of <20, 20–400 and >400 ng/mL were compared with Kaplan-Meier analysis. Subgroup analysis was performed according to tumour stage (7th edition UICC staging) and tumour size. The optimal cutoff value was determined by area under receiver operating characteristic curve.
Results
A total of 1,182 patients were included. Best overall (OS) and disease free survival (DFS) was observed in patients with AFP level <20 ng/mL. Progressively worse outcomes were seen for patients with increasing level of AFP. The median OS were 132.9, 77.2 and 38.4 months for patients with AFP <20, 20–400 and >400 ng/mL respectively (P<0.001). The median DFS for these three groups were 55.6, 25 and 8.4 months respectively (P<0.001). There was significant difference in both OS and DFS among all 3 groups. With subgroup analysis according to tumour stage (stage I and II versus stage III and IV) and tumour size (5 cm or less versus larger than 5 cm), such difference was still observed and remained statistically significant. Optimal cutoff value by discriminant analysis was 12,918.3 ng/mL for OS and 9,733.3 ng/mL for DFS.
Conclusions
This study demonstrates that AFP is a significant prognostic indicator in HCC. Despite tumour stage and size, high level of AFP is associated with poorer OS and DFS. Whether the level of AFP should be included in current staging systems, or treatment protocols, is yet to be determined.
Keywords: Alpha-fetoprotein (AFP), hepatocellular carcinoma (HCC), prognosis, cirrhosis, survival
Introduction
Alpha-fetoprotein (AFP) has been used as a tumour marker for hepatocellular carcinoma (HCC) since the 1970s (1). It is one kind of feto-specific proteins that is normally produced by the fetal yolk sac during the early embryonic life. Since its discovery, its use in diagnosing and detecting tumour recurrence after surgery was widely studied. In the systemic review by Tateishi et al. (2) in 2008, the sensitivity of AFP ranged from 49% to 71%, and specificity ranged from 49% to 86%, when the cutoff value was at 20 ng/mL. The AFP level can be normal in 32–59% of patients with HCC (3). With relatively low sensitivity and specificity, new markers such as lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) and des-γ-carboxy prothrombin (DCP), are now under study with promising results. Nevertheless, these new markers may not be easily available in every centre. AFP still remains to be the most commonly used marker.
Besides its use in diagnosis, AFP has also been studied for its prognostic value. The evidence so far has been contradictory. Blank et al. (4) studied the survival in patients with HCC according to 5 quintiles of pre-operative AFP levels, and concluded that the survival was better in groups with lower AFP levels. Another study done in Taiwan by Hsu et al. (5) showed that there was significant difference in survival when the cutoff was 20 and 400 ng/mL. However in another study by Shim et al. (6), it was shown that there was no significant difference in time-to-recurrence between AFP-positive and AFP-negative groups. The multivariable competing risks analysis also failed to reveal a significant correlation between baseline AFP level and HCC-specific mortality in the AFP-positive group. In this study, our aim was to investigate the prognostic significance of preoperative AFP in resectable HCC, and whether it will affect our current staging system.
Methods
The data of all patients who underwent initial liver resection for HCC at Queen Mary Hospital were prospectively collected and reviewed. Inclusion period was from March 1999 to March 2013. All operations were standardized and were operated by the same team of surgeons. Only those who had pathology proven HCC were included. Patients receiving palliative resection, re-resection, those who was found to have positive margin after pathological examination or distant metastasis were excluded from the study.
Preoperative work up and operative management
Diagnosis of HCC was based on the typical imaging finding (i.e., early arterial enhancement with early portovenous washout) on CT or magnetic resonance imaging and/or a serum AFP level >400 ng/mL as well as the patients’ hepatitis virus carrier status. Percutaneous needle biopsy was performed only for doubtful cases. AFP level was defined as the serum AFP level taken the day before operation.
Adequate hepatic functional reserve and absence of extrahepatic disease were prerequisites for liver resection. Moreover, the tumor had to be anatomically resectable as evaluated by imaging studies. Hepatic function assessment in terms of Child-Pugh classification and indocyanine green (ICG) clearance test was performed routinely. Child-Pugh class C was regarded as a contraindication to hepatectomy. Patients with an ICG retention rate ≤14% at 15 minutes were eligible for major hepatectomy and the test would be done after any sepsis was controlled. Our techniques of liver resection have been described in a previous report (7). A liver resection was classified as a major resection if ≥3 segments (according to the Couinaud classification) were resected. If <3 segments were resected, it was a minor resection. Staging was done according to the 7th edition Union for International Cancer Control (UICC) staging. Stage I and stage II were defined as early disease, where stage III and stage IV were defined as advanced disease. Tumour size was defined as the diameter of the largest tumour in the resected specimen. Small tumour was defined as the tumour size being 5 cm or less. Large tumour was defined as tumour size being larger than 5 cm.
Postoperative follow-up
All patients were followed up monthly in the first year and quarterly afterwards, with regular monitoring for HCC recurrence by AFP level check and CT of the liver. CT of the liver was performed one month after the operation and then every four to six months. Diagnosis of recurrence was based on the typical imaging finding. Since 2010, dual-tracer positron emission tomography was performed when indefinite recurrences were encountered (8). A standardized aggressive management protocol as described in a previous report was adopted to treat recurrences (9).
Statistical analysis
Patients were categorized according to their AFP levels into 3 groups: AFP <20, 20–400 and >400 ng/mL. Hospital mortality was defined as death occurring during the hospital stay for the primary operation. The overall survival (OS) and disease free survival (DFS) of the 3 groups were analyzed and compared. Subgroup analysis was also performed according to disease stage, tumour size and presence of microvascular invasion.
Pearson’s chi-squared test or Fisher’s exact test was used where appropriate to compare categorical variables. The Mann-Whitney U test was used to compare continuous variables. The Kaplan-Meier method was used in survival analyses, and the log-rank test was used to compare variables. P<0.05 denoted statistical significance, and all P values were two-tailed. Subgroup analyses were performed according to tumour stage (7th edition UICC staging) and tumour size. The optimal cutoff value was determined by area under receiver operating characteristic curve (AUROC curve). Analyses were performed by SPSS version 18.
To evaluate the effect of AFP level on current staging system, the survival outcomes of patients with different AFP levels were compared between different stages. Using the cutoff value derived from AUROC curve, the survival of patients with AFP value larger than the cutoff value was compared with the survival of patients one stage up.
Results
Patients’ characteristics
A total of 1,182 patients were included from the study period. Patient’s demographics were listed in Table 1. The median age of patients in 3 groups ranges from 55 to 60.5 years old. The AFP >400 ng/mL groups had a relatively younger age group (median 55 years; range 13–82 years; P<0.001). Male predominance was observe in all 3 groups, but it was less obvious in the AFP >400 ng/mL group (266:92) when compared with the AFP <20 ng/mL group (396:70) (P=0.001).
Table 1. Patients’ characteristics.
| Characteristic | AFP <20 ng/mL (n=466) | AFP 20–400 ng/mL (n=358) | AFP >400 ng/mL (n=358) | P value |
|---|---|---|---|---|
| Age (years) | 60.5 [26–84] | 58 [12–89] | 55 [13–82] | <0.001 |
| Sex (M:F) | 396:70 | 287:71 | 266:92 | 0.001 |
| Hepatitis status | 0.653 | |||
| No hepatitis | 67 (14.4%) | 37 (10.3%) | 40 (11.2%) | |
| Hepatitis B | 379 (81.3%) | 305 (85.2%) | 304 (84.9%) | |
| Hepatitis C | 16 (3.4%) | 14 (3.9%) | 12 (3.4%) | |
| Both | 4 (0.9%) | 2 (0.6%) | 2 (0.6%) | |
| Child Pugh grade | 0.978 | |||
| A | 450 (96.6%) | 345 (96.4%) | 346 (96.6%) | |
| B | 16 (3.4%) | 13 (3.6%) | 12 (3.4%) | |
| Presence of comorbid disease | 219 (47.0%) | 166 (46.4%) | 121 (33.8%) | <0.001 |
| Follow up (month) | 58.4 [0.3–195.1] | 53.3 [2.2–202.9] | 38.6 [0.6–202.2] | <0.001 |
AFP, alpha-fetoprotein.
Distribution in hepatitis status was similar across the 3 groups. Over 80% of patients in all three groups suffered from hepatitis B, which was a common pattern observed in Asia (10). The preoperative Child Pugh grade was also similar in the 3 groups, with around 96% of patients with Child Pugh A status. All Child Pugh C patients were deemed not suitable for resection and therefore not included. There was significant difference in presence of comorbid disease in the 3 groups, with less patient in the group with AFP >400 ng/mL (33.8%; P<0.001) suffered from comorbid disease. The median follow up period was also different between groups, with significant shorter period in the AFP >400 ng/mL group (38.6 months; P<0.001), attributed to the short survival.
Tumour characteristics
The group with highest AFP levels had significantly larger tumours. Size of the largest tumour was 4.65 cm (0.7–23 cm) in AFP <20 ng/mL group, 4.35 cm (0.7–28 cm) in AFP 20–400 ng/mL group, and 7 cm (0.8–27 cm) in AFP >400 ng/mL group (P<0.001). There were also significantly more patients with micro- and macrovascular invasion found in the specimen in the groups with higher AFP level. Up to 65.9% of patients were found with microvascular invasion and 14.8% of patients with macrovascular invasion in AFP >400 ng/mL group (P<0.001). Details of each group were listed in Table 2.
Table 2. Tumour characteristics.
| Characteristic | AFP <20 ng/mL (n=466) | AFP 20–400 ng/mL (n=358) | AFP >400 ng/mL (n=358) | P value |
|---|---|---|---|---|
| Size of largest tumour (cm) | 4.65 (0.7–23) | 4.35 (0.7–28) | 7 (0.8–27) | <0.001 |
| UICC 7th edition | <0.001 | |||
| Stage I | 236 (50.6%) | 145 (40.5%) | 92 (25.7%) | |
| Stage II | 145 (31.1%) | 135 (37.7%) | 113 (31.6%) | |
| Stage III | 84 (18%) | 76 (21.2%) | 151 (42.2%) | |
| Stage IVA | 1 (0.2%) | 2 (0.6%) | 2 (0.6%) | |
| Microvascular invasion | 177 (38%) | 175 (48.9%) | 236 (65.9%) | <0.001 |
| Macrovascular invasion | 13 (2.8%) | 18 (5%) | 53 (14.8%) | <0.001 |
AFP, alpha-fetoprotein; UICC, Union for International Cancer Control.
Survival outcomes
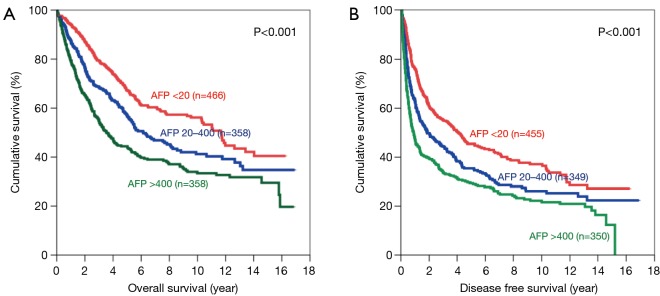
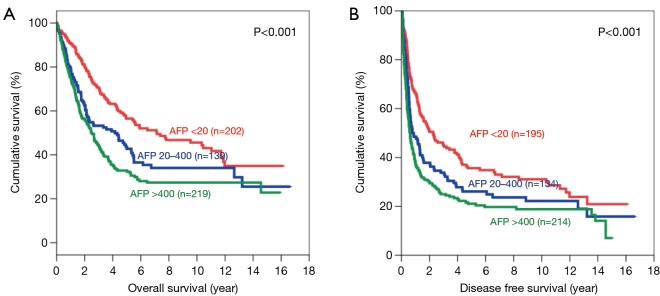
There was significant difference in both OS and DFS among all 3 groups (Figure 1). The 5-year OS were 67.3%, 56.6% and 44.4% for patients with AFP <20, 20–400 and >400 ng/mL respectively (P<0.001). The 5-year DFS for these three groups were 45.4%, 35.4% and 29.3% respectively (P<0.001). For both OS and DFS, there was statistically significant difference.
Figure 1.
Kaplan-Meier curves of overall (A) and disease-free survival (B) in all patients (AFP levels <20 ng/mL: red line; 20–400 ng/mL: blue line; and >400 ng/mL: green line). AFP, alpha-fetoprotein.
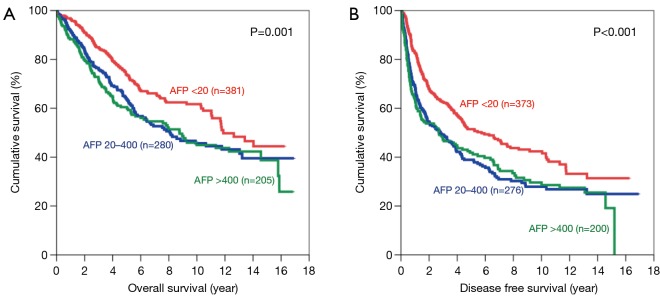
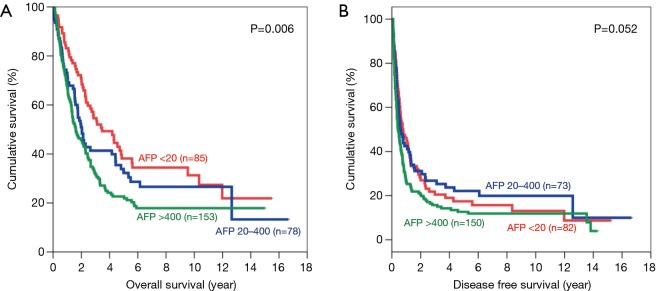
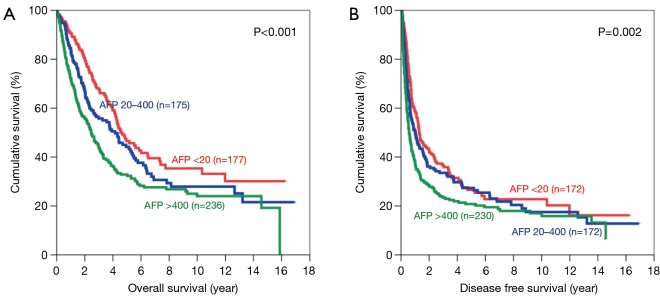
Subgroup analysis was performed according to stage of disease, tumour size and presence of microvascular invasion. For early disease patients, such difference was still observed in OS and DFS (Figure 2). Patients with AFP level <20 ng/mL have significantly better OS (5-year survival 73.8%; P=0.001) and DFS (5-year survival 51.4%; P<0.001). In advanced disease, such difference in OS became less but still remained significant (Figure 3). The 5-year OS for AFP <20 ng/mL group was 38.2%, and the percentage dropped to 32.2% for AFP 20–400 ng/mL group and 22.7% for AFP >400 ng/mL group (P=0.006). However such pattern was lost in DFS for advance disease, where there was no significant difference among the 3 groups.
Figure 2.
Kaplan-Meier curves of overall (A) and disease-free survival (B) in patients with early stage disease (AFP levels <20 ng/mL: red line; 20–400 ng/mL: blue line; and >400 ng/mL: green line). AFP, alpha-fetoprotein.
Figure 3.
Kaplan-Meier curves of overall (A) and disease-free survival (B) in patients with advanced stage disease (AFP levels <20 ng/mL: red line; 20–400 ng/mL: blue line; and >400 ng/mL: green line). AFP, alpha-fetoprotein.
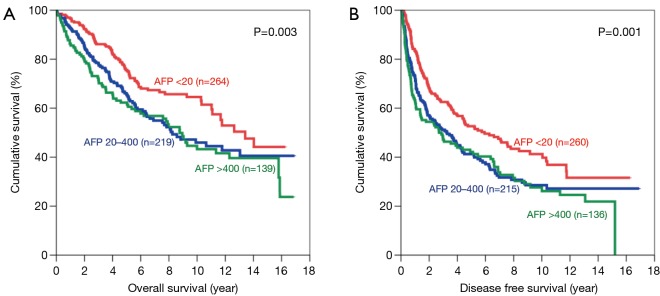
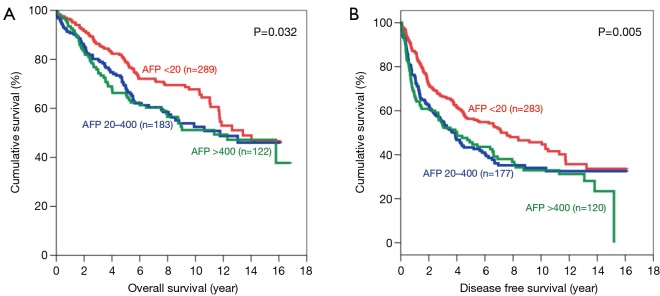
The same trend was observed after the patients were categorized according to tumour size. For patients with small tumours, both the OS and DFS demonstrated significant difference among all 3 groups, with AFP <20 ng/mL group having the best survival (Figure 4). The 5-year OS for AFP <20 ng/mL group was 75.6% (P=0.003) and the 5-year DFS was 52.6% (P=0.001). As for patients with large tumours, the AFP<20 ng/mL groups also had the longest survival among the three groups (Figure 5). The 5-year OS was 56.5% (P<0.001) and the 5-year DFS was 35.6% (P<0.001) in this group.
Figure 4.
Kaplan-Meier curves of overall (A) and disease-free survival (B) in patients with small tumours (AFP levels <20 ng/mL: red line; 20–400 ng/mL: blue line; and >400 ng/mL: green line). AFP, alpha-fetoprotein.
Figure 5.
Kaplan-Meier curves of overall (A) and disease-free survival (B) in patients with large tumours (AFP levels <20 ng/mL: red line; 20–400 ng/mL: blue line; and >400 ng/mL: green line). AFP, alpha-fetoprotein.
When the patients were categorized according to the presence of microvascular invasion in the tumour, the survival was again better in patients with lower AFP levels. For patients with microvascular invasion (Figure 6), the 5-year OS for AFP<20ng/mL group was 46.6% whereas those for AFP 20–400 ng/mL and AFP >400 ng/mL groups were 44.1% and 32.8% respectively (P<0.001). The 5-year DFS for AFP <20 ng/mL group was 26.6%, which was also significantly better than the AFP >400 ng/mL group (5-year DFS 20.8%, P=0.002). For patients without microvascular invasion (Figure 7), the 5-year OS was 79.1% for AFP <20 ng/mL group, 69.4% for AFP 20-400 ng/mL group and 66.3% for AFP >400 ng/mL group (P=0.032). Similarly, the DFS for AFP <20 ng/mL group was significantly better (5-year DFS 56.3%) when compared with AFP >400 ng/mL group (5-year DFS 45.7%; P=0.005).
Figure 6.
Kaplan-Meier curves of overall (A) and disease-free survival (B) in patients with microvascular invasion by tumour (AFP levels <20 ng/mL: red line; 20–400 ng/mL: blue line; and >400 ng/mL: green line). AFP, alpha-fetoprotein.
Figure 7.
Kaplan-Meier curves of overall (A) and disease-free survival (B) in patients without microvascular invasion by tumour (AFP levels <20 ng/mL: red line; 20–400 ng/mL: blue line; and >400 ng/mL: green line). AFP, alpha-fetoprotein.
Results of univariable and multivariable analysis were listed in Tables 3 and 4. Risk factors affecting OS by multivariate analysis include presence of ascites before operation, AFP >20 ng/mL, albumin level, tumour number and size, micro- and macrovascular invasion and invasion to other organs (Table 3). Risks factors affecting DFS by multivariate analysis include presence of ascites, AFP >20 ng/mL, albumin level, number and size of tumour, and presence of microvascular invasion (Table 4).
Table 3. Univariable analysis and multivariable analysis for overall survival.
| Characteristic | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Odds (95% CI) | P value | Odds (95% CI) | P value | ||
| Age | 1.004 (0.997–1.012) | 0.225 | |||
| Sex | 1.121 (0.91–1.38) | 0.284 | |||
| Comorbid | 1.035 (0.878–1.22) | 0.686 | |||
| Child Pugh grade | 0.788 (0.519–1.195) | 0.262 | |||
| Ascites | 3.082 (1.277–7.439) | 0.012 | 2.54 (1.05–6.19) | 0.04 | |
| AFP | |||||
| <20 | – | ||||
| 20–400 | 1.44 (1.17–1.77) | 0.001 | 1.29 (1.04–1.6) | 0.019 | |
| >400 | 1.96 (1.61–2.39) | <0.001 | 1.44 (1.17–1.77) | 0.001 | |
| Bilirubin | 1.006 (0.995–1.017) | 0.293 | |||
| Platelet | 1.001 (1.00001–1.002) | 0.048 | |||
| INR | 4.728 (2.109–10.6) | <0.001 | |||
| Albumin | 0.923 (0.907–0.939) | <0.001 | 0.95 (0.93–0.96) | <0.001 | |
| Preop ICG (>15) (101, 8.5% missing) | 1.241 (1.029–1.495) | 0.024 | |||
| No. of tumors (solitary vs. multiple) | 2.485 (2.101–2.94) | <0.001 | 1.89 (1.58–2.25) | <0.001 | |
| Size of largest tumor (cm) | 1.075 (1.059–1.091) | <0.001 | 1.02 (1.001–1.04) | 0.042 | |
| Magnitude of operation (major vs. minor) | 1.658 (1.402–1.96) | <0.001 | |||
| Intraop blood loss | 1.102 (1.058–1.147) | <0.001 | |||
| Blood transfusion | 1.845 (1.484–2.293) | <0.001 | |||
| Invasion into major branch/hepatic vein | 3.427 (2.654–4.424) | <0.001 | 1.59 (1.2–2.09) | 0.001 | |
| Invasion into adjacent organ | 2.496 (1.737–3.586) | <0.001 | 1.74 (1.19–2.54) | 0.004 | |
| Microvascular invasion | 2.518 (2.127–2.981) | <0.001 | 1.81 (1.5–2.19) | <0.001 | |
| Differentiation | |||||
| Well | – | – | |||
| Moderate | 1.399 (1.116–1.753) | 0.004 | |||
| Poor | 1.676 (1.289–2.18) | <0.001 | |||
| UICC staging (I/II vs. III/IV) | 2.855 (2.415–3.375) | <0.001 | |||
AFP, alpha-fetoprotein; ICG, indocyanine green; UICC, Union for International Cancer Control.
Table 4. Univariable analysis and multivariable analysis for disease-free survival.
| Characteristic | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Odds (95% CI) | P value | Odds (95% CI) | P value | ||
| Age | 1.001 (0.995–1.007) | 0.799 | |||
| Sex | 1.191 (0.991–1.431) | 0.062 | |||
| Comorbid | 1.081 (0.937–1.248) | 0.284 | |||
| Child Pugh grade | 1.262 (0.871–1.829) | 0.219 | |||
| Ascites | 2.989 (1.239–7.211) | 0.015 | 2.48 (1.02–6.02) | 0.045 | |
| AFP | |||||
| <20 | – | – | |||
| 20–400 | 1.36 (1.14–1.62) | 0.001 | 1.19 (0.99–1.42) | 0.065 | |
| >400 | 1.8 (1.52–2.13) | <0.001 | 1.34 (1.12–1.61) | 0.001 | |
| Bilirubin | 1.0006 (0.997–1.016) | 0.207 | |||
| Platelet | 1 (0.999–1.001) | 0.434 | |||
| INR | 3.848 (1.883–7.863) | <0.001 | |||
| Albumin | 0.943 (0.929–0.958) | <0.001 | 0.97 (0.95–0.98) | <0.001 | |
| Preop ICG (>15) | 1.207 (1.023–1.425) | 0.026 | |||
| No. of tumors (solitary vs. multiple) | 2.577 (2.214–2.998) | <0.001 | 2 (1.7–2.34) | <0.001 | |
| Size of largest tumor (cm) | 1.072 (1.058–1.086) | <0.001 | 1.03 (1.02–1.05) | <0.001 | |
| Magnitude of operation (major vs. minor) | 1.365 (1.183–1.575) | <0.001 | |||
| Intraop blood loss | 1.087 (1.047–1.129) | <0.001 | |||
| Blood transfusion | 1.507 (1.231–1.846) | <0.001 | |||
| Invasion into major branch/hepatic vein | 2.664 (2.087–3.402) | <0.001 | |||
| Invasion into adjacent organ | 1.925 (1.351–2.744) | <0.001 | |||
| Microvascular invasion | 2.202 (1.906–2.543) | <0.001 | 1.69 (1.44–1.97) | <0.001 | |
| Differentiation | |||||
| Well | – | – | |||
| Moderate | 1.317 (1.086–1.598) | 0.005 | |||
| Poor | 1.634 (1.303–2.048) | <0.001 | |||
| UICC staging (I/II vs. III/IV) | 2.66 (2.286–3.095) | <0.001 | |||
AFP, alpha-fetoprotein; ICG, indocyanine green; UICC, Union for International Cancer Control.
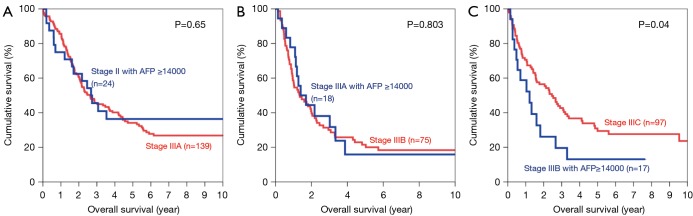
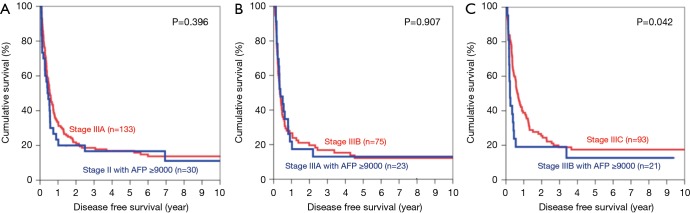
Optimal cutoff value by AUROC was 14,000 ng/mL for OS (area =0.687) and 9,000 ng/mL for DFS (area =0.705). Using these cutoff, it was found that the 5-year OS of patients with AFP >14,000 ng/mL was comparable to one stage up (Figure 8). It was impossible to compare using patients of stage I and IVA, as the numbers of patients in these groups were too small. When the cutoff value of AFP 9,000 ng/mL was used, the patients with stage II to IIIB disease and AFP level higher than the cutoff all showed similar 5-year DFS when compared with those patients one stage above (Figure 9). In patients with stage IIIB disease, the 5-year DFS was 12.7% in patients with AFP >9,000 ng/mL whereas the 5-year DFS for those with stage IIIC disease was 17.5% (P=0.042).
Figure 8.
Kaplan-Meier curves of overall survival in patients with AFP >14,000 ng/mL, compared with patients one stage up (A: stage II versus stage IIIA; B: stage IIIA versus stage IIIB; C: stage IIIB versus stage IIIC). AFP, alpha-fetoprotein.
Figure 9.
Kaplan-Meier curves of disease-free survival in patients with AFP >9,000 ng/mL, compared with patients one stage up (A: stage II versus stage IIIA; B: stage IIIA versus stage IIIB; C: stage IIIB versus stage IIIC). AFP, alpha-fetoprotein.
Discussion
As early as in 1989, the prognostic value of AFP has already been studied. In the paper published by Nomura et al. (11), survival rates of 606 patients were compared among 4 groups according to AFP levels in size matched cases. It was found that the group with higher AFP levels was associated with worse prognosis. Over the years, there were a number of other studies which showed the same conclusion (4,5,12,13). In this study, it has once again demonstrated the relationship between AFP level and prognosis, even in subgroup analysis according to tumour size, presence of microvascular invasion and stage of disease.
With growing evidence in its prognostic value, the next question will be whether it will have an impact on our current clinical practice, namely the staging system. There are a number of staging systems besides the TNM system. Some of them already incorporated the level of AFP, such as the Cancer of the Liver Italian Program score (CLIP score) proposed by the Italian group in 1998 (14). There was also proposition of adding biomarkers to current available system, such as the one suggested by Kitai et al. (15). The Japanese group combined the Japan Integrated Staging (JIS) and three tumour markers (AFP, AFP-L3 and DCP) to become a new system called the Biomarker combined JIS (bm-JIS). The author showed that the new staging system had a better stratification value than the conventional JIS system. With the results from this study, it is possible that by including AFP level in the TNM system, the prognostic ability of the current system could be refined. Some other researchers even proposed to incorporate preoperative AFP level into Barcelona Clinic Liver Center (BCLC) staging guidelines in order to aid in the choice of the most optimal therapy for HCC (16,17). For most patients, resection will still remain to be the best treatment option if the lesion is resectable. However if the patient is having a relatively high surgical risk, a high preoperative AFP level may prompt the surgeons to consider other treatment options, knowing that there may be a higher recurrence rate and a poorer survival even with complete resection.
The findings of this study may also have an impact on our criteria for liver transplantation. Currently only patients with T2 disease or below were eligible to liver transplantation. If a high AFP level warrants an upstage of the disease, there may be a group of patients who will probably be excluded from liver transplantation. This echoes with another study conducted by Hameed et al. (18), which concluded that an AFP level of >1,000 ng/mL was the strongest pre-transplant variable predicting HCC recurrence as well as vascular invasion found in the explant. Therefore the author proposed that patients with AFP level >1,000 ng/mL should be excluded from liver transplantation even they were within the Milan Criteria. In some countries like the United Kingdom, a high AFP level (>1,000 IU/mL) is considered to be an absolute contraindication to liver transplantation. This is particularly important to locality with relatively low deceased donor rate like Hong Kong. Whether this group of patients should undergo living donor liver transplantation is still unknown, but it is the surgeon’s duty to inform the donor and the recipient beforehand about the implication of a high AFP level.
Another topic which is worth further study is adjuvant therapy. It was shown in several studies (19,20) that there might be potential benefit by adjuvant therapy, such as transcatheter arterial chemoembolization, in a selected group of patients who received curative resection. In a randomized controlled trial by Zhong et al. (21), it was shown that hepatectomy with adjuvant TACE was able to improve both the OS when compared with hepatectomy alone in patients with Stage IIIA disease (UICC 6th edition). They found that although the DFS was similar, those with recurrence tend to be more of single lesion, and thus more proportion of patients could receive curative therapy. It was also shown that those receiving adjuvant TACE had a significantly better OS. In other studies (22-24), different selection criteria were examined, including tumour size, microvascular invasion and portal vein thrombus, and they showed promising results from adjuvant TACE. It is worth investigating whether AFP level should be included as one of the selection criteria, given its prognostic ability and its potential impact on staging.
There are certain limitations in the study, one of which being that a majority of patients were suffering from HBV infection. This may affect the general applicability of the results, particularly to the Western countries. As for the optimal cutoff value, further external validation is still required.
To conclude, AFP level before curative resection is a significant prognostic factor for predicting survival. A high level of AFP may warrant an upstage of the disease. Further studies should investigate for a need to refine our current management strategy according to AFP level.
Acknowledgments
None.
Ethical Statement: The HKU/HA HKW IRB is authorized by a joint agreement of the University of Hong Kong and Hospital Authority Hong Kong West Cluster to review and monitor clinical research (IRB Reference Number: UW 19-428) and informed consent was taken from all the patients. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Johnson PJ. Role of alpha-fetoprotein in the diagnosis and management of hepatocellular carcinoma. J Gastroenterol Hepatol 1999;14 Suppl:S32-6. 10.1046/j.1440-1746.1999.01873.x [DOI] [PubMed] [Google Scholar]
- 2.Tateishi R, Yoshida H, Matsuyama Y, et al. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int 2008;2:17-30. 10.1007/s12072-007-9038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biselli M, Conti F, Gramenzi A, et al. A new approach to the use of alpha-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br J Cancer 2015;112:69-76. 10.1038/bjc.2014.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blank S, Wang Q, Fiel MI, et al. Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: normal is not the new normal. Ann Surg Oncol 2014;21:986-94. 10.1245/s10434-013-3357-z [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, Liu PH, Lee YH, et al. Using serum alpha-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PLoS One 2015;10:e0118825. 10.1371/journal.pone.0118825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shim JH, Yoon DL, Han S, et al. Is serum alpha-fetoprotein useful for predicting recurrence and mortality specific to hepatocellular carcinoma after hepatectomy? A test based on propensity scores and competing risks analysis. Ann Surg Oncol 2012;19:3687-96. 10.1245/s10434-012-2416-1 [DOI] [PubMed] [Google Scholar]
- 7.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322. 10.1097/00000658-199903000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung TT, Chan SC, Ho CL, et al. Can positron emission tomography with the dual tracers [11C] acetate and [18F] fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transplantation 2011;17:1218-25. 10.1002/lt.22362 [DOI] [PubMed] [Google Scholar]
- 9.Chok KS, Chan SC, Poon RT, et al. Re-resection for metachronous primary hepatocellular carcinoma: is it justified? ANZ J Surg 2012;82:63-7. 10.1111/j.1445-2197.2011.05931.x [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology 2012;142:1264-73.e1. 10.1053/j.gastro.2011.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer 1989;64:1700-7. [DOI] [PubMed] [Google Scholar]
- 12.Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004;15:796-802. 10.1002/cncr.20426 [DOI] [PubMed] [Google Scholar]
- 13.Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol 2013;11:212. 10.1186/1477-7819-11-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manghisi G, Elba S, Mossa A, et al. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology 1998;28:751-5. 10.1002/hep.510280322 [DOI] [PubMed] [Google Scholar]
- 15.Kitai S, Kudo M, Minami Y, et al. A new prognostic staging system for hepatocellular carcinoma: value of the biomarker combined Japan integrated staging score. Intervirology 2008;51:86-94. 10.1159/000122599 [DOI] [PubMed] [Google Scholar]
- 16.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol 2000;31:302-8. 10.1097/00004836-200012000-00007 [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Rodriguez R, Romero-Gutierrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012;104:298-304. 10.4321/S1130-01082012000600003 [DOI] [PubMed] [Google Scholar]
- 18.Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-51. 10.1002/lt.23904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong JH, Li H, Li LQ, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol 2012;38:286-95. 10.1016/j.ejso.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 20.Hong Y, Wu LP, Ye F, et al. Adjuvant Intrahepatic Injection Iodine-131-Lipiodol Improves Prognosis of Patients with Hepatocellular Carcinoma After Resection: a Meta-Analysis. Indian J Surg 2015;77:1227-32. 10.1007/s12262-015-1261-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong C, Guo RP, Li JQ, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol 2009;135:1437-45. 10.1007/s00432-009-0588-2 [DOI] [PubMed] [Google Scholar]
- 22.Ren ZG, Lin ZY, Xia JL, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol 2004;10:2791-4. 10.3748/wjg.v10.i19.2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Sun L, Xu J, et al. Clinical efficacy of postoperative adjuvant transcatheter arterial chemoembolization on hepatocellular carcinoma. World J Surg Oncol 2016;14:100. 10.1186/s12957-016-0855-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun JJ, Wang K, Zhang CZ, et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol 2016;23:1344-51. 10.1245/s10434-015-5008-z [DOI] [PubMed] [Google Scholar]