Abstract
Purpose
Oxymatrine, an alkaloid extracted from the Chinese herb Sophora flavescens Aiton, possesses anti-inflammatory, anti-immune, anti-hepatic fibrosis, and anti-cancer properties. However, the effects of oxymatrine on epithelial-mesenchymal transition (EMT) of breast cancer cells are still unclear.
Aim
The present study was performed to investigate whether oxymatrine reverses EMT in breast cancer cells and to explore the underlying molecular mechanisms.
Materials and methods
MTT assay was performed to evaluate cell viability. Wound-healing assay and transwell chamber assay were used to assess cell migration and invasion, respectively. Immunofluorescence and Western blot were used to study the expression of EMT-related molecules and αⅤβ3 integrin/focal adhesion kinase (FAK)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling transduction. Fibronectin, a physiologic ligand of αⅤβ3 integrin, was used to stimulate αⅤβ3 integrin signaling.
Results
Our results demonstrated that oxymatrine effectively suppressed the viability of MDA-MB-231 and 4T1 breast cancer cells, and oxymatrine showed less cytotoxicity on normal breast mammary epithelial MCF-10A cells. In addition, oxymatrine reversed EMT in the MDA-MB-231 and 4T1 cells at nontoxic concentrations. Oxymatrine significantly inhibited cell migration and invasion, downregulated the expression of N-cadherin, vimentin, and Snail in MDA-MB-231 and 4T1 cells, but upregulated the expression of E-cadherin in 4T1 cells. The mechanism revealed that oxymatrine decreased the expression of αⅤ and β3 integrin and their co-localization. It also inhibited αⅤβ3 integrin downstream activation by suppressing the phosphorylation of FAK, PI3K, and Akt. Furthermore, oxymatrine prevented fibronectin-induced EMT and αⅤβ3 integrin/FAK/PI3K/Akt signaling activation.
Conclusion
Our results revealed that oxymatrine effectively reversed EMT in breast cancer cells by depressing αⅤβ3 integrin/FAK/PI3K/Akt signaling. Thus, oxymatrine could be a potential therapeutic candidate with anti-metastatic potential for the treatment of breast cancer.
Keywords: oxymatrine, breast cancer, αⅤβ3 integrin, epithelial-mesenchymal transition
Introduction
Breast cancer is one of the most common malignancy occurring among women worldwide.1 More than 90% of breast cancer-related deaths are not caused by the primary tumors, but by distant metastases.2 In spite of great improvements in the early detection and diagnosis, no gold standard treatment has been defined or validated for metastatic breast cancer so far.3 Once breast cancer progresses to the metastatic stage, the clinical prognosis is guarded and the five-year relative survival rate is poor.4 Therefore, new therapeutic strategies constraining metastasis in breast cancer are still warranted. Metastasis is accomplished by a shift of the polarized epithelial tumor cells to mesenchymal cells, which is known as epithelial to mesenchymal transition (EMT).5 Aberrant activation of EMT program powers the cancer cells to lose cell-cell adhesion and acquire enhanced migratory and invasive behavior so that they can navigate through the natal sites and eventually lead to distant metastases.6 Thus, reversal or suppression of EMT may prevent the metastatic spread of breast cancer cells.
Integrins are responsible for cellular adhesion to extracellular matrix proteins though forming a heterodimer of α and β subunits.7 They allow the cells to interact with their environment and transmit bidirectional signaling as “outside-in” and “inside-out” thereby modulating cell proliferation, cytoskeletal organization, adhesion, migration, and differentiation.8 In addition, integrins transmit signals through crosstalk with several growth factor receptors or directly recruit and activate focal adhesion kinase (FAK) and Src-family kinases, and subsequently stimulated downstream signaling cascades, including the phosphoinositide kinase (PI3K)/Akt, mitogen-activated protein kinases (MAPK), and extracellular signal-regulated kinase (ERK).9
Abnormal high expression of αⅤβ3 integrin significantly correlates with tumor progression in several cancers.10 In breast cancer, αⅤβ3 integrin is strongly expressed in the invasive tumors and distant metastatic sites within the tumors and is an indicator of poor prognosis.11,12 It has been revealed that αⅤβ3 integrin promotes several steps of breast cancer metastasis. As an adhesion receptor, αⅤβ3 integrin supports the tumor cells to attach to the extracellular matrix, and interact with platelets to arrest within the capillaries during blood flow.13 It also induces metastatic phenotype of breast cancer cells by intensifying their migratory and invasive behavior.14 In addition, αⅤβ3 integrin is required for enhancing the EMT process in breast cancer cells.15 Gene silencing of αⅤβ3 integrin leads to decreased fibroblast growth factor 1-enhanced N-cadherin expression and cell motility in breast cancer cells.16 Knockout of αⅤβ3 integrin transcription factor HoxD3 was found to significantly suppress lipopolysaccharide-induced EMT in breast cancer cells.17 Thus, these results suggest that αⅤβ3 integrin may be a potential therapeutic target for reversing EMT in breast cancer, thereby providing an alternative avenue for the treatment of patients having a metastatic risk.
Oxymatrine is an alkaloid derived from the traditional Chinese herb Sophora flavescens Aiton.18 It exhibits multiple pharmacological activities, such as anti-viral, anti-fibrotic, anti-inflammatory, anti-allergic, and cardiovascular protective effects.19 In addition, oxymatrine also possesses anti-tumor effects by targeting multiple pathways through inhibiting cell growth and angiogenesis, inducing apoptosis and cell cycle arrest, reducing cancer metastasis and invasion, and reversing multidrug resistance in cancer cells.20 It has also been considered as a promising anticancer agent in breast cancer. Oxymatrine significantly suppresses proliferation and promotes S-phase cell cycle arrest through mitochondria-mediated apoptosis in MDA-MB-231 and MCF-7 breast cancer cells.21 Furthermore, oxymatrine inhibits proliferation of cancer stem-like cells in MCF-7 cells via suppression of Wnt/β-catenin signaling pathway.22 However, it is still unclear whether oxymatrine reverses EMT in breast cancer cells.
In this study, we used two breast cancer cell lines, MDA-MA-231 and 4T1, exhibiting highly invasive and metastatic potentials,23,24 to evaluate whether oxymatrine could reverse EMT in these two types of cells. We also explored the mechanisms related to the effects of oxymatrine on αⅤβ3 integrin/FAK/PI3K/Akt signaling pathway.
Materials and methods
Materials
Oxymatrine was purchased from Zelang Pharmaceutical Technology Co., Ltd (Nanjing, Jiangsu, China). Cyclo (Arg-Gly-Asp-d-Phe-Lys, -RGDfK, #A8164) was obtained from WeiHuan Biotech Co., Ltd (Shanghai, China). Fibronectin (FN, #F2006) was obtained from Sigma-Aldrich (Saint Louis, MO, USA). Matrigel (#356237) was obtained from BD Biosciences (Bedford, MA, USA). 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, #804W059) was obtained from Solarbio Co., Ltd (Beijing, China). The primary antibodies against E-cadherin (#BS1098, 1:800), vimentin (#BS1491, 1:800), N-cadherin (#BS2224, 1:800), phosphorylated-PI3K (#AP0152, 1:800), PI3K (#BS3006, 1:1000), phosphorylated-FAK (#BS4684, 1:1000), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, #MB001, 1:5000) were obtained from Bio-World (Dublin, OH, USA). The primary antibody against Akt (#BA2318, 1:300) was obtained from Boster Biological Technology (Wuhan, Hubei, China). The primary antibodies against phosphorylated-Akt (#4058S, 1:800) and Snail (#3879, 1:800) were obtained from Cell Signaling Technology (Beverly, MA, USA). The primary antibodies against integrin β3 (#sc-46655 1:1000) and FAK (#sc-557, 1:1000) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The anti-αⅤ integrin antibody (#ab179475, 1:1000) was purchased from Abcam, Inc. (Cambridge, MA, USA). Horseradish peroxidase-labeled goat anti-mouse (#BS12478, 1:3000) and goat anti-rabbit (#BS13278, 1:3000) secondary antibodies were purchased from Bio-World. Fluorescein isothiocyanate (FITC)-labeled goat anti-mouse antibody (#BS11502, 1:100) and goat anti-rabbit secondary antibody (#ab150077, 1:400) were purchased from Bio-World and Abcam, respectively.
Cell culture
MDA-MB-231 and 4T1 breast cancer cell lines were purchased from Kunming Cell Biological Institute of the Chinese Academy of Science (Kunming, Yunnan, China). The cells were cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12, Gibco, Carlsbad, CA, USA). The medium was supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco). The MCF-10A cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured in DMEM/F-12 with 5% horse serum (HS; 1312396, Gibco), 20 ng/mL epidermal growth factor (AF-100-15, PeproTech, London, United Kingdom), 0.1 μg/mL cholera enterotoxin (C8052, Sigma), 10 μg/mL insulin (I6634, Sigma), 0.5 μg/mL hydrocortisone (H0888, Sigma). These cells were incubated in presence of 5% CO2 at 37°C.
MTT assay
The cells were digested, collected, and seeded in 96-well plates for 24 h. The cells were treated with 0, 1.01, 1.68, 2.8, 4.67, 7.78, 12.96, 21.60, 36, 60, and 100 μmol/mL oxymatrine for 24, 48, or 72 h. MTT solution (5 mg/mL, 20 μl/well) was then added and the cells were continuously incubated for 4 h. The medium was subsequently replaced with DMSO (100 μl/well). The optical density (OD) was detected with a spectrophotometer at 570 nm by a microplate reader (ELx800, General Electric, Fairfield, CT, USA).
Wound healing assay
The cells were seeded into 12-well plates and grown to a monolayer until it reached 90% confluence. The cell monolayer was scratched by a 10 μl pipette tip to form a straight wound. The cells were then treated with oxymatrine in a medium containing 1% FBS for 48 h. The images were captured by a Leica DMI 1 microscope at 0 and 48 h (Leica, Wetzlar, Germany) at 50× magnification.
Invasion assay
The cell invasion was detected by Matrigel-coated transwell chamber (8-μm pore size, Millipore, Billerica, MA, USA) assay. After treatment, the cells were resuspended in a serum-free media (5 × 104 cells/well) and were added into the upper chambers. The medium containing 10% FBS was placed in the bottom chamber as a chemotaxin. After 24 h, the membrane was lightly washed with phosphate-buffered saline and fixed with 4% paraformaldehyde. The non-invasive cells on the upside of the membrane were removed and the invasive cells on the downside of the membrane were stained with Hematoxylin and Eosin (H&E, Beyotime). The images were captured by a Leica DMI 1 microscope (Leica, 100× magnification).
Western blotting
The collected cells were lysed using a lysis buffer (Beyotime). The lysates were centrifuged at 4 °C for 20 min at 12,000 g. The protein concentration was estimated by bicinchoninic acid protein assay kit (Thermo, Waltham, MA, USA). The total protein was separated by electrophoresis in sodium dodecyl sulfate-10% polyacrylamide gel and transferred on a polyvinylidene fluoride membrane (Millipore). After blocking with 5% non-fat milk for 1 h, the membrane was incubated with the appropriate primary antibodies overnight at 4 ºC and subsequently incubated with the secondary antibodies for 1.5 h at 37 ºC. The results were analyzed using the ChemiDoc XRS + system and Image Lab Software (Version 5.2, Bio-Rad).
Immunofluorescence microscopy
The coverslips were fixed with 4% paraformaldehyde (15 min), permeabilized with 0.2% Triton X-100 (15 min), and blocked with goat serum (1 h). Then coverslips were incubated with primary antibodies for αⅤ integrin (1:300), β3 integrin (1:100), N-cadherin (1:100) and vimentin (1:400) overnight at 4 °C. The coverslips were subsequently incubated with FITC-conjugated secondary antibodies for 1 h and counterstained with 4,6’-diamidino-2- phenylindole dihydrochloride (DAPI, 1 μg/mL) for 40 min. The images were captured by using a DMi8 fluorescence microscope and Leica X software (Leica, 400×magnification).
Statistical analyses
All data are represented as the mean ± standard error of the mean (SEM), and the experiments were performed in triplicates. One-way analysis of variance and Bonferroni post-hoc test was performed using GraphPad Prism 5. A P-value of <0.05 was considered statistically significant.
Results
Oxymatrine inhibited the viability of MDA-MB-231 and 4T1 cells
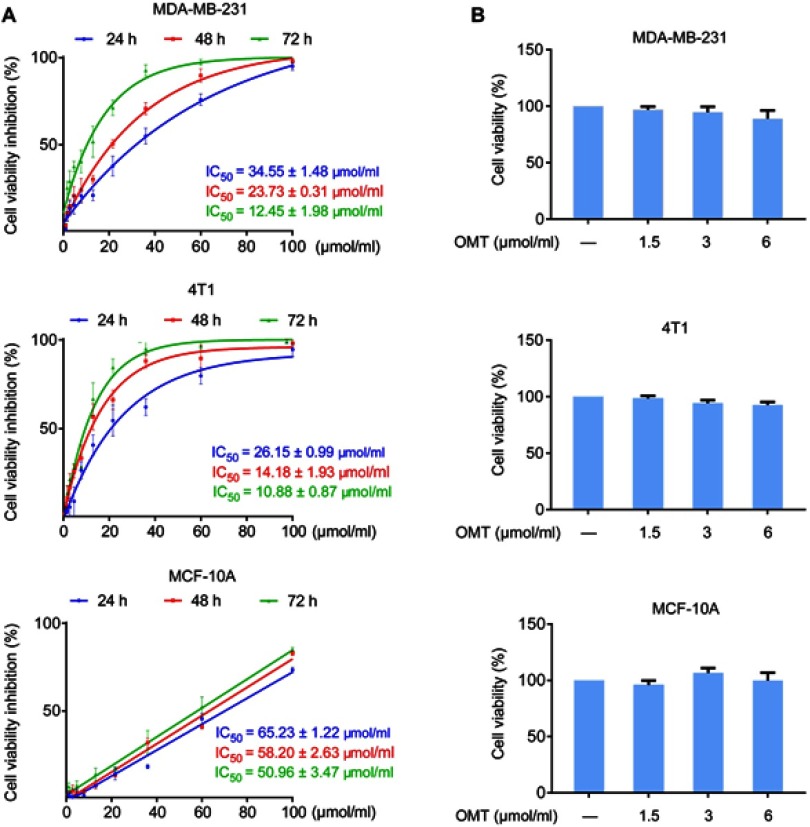
MTT assay was performed to evaluate the effects of oxymatrine on cell viability in breast cancer MDA-MB-231 and 4T1 cells, and normal breast mammary epithelial MCF-10A cells at different time points. Oxymatrine significantly suppressed the viability of MDA-MB-231 and 4T1 cells. The IC50 values of oxymatrine in MDA-MB-231 cells at 24, 48, and 72 h were 34.55±1.48, 23.71±0.31, and 12.45±1.98 μmol/mL, respectively (Figure 1A). Additionally, the IC50 values of oxymatrine on 4T1 cells at 24, 48, and 72 h were 26.15±0.99, 14.18±1.93, and 10.88±0.87 μmol/mL, respectively (Figure 1A). Oxymatrine showed less toxicity on MCF-10A cells with IC50 values for 24-, 48-, and 72-h treatments of 65.23±1.22 μmol/mL, 58.20±2.63 μmol/mL, and 50.96±3.47 μmol/mL, respectively. In addition, the results demonstrated that treatment with oxymatrine at concentrations of 1.5, 3, and 6 μmol/mL for 48 h had no obvious cytotoxicity in MDA-MB-231, 4T1 and MCF-10A cells (Figure 1B). Therefore, these non-cytotoxic concentrations of oxymatrine were used in the subsequent experiments.
Figure 1.
Effects of oxymatrine on the viability of MDA-MB-231, 4T1 and MCF-10A cells.
Notes: (A) Inhibition of cell viability and IC50 values for oxymatrine in MDA-MB-231, 4T1 and MCF-10A cells. The cells were treated with various concentrations of oxymatrine (0–100 μmol/mL) for 24, 48, and 72 h. (B) The viability of MDA-MB-231, 4T1 and MCF-10A cells after 1.5, 3, and 6 μmol/mL oxymatrine treatment for 48 h. The values represent the mean ± SEM. The statistical data is provided from 3 independent experiments.
Abbreviation: OMT, oxymatrine.
Oxymatrine inhibited the migration and invasion of MDA-MB-231 and 4T1 cells
We next assessed the inhibitory activities of oxymatrine on cell migratory and invasive abilities by wound-healing assay and transwell chamber assay, respectively. The results showed that oxymatrine significantly inhibited the migratory abilities of MDA-MB-231 and 4T1 cells as compared to the control cells (Figure 2A). After treatment with oxymatrine at 1.5, 3, or 6 μmol/mL for 48 h, the migration rate decreased to 91.8±5.6%, 74.6±10.6%, and 59.8±6.6% in the MDA-MB-231 cells, and 70.9±8.1%, 58.4±6.2% and 46.1±5.4% in the 4T1 cells, respectively. As shown in Figure 2B, oxymatrine also dramatically decreased cell invasive ability. Treatment with 1.5, 3, and 6 μmol/mL oxymatrine reduced the invasive cells to 82.9±6.8%, 47.4±4.5%, and 33.1±6.5% in the MDA-MB-231 cells and 88.0±10.9%, 51.1±5.9%, and 19.7±4.9% in the 4T1 cells, respectively.
Figure 2.
The effects of oxymatrine on cell migration and invasion.
Notes: (A) The cell migration was estimated using a wound healing assay. The images were captured at 0 and 48 h after wounding (magnification, 50×; scale bars, 100 μm). (B) The cell invasion was investigated using the Matrigel-coated transwell model (magnification, 100×; scale bars, 75 μm). The values represent the mean ± SEM, *P<0.05, as compared to the control group. The statistical data is provided from 3 independent experiments.
Abbreviation: OMT, oxymatrine.
Oxymatrine reversed the expression of EMT-related markers in MDA-MB-231 and 4T1 cells
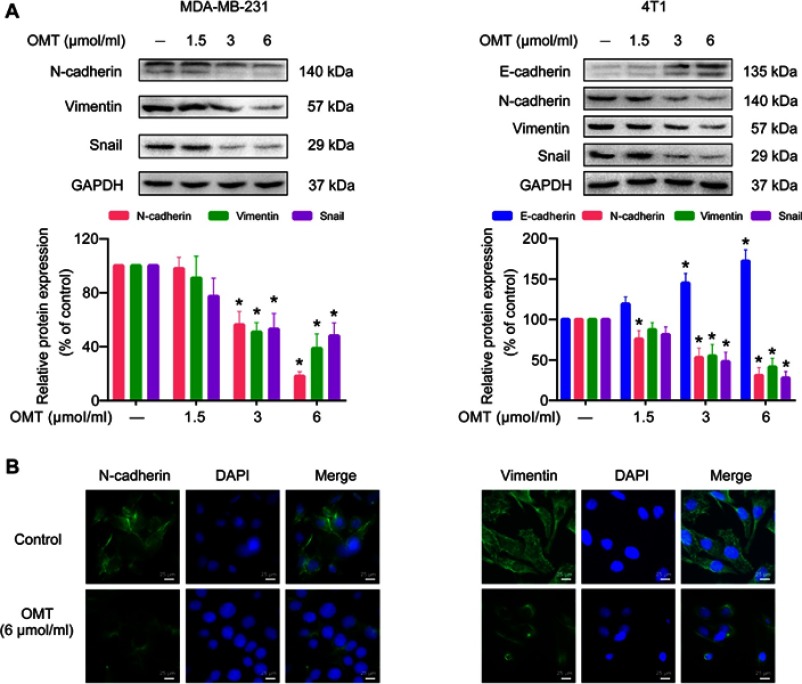
We continued to examine whether oxymatrine affected the expression of EMT-related makers by Western blot and immunofluorescence microscopy. Oxymatrine significantly enhanced E-cadherin expression in 4T1 cells and reduced the expression of N-cadherin, vimentin, and Snail in MDA-MB-231 and 4T1 cells as compared to the control (Figure 3A). Similarly, immunofluorescence results also confirmed that N-cadherin and vimentin decreased in MDA-MB-231 cells after oxymatrine treatment (Figure 3B). In addition, the morphological changes of vimentin were also observed after oxymatrine treatment. The cells in the control group showed extended filamentous of vimentin, which was obviously decreased in the oxymatrine-treated group (Figure 3B).
Figure 3.
The effects of oxymatrine on the expression of EMT markers in MDA-MB-231 and 4T1 cells.
Notes: (A) The expression of E-cadherin, N-cadherin, vimentin, and Snail in MDA-MB-231 and 4T1 cells were detected by Western blot. Densitometric analysis of EMT-related protein expression normalized with GAPDH. The values represent the mean ± SEM. *P<0.05 as compared to the control group. (B) The expression and distribution of N-cadherin and vimentin in MDA-MB-231 cells were confirmed by immunofluorescence microscopy (magnification, 400×; scale bars, 25 μm). The statistical data is provided from 3 independent experiments.
Abbreviations: OMT, oxymatrine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Oxymatrine suppressed αⅤβ3 integrin expression and its downstream FAK/ PI3k/Akt signaling activation
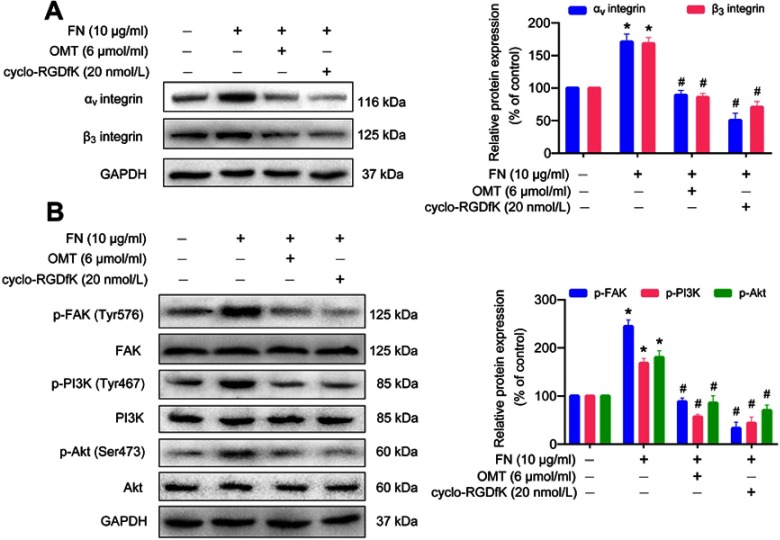
We explored the underlying molecular mechanisms of oxymatrine on EMT suppression. It was demonstrated that oxymatrine significantly inhibited the expression of αⅤ and β3 integrin in MDA-MB-231 and 4T1 cells as compared to the control (Figure 4A). We also confirmed the influence of oxymatrine on the expression and co-localization of αⅤ (green) and β3 (red) integrin in MDA-MB-231 cells using immunofluorescence staining. The yellow immunofluorescence represented co-localization of the two molecules. It was observed that oxymatrine reduced the αⅤ and β3 integrin-related fluorescence intensity and decreased the co-localization of αⅤ and β3 integrin in the cytoplasm (Figure 4B). In addition, increased αⅤβ3 integrin expression has been reported to stimulate FAK/PI3K/Akt signaling in breast cancer.25 Thus, we continually estimated the effects of oxymatrine on the phosphorylation of FAK, PI3K, and Akt. It was demonstrated that oxymatrine significantly downregulated the phosphorylation of FAK (Tyr576) but did not change the total FAK expression in MDA-MB-231 cells (Figure 4C). Meanwhile, oxymatrine inhibited the phosphorylation of PI3K (p85α Tyr467) and Akt (Ser473) (Figure 4C). The total protein expression of PI3K and Akt also did not change after oxymatrine treatment.
Figure 4.
The inhibitory effects of oxymatrine on αⅤβ3 integrin/FAK/PI3K/Akt signaling.
Notes: MDA-MB-231 and 4T1 cells were treated with or without oxymatrine (1.5, 3 and 6 μmol/mL) for 48 h. (A) The expression of αⅤ and β3 integrin was analyzed by Western blot. (B) The expression and co-localization of αⅤ and β integrin were evaluated by immunofluorescence microscopy (magnification, 400×; scale bars, 25 μm). (C) The expression of phosphorylated and total FAK, PI3K, and Akt were detected by Western blot. The values represent the mean ± SEM. *P<0.05 compared with the control group. The statistical data is provided from 3 independent experiments.
Abbreviations: OMT, oxymatrine; FAK, focal adhesion kinase; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Oxymatrine inhibited FN-enhanced αⅤβ3 integrin/FAK/PI3K/Akt signaling activation
We used αⅤβ3 integrin ligand FN and its specific antagonist cyclo-RGDfK to further investigate the inhibitory effects of oxymatrine on αⅤβ3 integrin signaling. FN obviously augmented the expression of αⅤ and β3 integrin in MDA-MB-231 cells as compared to the control group (Figure 5A). However, oxymatrine significantly inhibited FN-promoted αⅤ and β3 integrin expression in a manner similar to cyclo-RGDfK. Meanwhile, FN evidently stimulated the downstream FAK/PI3K/Akt signaling activation accompanied by increased phosphorylated FAK (Tyr576), PI3K p85α (Tyr467), and Akt (Ser473) (Figure 5B). Furthermore, pretreatment with oxymatrine and cyclo-RGDfK inhibited the phosphorylation of FAK, PI3K, and Akt (Figure 5B). These results indicated that oxymatrine possesses inhibitory effects on αⅤβ3/FAK/PI3K/Akt signaling activation.
Figure 5.
The effects of oxymatrine on FN-induced αⅤβ3 integrin up-regulation and FAK/PI3K/Akt signaling activation in MDA-MB-231 cells.
Notes: The cells were pretreated with oxymatrine and cyclo-RGDfK for 1 h, and then stimulated with FN for 48 h (serum-free condition). (A) The expression of αⅤ and β3 integrin was analyzed by Western blot. (B) The expression of phosphorylated and total FAK, PI3K, and Akt was detected by Western blot. The data are shown as the mean ± SEM. *P<0.05 as compared to the control group, #P<0.05 as compared to the FN group. The statistical data is provided from 3 independent experiments.
Abbreviations: OMT, oxymatrine; FN, fibronectin; FAK, focal adhesion kinase; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Oxymatrine inhibited FN-induced EMT in MDA-MB-231 cells
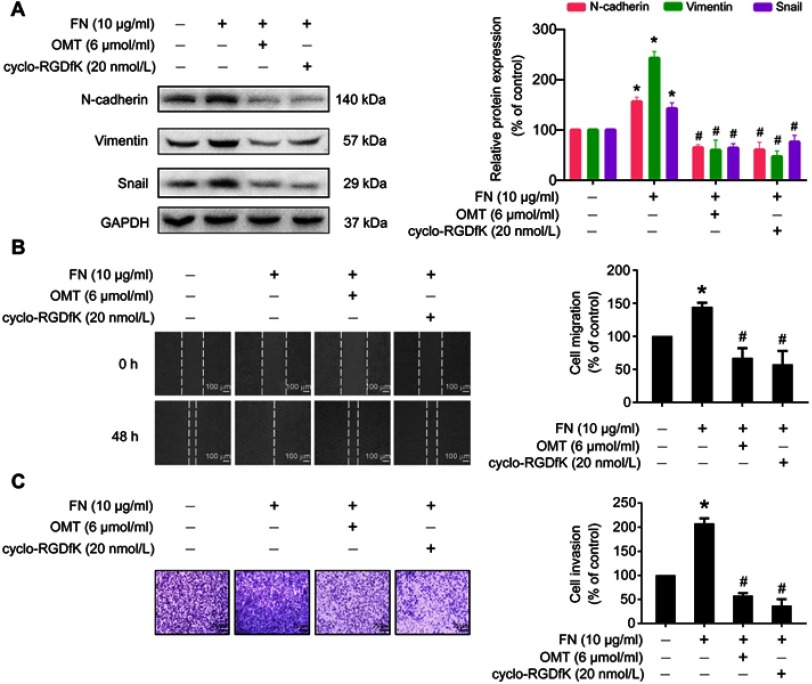
To further explore whether oxymatrine suppressed EMT via inhibition of αⅤβ3 integrin, we assessed the effects of oxymatrine on FN-induced changes of EMT-related proteins, cellular migration, and invasion in MDA-MB-231 cells. Upon FN stimulation in MDA-MB-231 cells for 48 h, N-cadherin, vimentin and Snail were upregulated (Figure 6A). However, pretreatment with oxymatrine or cyclo-RGDfK significantly decreased the expression of N-cadherin, vimentin, and Snail as compared to the FN-treated group (Figure 6A). In addition, FN facilitated the migration and invasion of MDA-MB-231 cells, but oxymatrine and cyclo-RGDfK pretreatment also depressed FN-enhanced cell migration and invasion (Figure 6B and C). It suggested that oxymatrine reversed EMT in breast cancer cells by suppressing αⅤβ3 integrin.
Figure 6.
The effects of oxymatrine on FN-induced cell migration, invasion, and EMT-related protein expression.
Notes: The cells were pretreated with oxymatrine and cyclo-RGDfK for 1 h, and then stimulated with FN for 48 h (serum-free condition). (A) The effects of oxymatrine on FN-induced EMT-related protein expression. (B) The effects of oxymatrine on FN-induced cell migration by wound healing assay. The images were captured at 0 and 48 h after wounding (magnification, 50×, scale bars, 100 μm). (C) The effects of oxymatrine on FN-induced cell invasion by Matrigel-coated transwell assay. The images were captured by an inverted microscope (magnification, 100×, scale bars, 75 μm). The values represent the mean ± SEM. *P<0.05 compared with the control group; #P<0.05 compared with the FN group. The statistical data is provided from 3 independent experiments.
Abbreviations: OMT, oxymatrine; FN, fibronectin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
New generations of drugs targeting metastasis for the treatment of breast cancer are required.26 Natural products are an important source of new drug.27 Oxymatrine, a natural bioactive compound, has been approved as an anti-hepatitis B drug by the State Food and Drug Administration of China.18 Oral administration of oxymatrine has demonstrated potential benefits and safety in chronic hepatitis B patients without causing serious adverse events.28 Additionally, increasing pieces of evidence have confirmed that oxymatrine possesses anti-tumor efficacies against several types of cancer, including breast cancer.29 Drug repurposing, which deals with novel uses of existing drugs, is a practical and economical method to develop an anticancer drug.30 Thus, evaluation of the anti-metastatic effects of oxymatrine may be an alternative promising strategy for inhibiting metastasis in breast cancer patients.
In our present study, we found that oxymatrine markedly reversed EMT in high invasive and metastatic breast cancer MDA-MB-231 and 4T1 cells. It significantly suppressed cell migration and invasion in the two cell lines. Beside increased migratory and invasive capacities, the EMT process is accomplished by loss of epithelial markers as well as the elevation of mesenchymal markers and EMT-activating transcription factors.31 The epithelial marker E-cadherin is a critical cell adhesion molecule that maintains the integrity of the epithelial cells and cell junction and inhibits cancer cell movement.32 High expression of N-cadherin and vimentin contributes to directly enhancing the invasiveness and metastatic phenotype of cancer cells.33 Snail, one of EMT-inducing transcription factor, is activated early in EMT,34 and is known to activate the transcription of genes contributing to the mesenchymal phenotype.35 Here, oxymatrine was found to reduce the expression of N-cadherin, vimentin, and Snail in MDA-MB-231 and 4T1 cells, and accelerate E-cadherin expression in 4T1 cells. These results suggested that oxymatrine might be a promising candidate to inhibit breast cancer metastasis by reversing EMT.
The integrins are presented as attractive therapeutic targets of drug designing for cancer metastasis. In recent decades, several integrin-based antibodies or antagonists are currently tested in clinical trials. Although some anti-integrin agents did not demonstrate any efficacy, others such as MK-0429 (αⅤβ3 antagonist) or GLPG0187 (multi-integrin antagonist) are still under clinical investigation and showed promising results for the treatment of metastatic prostate cancer.36 In addition, preclinical investigations have revealed that integrin αⅤβ3 antagonists (cilengitide) or antibody (CNTO 95) significantly reduced the metastatic tumor burden in the bone, brain, or lungs of rats bearing highly metastatic breast cancer cell xenografts.37–39 It was indicated that breast cancer patients with metastatic risk might benefit from anti-integrin αⅤβ3 treatment. Our results demonstrated that oxymatrine significantly decreases the expression of αⅤ and β3 integrin. It also suppressed the co-localization of αⅤ and β3 integrin in the cytoplasm, which indicates that the frequency of their combination and formation of heterodimer were reduced. FN, a major extracellular matrix glycoprotein, stimulates biochemical and mechanical signaling though integrin.40 It contains an arginine-glycine-aspartate (RGD) motif, which can be attached by αⅤβ3 integrin.41 Thus, we used FN as a stimulator to confirm the role of αⅤβ3 integrin on the oxymatrine-induced reversal of EMT. The results demonstrated that oxymatrine, similar to an αⅤβ3 integrin antagonist cyclo-RGDfK, suppressed FN-enhanced αv and β3 integrin expression. Oxymatrine also decreased FN-induced cell migration, invasion, and EMT marker changes. Thus, these results indicated that oxymatrine reversed EMT features of breast cancer via αⅤβ3 integrin suppression.
FAK is a main component of adhesion complex and acts as a central downstream effector of integrin signaling transduction which influences multiple cellular processes, such as survival, migration, invasion, and tissue remodeling.42 Most integrins activate FAK through inducing its autophosphorylation at Tyr397 that produces a binding site for Src kinase. Following this, FAK is phosphorylated at Tyr576 and Tyr577 by Src, which displays the maximal catalytic activity.9 As a non-receptor tyrosine kinase, activated FAK stimulates the downstream kinase PI3K by binding and phosphorylating the p85α subunit of PI3K (regulatory subunit of PI3K) and subsequently activates Akt via phosphorylation.43 Moreover, the FAK and PI3K/Akt are the common pathways involved in facilitating tumor cell EMT and metastasis.44 Recent investigations have also confirmed that FAK/PI3K/Akt is the downstream signaling pathway for αⅤβ3 integrin-mediated breast cancer cell EMT, which is induced by 14,15-epoxyeicosatrienoic acid.45 Here, we have found that oxymatrine markedly depressed the phosphorylation of FAK (Try576), PI3K p85α (Tyr467), and Akt (Ser473) in MDA-MB-231 breast cancer cells. Furthermore, oxymatrine also blocked FN-induced activation of FAK, PI3K, and Akt similar to the actions of cyclo-RGDfK. These results demonstrated that oxymatrine exerts inhibitory effects on the αvβ3 integrin/FAK/PI3K/Akt signaling activation.
Limitations
There are some further issues that need to be addressed by future investigations. It remains to be evaluated whether oxymatrine could suppress the metastases of breast cancer in vivo. It is also required to explore how oxymatrine affects αⅤβ3 integrin and whether oxymatrine interrupts its conformational rearrangements or binding with paxillin and talin. Moreover, the expression of αⅤβ3 integrin is normally found in the activated tumor endothelial cells, but not in the resting endothelial cells, and hence, it is required for forcing tumor cells to form new vessels under vascular endothelial growth factor stimulation.46 Thus, αⅤβ3 integrin is considered an appropriate therapeutic target for the treatment of tumor angiogenesis, which is an essential step in cancer metastasis. It is possible that oxymatrine exerts inhibitory effects on angiogenesis of breast cancer, although further investigations are required in this regard.
Conclusion
In view of our results, oxymatrine effectively reversed EMT in breast cancer cells through inhibition of αⅤβ3 integrin signaling. This provides an important basis to further explore the potential mechanisms and beneficial effects of oxymatrine for the prevention of breast cancer metastasis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81560598, 81860648), the High Level Innovation Talents of Guizhou Province (grant number 71000305), the Science and Technology Innovation Advanced Individual of Guizhou Educational Department (grant number QJHKY[2018]048), the Science Foundation of Guiyang Science and Technology Bureau (grant number [20141001]06), Postdoctoral Science Foundation of China (grant number 2015M582749XB), the Foundation for Training Programs of Innovation and Entrepreneurship for Undergraduates of Guizhou Medical University (grant number 2018520337), the Key Project for Natural Science Foundation of Guizhou Province (grant number QKHJZ[2015]2002), the Innovation Team of Guizhou Province (grant number 2015-4025), the High Level Innovation Talents (grant number 2015‑4029), the International Scientific and Technological Cooperation Base of Guizhou Province (grant number 2017‑5802), the Natural Science Foundation of Guizhou Province Science and Technology Agency and Guizhou Medical University (grant number QKHLH[2015]7365), the Natural Science Foundation of Guizhou Province (grant number QKHJ[2012]2028), the Social Development Supportive Program of Science & Technology Department of Guizhou Province (grant number [2018]2767), the Foundation of Basic Science Research Project of Guizhou Province (grant number [2019]1279), the Foundation of Key Laboratory of Basic Pharmacology of Ministry of Education in Zunyi Medical University (grant number QJHKY[2017]379), and the Foundation from Administration of Traditional Chinese Medicine of Guizhou Province (grant number QZYY-2018-086).
Author contributions
All authors contributed to data analysis, drafting and revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Arora R, Yates C, Gary BD, et al. Panepoxydone targets NF-kB and FOXM1 to inhibit proliferation, induce apoptosis and reverse epithelial to mesenchymal transition in breast cancer. PLoS One. 2014;9(6):e98370. doi: 10.1371/journal.pone.0098370 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Kozlowski J, Kozlowska A, Kocki J. Breast cancer metastasis - insight into selected molecular mechanisms of the phenomenon. Postepy Hig Med Dosw (Online). 2015;69:447–451. [DOI] [PubMed] [Google Scholar]
- 3.Bonotto M, Gerratana L, Poletto E, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. 2014;19(6):608–615. doi: 10.1634/theoncologist.2014-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiely BE, Soon YY, Tattersall MH, Stockler MR. How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol. 2011;29(4):456–463. doi: 10.1200/JCO.2010.30.2174 [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savagner P. Epithelial-mesenchymal transitions: from cell plasticity to concept elasticity. Curr Top Dev Biol. 2015;112:273–300. doi: 10.1016/bs.ctdb.2014.11.021 [DOI] [PubMed] [Google Scholar]
- 7.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215. doi: 10.1186/gb-2007-8-5-r81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334 [DOI] [PubMed] [Google Scholar]
- 9.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549 [DOI] [PubMed] [Google Scholar]
- 10.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liapis H, Flath A, Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol. 1996;5(2):127–135. [DOI] [PubMed] [Google Scholar]
- 12.Gasparini G, Brooks PC, Biganzoli E, et al. Vascular integrin alpha(v)beta3: a new prognostic indicator in breast cancer. Clin Cancer Res. 1998;4(11):2625–2634. [PubMed] [Google Scholar]
- 13.Felding-Habermann B, O’Toole TE, Smith JW, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98(4):1853–1858. doi: 10.1073/pnas.98.4.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh S, Kim H, Nam K, Shin I. Glut1 promotes cell proliferation, migration and invasion by regulating epidermal growth factor receptor and integrin signaling in triple-negative breast cancer cells. BMB Rep. 2017;50(3):132–137. doi: 10.5483/bmbrep.2017.50.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill BS, Sarnella A, Capasso D, et al. Therapeutic potential of a novel alphavbeta(3) antagonist to hamper the aggressiveness of mesenchymal triple negative breast cancer sub-type. Cancers (Basel). 2019;11(2):139. doi: 10.3390/cancers11020139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori S, Kodaira M, Ito A, et al. Enhanced expression of integrin alphavbeta3 induced by TGF-beta is required for the enhancing effect of Fibroblast Growth Factor 1 (FGF1) in TGF-beta-induced Epithelial-Mesenchymal Transition (EMT) in mammary epithelial cells. PLoS One. 2015;10(9):e0137486. doi: 10.1371/journal.pone.0137486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo BP, Luo J, Hu YB, Yao XW, Wu FH. Cyclin D1b splice variant promotes alphavbeta3-mediated EMT induced by LPS in breast cancer cells. Curr Med Sci. 2018;38(3):467–472. doi: 10.1007/s11596-018-1902-5 [DOI] [PubMed] [Google Scholar]
- 18.He M, Wu Y, Wang M, Chen W, Yuan W, Jiang J. The clinical value of oxymatrine in preventing lamivudine induced YMDD mutation: a meta-analysis. Evid Based Complement Alternat Med. 2015;2015:971616. doi: 10.1155/2015/971616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, You RL, Qin WJ, et al. Anti-tumor activities of active ingredients in compound kushen injection. Acta Pharmacol Sin. 2015;36(6):676–679. doi: 10.1038/aps.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Xu Y, Ji W, et al. Anti-tumor activities of matrine and oxymatrine: literature review. Tumour Biol. 2014;35(6):5111–5119. doi: 10.1007/s13277-014-1680-z [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Cai Y, Li M, Zhang Y, Li H, Tan Z. Oxymatrine promotes S-phase arrest and inhibits cell proliferation of human breast cancer cells in vitro through mitochondria-mediated apoptosis. Biol Pharm Bull. 2017;40(8):1232–1239. doi: 10.1248/bpb.b17-00010 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Piao B, Zhang Y, et al. Oxymatrine diminishes the side population and inhibits the expression of beta-catenin in MCF-7 breast cancer cells. Med Oncol. 2011;28(Suppl 1):S99–S107. doi: 10.1007/s12032-010-9721-y [DOI] [PubMed] [Google Scholar]
- 23.Kia V, Mortazavi Y, Paryan M, Biglari A, Mohammadi-Yeganeh S. Exosomal miRNAs from highly metastatic cells can induce metastasis in non-metastatic cells. Life Sci. 2019;220:162–168. doi: 10.1016/j.lfs.2019.01.057 [DOI] [PubMed] [Google Scholar]
- 24.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oudart JB, Doue M, Vautrin A, et al. The anti-tumor NC1 domain of collagen XIX inhibits the FAK/ PI3K/Akt/mTOR signaling pathway through alphavbeta3 integrin interaction. Oncotarget. 2016;7(2):1516–1528. doi: 10.18632/oncotarget.6399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson RL, Balasas T, Callaghan J, et al. A framework for the development of effective anti-metastatic agents. Nat Rev Clin Oncol. 2019;16(3):185–204. doi: 10.1038/s41571-018-0134-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seabrooks L, Hu L. Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharm Sin B. 2017;7(4):409–426. doi: 10.1016/j.apsb.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song WJ, Luo J, Wu T, Yao SK. Oral oxymatrine preparation for chronic hepatitis B: a systematic review of randomized controlled trials. Chin J Integr Med. 2016;22(2):141–149. doi: 10.1007/s11655-015-2143-0 [DOI] [PubMed] [Google Scholar]
- 29.Lu ML, Xiang XH, Xia SH. Potential signaling pathways involved in the clinical application of oxymatrine. Phytother Res. 2016;30(7):1104–1112. doi: 10.1002/ptr.5632 [DOI] [PubMed] [Google Scholar]
- 30.Cha Y, Erez T, Reynolds IJ, et al. Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol. 2018;175(2):168–180. doi: 10.1111/bph.13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banyard J, Bielenberg DR. The role of EMT and MET in cancer dissemination. Connect Tissue Res. 2015;56(5):403–413. doi: 10.3109/03008207.2015.1060970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65(23):3756–3788. doi: 10.1007/s00018-008-8281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Tao D, Xu Q, Gao Z, Tang D. Expression of E-cadherin and vimentin in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8(3):3150–3154. [PMC free article] [PubMed] [Google Scholar]
- 34.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci. 2013;116:317–336. doi: 10.1016/B978-0-12-394311-8.00014-5 [DOI] [PubMed] [Google Scholar]
- 36.Philippe CL. Therapeutic value of an integrin antagonist in prostate cancer. Curr Drug Targets. 2016;17(3):321–327. [DOI] [PubMed] [Google Scholar]
- 37.Cheng C, Komljenovic D, Pan L, Dimitrakopoulou-Strauss A, Strauss L, Bauerle T. Evaluation of treatment response of cilengitide in an experimental model of breast cancer bone metastasis using dynamic PET with 18F-FDG. Hell J Nucl Med. 2011;14(1):15–20. [PubMed] [Google Scholar]
- 38.Wu YJ, Muldoon LL, Gahramanov S, Kraemer DF, Marshall DJ, Neuwelt EA. Targeting alphaV-integrins decreased metastasis and increased survival in a nude rat breast cancer brain metastasis model. J Neurooncol. 2012;110(1):27–36. doi: 10.1007/s11060-012-0942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu YJ, Pagel MA, Muldoon LL, Fu R, Neuwelt EA. High alphav integrin level of cancer cells is associated with development of brain metastasis in athymic rats. Anticancer Res. 2017;37(8):4029–4040. doi: 10.21873/anticanres.11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benito-Jardon M, Klapproth S, Gimeno LI, et al. The fibronectin synergy site re-enforces cell adhesion and mediates a crosstalk between integrin classes. Elife. 2017;6:e22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberhauser AF, Badilla-Fernandez C, Carrion-Vazquez M, Fernandez JM. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol. 2002;319(2):433–447. doi: 10.1016/S0022-2836(02)00306-6 [DOI] [PubMed] [Google Scholar]
- 42.Burridge K. Focal adhesions: a personal perspective on a half century of progress. Febs J. 2017;284(20):3355–3361. doi: 10.1111/febs.14195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flamini MI, Uzair ID, Pennacchio GE, et al. Thyroid hormone controls breast cancer cell movement via integrin alphaV/beta3/SRC/FAK/PI3-Kinases. Horm Cancer. 2017;8(1):16–27. doi: 10.1007/s12672-016-0280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang WG, Sanders AJ, Katoh M, et al. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35(Suppl):S244–S275. doi: 10.1016/j.semcancer.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 45.Luo J, Yao JF, Deng XF, et al. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin alphavbeta3 and activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37(1):23. doi: 10.1186/s13046-018-0694-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V, Chen X. Integrin alpha v beta 3 antagonists for anti-angiogenic cancer treatment. Recent Pat Anticancer Drug Discov. 2007;2(2):143–158. [DOI] [PubMed] [Google Scholar]