Abstract
Estradiol (E2) is a first-line drug for osteoporosis (OP) treatment via promotion of osteoblastic proliferation and differentiation. However, a long-term use of E2 would produce side effects thus, it is imperative to discover safer and more effective drugs. Pinoresinol (PINO) has a similar chemical structure to E2. The present study aimed to investigate whether PINO could promote osteoblastic proliferation and differentiation and the potential mechanisms. After treatment with 0.1 µg/l PINO for 2 days, MC3T3-E1 cell migration was assessed by wound healing assay. Estrogen (E2) treatment served as a positive control. RT-qPCR and western blotting were used for mRNA and protein expression analyses. Alkaline phosphatase (ALP) activity assay and Alizarin red staining were performed to investigate the calcification and mineralization, and the cyclic AMP (cAMP) level was detected by enzyme-linked immunosorbent assay (ELISA). H89, an inhibitor of protein kinase A (PKA), was introduced to verify the role of cAMP/PKA in the effect of PINO on MC3T3-E1 cells. Cell viability was the highest under 48 h of 0.1 µg/l PINO treatment. After treatment with PINO, a significant increase was observed in the migration rate and the expression of collagen type I (Col-I), ALP, osteopontin (OPN), runt-related transcription factor 2 (Runx2) and bone morphogenetic protein-2 (BMP-2) (P<0.01). The ALP activity and Alizarin red size in PINO and E2 groups were notably increased. The increased cAMP, PKA and phosphorylated cAMP response element-binding protein (CREB) levels were also observed in the PINO group. Furthermore, H89 co-treatment abolished the positive effects of PINO on cell viability and migration. PINO had similar effects to E2 on the osteoblastic proliferation and differentiation, and these positive effects may be attributed to the regulation of the cAMP/PKA signaling pathway.
Keywords: estrogen, alkaline phosphatase, mineralization, bone morphogenetic protein-2
Introduction
Osteoporosis (OP), which is a type of body-wide metabolic osteopathy, is characterized by osteopenia, skeletal fragility, and microarchitectural deterioration. The main cause of OP is the imbalance between bone formation and resorption (1). The chronic pain, malformation and pathological fracture induced by OP severely affect the quality of life of the elderly, postmenopausal or estrogen-deficient women, and it even leads to death (2,3). With the increase of the aging population, OP has gradually attracted the attention of many countries and society. It has been reported that the balance of bone metabolism is maintained by osteoclasts and osteoblasts, and the weakness of osteoblast proliferation is one of the most important pathogenesis of OP (4,5). Therefore, enhancing the proliferation and differentiation of osteoblasts is vital to develop OP treatment strategies. To date, the main treatments of OP were based on drug therapies, including calcitonin, and bisphosphonates which functioned as bone resorption inhibitors by blocking the function of osteoclasts (6). Estrogens could directly regulate the expression of bone formation-related genes by binding to estrogen receptor (ER) in osteoblasts (4,7). However, a long-term use of these agents has been associated with severe side effects (8,9). For instance, a long term use of bisphosphonates in clinical practice could lead to gastrointestinal intolerance, osteonecrosis of the jaw, atypical femur fractures, oesophageal cancer, atrial fibrillation and chronic musculoskeletal pain (10). In addition, although estrogen (E2) therapy was believed to be the most effective treatment for postmenopausal osteoporosis (PMOP) via the notch signaling pathway (11), recent research has indicated that the prolonged use of E2 could increase the risk of endometrial and breast cancer (12,13). Hence, it is necessary to identify safer and more effective natural compounds for OP treatment.
Bone formation contains a complex series of events including osteoblastic differentiation. In the process of osteoblastic differentiation, bone morphogenetic protein-2 (BMP-2), a member of the BMP subfamily, is responsible for the production of bone-specific matrix proteins (14). BMP-2 participates in osteoblastic differentiation by activating osteoblast-associated transcriptional factors such as Osterix, runt-related transcription factor 2 (Runx2) and osteocalcin (15,16). Notably, numerous studies have indicated that protein kinase A (PKA) plays a crucial role in osteoblast differentiation and could regulate several osteoblast-specific transcriptional factors (17,18). However, the functional role of PKA still requires further exploration.
Pinoresinol (PINO), a high-value plant-derived lignan, is the third most abundant phenolic compound in virgin olive oil (VOO) (19). PINO was demonstrated to be able to notably inhibit the proliferative ability of human leukemia cells (20), and attenuate colon cancer progression by inducing tumor cell cycle arrest and apoptosis (21). Furthermore, a previous study indicated that the phenolic compounds of VOO could promote osteoblast proliferation, however, the signaling pathways involved in this treatment effect of VOO are still unclear (22). PINO is a phytoestrogen and has a chemical structure that is similar to that of E2, therefore, it was speculated that PINO could have similar functional effects as estrogen on OP. The aim of the present study was to investigate the effects of PINO on osteoblastic proliferation and differentiation and the underlying mechanism.
Materials and methods
Cell culture and viability detection
The mouse osteoblast MC3T3-E1 cell line (cat. no. CRL-2595; ATCC) was cultured in α-Minimum Essential Medium (α-MEM, cat. no. A1049001; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; cat. no. 30-2020; ATCC), 1% penicillin and 1% streptomycin at 37°C in the presence of 5% CO2. The medium was refreshed every 3 days. The morphology of MC3T3-E1 cells was observed using an inverted microscope (Nikon Eclipse TC 100; Nikon Corporation).
MC3T3-E1 cell viability was examined using Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Inc.). Briefly, MC3T3-E1 cells were embedded in 96-well plates at a cell density of 4×103 cells/well for 24 h at 37°C with 5% CO2. Then, the cells were treated with 0, 10−7, 10−6, 10−5, 10−4, 10−3, 10−2, 0.1, 1 and 100 µg/l PINO (CAS no. 487-36-5; Sigma-Aldrich; Merck KGaA) concentrations. The treatment of 0.01 µmol/l E2 served as a positive control. After 24 and 48 h of incubation, 10 µl CCK-8 reagent was added to each well and cultured for another 1 h. Absorbance at 450 nm was examined using a microplate reader (Bio-Rad Laboratories, Inc.).
Cell migration assay
The logarithmic phase of MC3T3-E1 cells was trypsinized and collected by centrifugation at 3,000 × g for 5 min. The cells were randomly divided into five groups and incubated in 96-well plates (3×104 cells/well) at 37°C with 5% CO2, including a control group (untreated cells), PINO group (cells were treated with 0.1 µg/l PINO for 2 days) and E2 group. The treated MC3T3-E1 cells were plated in 6-cm culture dishes and maintained in the presence of 5% CO2 at 37°C. After cell confluence reached >90%, a cell-free line was created by a 200 µl sterile pipette tip. At 48 h after the scratch, wound healing images were captured on a light microscope and the migration rates were calculated based on the changes of the width of wound closure.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA from MC3T3-E1 cells was extracted using TRIzol reagent (Thermo Fisher Scientific, Inc.). Total extract (1 µg) was reversely-transcribed by iScript™ reverse transcription (Bio-Rad Laboratories, Inc.). RT-qPCR was performed using the SYBR Green real-time PCR Master mix (Toyobo Co., Ltd.) on an ABI PRISM 7000 Sequence 10 Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.). Reaction mixture (20 µl) consisted of each primer (10 µM), 10 µl SYBR fluorescent dye, 2 µl cDNA and RNase-Free dH2O. The basic protocol used for the RT-PCR analysis was an initial denaturation at 95°C for 10 min, followed by 45 cycles at 95°C for 15 sec, 56°C for 30 sec and elongation at 72°C for 2 min. The mRNA expression levels were normalized against that of GAPDH, and the levels of the transcripts were quantified using the 2−ΔΔCq method (23). All primers are listed in Table I.
Table I.
Primers for reverse transcription-quantitative PCR.
| Gene name | Primer sequences |
|---|---|
| Col-I | F: 5′-GAGCGGAGTACTGGATCG-3′ |
| R: 5′-GCTTCTTTTCCTTGGGGTT-3′ | |
| ALP | F: 5′-GATCATTCCCACGTTTTCACATT-3′ |
| R: 5′-TTCACCGTCCACCACCTTGT-3′ | |
| OPN | F: 5′- GCCACAAGTTTCACAGCCAC-3′ |
| R: 5′-AAAATGCAGTGGCCGTTTGC-3′ | |
| Runx2 | F: 5′-AGCGGCAGAATGGATGAGTC-3′ |
| R: 5′-ACCAGACAACACCTTTGACG-3 | |
| BMP-2 | F: 5′-TGAGCAAAGTGCTTGCACAC-3′ |
| R: 5′-AGCCCCCTGGAAGGGATTAT-3′ | |
| GAPDH | F: 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| R: 5′-GGGGTCGTTGATGGCAACA-3 |
Col-I, collagen type I; ALP, alkaline phosphatase; OPN, osteopontin; Runx2, runt-related transcription factor 2; BMP-2, bone morphogenetic protein-2; F, forward; R, reverse.
Western blotting
The treated MC3T3-E1 cells were lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology). The protein concentrations were quantified by BCA Protein Assay reagent (Pierce; Thermo Fisher Scientific, Inc.). Total protein (20 µg) was run on 12% sodium dodecyl sulfate-polyacrylamide gel and electro-transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.), which were then blocked with 5% non-fat dry milk in Tris-buffered saline, followed by the incubation with the primary antibodies anti-collagen type I (Col-I; 1:5,000; 130 kDa; cat. no. ab34710), anti-ALP (1:500; 39 kDa, cat. no. ab83259), anti-osteopontin (OPN; 1:1,000; 66 kDa, cat. no. ab8448), anti-Runx2 (1:1,000; 57 kDa; cat. no. ab23981), and anti-BMP-2 (1:1,000; 45 kDa; cat. no. ab14933; all from Abcam), anti-PKA (1:1,000; 42 kDa; cat. no. 4782), anti-p-cAMP response element-binding protein (CREB; 1:1,000; 43 kDa; cat. no. 9198) and anti-CREB (1:1,000; 43 kDa; cat. no. 9197; all from Cell Signaling Technology (CST)) overnight at 4°C. Following the primary antibodies, the membranes were incubated with goat anti-rabbit HRP-conjugated secondary antibodies (cat. no. ab205718; Abcam) at 4°C for 1 h. Protein bands were detected with an enhanced chemiluminescence detection system (EMD Millipore), and GAPDH (1:1,000; 36 kDa; cat. no. ab8245; Abcam) was used as the internal control.
Alkaline phosphatase (ALP) activity assay
The cells from all the groups were embedded into 24-well plates and maintained in 5% CO2 at 37°C for 2 days. The cells were washed 2 times with phosphate-buffered saline (PBS) and lysed in 0.2% Triton X-100. Then, cell lysates were subjected to a 5-min centrifugation at 10,000 × g. The supernatant was mixed with p-nitrophenyl phosphate (pNPP) (Sigma-Aldrich; Merck KGaA) and maintained at 37°C for 15 min. NaOH (2 mol/l) was used for terminating the reaction. ALP activity at 405 nm was examined under a microplate reader.
Mineralization detection
Alizarin red staining was used to determine the mineralization level of MC3T3-E1 cells. Firstly, Alizarin red S could selectively bind to calcium and produce a dark red stain. Each group of cells (2×105) was respectively cultured in 24-well plates for 14 days and the medium was changed every 3 days. After wiping off the medium, the cells were then stained with 40 mM Alizarin red S (pH 4.2; Sigma-Aldrich; Merck KGaA) for 15 min with gentle agitation, and the images of calcified matrices were captured by light microscope.
Enzyme-linked immunosorbent assay (ELISA)
All treated MC3T3-E1 cells were resuspended by cell extraction buffer (Thermo Fisher Scientific, Inc.) containing 1 mM PMSF and protease inhibitor cocktail. Total cAMP levels in the supernatant of each group of cells were assessed by commercially available cAMP ELISA kits (cat. no. STA-501; Cell Biolabs, Inc.). In this assay, these plates were coated overnight with specific antibodies to cAMP (cat. no. ab134901; 1:1,000; Abcam), and the following morning, the plates were washed and blocked for 2 h. The prepared standard liquid and diluted supernatant (supernatant: diluent, 1:4) were added to an ELISA plate. After being washed by PBS, the streptavidin-horseradish peroxidase-conjugated (HRP) antibody-labeled secondary cAMP (cat. no. ab205719; Abcam) was added to each well at room temperature for 1 h of incubation. After an additional washing step, tetramethylbenzidine substrate solution (Clinical Science Products, Inc.) was added to produce a substrate solution. The enzymatic activities were then quantified by detecting the optical density at 450 nm with a microplate reader.
Pathway inhibition
To further explore whether the cAMP/PKA signaling pathway participated in the functional effects of PINO on osteoblast proliferation and differentiation, H89, an effective inhibitor of PKA, was used for co-treatment with PINO. The effects on the MC3T3-E1 cells were assessed by CCK-8 kit and wound healing assay.
Statistical analysis
Data are expressed as the mean ± SD, and statistical analysis among three and more groups was performed by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
PINO treatment enhances cell viability and migratory capacity of MC3T3-E1 cells
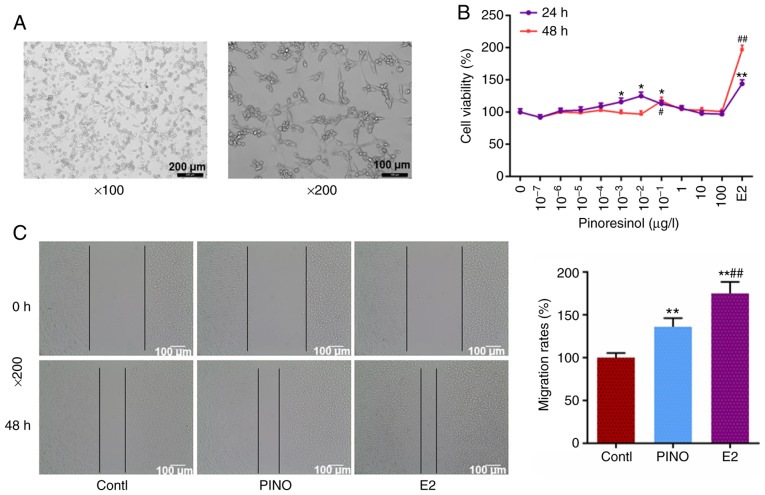
The morphology of MC3T3-E1 cells was observed under a light microscope, and as revealed in Fig. 1A, the MC3T3-E1 cells were cuboidal or fusiform. After treatment with various concentrations of PINO, it was revealed (Fig. 1B) that low concentrations of PINO had a positive effect on MC3T3-E1 cell viability, and PINO treatment at 0.1 µg/l for 48 h had the most significant effect on cell viability. Hence, PINO at a concentration of 0.1 µg/l was selected for the following experiments. To further investigate the functional effects of PINO on the MC3T3-E1 cells, the migratory capacity was assessed by wound healing assay. As revealed in Fig. 1C, E2 was set as the positive control, and the migration rate in the E2 group was greater than that in the control group (P<0.01). PINO treatment had similar effects to E2 on MC3T3-E1 cells and it significantly enhanced the migratory ability. Collectively, the results revealed that PINO had similar effects to E2 on osteoblastic viability and migration.
Figure 1.
PINO treatment enhances cell viability and migratory capacity of MC3T3-E1 cells. (A) The cellular morphology of MC3T3-E1 cells was observed under a light microscope (magnification, ×100 and ×200). (B) MC3T3-E1 cells were treated with various concentrations of PINO (0, 10−7, 10−6, 10−5, 10−4, 10−3, 10−2, 0.1, 1 and 100 µg/l). E2 treatment at the concentration of 0.01 µmol/l served as the positive control. Following incubation for 24 and 48 h, the changes of cell viability with PINO treatment were detected by CCK-8 kit. *P<0.05, **P<0.01 vs. 0 µg/l PINO group (24 h); #P<0.05, ##P<0.01 vs. 0 µg/l PINO group (48 h). (C) The changes in the migration rates of MC3T3-E1 cells, with treatment of 0.1 µg/l PINO or 0.01 µmol/l E2. Each value was represented by the mean ± SEM (n=3). **P<0.01 vs. the control group; ##P<0.01 vs. the PINO group. PINO, pinoresinol; E2, estrogen.
PINO treatment promotes the expression of differentiation-associated factors in MC3T3-E1 cells
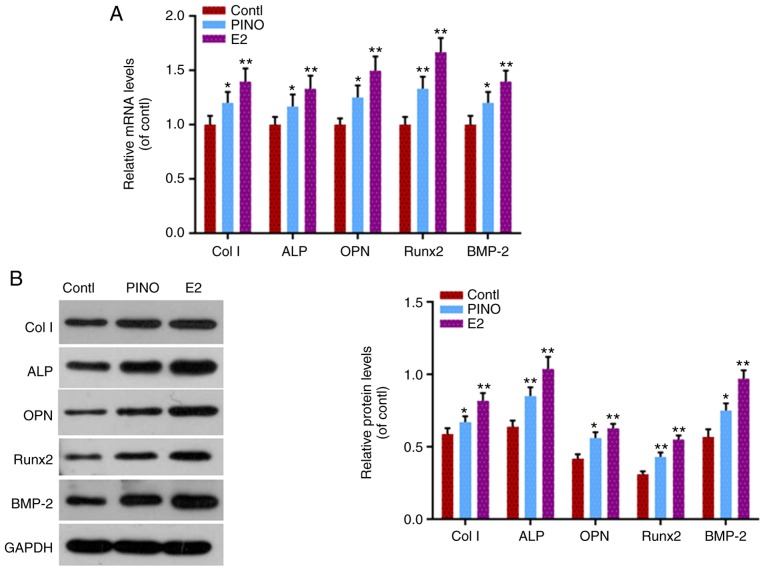
For the assessment of osteoblastic differentiation, the expression levels of several osteoblast-specific genes (Col-I, ALP, OPN, Runx2, and BMP-2) were detected in MC3T3-E1 cells. As one of the first-line drugs for OP treatment, E2 significantly increased the mRNA and protein levels of these osteoblastic differentiation-related genes (P<0.01, Fig. 2). In addition, the present results revealed that PINO had similar effects to E2. Although the effects of PINO on inducing Col-I, ALP, OPN, Runx2 and BMP-2 expression were not as strong as those of E2, the mRNA and protein levels of these genes were also significantly upregulated under PINO treatment. Therefore, the results indicated that PINO had the ability to promote osteoblastic differentiation.
Figure 2.
PINO treatment promotes the expression of differentiation-associated factors in MC3T3-E1 cells. (A) In order to assess the effects of PINO treatment on the differentiation of MC3T3-E1 cells, the mRNA levels of several osteoblast-specific factors (Col-I, ALP, OPN, Runx2, and BMP-2) were detected by RT-qPCR. (B) The protein levels of these genes were detected by western blotting. Each value was represented by the mean ± SEM (n=3). GAPDH was used as an internal control. *P<0.05 and **P<0.01 vs. the control group. PINO, pinoresinol; Col-I, collagen type I; ALP, alkaline phosphatase; OPN, osteopontin; Runx2, runt-related transcription factor 2; BMP-2, bone morphogenetic protein-2.
PINO stimulates ALP activity and mineralization in MC3T3-E1 cells
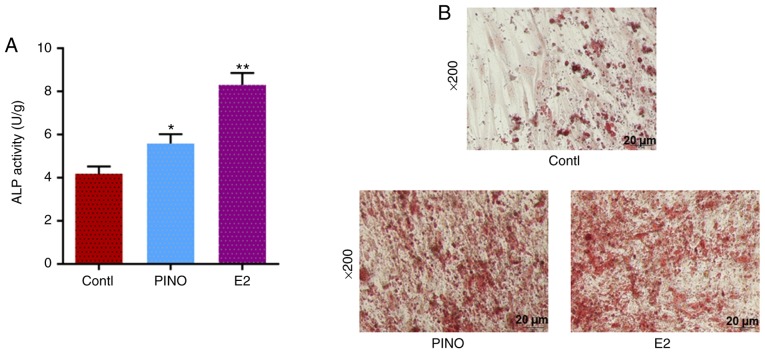
ALP activity and mineralization were also detected to further determine MC3T3-E1 cell differentiation. After incubation with α-MEM in the presence of PINO and E2, pNPP was used as a substrate for ALP activity detection. In comparison to the control group, the ALP activity in the E2 group was the highest and the PINO group also had a significant increase (P<0.05), as revealed in Fig. 3A. After Alizarin red staining, the red region in the PINO and E2 groups were denser than in the control group (Fig. 3B), indicating that both PINO and E2 contributed to the mineralization of MC3T3-E1 cells. Collectively, our findings indicated that PINO treatment could promote the differentiation of osteoblastic MC3T3-E1 cells.
Figure 3.
PINO stimulates ALP activity and mineralization in MC3T3-E1 cells. (A) In order to verify the effects of PINO or E2 treatment on osteoblast differentiation, ALP activity detection was measured after treatment. (B) The mineralization of MC3T3-E1 cells was reflected by Alizarin red staining (magnification, ×200). Each value was represented by the mean ± SEM (n=3). *P<0.05 and **P<0.01 vs. the control group. PINO, pinoresinol; ALP, alkaline phosphatase; E2, estrogen.
The cAMP/PKA signaling pathway participates in the promotive effects of PINO on osteoblastic differentiation
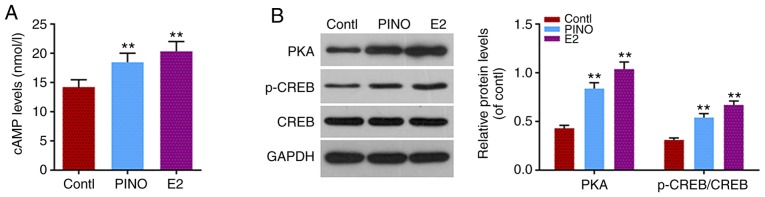
As aforementioned, the cAMP/PKA signaling pathway was involved in the regulation of multiple osteoblast-specific transcriptional factors. The protein levels of cAMP, PKA, phosphorylated and unphosphorylated CREB were assessed to further study the molecular mechanism of the effect of PINO on MC3T3-E1 cells. As revealed in Fig. 4A, a significantly increased cAMP level was revealed in the PINO and E2 groups, compared to the control group (P<0.01), and the cAMP level in the PINO group was slightly lower than that in the E2 group. Similarly, the protein level of PKA was also notably upregulated under the treatment of PINO or E2 (P<0.01). PINO had limited effect on unphosphorylated CREB expression, however, the phosphorylation levels of CREB were markedly increased (P<0.01, Fig. 4B). Moreover, the effects of PINO on cAMP/PKA signals were not as strong as E2. Collectively, the promoting effect of PINO on osteoblastic differentiation may partially rely on the activation of cAMP/PKA signals.
Figure 4.
The cAMP/PKA signaling pathway participates in the positive effects of PINO on osteoblastic differentiation. (A) In order to investigate the mechanism underlying the promoting effects of PINO on osteoblast differentiation, the cAMP level was detected by ELISA. (B) The activation of the cAMP/PKA pathway was reflected by the protein levels of PKA, CREB and p-CREB assessed by western blotting. Each value was represented by the mean ± SEM (n=3). GAPDH was used as an internal control. **P<0.01 vs. the control group. cAMP, cyclic AMP; PKA, protein kinase A; PINO, pinoresinol; CREB, cAMP response element-binding protein; p-CREB, phosphorylated CREB.
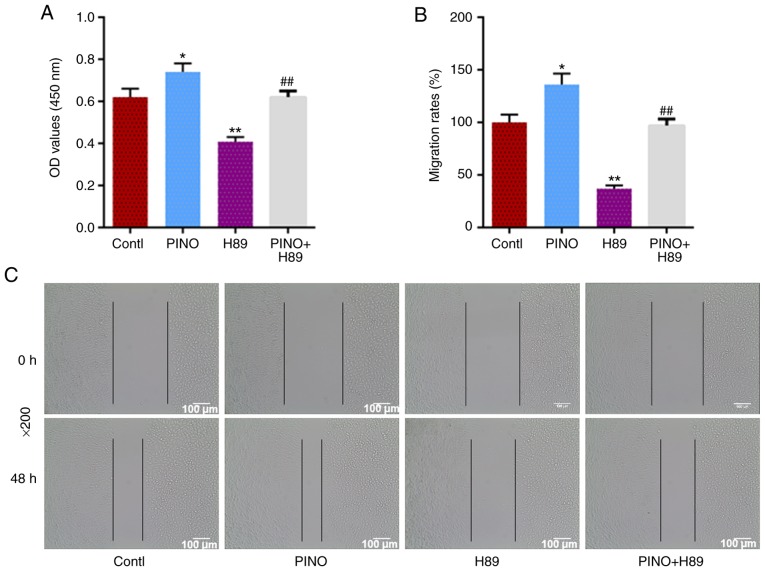
H89 co-treatment reverses the positive effects of PINO on the viability and migration of MC3T3-E1 cells
The H89 solution was used as an inhibitor of the cAMP/PKA signaling pathway to further verify the role of cAMP/PKA in the functional effects of PINO on MC3T3-E1 cells. In comparison to the control, H89 treatment alone significantly decreased cell viability (P<0.01, Fig. 5A). When cells were co-treated with H89, the positive effects of PINO on MC3T3-E1 cell viability were also abolished (P<0.01). In addition, H89 treatment alone could significantly decrease the migration rate by less than half of the control group. Furthermore, H89 co-treatment could also significantly limit the capacity of PINO in enhancing the migration of MC3T3-E1 cells (P<0.01, Fig. 5B and C). Therefore, the present results indicated that the functional effects of PINO on osteoblastic differentiation and proliferation may be attributed to the activation of cAMP/PKA signals.
Figure 5.
H89 co-treatment reverses the positive effects of PINO on the viability and migration of MC3T3-E1 cells. (A) H89 is an inhibitor of the cAMP/PKA signaling pathway. In order to confirm the involvement of the cAMP/PKA signaling pathway in the mechanisms of the promoting effects of PINO on osteoblast differentiation, MC3T3-E1 cells were co-treated with PINO and H89, and the changes in cell viability of each experimental group were assessed by CCK-8 kit. (B and C) The changes of migration ability were detected by wound healing assay (magnification, ×200). Each value was represented by the mean ± SEM (n=3). *P<0.05 and **P<0.01 vs. the control group; ##P<0.01 vs. the PINO group.
Discussion
It is recognized that osteoblasts occupy a fundamental place in bone formation and remodeling, therefore, enhancing osteoblastic proliferation and differentiation can radically improve OP treatment (24,25). E2, one of the first-line drugs for OP treatment, could effectively enhance osteoblastic proliferation and differentiation, however, the side effects of a long-term use of E2 make it imperative to identify safer and more effective drugs. In the present study, E2 treatment was set as the positive control to assess whether PINO treatment had the ability to promote osteoblastic proliferation and differentiation in MC3T3-E1 cells. Although a previous study revealed that PINO did not affect cell proliferation of osteoblasts (22), the result was not in conflict with our study due to the differences in the concentration of PINO. The present results indicated that only PINO at a concentration of 1×10−2 µg/l could induce a slight upregulation of MC3T3-E1 cell viability, and promote cell migration. In addition, PINO had a positive effect on osteoblastic differentiation by inducing the expression of multiple differentiation-associated genes. Moreover, the involvement of cAMP/PKA signaling pathway played a vital role in the molecular mechanism of the effect of PINO on osteoblastic proliferation and differentiation. Although the positive effects of PINO on osteoblastic proliferation and differentiation were not as strong as those of E2, the present results indicated that PINO has the potential to replace E2 in terms of OP treatment.
Osteoblastic differentiation is a complex process mediated by a variety of regulators such as Col-I, ALP, OPN, Runx2, and BMP-2. In the present study, when treating with PINO, a significant increase of these differentiation-associated genes was revealed in MC3T3-E1 cells. The Col-I protein is a fundamental component of the bone extracellular matrix and it is responsible for connecting the cell surface integrins with other extracellular matrix proteins (26). ALP can hydrolyze organic phosphate and pyrophosphate, subsequently promoting bone mineral formation and mineralization (27). In addition, ALP and Col-I both are recognized as the earliest biomarkers of osteoblastic differentiation (28,29). A previous study indicated that the treatment of fermented red ginseng extract (FRGE) could enhance the ALP level and Col-I expression, ultimately improving bone formation and MC3T3-E1 cell differentiation (30). Therefore, the increased ALP activity, mineralization and Col-I in the present results indicated that PINO treatment could promote osteoblastic MC3T3-E1 cell differentiation. OPN is a type of acidic protein secreted by osteoblasts and plays a crucial role in biomineralization and bone remodeling (31). As another specific marker of bone formation, increased OPN expression in the present study also indicated that PINO treatment contributed to the improvement of osteoblastic differentiation. It is recognized that BMPs can induce undifferentiated mesenchymal cells to gather and differentiate to osteoblasts and chondrocytes, finally promoting osteogenic activity (32). In addition, BMP-2 expression induced the activated downstream transcriptional factors (Runx2 and Osterix) to bind to the promoter regions of ALP and OPN (33). Aucubin, an iridoid glucoside separated from multiple Chinese herbs, was reported to inhibit titanium particle-induced osteoblast apoptosis and promote bone formation by activating the BMP-2/Runx2 pathway (34). Collectively, the promoting effect of PINO treatment on osteoblastic differentiation and osteogenesis may rely on the regulation of BMP-2/Runx2 pathway.
In the cAMP/PKA signaling pathway, PKA functions as a second messenger to mediate the various functions of cAMP in mammalian cells. A previous study demonstrated that PKA played an essential role in the process of the promotion of osteoblastic differentiation (35). In addition, another study demonstrated that cAMP could promote drosophila mothers against decapentaplegic protein (SMAD)-mediated BMP-2/Runx2 signaling via the PKA pathway (36). Hence, the potential relationship between the cAMP/PKA and BMP-2/Runx2 signaling pathways was surmised based on this research. Recent research revealed that linarin (LIN), a natural flavonoid compound in Flos Chrysanthemi Indici (FCI), could enhance the expression of BMP-2/Runx2 signals via the activation of the cAMP/PKA signaling pathway. However, when the cells were treated with H89, the positive effects of LIN were effectively abrogated (37). This research was basically consistent with the present results. After co-treatment with a PKA inhibitor (H89), the increased cAMP, PKA and phosphorylated CREB expression levels in the PINO group were nearly decreased to the control levels (data not shown). Furthermore, PINO was also reported to be able to inhibit cell viability and promote apoptosis of human leukemia cell line (HL60) and colorectal cancer (CRC) cell lines through the upregulation of the CDK inhibitor p21Waf1/Cip1 or ATM-p53 cascade (20,21). However, there are many differences in the gene expression, metabolism and other cellular morphological and phenotypic profiles between HL60 or CRC cells and MC3T3-E1 cells due to cell heterogeneity. This does not signify that PINO could also upregulate the p21 and ATM-p53 cascade, and subsequently, inhibit cell proliferation and differentiation in MC3T3-E1 cells. In the present study, the data demonstrated a promoting effect of PINO on osteoblast proliferation and differentiation. Collectively, the present study indicated that PINO-induced osteoblastic differentiation was largely attributed to the regulation of the cAMP/PKA signaling pathway.
There were some limitations in our study. The duration of PINO treatment was too short to completely reflect the effects of PINO on the growth and differentiation of osteoblasts. Moreover, the effects of PINO on the ER pathway require further investigation.
In summary, the present study revealed that PINO had a similar function to E2 in OP treatment. PINO induced osteoblast differentiation and mineralization by regulating BMP-2/Runx2 signaling via activation of the cAMP/PKA pathway. Collectively, PINO may be considered as an alternative to E2 in OP treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Qiqihar Science and Technology Plan Project (grant no. SFZD-2015037).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
XJ and WC substantially contributed to the conception and design of the study. FS, JX, HG, WS, HS and WX performed the data acquisition, data analysis and interpretation. XJ and WC drafted the study and critically revised it for important intellectual content. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tang D, Ju C, Liu Y, Xu F, Wang Z, Wang D. Therapeutic effect of icariin combined with stem cells on postmenopausal osteoporosis in rats. J Bone Miner Metab. 2018;36:180–188. doi: 10.1007/s00774-017-0831-x. [DOI] [PubMed] [Google Scholar]
- 2.Krege JH, Wan X. Teriparatide and the risk of nonvertebral fractures in women with postmenopausal osteoporosis. Bone. 2012;50:161–164. doi: 10.1016/j.bone.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Hannafon F, Cadogan MP. Recognition and treatment of postmenopausal osteoporosis. J Gerontol Nurs. 2014;40:10–14. doi: 10.3928/00989134-20140204-01. [DOI] [PubMed] [Google Scholar]
- 4.Ming LG, Lv X, Ma XN, Ge BF, Zhen P, Song P, Zhou J, Ma HP, Xian CJ, Chen KM. The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro. Endocrinology. 2013;154:1202–1214. doi: 10.1210/en.2012-2086. [DOI] [PubMed] [Google Scholar]
- 5.Ke K, Li Q, Yang X, Xie Z, Wang Y, Shi J, Chi L, Xu W, Hu L, Shi H. Asperosaponin VI promotes bone marrow stromal cell osteogenic differentiation through the PI3K/AKT signaling pathway in an osteoporosis model. Sci Rep. 2016;6:35233. doi: 10.1038/srep35233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antebi B, Pelled G, Gazit D. Stem cell therapy for osteoporosis. Curr Osteoporos Rep. 2014;12:41–47. doi: 10.1007/s11914-013-0184-x. [DOI] [PubMed] [Google Scholar]
- 7.Vico L, Vanacker JM. Sex hormones and their receptors in bone homeostasis: Insights from genetically modified mouse models. Osteoporos Int. 2010;21:365–372. doi: 10.1007/s00198-009-0963-5. [DOI] [PubMed] [Google Scholar]
- 8.Reginster JY, Pelousse F, Bruyere O. Safety concerns with the long-term management of osteoporosis. Expert Opin Drug Saf. 2013;12:507–522. doi: 10.1517/14740338.2013.793669. [DOI] [PubMed] [Google Scholar]
- 9.Cosman F. Long-term treatment strategies for postmenopausal osteoporosis. Curr Opin Rheumatol. 2018;30:420–426. doi: 10.1097/BOR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 10.Lewiecki EM. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs. 2011;71:791–814. doi: 10.2165/11585470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Fan JZ, Yang L, Meng GL, Lin YS, Wei BY, Fan J, Hu HM, Liu YW, Chen S, Zhang JK, et al. Estrogen improves the proliferation and differentiation of hBMSCs derived from postmenopausal osteoporosis through notch signaling pathway. Mol Cell Biochem. 2014;392:85–93. doi: 10.1007/s11010-014-2021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkman RT. Hormone replacement therapy. Current controversies. Minerva Ginecol. 2003;55:107–116. [PubMed] [Google Scholar]
- 13.Komm BS, Morgenstern D, A Yamamoto L, Jenkins SN. The safety and tolerability profile of therapies for the prevention and treatment of osteoporosis in postmenopausal women. Expert Rev Clin Pharmacol. 2015;8:769–784. doi: 10.1586/17512433.2015.1099432. [DOI] [PubMed] [Google Scholar]
- 14.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 15.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–646. doi: 10.1359/jbmr.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B, Lin X, Tan J, She X, Liu Y, Kuang H. Root bark of Sambucus Williamsii Hance promotes rat femoral fracture healing by the BMP-2/Runx2 signaling pathway. J Ethnopharmacol. 2016;191:107–114. doi: 10.1016/j.jep.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 17.He S, Choi YH, Choi JK, Yeo CY, Chun C, Lee KY. Protein kinase A regulates the osteogenic activity of Osterix. J Cell Biochem. 2014;115:1808–1815. doi: 10.1002/jcb.24851. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Jeong HM, Choi YH, Kim JH, Choi JK, Yeo CY, Jeong HG, Jeong TC, Chun C, Lee KY. Protein kinase a phosphorylates Dlx3 and regulates the function of Dlx3 during osteoblast differentiation. J Cell Biochem. 2014;115:2004–2011. doi: 10.1002/jcb.24872. [DOI] [PubMed] [Google Scholar]
- 19.Owen RW, Mier W, Giacosa A, Hull WE, Spiegelhalder B, Bartsch H. Identification of lignans as major components in the phenolic fraction of olive oil. Clin Chem. 2000;46:976–988. [PubMed] [Google Scholar]
- 20.Sepporta MV, Mazza T, Morozzi G, Fabiani R. Pinoresinol inhibits proliferation and induces differentiation on human HL60 leukemia cells. Nutr Cancer. 2013;65:1208–1218. doi: 10.1080/01635581.2013.828089. [DOI] [PubMed] [Google Scholar]
- 21.Fini L, Hotchkiss E, Fogliano V, Graziani G, Romano M, De Vol EB, Qin H, Selgrad M, Boland CR, Ricciardiello L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis. 2008;29:139–146. doi: 10.1093/carcin/bgm255. [DOI] [PubMed] [Google Scholar]
- 22.García-Martínez O, De Luna-Bertos E, Ramos-Torrecillas J, Ruiz C, Milia E, Lorenzo ML, Jimenez B, Sánchez-Ortiz A, Rivas A. Phenolic compounds in extra virgin olive oil stimulate human osteoblastic cell proliferation. PLoS One. 2016;11:e0150045. doi: 10.1371/journal.pone.0150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Luo Z, Liu M, Sun L, Rui F. Icariin recovers the osteogenic differentiation and bone formation of bone marrow stromal cells from a rat model of estrogen deficiency-induced osteoporosis. Mol Med Rep. 2015;12:382–388. doi: 10.3892/mmr.2015.3369. [DOI] [PubMed] [Google Scholar]
- 25.Zhu C, Bao N, Chen S, Zhao J. Dioscin enhances osteoblastic cell differentiation and proliferation by inhibiting cell autophagy via the ASPP2/NF-κβ pathway. Mol Med Rep. 2017;16:4922–4926. doi: 10.3892/mmr.2017.7206. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Hu RY, Sun L, Luo R, Lu KH, Tian XB. Potential role of andrographolide in the proliferation of osteoblasts mediated by the ERK signaling pathway. Biomed Pharmacother. 2016;83:1335–1344. doi: 10.1016/j.biopha.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Ma XN, Ma CX, Shi WG, Zhou J, Ma HP, Gao YH, Xian CJ, Chen KM. Primary cilium is required for the stimulating effect of icaritin on osteogenic differentiation and mineralization of osteoblasts in vitro. J Endocrinol Invest. 2017;40:357–366. doi: 10.1007/s40618-016-0568-8. [DOI] [PubMed] [Google Scholar]
- 28.Shao X, Cao X, Song G, Zhao Y, Shi B. Metformin rescues the MG63 osteoblasts against the effect of high glucose on proliferation. J Diabetes Res. 2014;2014:453940. doi: 10.1155/2014/453940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fusaro M, Crepaldi G, Maggi S, D'Angelo A, Calo L, Miozzo D, Fornasieri A, Gallieni M. Bleeding, vertebral fractures and vascular calcifications in patients treated with warfarin: Hope for lower risks with alternative therapies. Curr Vasc Pharmacol. 2011;9:763–769. doi: 10.2174/157016111797484134. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqi MZ, Siddiqi MH, Kim YJ, Jin Y, Huq MA, Yang DC. Effect of fermented red ginseng extract enriched in ginsenoside Rg3 on the differentiation and mineralization of preosteoblastic MC3T3-E1 cells. J Med Food. 2015;18:542–548. doi: 10.1089/jmf.2014.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi ST, Kim JH, Kang EJ, Lee SW, Park MC, Park YB, Lee SK. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology (Oxford) 2008;47:1775–1779. doi: 10.1093/rheumatology/ken385. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Park Y, Song M, Srikanth S, Kim S, Kang MK, Gwack Y, Park NH, Kim RH, Shin KH. Orai1 mediates osteogenic differentiation via BMP signaling pathway in bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2016;473:1309–1314. doi: 10.1016/j.bbrc.2016.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem. 2012;151:247–254. doi: 10.1093/jb/mvs004. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Z, Xie Q, Huang Y, Zhang S, Chen Y. Aucubin suppresses Titanium particles-mediated apoptosis of MC3T3-E1 cells and facilitates osteogenesis by affecting the BMP2/Smads/RunX2 signaling pathway. Mol Med Rep. 2018;18:2561–2570. doi: 10.3892/mmr.2018.9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo KW, Kan HM, Ashe KM, Laurencin CT. The small molecule PKA-specific cyclic AMP analogue as an inducer of osteoblast-like cells differentiation and mineralization. J Tissue Eng Regen Med. 2012;6:40–48. doi: 10.1002/term.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohta Y, Nakagawa K, Imai Y, Katagiri T, Koike T, Takaoka K. Cyclic AMP enhances Smad-mediated BMP signaling through PKA-CREB pathway. J Bone Miner Metab. 2008;26:478–484. doi: 10.1007/s00774-008-0850-8. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Hao L, Wu J, Zhang J, Su J. Linarin promotes osteogenic differentiation by activating the BMP-2/RUNX2 pathway via protein kinase A signaling. Int J Mol Med. 2016;37:901–910. doi: 10.3892/ijmm.2016.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.