Abstract
Chronic hypoxia is one of the most common causes of secondary pulmonary hypertension, the mechanisms of which remain unclear. MicroRNAs (miRNAs) are small, noncoding RNAs that inhibit the translation or accelerate the degradation of mRNA. Previous studies have demonstrated that deregulated miRNA expression contributes to various cellular processes including cell apoptosis and proliferation, which are mediated by hypoxia. In the present study, the expression of miR-98 was identified to be decreased in the lung tissue of a hypoxic pulmonary hypertension (HPH) rat model and pulmonary artery (PA) smooth muscle cells (PASMCs), which was induced by hypoxia. By transfecting miR-98 mimics into PASMCs, the high expression of miR-98 inhibited cell proliferation, but upregulated hypoxia-induced PASMCs apoptosis. However, these effects of miR-98 mimics on PASMCs were reversed by ALK1 (activin receptor-like kinase-1) overexpression. ALK1 was identified as a candidate target of miR-98. In addition, overexpressing miR-98 markedly decreased the pulmonary artery wall thickness and the right ventricular systolic pressure in rats induced by hypoxia. These results provided clear evidence that miR-98 was a direct regulator of ALK1, and that the downregulation of miR-98 contributed to the pathogenesis of HPH. These results provide a novel potential therapeutic strategy for the treatment of HPH.
Keywords: pulmonary hypertension, microRNA-98, activin receptor-like kinase-1, hypoxia
Introduction
Pulmonary hypertension (PH) is a physiological pathological disorder with several clinical manifestations, which finally leads to cardiovascular and respiratory diseases (1). According to several data, the current worldwide prevalence of PH is ~97/1,000,000 individuals, and the mortality rate 5–10/100,000 (2,3). PH is a pan-vasculopathy involving several cell types, including endothelial cells, smooth muscle cells (SMCs) and fibroblasts, all of which constitute vascular cells (4). A wide spectrum of genetic factors and environmental stimuli, including hypoxia, are the pathological factors of PH. Pulmonary artery (PA) SMCs (PASMCs), the major component of the vasculature, serve a key role in the response to hypoxia, and the dysregulation of their functions is closely associated with the occurrence and development of PH (5). Recently, several studies demonstrated that the inhibited apoptosis and enhanced proliferation of PASMCs, induced by chronic exposure to hypoxia, were major contributors to the development of hypoxic PH (HPH) (6). However, the molecular mechanisms of the hypoxia-induced dysfunction of PASMCs remain unclear.
MicroRNAs (miRNAs) are small endogenous noncoding RNAs and they serve an important role in regulating gene expression by targeting the 3′-untranslated region (3′-UTR) of mRNA to repress its translation or accelerate its degradation (7,8). miRNAs have been identified to serve important roles in diverse biological processes, including cellular development, differentiation and proliferation by several previous studies (8,9). Several cardiovascular diseases, including PH, exhibit a decreased miRNA expression, resulting in disease progression (10–12). However, the molecular role of miRNAs in these pathologies has not yet been fully elucidated.
Transforming growth factor-β1 (TGF-β1), which is a multifunctional cytokine, serves an important role in regulating cell differentiation, proliferation and extracellular matrix deposition, which is directly associated with the occurrence and development of pulmonary hypertension (13–15). The TGF-β1 pathway signals are mediated by specific type I and II serine/threonine kinase receptors. Activin receptor-like kinase-1 (ALK1) is a TGF-β-type I receptor that transmits signals via the ALK1/Smad1/5 pathways (16). Although several studies have suggested that ALK1 is expressed primarily in endothelial cells in the vasculature (13,17–19), ALK1 mRNA and protein expression in PASMCs in vitro and in mouse pup lungs in vivo were also detected in other studies (20,21). miRNA expression has been demonstrated to be associated with the activation of the TGF-β1 pathway (22); however, the association between miRNA and TGF-β1 in the pathogenesis of HPH has not yet been fully elucidated.
In the present study, the role of miR-98 in HPH was examined. It was identified that the levels of miR-98 expression were decreased in the lung tissues of HPH rat models and rat PASMCs under hypoxia, as compared with those of the controls. Furthermore, the downregulation of miR-98 was involved in the hypoxia-induced proliferation and apoptosis of PASMCs. In addition, it was demonstrated that ALK1 was the direct target of miR-98. The conclusions of the present study provided novel insights into the pathogenesis of HPH and potential targets for its clinical diagnosis and treatment.
Materials and methods
HPH rat model and lung tissue preparation
Adult male Wistar rats (weighing 200±10 g; age, ~8 weeks) were obtained from the Animal Experimental Centre of Nanjing Medical University (Nanjing, China). A total of ~60 rats were used in the experimental model, and were fed with standard rat chow and allowed water ad libitum. The animals were maintained under a 12:12 light: dark cycle. The 60 rats were distributed into five groups: The normoxia group (10 rats); hypoxia group (10 rats); normoxia control (N-control, 10 rats); hypoxia control (H-control, 15 rats); and hypoxia + miR-98 agomir, (H + miR-98 agomir, 15 rats). miR-98 agomir (Guangzhou RiboBio Co., Ltd.; sequence, 5′-UGAGGUAGUAAGUUGUAUUGUU-3′) was used to mimic the overexpression of miR-98. miR-98 agomir (250 µg/kg at the volume of 100 µl) was injected into the tail vein. For hypoxic exposure, rats were placed into a normobaric hypoxic chamber (percentage of inspired O2=10%) for 8 h/day. For the control groups, the rats were maintained in a similar normoxic chamber for 3 weeks, as described previously (23). Right ventricular systolic pressure (RVSP) was measured to confirm the successful establishment of the HPH rat model. At the end of the 3 week exposure period, each rat was treated with an intraperitoneal (i.p.) injection of pentobarbital (120 mg/kg). The thorax was then opened and the heart and lungs removed and placed onto a flat plate. The study was approved by the Ethical Committee of Xuzhou Medical University (Xuzhou, China).
Cell culture
PA segments were collected from a group of 5 rats not included in the HPH rat model immediately following sacrifice. The samples were then minced into small fragments using ophthalmic scissors, followed by digesting with 0.25% trypsin (Thermo Fisher Scientific, Inc.) at 37°C for 40 min. Next, 0.2% collagenase (Merck KGaA) was added to treat the fragments at 37°C for an additional 4 h. Cell suspension was filtered and dissociated with a 40 µm cell strainer (BD Biosciences), followed by centrifugation at 850 × g for 10 min at 4°C. Rat PASMCs were incubated in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) supplied with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified incubator. The cell line 293 was obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Science and maintained in DMEM with 10% FBS at 37°C in a humidified incubator.
Prediction of miR-98 target genes
Genes targeted by miR-98 were predicted using PicTar (version 2; http://pictar.bio.nyu.edu) (24), TargetScan (version 5.1; http://www.targetscan.org/) combined with miRanda (version 3.3a; http://www.microrna.org/) as previously described (25).
Hypoxia treatment of PASMCs
PAMSCs at ~60% confluence were exposed to difference oxygen concentrations (0, 3 and 5%) by connecting the incubator with a chamber that was equilibrated with a water-saturated gas mixture of (0, 3 and 5%) O2, 5% CO2 and (95, 92 and 90%) N2 at 37°C for 24–48 h, as described previously (26).
Transfection
A total of 24 h prior to transfection, suspended rat PASMCs at 50% confluence were seeded onto 12-well plates. The miR-98 mimics (5′-UGAGGUAGUAAGUUGUAUUGUU-3′), inhibitors (5′-AACAAUACAACUUACUACCUC-3′) or negative control (NC) sequence, (5′-GUGUAACACGUCUAUACGCCCA-3) were purchased from Guangzhou RiboBio Co., Ltd. Mimics or inhibitors were transfected into PASMCs with Lipofectamine® 3000 reagent (Thermo Fisher Scientific, Inc.). The final concentration of miRNAs was 60 nM. To overexpress ALK1 in rat primary PASMCs, coding sequences of ALK1 were cloned into a lentiviral vector (GeneCopoeia, Inc.) (lenti-ALK1) and packaged into lentiviruses particles by Obio Technology (Shanghai) Co., Ltd. The transfected cells (60% confluence, 6 h after transfection) were incubated with lentiviruses for ~6 h at 37°C, then the media was replaced with normal media. After 24, 48 or 72 h, the cells were harvested for subsequent experiments.
RNA extraction and reverse transcription quantitative polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Thermo Fisher Scientific, Inc.) was used to extract total RNA from lung tissues or PASMCs according to the manufacturer's protocol. RNAs (1 µg) was used for cDNA reverse transcription using an M-MLV kit (Takara Biotechnology Co., Ltd.). RT-qPCR was performed for the detection of ALK1 and ALK4 gene expression. The forward and reverse primers were as follows: Rat ALK1 forward, 5′-ACCCAAACTCCTTCGGAGGAG-3′; rat ALK1 reverse, 5′-CGCTGCTTCTCCTGCCTTC-3′; rat ALK4 forward, 5′-TGACCTGAGGGTGCCCAGTG-3′; rat ALK4 reverse, 5′-TGAGGGGTCCTCCATGTCCAG-3′; β-actin forward, 5′-GAGTACGATGAGTCCGGCCCC-3′; and β-actin reverse, 5′-GCAGCTCAGTAACAGTCCGCCT-3′. β-actin was used as an internal control. The relative expression levels of ALK1 and ALK4 were quantified using the Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific, Inc.) with Power SYBR1 Green PCR Master Mix (Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: Initial denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The relative mRNA expression levels were analyzed through using the expressed relative to the threshold cycle values (ΔCq), and then converted to fold changes using the 2−ΔΔCq method (27).
The expression levels of miR-98 were analyzed and quantified separately using a stem-loop RT-PCR assay, as described previously (28,29). RNA was extracted from tissues or cells using the TRIzol® reagent (Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was used for cDNA reverse transcription using a Mir-X™ miRNA First Strand Synthesis Kit (cat. no. 638315; Takara Biotechnology Co., Ltd.). For the RT reaction, the samples were incubated for 1 h at 37°C, and the reaction was terminated at 85°C for 5 min. qPCR was performed for the detection of the expression levels of miR-98. The primers used for qPCR analysis were the following: miR-98 forward, 5′-TGAGGTAGTAAGTTGTATTGTT-3′; qPCR miR-98 reverse, 5′-GCTGTCAACGATACGCTACGTAACG-3′; qPCR 5S forward, 5′-GTCTACGGCCATACCACCCIGAAC; and qPCR 5S reverse, 5′-CTGTCAACGATACGCTACGTAACG. RNA input was normalized to the level of rat 5S rRNA. RT-qPCR was performed using SYBR1 Green PCR Master Mix (Thermo Fisher Scientific, Inc.) on a 7500 system (Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: Initial denaturation at 95°C for 10 min and 40 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The expression level of miR-98 were normalized to the expression levels of 5S rRNA, and fold changes were calculated by relative quantification (2−ΔΔCq) (27).
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8; Nanjing KeyGen Biotech, Co., Ltd.) assay was used to analyze cell proliferation, as described previously (26). PASMCs were seeded in 96-well plates at a density of 3×103 cells per well and cultured at 37°C in 5% CO2 for 24 h. Following treatment with 0, 24 and 48 h, CCK-8 solution (10% combined with DMEM) was added to each well and incubated for an additional 2 h. Cell proliferation was detected and analyzed by scanning with a microplate reader (Bio-Rad Laboratories, Inc.) at 450 nm. Each experiment was performed in triplicate.
Cell apoptosis assay
Apoptosis was analyzed using a flow cytometer (BD Biosciences). Briefly, an annexin-V fluorescein isothiocyanate and propidium iodide double-stain assay was performed in accordance with the manufacturer's protocol (FITC Annexin V Apoptosis Detection Kit I; BD Biosciences). The results were analyzed using FlowJo software (version 7.5.5; Tree Star, Inc.). Each experiment was performed in triplicate.
Measurement of RVSP
A total of 10 rats from each group was used to measure RVSP by right heart catheterization, as previously described (30). Briefly, rats were anesthetized by an i.p. injection of 35 mg/kg pentobarbital sodium. An additional dose of pentobarbital sodium (17.5 mg/kg) was administered when further anesthesia was necessary. A 1.2 French Pressure Catheter (Transonic Systems, Inc.) was connected to the Scisense FA-404 recorder (Transonic Systems, Inc.). Following exposure of the right jugular vein, the catheter was inserted into the vein, advanced into the superior vena cava, and finally into the RV. RVSP was continuously recorded for 45 min.
Luciferase reporter assay
The 3′-UTR-Luc reporter of ALK1 was constructed by the ligation of ALK1 3′-UTR PCR product into the Xhol and BamH I sites of the pGL3 basic vector (Promega Corporation). The mutant reporter was generated from pGL3-wild type (WT)-ALK1 3′-UTR-Luc by replacing the binding site of miR-98 with restriction enzyme cutting site of BamH I: CGGATCCG. For the luciferase reporter assay, cells were cultured in 96-well plates and then transfected with WT or mutant luciferase reporter plasmids, and then miR-98 mimics with Lipofectamine® 3000 reagent (Thermo Fisher Scientific, Inc.). After incubation for 24 h, luciferase activity of each well was measured using a Dual-Luciferase Reporter Assay System (Promega Corporation) according to the protocol of the manufacturer (Promega Corporation). Renilla luciferase activity was utilized as an internal control.
Western blot analysis
For western blot analysis, total protein was extracted using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology) and quantified using the Bradford method. A total of ~30 µg total protein from each sample was loaded and separated by 10% SDS-PAGE and then transferred onto polyvinylidene fluoride membranes (EMD Millipore). Following blocking with 5% non-fat milk in PBST buffer at 37°C for 1 h, membranes were incubated with the primary antibody at 4°C overnight. Subsequently, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.; cat. no. sc-2004; 1:2,000 dilution) for 1 h at 37°C and finally detected using an ECL substrate kit (Tanon Science and Technology Co., Ltd.). Chemiluminescence was detected using the ChemiDoc XRS+ (Bio-Rad Laboratories, Inc.). The secondary antibodies conjugated with HRP (cat. no. sc-2004; 1:2,000 dilution) and primary antibodies against β-actin (cat. no. sc-70319; 1:1,000 dilution) were purchased from Santa Cruz Biotechnology, Inc., ALK1 (cat. no. ab108207; 1:1,000 dilution), cleaved caspase 3 (cat. no. ab13847; 1:1,000 dilution), proliferating cell nuclear antigen (PCNA; cat. no. ab92552; 1:1,000 dilution), smad1 (cat. no. ab66737; 1:1,000 dilution) and P-smad1 (cat. no. ab73211; 1:1,000 dilution) primary antibodies were purchased from Abcam. The densitometric was analyzed using ImageJ (version 1.51J8; National Institutes of Health).
Histology
After the rats were anesthetized, the lung tissues were obtained immediately, then the tissues were sliced into tissue blocks, and immersed in 4% paraformaldehyde for overnight fixation at 37°C. Fixed tissues were then dehydrated in ascending alcohol series, cleared, and embedded in paraffin wax. The tissues were cut into 4-µm thick sections using a Leica slicer (Leica Microsystems, Inc.) and stained with hematoxylin and eosin (H&E) using a standard method as described previously (31). Additionally, Masson staining and PCNA immunohistochemistry were used to study the effect of the miR-98 agomir on the development of HPH, as described previously (32). For immunohistochemistry, the slides were boiled (~100°C) in citrate antigen retrieval solution (Beyotime Institute of Biotechnology) for 1.5 min and cooled at room temperature for 30 min. After a 15-min incubation in 3% hydrogen peroxide, sections were blocked with 10% goat serum (Beyotime Institute of Biotechnology; cat. no. C0265) for 60 min at 37°C and then incubated with primary antibody against PCNA (cat. no. ab92552; Abcam; 1:100 dilution) overnight at 4°C. After washing with PBST buffer for three times, sections were incubated with HRP-conjugated anti-rabbit (cat. no. ZDR-5306; ZSBio; OriGene Technologies, Inc.; 1:500 dilution) for 60 min at 37°C. Localization of peroxidase conjugates was determined using a 3′-diaminobenzidine kit (cat. no. ZLI-9018; ZSBio; OriGene Technologies, Inc.) as a chromogen and H&E for counterstaining. Samples were photographed using a light microscope (magnification, ×400).
Statistical analysis
All data are presented as the mean ± standard deviation. GraphPad Prism 6.0 software (GraphPad Software, Inc.) was used for statistical analysis. Unpaired two-tailed Student's t-test and one-way ANOVA followed by Tukey-Kramer post-hoc test were used to determine statistical significance. P<0.05 was considered to indicate a statistically significant difference.
Results
miR98 is downregulated by hypoxia in an HPH rat model and PASMCs
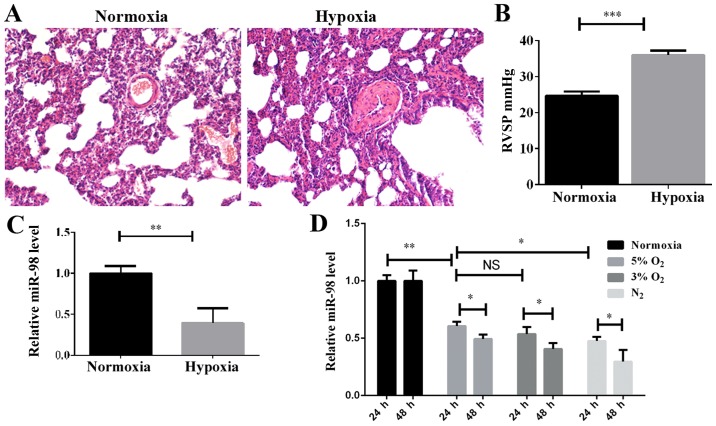
The HPH rat model was established by subjecting the rats to a hypoxic assault with 10% O2 for 3 weeks, as described previously (23), and PH indicators, including the morphology of pulmonary vessels and the RVSP, were detected. H&E staining indicated that the pulmonary vascular smooth muscle layer was significantly thickened in the hypoxic-induced group compared with the normoxic group (Fig. 1A), and the vascular lumen was significantly narrowed. Furthermore, the RVSP was increased significantly in the hypoxic-induced rats (Fig. 1B). These results demonstrated that the HPH rat model had been successfully established. In order to examine the expression levels of miR-98 in the HPH rat model, the total lung tissue RNA was extracted and examined by RT-qPCR. As indicated in Fig. 1C, the level of miR-98 was significantly decreased in the HPH model compared with the control. To investigate the effect of hypoxia on the expression of miR-98 in PASMCs, the optimal hypoxic stimulation concentration was first selected by determining the O2 concentration gradient for cell induction (data not shown). As demonstrated in Fig. 1D, the expression of miR-98 in rat primary PASMCs was decreased as the O2 concentration gradually decreased. The expression of miR-98 was significantly downregulated after 24 h of hypoxia stimulation, and further downregulated after 48 h (Fig. 1D). These results revealed that the downregulation of miR-98 may be associated with the hypoxia-induced development of pulmonary hypertension.
Figure 1.
Effect of hypoxia on miR-98 expression in vivo and in vitro. (A) Morphology of the lung PA from normoxia and hypoxic rats was examined with hematoxylin and eosin staining (magnification, ×200). (B) Measurement of RVSP. ***P<0.001 (n=10). (C) The expression of miR-98 in lung pulmonary arteries of HPH rats was examined by RT-qPCR. **P<0.01 (n=10). (D) The miR-98 expression in rat primary PASMCs stimulated with different concentrations of O2 at 24 and 48 h were examined by RT-qPCR. *P<0.05 and **P<0.01. (n=3). miR, microRNA; PA, pulmonary artery; RVSP, right ventricular systolic pressure; HPH, hypoxic pulmonary hypertension; RT-qPCR; reverse transcription quantitative polymerase chain reaction; NS, not significant.
Effect of miR-98 on PASMC proliferation and apoptosis
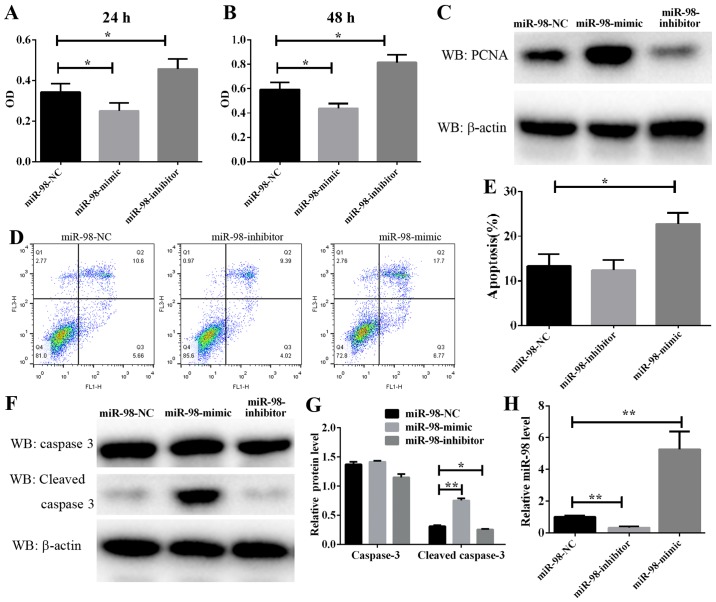
PASMC proliferation is a well-known mechanism of vascular remodeling in HPH. In the present study, a CCK-8 assay was used to assess the effect of miR-98 on PASMC proliferation. As demonstrated in Fig. 2A and B, PASMC proliferation was significantly enhanced following the transfection of cells exposed to hypoxia for 24 and 48 h with anti-miR-98 inhibitors. Conversely, transfection with miR-98 mimics significantly inhibited the hypoxia-induced enhancement of rat primary PASMC proliferation. Furthermore, a cell proliferation marker, PCNA, was analyzed. The protein level of PCNA was markedly downregulated in cultured miR-98 mimic-transfected cells and upregulated in anti-miR-98 inhibitor-transfected cells (Fig. 2C). The RT-qPCR results suggested a high efficiency of miR-98 inhibitors and mimics in PASMCs (Fig. 2H). These data suggested that the downregulation of miR-98, which resulted in PASMC proliferation, was induced by hypoxia and accelerated vascular remodeling in HPH.
Figure 2.
Effect of miR-98 on PASMCs proliferation and apoptosis. Following transfection with miR-98 mimics or inhibitors, the hypoxia-induced PASMC proliferation for (A) 24 or (B) 48 h was analyzed by Cell Counting Kit-8 assay. (C) The protein level of PCNA was determined by western blot analysis following PASMC transfection with miR-98 mimics or inhibitors and subsequent exposure to hypoxia for 24 hz (D) Representative FACS analysis of Annexin V and PI staining. (E) Percentage of apoptotic cells analyzed by FACS. (F) The protein level of cleaved caspase 3 was determined by western blot analysis following PASMC transfection with miR-98 mimics or inhibitors and subsequent exposure to hypoxia for 48 h. (G) Densitometry analysis of the western blots of caspase 3 and cleaved caspase 3. (H) The miR-98 expression in PASMCs transfected with miR-98 mimics or inhibitors was examined by reverse transcription quantitative polymerase chain reaction. *P<0.05 and **P<0.01 (n=3). miR, microRNA; PASMCs, pulmonary artery smooth muscle cells; PCNA, proliferating cell nuclear antigen; PI, propidium iodide; FACS, fluorescence-activated cell sorting; NC, negative control.
Furthermore, to explore the molecular mechanism underlying the modulation of miR-98 in PASMC proliferation, PASMC apoptosis was analyzed by flow cytometry, and the results indicated that the overexpression of miR-98 significantly induced cell apoptosis, whereas transfection with anti-miR-98 inhibitors prevented hypoxia-induced apoptosis (Fig. 2D and E). As caspase 3 serves a key role in the process of apoptosis, its cleaved form is often used as an indicator of apoptosis levels. In the present study, the protein level of cleaved caspase 3 in PASMCs transfected with miR-98 mimics or inhibitors and then exposed to hypoxia was examined. It was identified that the protein level of cleaved caspase 3 was consistently decreased in cells transfected with miR-98 inhibitors (Fig. 2F and G). By contrast, transfection of cells with miR-98 mimics increased cleaved caspase 3 activity. These results suggested that miR-98 upregulation leads to PASMC apoptosis.
ALK1 is a direct target of miR-98
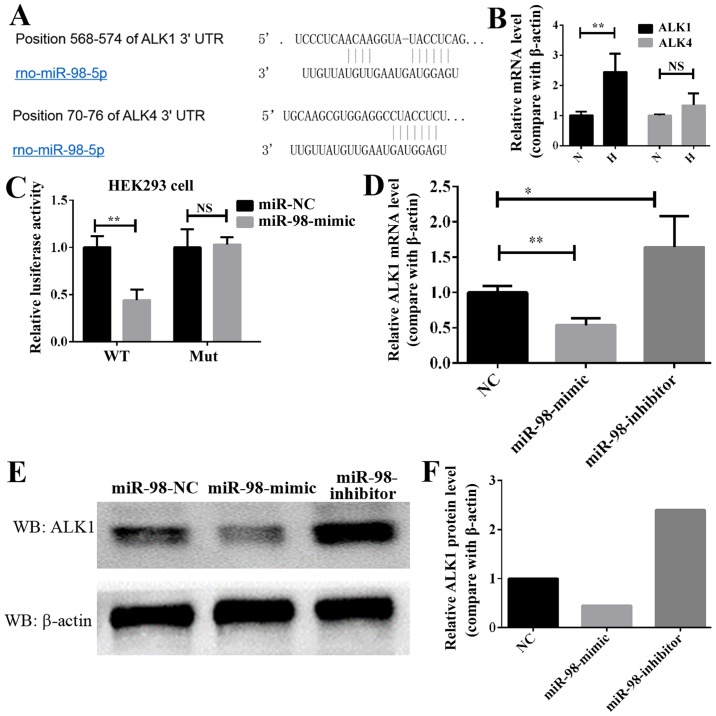
In order to elucidate the molecular mechanism through which miR-98 affects HPH, TargetScan and MiRanda were used to identify putative binding sites for miR-98. A number of mRNAs may be targets of miR-98, including the ALK gene family, which is associated with the occurrence and progression of HPH (13). The bioinformatics analysis of the potential targets of miR-98 indicated that the ALK family genes, including ALK1 and ALK4, may be targets of miR-98 (Fig. 3A). Next, the expression of these two genes, ALK1 and ALK4, was analyzed in HPH lung tissue. As demonstrated in Fig. 3B, the expression of ALK1 in hypoxia-induced HP was significantly increased, while that of ALK4 was increased, but not significantly. Therefore, ALK1 was selected as the potential target gene of miR-98 in HPH. To additionally confirm whether ALK1 is the target of miR-98, luciferase reporter plasmids carrying the 3′-UTR miR-98 wild type or miR-98 mutant-binding sites of ALK1 was constructed, and 293 cells were co-transfected with either miR-98 mimics or NC. Compared with the control, transfection with miR-98 mimics decreased the luciferase activities significantly (Fig. 3C). However, the miR-98 mimics did not affect the luciferase activity in the mutant construct, and their luciferase activities did not exhibit any significant differences when compared with the control (Fig. 3C). In addition, transfection with miR-98 mimics significantly decreased the mRNA level of ALK1 in PASMCs, while transfection with miR-98 inhibitors increased it (Fig. 3D). Western blot analysis data indicated that the protein level of ALK1 in PASMCs was decreased when transfected with miR-98 mimics. Conversely, the protein level of ALK1 was increased in the cells expressing anti-miR-98 (Fig. 3E and F). These data indicated that miR-98 directly modulated the ALK1 expression by binding to the 3′-UTR of ALK1.
Figure 3.
ALK1 is a direct target of miR-98 in PASMCs. (A) Diagram of miR-98 seed sequence, indicating that it matched the 3′-UTR of the ALK1 and ALK4 genes. (B) RT-qPCR analysis of the ALK1 and ALK4 expression in lung pulmonary arteries from H and N rats. **P<0.01 (n=10). (C) Luciferase reporter assays in 293 cells, following co-transfection of cells with WT or mut 3′-UTR ALK1 and miR-98 mimics. (D) RT-qPCR analysis of the ALK1 expression 24 h following transfection with miR-98 mimics, inhibitors or NC. (E) Western blot analysis results of the ALK1 expression in PASMCs transfected with miR-98 mimics, inhibitors or NC. (F) Densitometric analysis of the western blot analysis of ALK1. All experiments were repeated 3 times. *P<0.05, **P<0.01. ALK, activin receptor-like kinase; miR, microRNA; UTR, untranslated region; PASMCs, pulmonary artery smooth muscle cells; RT-qPCR; reverse transcription quantitative polymerase chain reaction; N, normoxic; H, hypoxic; NS, not significant; WT, wild type; mut, mutant; NC, negative control.
Confirmation of miR-98 functions by targeting ALK1
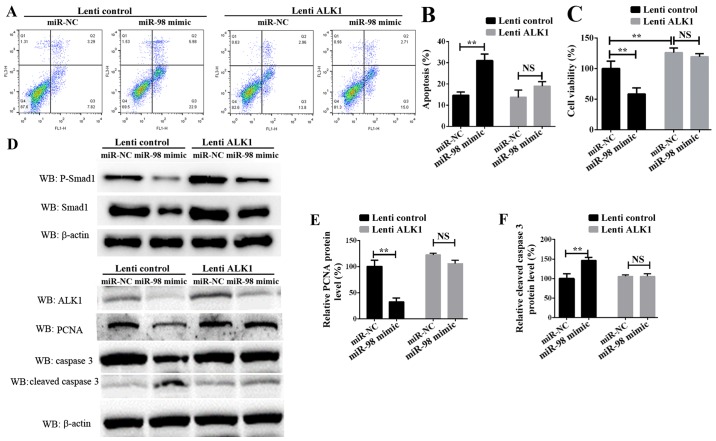
To additionally examine the role of ALK1 in mediating the function of miR-98, rescue experiments were conducted. PASMCs were transiently transfected with miR-98 mimics and a ALK1 overexpression vector carried by lentiviral particles (lenti-ALK1), and subsequently exposed to hypoxia for 24 h or 72 h. Cell survival, proliferation and apoptosis assays indicated that the re-introduction of ALK1 into the miR-98-overexpressing cells inhibited PASMC apoptosis (Fig. 4A and B), and enhanced cell survival and proliferation (Fig. 4C). It must be noted that the time of PASMCs exposure to hypoxia in these 2 experiments was different; the data in Fig. 4B was obtained 24 h after transfection, and the data from Fig. 4C was measured 72h after transfection. Western blot analysis results suggested that ALK1 overexpression inhibited the protein level of cleaved caspase 3. Concomitantly, the protein levels of phosphorylated Smad1, one of the target signaling pathways of ALK1, and the proliferation-associated protein PCNA were significantly increased (Fig. 4D-F). These results suggested that miR-98 was significantly downregulated in HPH, leading to an increase in the levels of ALK1 protein, which promoted the activation of Smad signaling pathways, thereby inhibiting SMC apoptosis, promoting cell proliferation and leading to the development of HPH.
Figure 4.
Confirmation of miR-98 functions by targeting ALK1 in PASMCs. (A) Representative FACS analysis of Annexin V and PI staining of PASMCs following transfection with miR-98 mimics and lenti-ALK1 particles, and subsequent exposure to hypoxia for 24 h. (B) Percentage of apoptotic cells analyzed by FACS. (C) Cell Counting Kit-8 assay was used to analyze PASMC proliferation following transfection of PASMCs and exposure to hypoxia for 72 h. (D) Upper panel, western blot analysis of phosphorylated Smad1 and total Smad1; lower panel, western blot analysis of ALK1, cleaved caspase 3 and PCNA levels in PASMCs transfected with miR-98 mimics and ALK1 overexpression vector and subsequent exposure to hypoxia for 24 h. Densitometric analysis of (E) PCNA and (F) cleaved caspase 3 levels. All experiments were repeated 3 times. **P<0.01. miR, microRNA; ALK1, activin receptor-like kinase-1; PASMCs, pulmonary artery smooth muscle cells; PI, propidium iodide; NS, not significant; CCK-8, Cell Counting Kit-8; lenti-ALK1, ALK1 overexpression vectors carrying lentiviral particles; FACS, fluorescence-activated cell sorting; PCNA, proliferating cell nuclear antigen.
In vivo effect of miR-98 agomir treatment on the expression of ALK1 and hypoxia-induced pulmonary vasoconstriction
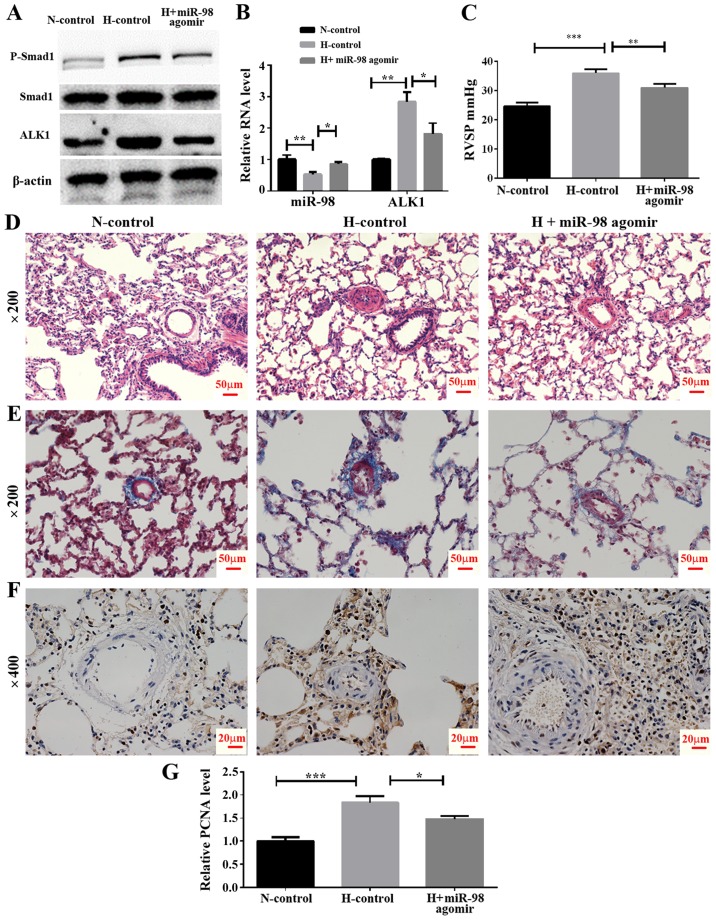
To examine the effect of miR-98 agomir, a chemically-modified miR-98 mimic, on the expression of ALK1 and the development of HPH, an animal model of HPH was established by exposing rats to hypoxia and a tail vein injection of miR-98 agomir or its control. The rats were divided into three groups, including normoxia control (N-control), hypoxia control (H-control) and hypoxia + miR-98 agomir (H + miR-98 agomir). The results demonstrated that the miR-98 agomir injection significantly increased miR-98 in the lung tissue, as compared with the untreated group. In addition, the mRNA level of ALK1 was significantly decreased following the tail injection of miR-98 mimics (Fig. 5B). Furthermore, western blot analysis results indicated that the miR-98 agomir significantly decreased the levels of ALK1 protein expression, and inhibited the phosphorylation of Smad1 (Fig. 5A). To explore the biological effect of miR-98, the RVSP was measured. It was identified that the RVSP was markedly decreased in miR-98 agomir-treated rats under hypoxia (Fig. 5C). Furthermore, H&E staining demonstrated that the thickening of the smooth muscle layer of the pulmonary small blood vessels was improved by miR-98 agomir treatment (Fig. 5D). The results of the Masson staining protocol suggested that the collagen deposition in the pulmonary muscle arterioles induced by hypoxia was also improved by miR-98 agomir treatment (Fig. 5E). Hyperproliferation of SMCs induced by hypoxia was also inhibited following miR-98 agomir treatment, as the protein level of PCNA was decreased in the H + miR-98 agomir group compared with the H-control group (Fig. 5F and G). All of these results indicated that treatment with miR-98 agomir decreased the expression of ALK1 and attenuated hypoxia-induced vascular remodeling.
Figure 5.
Effect of miR-98 agomir treatment on hypoxia-induced pulmonary vasoconstriction in the HPH rat model. (A) Western blot analysis of ALK1 and phosphorylated Smad1 levels in lung pulmonary arteries from normoxic and hypoxic rats with or without miR-98 agomir treatment. (B) Reverse transcription quantitative polymerase chain reaction analysis of ALK1 and miR-98 expression in lung pulmonary arteries from N and H rats with or without miR-98 agomir treatment. (C) Measurement of RVSP. (D) Representative images of hematoxylin and eosin staining of lung pulmonary arteries (magnification, ×200). (E) Representative images of Masson staining of lung pulmonary arteries (magnification, ×200). (F) Representative immunohistochemical staining of PCNA in N-control, H-control and H + miR-98 agomir treatment groups (magnification, ×400). (G) Semi-quantitative analysis of immunohistochemical staining of PCNA (analyzed by ImageJ). *P<0.05, **P<0.01 and ***P<0.001. All experiments were repeated 3 times. miR, microRNA; HPH, hypoxic pulmonary hypertension; ALK1, activin receptor-like kinase-1; N, normoxia; H, hypoxia; RVSP, right ventricular systolic pressure; PCNA, proliferating cell nuclear antigen; N-control, normoxic control group; H-control, hypoxia induced group; H + miR-98 agomir, hypoxia induced with miR-98 agomir.
Discussion
Although the mechanisms of PH have been studied for several decades, they remain unclear. Chronic hypoxia involving multiple molecular signaling pathways has been reported to be an important reason for the occurrence and development of PH. In previous decades, several studies have demonstrated that the association between miRNAs and vascular remodeling, the development of hypertrophy, failure in the heart muscle function and vasculature, all are relevant (33,34). In addition, several miRNAs have been suggested to be aberrantly expressed and involved in the development of PH (10,35,36). Although great progress has been made with regards to the role of miRNAs in PH, these studies have not fully clarified their mechanisms in HPH, which require additional study.
The present study revealed that the expression of miR-98 was significantly decreased in the lung tissues of the HPH rat model and PASMCs under hypoxia compared with the controls. However, the association between miR-98 and the pathological process of HPH was not examined. Based on all of these results, it was reasonable to hypothesize that miR-98 may serve an important role in the development of HPH. In order to examine our hypothesis, an HPH animal model and primary rat PASMCs were used to examine the function of miR-98 in the development of HPH. As expected, miR-98 overexpression inhibited hypoxia-induced PASMC proliferation. Rat PASMC apoptosis was also markedly increased following miR-98 transfection. These results suggested that miR-98 may serve an important role in hypoxia-induced PASMC hyperproliferation. miRNAs exert their functions via targeting different target genes; for example miR-328 regulates HPH by targeting insulin growth factor 1 receptor and l-Type calcium channel-α1C (37). Therefore, the present study hypothesized that miR-98 regulated several target genes to modify the regulation network and inhibit PASMC proliferation in the present study. Using online bioinformatics software, including TargetScan, MiRanda and PicTar, several candidate targets for miR-98 were predicted.
Though gain-of-function approaches, it was confirmed that ALK1 is a direct target gene of miR-98. Firstly, the mRNA expression of ALK1 was identified to be increased in the lung tissues of HPH rats, while that of ALK4, which was revealed to be a target of miR-98 in breast cancer cells, was not (38). Secondly, data from the present study demonstrated that the overexpression of miR-98 notably decreased the relative luciferase activity of the WT but not the mutant vector containing ALK1 3′-UTR. Thirdly, the overexpression of miR-98 inhibited the mRNA and protein expression of ALK1. Finally, miR-98 overexpression inhibited cell proliferation and promoted apoptosis in PASMCs; however, the levels of proliferation were restored and apoptosis was inhibited when ALK1 was overexpressed.
However, it must be noted that when the PASMCs transfected with miR-98 mimics and ALK1 overexpression lenti-ALK1 particles were exposed to hypoxia for 24 h, and then analyzed ~30 h after transfection, it is possible that the cells had not completely recovered from the toxicity of transfection, and that the levels of overexpression of ALK1 were not high. This may explain why the overexpression of ALK1 did not change the levels of cell apoptosis at first. However, the results from the present study indicated that, after transfection for 72 h, the overexpression of ALK1 significantly inhibited the levels of apoptosis.
In addition, it was indicated that the forced expression of ALK1 was also degraded by overexpression of miR-98; therefore, it may be better to use resistant mutants of ALK1 in future studies. It was concluded that ALK1 is a direct and pivotal target gene of miR-98 in the development of HPH.
ALK1 is a specific type I receptor that transmits signals via the ALK1/Smad1/5 pathways. Several studies have demonstrated that the TGF-β/ALK1 signaling pathway serves a key role in idiopathic pulmonary arterial hypertension and experimental hypoxic PH via a direct effect on pulmonary endothelial cells, leading to the overproduction of growth factors and inflammatory cytokines that are involved in the pathogenesis of PAH (13,18,39–41). In addition, several previous data have indicated that ALK1 is essential for hypoxia-induced PASMC proliferation (13,42). The present study demonstrated that ALK1 overexpression restored the proliferation of PASMC, which was inhibited by miR-98 mimics.
In conclusion, to the best of our knowledge, the present study was the first to provide evidence that miR-98 serves an important role in vasoconstriction and remodeling in the pathogenesis of HPH. The inhibitory effect of miR-98 on vasoconstriction may be attributable to the hypoxia-induced inhibition of the ALK1 expression. These results provide novel insight into the molecular mechanisms of HPH and suggest a potential target for treatment of HPH. However, these results require verification through additional clinical studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from The Science and Technology Bureau of Xuzhou (grant no. BK2016109).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XiaZ conceived and designed the experiments. QL and XinZ performed the experiments, and XiaZ and QL analyzed the data and wrote the manuscript.
Ethics approval and consent to participate
All aspects of the present study were approved by the Ethics Committee of the Municipal Hospital Affiliated to Xuzhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Kumar RK, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. Turk Kardiyol Dern Arsivi (Turkish) 2014;42(Suppl):S45–S54. [PubMed] [Google Scholar]
- 2.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Sitbon O, Badesch DB, Barst RJ, Black C, Galiè N, Rainisio M, Simonneau G, Rubin LJ. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005;25:244–249. doi: 10.1183/09031936.05.00054804. [DOI] [PubMed] [Google Scholar]
- 4.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44:14–30. doi: 10.1016/j.yjmcc.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang H, Desai AA, Yuan JX. Genetic insights into pulmonary arterial hypertension. Application of whole-exome sequencing to the study of pathogenic mechanisms. Am J Respir Crit Care Med. 2016;194:393–397. doi: 10.1164/rccm.201603-0577ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Negash S, Liu J, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: Role of cGMP-dependent protein kinase and myocardin. Am J Physiol Lung Cell Mol Physiol. 2009;296:L780–L789. doi: 10.1152/ajplung.90295.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 9.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 10.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, Saggar R, Wallace WD, Ross DJ, Vargas SO, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124:3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al. A role for miR-145 in pulmonary arterial hypertension: Evidence from mouse models and patient samples. Circ Res. 2012;111:290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 12.Potus F, Graydon C, Provencher S, Bonnet S. Vascular remodeling process in pulmonary arterial hypertension, with focus on miR-204 and miR-126 (2013 Grover Conference series) Pulm Circ. 2014;4:175–184. doi: 10.1086/675980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gore B, Izikki M, Mercier O, Dewachter L, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Lebrin F, Eddahibi S. Key role of the endothelial TGF-beta/ALK1/endoglin signaling pathway in humans and rodents pulmonary hypertension. PLoS One. 2014;9:e100310. doi: 10.1371/journal.pone.0100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage-Dunn C. TGF-beta signaling. WormBook. 2005;9:1–12. doi: 10.1895/wormbook.1.22.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Y, Wang XJ, Li SQ, Yang SH, Lv ZC, Wang LT, He YY, Jiang X, Wang Y, Jing ZC. Elevated levels of plasma transforming growth factor-β1 in idiopathic and heritable pulmonary arterial hypertension. Int J Cardiol. 2016;222:368–374. doi: 10.1016/j.ijcard.2016.07.192. [DOI] [PubMed] [Google Scholar]
- 16.Massague J. TGF-beta signal transduction. Ann Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 17.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uznanska-Loch B, Wiklo K, Kulczycka-Wojdala D, Szymańska B, Chrzanowski Ł, Wierzbowska-Drabik K, Trzos E, Kasprzak JD, Kurpesa M. Genetic variants in a Polish population of patients with pulmonary arterial hypertension: Sequencing of BMPR2, ALK1, and ENG genes. Kardiol Pol. 2018;76:852–859. doi: 10.5603/KP.a2018.0034. [DOI] [PubMed] [Google Scholar]
- 19.Chida A, Shintani M, Yagi H, Fujiwara M, Kojima Y, Sato H, Imamura S, Yokozawa M, Onodera N, Horigome H, et al. Outcomes of childhood pulmonary arterial hypertension in BMPR2 and ALK1 mutation carriers. Am J Cardiol. 2012;110:586–593. doi: 10.1016/j.amjcard.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Du L, Zhong Y, Flanders KC, Roberts JD., Jr Transforming growth factor-β stimulates Smad1/5 signaling in pulmonary artery smooth muscle cells and fibroblasts of the newborn mouse through ALK1. Am J Physiol Lung Cell Mol Physiol. 2017;313:L615–L627. doi: 10.1152/ajplung.00079.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu D, Mackenzie NC, Shanahan CM, Shroff RC, Farquharson C, MacRae VE. BMP-9 regulates the osteoblastic differentiation and calcification of vascular smooth muscle cells through an ALK1 mediated pathway. J Cell Mol Med. 2015;19:165–174. doi: 10.1111/jcmm.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Shi JY, Zhu GQ, Shi B. MiR-17-92 cluster regulates cell proliferation and collagen synthesis by targeting TGFB pathway in mouse palatal mesenchymal cells. J Cell Biochem. 2012;113:1235–1244. doi: 10.1002/jcb.23457. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Yan C, Xu X, Dong L, Su H, Hu Y, Zhang R, Ying K. Long noncoding RNA expression profiles of hypoxic pulmonary hypertension rat model. Gene. 2016;579:23–28. doi: 10.1016/j.gene.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 24.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 25.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Sun L, Cao C, Wu P, Li M, Sun G, Fei G, Ding X, Wang R. Hypoxia-induced microRNA-26b inhibition contributes to hypoxic pulmonary hypertension via CTGF. J Cell Biochem. 2018;119:1942–1952. doi: 10.1002/jcb.26355. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X. A PCR-based platform for microRNA expression profiling studies. RNA. 2009;15:716–723. doi: 10.1261/rna.1460509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Y, Jones JE, Beppu H, Keaney JF, Jr, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008: pdb prot4986. 2008 doi: 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Hu H, Wang X, Ye W, Su H, Hu Y, Dong L, Zhang R, Ying K. Involvement of CapG in proliferation and apoptosis of pulmonary arterial smooth muscle cells and in hypoxia-induced pulmonary hypertension rat model. Exp Lung Res. 2016;42:142–153. doi: 10.3109/01902148.2016.1160304. [DOI] [PubMed] [Google Scholar]
- 33.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, et al. MicroRNAs in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 34.Mann DL. MicroRNAs and the failing heart. N Eng J Med. 2007;356:2644–2645. doi: 10.1056/NEJMcibr072068. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol. 2010;299:L861–L871. doi: 10.1152/ajplung.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Côté J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 38.Siragam V, Rutnam ZJ, Yang W, Fang L, Luo L, Yang X, Li M, Deng Z, Qian J, Peng C, Yang BB. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget. 2012;3:1370–1385. doi: 10.18632/oncotarget.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trembath RC. Mutations in the TGF-beta type 1 receptor, ALK1, in combined primary pulmonary hypertension and hereditary haemorrhagic telangiectasia, implies pathway specificity. J Heart Lung Transplant. 2001;20:175. doi: 10.1016/S1053-2498(00)00352-1. [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara M, Yagi H, Matsuoka R, Akimoto K, Furutani M, Imamura S, Uehara R, Nakayama T, Takao A, Nakazawa M, Saji T. Implications of mutations of activin receptor-like kinase 1 gene (ALK1) in addition to bone morphogenetic protein receptor II gene (BMPR2) in children with pulmonary arterial hypertension. Circ J. 2008;72:127–133. doi: 10.1253/circj.72.127. [DOI] [PubMed] [Google Scholar]
- 41.Jerkic M, Kabir MG, Davies A, Yu LX, McIntyre BA, Husain NW, Enomoto M, Sotov V, Husain M, Henkelman M, et al. Pulmonary hypertension in adult Alk1 heterozygous mice due to oxidative stress. Cardiovasc Res. 2011;92:375–384. doi: 10.1093/cvr/cvr232. [DOI] [PubMed] [Google Scholar]
- 42.Soubrier F, Chung WK, Machado R, Grünig E, Aldred M, Geraci M, Loyd JE, Elliott CG, Trembath RC, Newman JH, Humbert M. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D13–D21. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.