Abstract
Background:
Breakthroughs in the treatment of preterm birth approximately 40 years ago have enabled a generation of preterm survivors to now reach mid-adulthood. Understanding their health sequelae is essential for guiding their long-term care. We conducted the first study to examine preterm birth in relation to mortality into mid-adulthood.
Methods:
A national cohort study was conducted of all 4,296,814 singleton live births in Sweden in 1973–2015, who were followed up for mortality through 2017 (maximum age 45 years). Cox regression was used to examine gestational age at birth in relation to all-cause and cause-specific mortality, and co-sibling analyses assessed for potential confounding by shared familial (genetic and/or environmental) factors.
Findings:
There were 43,916 (1·0%) deaths in 103·5 million person-years of follow-up. Gestational age at birth was inversely associated with mortality from infancy into mid-adulthood. Relative to full-term birth (39–41 weeks), adjusted hazard ratios for mortality associated with extremely preterm (22–27 weeks), very preterm (28–33 weeks), late preterm (34–36 weeks), and early term (37–38 weeks) birth were 66·14 (95% CI, 63·09–69·34), 8·67 (8·32–9·03), 2·61 (2·52–2·71), and 1·34 (1·30–1·37), respectively, at ages 0–45 years; and 2·04 (95% CI, 0·92–4·55), 1·48 (1·17–1·87), 1·22 (1·07–1·39), and 1·16 (1·08–1·25), respectively, at ages 30–45 years. Preterm birth accounted for more deaths among males than females (additive interaction, P<0·001). Multiple underlying causes were identified, including congenital anomalies; respiratory, endocrine, cardiovascular, and neurological diseases; cancer; and external causes. Co-sibling analyses suggested that the observed associations were not due to shared genetic or environmental factors in families.
Interpretation:
Preterm and early term birth should be recognized as chronic conditions that require long-term follow-up for adverse health sequelae in adulthood.
Funding:
National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL139536).
INTRODUCTION
Preterm birth (gestational age <37 completed weeks) has a worldwide prevalence of 11%,1 and most preterm infants now survive into adulthood.2–4 The biggest breakthroughs in preterm birth survival occurred with treatment advances in the 1970s-80s, including antenatal corticosteroids, surfactant therapy, and high-frequency ventilation.5 As a result, the earliest generation of preterm infants who survived because of those advances have now reached their mid-40s. Preterm birth has previously been linked with increased mortality in childhood as well as young adulthood (ages 18–36 years).3 However, no studies to date have examined longer-term mortality into mid-adulthood. Clinicians will increasingly encounter adults of all ages who were born preterm, and will need to understand the long-term health sequelae to enable better prevention, detection, and treatment.
We conducted a national cohort study of over 4 million births in Sweden to examine gestational age at birth in relation to all-cause and cause-specific mortality from infancy up to age 45 years. This study advances prior knowledge by: (1) extending follow-up by nearly a decade further, thus enabling assessment of mortality for the first time in the mid-adulthood period; (2) examining sex-specific differences in associations between gestational age at birth and mortality; and (3) using co-sibling analyses to assess for potential confounding effects of shared genetic and environmental factors in families. Our overarching goal is to understand the long-term outcomes of preterm birth and help improve care for these patients across the life course.
METHODS
Study Population
We identified 4,305,460 singleton live births in Sweden during 1973–2015 using the Swedish Birth Registry. This registry contains prenatal and birth information for nearly all births nationwide since 1973. We excluded 8,646 (0·2%) births that had missing information for gestational age, leaving 4,296,814 births (99·8% of the original cohort) for inclusion in the study. This study was approved by the ethics committee of Lund University in Sweden.
Ascertainment of Gestational Age at Birth and Mortality
Gestational age at birth was identified from the Swedish Birth Registry based on maternal report of last menstrual period in the 1970s and ultrasound estimation starting in the 1980s and later. This was analyzed alternatively as a continuous variable or categorical variable with 6 groups: extremely preterm (22–27 weeks), very preterm (28–33 weeks), late preterm (34–36 weeks), early term (37–38 weeks), full-term (39–41 weeks, used as the reference group), and post-term (≥42 weeks). These categories were chosen to facilitate comparisons with prior studies and to examine mortality for specific gestational age groups with sufficient power. Early term birth (37–38 weeks) was examined as a separate category because it has previously been associated with increased mortality in young adulthood relative to later term birth.4 In addition, the first 3 groups were combined to provide summary estimates for preterm birth (<37 weeks).
The study cohort was followed up for all deaths through 2017 (maximum age 45 years), identified using the Swedish Death Registry. This registry began in 1960 and includes all deaths in Sweden with compulsory reporting nationwide. Cause of death is classified according to the International Classification of Diseases (ICD), revisions 8, 9, and 10 (Table S1, Appendix, page 2).
Other Study Variables
Other perinatal and demographic characteristics that may be associated with gestational age at birth and mortality were identified using the Swedish Birth Registry and national census data, which were linked using an anonymous personal identification number. The following were included as adjustment variables: birth year (continuous variable), sex, birth order (1, 2, ≥3), maternal age at delivery (<20, 20–24, 25–29, 30–34, 35–39, ≥40 years), maternal education level (<9, 10–11, ≥12 years), and maternal smoking at the beginning of prenatal care (0, 1–9, ≥10 cigarettes/day). Missing data were infrequent for birth order (<0·1%), maternal age (<0·1%), and maternal education (0·7%), whereas 25·6% of mothers lacked smoking data. Mothers without smoking data were more likely to have the lowest education level compared to those with such data (20·6% vs·10·7%). Missing data for each covariate were imputed using a standard multiple imputation procedure based on the variable’s relationship with all other covariates and mortality, to produce standard errors that account for the uncertainty in imputations and support valid inferences.6
Statistical Analysis
Cox proportional hazards regression was used to determine hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between gestational age at birth and all-cause or cause-specific mortality at ages 0–45 years and in narrower age ranges (0 to <1, 1–9, 10–19, 20–29, 30–45 years) among persons still alive at the beginning of the respective age range. These age intervals were chosen to examine these associations in different stages of life from childhood into mid-adulthood. Attained age was used as the Cox model time axis. Individuals were censored at the date of emigration (n=260,079; 6·1%), determined by absence of a Swedish residential address in census data. Emigrants and non-emigrants had a similar gestational duration (median, 40 1/7 weeks for both groups), and thus it was unlikely that emigration introduced any substantial bias.
Analyses were conducted both unadjusted and adjusted for covariates (as above). In secondary analyses, we further adjusted for fetal growth (birth weight standardized for gestational age and sex based on Swedish reference growth curves7) to explore the effects of gestational age at birth on mortality independent of fetal growth. Cause-specific mortality was examined for all ICD categories with ≥500 total deaths to enable sufficient statistical power for each outcome. The proportional hazards assumption was assessed by examining log-log plots, and was met in each model (e.g., Figures S1a and S1b in Appendix, page 6).
Potential interactions between preterm or early term birth and sex in relation to mortality were examined on the additive and multiplicative scale. Additive interactions were tested using the “relative excess risk due to interaction” (RERI), a measure of departure from additivity of effects on a relative risk scale.8 Multiplicative interactions were tested using the ratio of the HR for the effect of both factors considered together to the product of HRs for their effects considered separately.8
Co-sibling analyses were performed to assess for potential confounding effects of unmeasured shared familial (genetic and/or environmental) factors. Shared environmental factors in families may potentially include lifestyle factors such as diet, or ambient exposures such as passive smoking or air pollution. These analyses used stratified Cox regression with a separate stratum for each family as identified by the mother’s anonymous identification number, so that comparisons are made among siblings that control for their shared exposures. A total of 3,562,267 individuals (82·9% of the cohort) had at least one sibling and were included in these analyses. In the stratified Cox model, each set of siblings has its own baseline hazard function that reflects the family’s shared genetic and environmental factors, and thus comparisons of different gestational ages at birth are made within the family. In addition, these analyses were further adjusted for the same covariates as in the main analyses.
Sensitivity analyses were performed by repeating the main analyses after excluding deaths due to congenital anomalies and other perinatal conditions, or deaths from external causes (i.e., unnatural causes, including accidents, homicides, and suicides). All statistical tests were 2-sided and used an α-level of 0·05. All analyses were conducted using Stata version 15·1.
Role of the Funding Source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
The overall prevalence of preterm birth in Sweden during 1973–2015 was 4·9% (4·6% in the 1970s, 5·4% in the 1980s, 5·0% in the 1990s and 2000s, and 4·6% in the 2010s). Prevalences were 0·2% for extremely preterm (22–27 weeks), 1·0% for very preterm (28–33 weeks), 3·7% for late preterm (34–36 weeks), 17·6% for early term (37–38 weeks), 69·2% for full-term (39–41 weeks), and 8·3% for post-term (≥42 weeks) births. Preterm infants were more likely than full-term infants to be male or first-born; and their mothers were more likely to be at the extremes of age, have low education level, or smoke (Table 1).
Table 1.
Characteristics of study participants by gestational age at birth, Sweden, 1973–2015.
| Extremely preterm (22-27 weeks) |
Very preterm (28-33 weeks) |
Late preterm (34-36 weeks) |
Early term (37-38 weeks) |
Full-term (39-41 weeks) |
Post-term (≥42 weeks) |
|
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Total | 8,426 (100·0) | 44,567 (100·0) | 159,307 (100·0) | 756,442 (100·0) | 2,973,708 (100·0) | 354,364 (100·0) |
| Sex | ||||||
| Male | 4,615 (54·8) | 24,895 (55·9) | 86,744 (54·4) | 389,108 (51·4) | 1,511,124 (50·8) | 193,175 (54·5) |
| Female | 3,811 (45·2) | 19,672 (44·1) | 72,563 (45·6) | 367,334 (48·6) | 1,462,584 (49·2) | 161,189 (45·5) |
| Birth order | ||||||
| 1 | 4,199 (49·8) | 22,904 (51·4) | 78,932 (49·6) | 304,260 (40·2) | 1,253,685 (42·2) | 176,327 (49·8) |
| 2 | 2,396 (28·4) | 12,623 (28·3) | 47,822 (30·0) | 276,917 (36·6) | 1,115,055 (37·5) | 114,202 (32·2) |
| ≥3 | 1,831 (21·7) | 9,040 (20·3) | 32,553 (20·4) | 175,265 (23·2) | 604,968 (20·3) | 63,835 (18·0) |
| Maternal age (years) | ||||||
| <20 | 362 (4·3) | 2,084 (4·7) | 6,502 (4·1) | 22,257 (2·9) | 84,747 (2·8) | 13,046 (3·7) |
| 20–24 | 1,602 (19·0) | 9,018 (20·2) | 33,546 (21·1) | 141,176 (18·7) | 590,497 (19·9) | 77,240 (21·8) |
| 25–29 | 2,466 (29·3) | 13,791 (30·9) | 51,927 (32·6) | 248,325 (32·8) | 1,043,905 (35·1) | 123,872 (35·0) |
| 30–34 | 2,290 (27·2) | 11,892 (26·7) | 42,119 (26·4) | 216,965 (28·7) | 847,481 (28·5) | 95,651 (27·0) |
| 35–39 | 1,337 (15·9) | 6,175 (13·9) | 20,438 (12·8) | 103,813 (13·7) | 343,929 (11·6) | 38,363 (10·8) |
| ≥40 | 369 (4·4) | 1,607 (3·6) | 4,775 (3·0) | 23,906 (3·2) | 63,149 (2·1) | 6,192 (1·7) |
| Maternal education (years) | ||||||
| ≤9 | 1,439 (17·1) | 7,405 (16·6) | 24,783 (15·6) | 106,638 (14·1) | 378,085 (12·7) | 49,769 (14·0) |
| 10–12 | 3,977 (47·2) | 21,197 (47·6) | 75,084 (47·1) | 344,694 (45·6) | 1,331,927 (44·8) | 159,696 (45·1) |
| >12 | 3,010 (35·7) | 15,965 (35·8) | 59,440 (37·3) | 305,110 (40·3) | 1,263,696 (42·5) | 144,899 (40·9) |
| Maternal smoking (cigarettes/day) | ||||||
| 0 | 6,176 (73·3) | 31,563 (70·8) | 116,493 (73·1) | 580,120 (76·7) | 2,290,045 (77·0) | 255,160 (72·0) |
| 1–9 | 1,751 (20·8) | 10,245 (23·0) | 33,795 (21·2) | 139·123 (18·4) | 570,028 (19·2) | 88,190 (24·9) |
| ≥10 | 499 (5·9) | 2,759 (6·1) | 9,019 (5·7) | 37,199 (4·9) | 113,385 (3·8) | 11,014 (3·1) |
All-Cause Mortality
A total of 43,916 (1·0%) deaths occurred in 103·5 million person-years of follow-up, yielding an overall mortality rate of 42·42 per 100,000 person-years across the entire age range examined (0–45 years). The corresponding mortality rates were 172·79 among those born preterm, 44·14 among those born at early term, and 33·45 among those born at full-term (Table 2).
Table 2.
Adjusted hazard ratios for all-cause mortality by gestational age at birth, Sweden, 1973–2017.
| All | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Ratea | HR (95% CI)b | P | Deaths | Ratea | HR (95% CI)b | P | Deaths | Ratea | HR (95% CI)b | P | |
| Attained ages 0–45 years | ||||||||||||
| Preterm (<37 wks) | 10,392 | 172·79 | 5·01 (4·88, 5·15) | <0·001 | 6,197 | 188·78 | 4·44 (4·30, 4·60) | <0·001 | 4,195 | 153·31 | 6·14 (5·89, 6·40) | <0·001 |
| Extremely preterm (<28 wks) | 2,937 | 2119·13 | 66·14 (63·09, 69·34) | <0·001 | 1,697 | 2363·28 | 60·68 (57·06, 64·54) | <0·001 | 1,240 | 1852·61 | 76·36 (70·95, 82·18) | <0·001 |
| Very preterm (28–33 wks) | 3,734 | 298·12 | 8·67 (8·32, 9·03) | <0·001 | 2,251 | 323·90 | 7·67 (7·27, 8·08) | <0·001 | 1,483 | 265·85 | 10·70 (10·02, 11·42) | <0·001 |
| Late preterm (34–36 wks) | 3,721 | 89·93 | 2·61 (2·52, 2·71) | <0·001 | 2,249 | 99·75 | 2·35 (2·24, 2·46) | <0·001 | 1,472 | 78·00 | 3·13 (2·95, 3·33) | <0·001 |
| Early term (37–38 wks) | 7,502 | 44·14 | 1·34 (1·30, 1·37) | <0·001 | 4,747 | 53·69 | 1·30 (1·26, 1·35) | <0·001 | 2,755 | 33·73 | 1·39 (1·33, 1·46) | <0·001 |
| Full-term (39–41 wks) | 22,429 | 33·45 | Reference | 14,161 | 41·79 | Reference | 8,268 | 24·82 | Reference | |||
| Post-term (≥42 wks) | 3,593 | 39·76 | 1·09 (1·06, 1·14) | <0·001 | 2,255 | 47·48 | 1·06 (1·02, 1·11) | 0·009 | 1,388 | 31·08 | 1·16 (1·09, 1·23) | <0·001 |
| Per additional week (trend test) | 0·78 (0·78, 0·78) | <0·001 | 0·79 (0·78, 0·79) | <0·001 | 0·76 (0·76, 0·77) | <0·001 | ||||||
| Attained ages 0 to <1 year | ||||||||||||
| Preterm (<37 wks) | 8,545 | 2840·39 | 17·15 (16·50, 17·82) | <0·001 | 4,989 | 3028·49 | 16·99 (16·14, 17·88) | <0·001 | 3,556 | 2613·72 | 17·35 (16·37, 18·40) | <0·001 |
| Extremely preterm (<28 wks) | 2,884 | 34437·6 | 236·32 (223·81, 249·54) | <0·001 | 1,668 | 37585·0 | 241·14 (224·40, 259·13) | <0·001 | 1,216 | 30831·4 | 230·09 (211·71, 250·07) | <0·001 |
| Very preterm (28–33 wks) | 3,284 | 5235·20 | 32·10 (30·49, 33·79) | <0·001 | 1,969 | 5639·36 | 32·27 (30·17, 34·51) | <0·001 | 1,315 | 4729·20 | 31·80 (29·37, 34·43) | <0·001 |
| Late preterm (34–36 wks) | 2,377 | 1118·07 | 6·75 (6·39, 7·13) | <0·001 | 1,352 | 1155·39 | 6·47 (6·01, 6·96) | <0·001 | 1,025 | 1073·58 | 7·14 (6·57, 7·76) | <0·001 |
| Early term (37–38 wks) | 2,840 | 309·65 | 1·95 (1·86, 2·05) | <0·001 | 1,616 | 337·15 | 1·95 (1·83, 2·09) | <0·001 | 1,224 | 280·54 | 1·95 (1·81, 2·10) | <0·001 |
| Full-term (39–41 wks) | 5,715 | 162·87 | Reference | 3,120 | 175·52 | Reference | 2,595 | 149·81 | Reference | |||
| Post-term (≥42 wks) | 1,012 | 227·37 | 1·25 (1·16, 1·35) | <0·001 | 549 | 224·49 | 1·19 (1·07, 1·31) | 0·001 | 463 | 230·83 | 1·34 (1·20, 1·49) | <0·001 |
| Per additional week (trend test) | 0·69 (0·69, 0·69) | <0·001 | 0·69 (0·68, 0·69) | <0·001 | 0·69 (0·69, 0·69) | <0·001 | ||||||
| Attained ages 1–9 years | ||||||||||||
| Preterm (<37 wks) | 547 | 33·93 | 2·22 (2·03, 2·44) | <0·001 | 314 | 35·65 | 2·08 (1·85, 2·34) | <0·001 | 233 | 31·86 | 2·44 (2·12, 2·80) | <0·001 |
| Extremely preterm (<28 wks) | 24 | 60·81 | 4·52 (3·02, 6·75) | <0·001 | 14 | 67·17 | 4·52 (2·67, 7·65) | <0·001 | 10 | 53·70 | 4·52 (2·42, 8·41) | <0·001 |
| Very preterm (28–33 wks) | 160 | 49·38 | 3·26 (2·78, 3·82) | <0·001 | 91 | 50·61 | 2·99 (2·42, 3·69) | <0·001 | 69 | 47·85 | 3·67 (2·88, 4·67) | <0·001 |
| Late preterm (34–36 wks) | 363 | 29·08 | 1·90 (1·70, 2·11) | <0·001 | 209 | 30·73 | 1·78 (1·54, 2·06) | <0·001 | 154 | 27·09 | 2·06 (1·75, 2·44) | <0·001 |
| Early term (37–38 wks) | 1,049 | 17·63 | 1·20 (1·12, 1·28) | <0·001 | 582 | 18·97 | 1·14 (1·04, 1·25) | 0·006 | 467 | 16·20 | 1·27 (1·15, 1·42) | <0·001 |
| Full-term (39–41 wks) | 3,481 | 14·84 | Reference | 1,995 | 16·75 | Reference | 1,486 | 12·87 | Reference | |||
| Post-term (≥42 wks) | 505 | 17·74 | 1·10 (1·01, 1·21) | 0·04 | 273 | 17·73 | 1·00 (0·88, 1·13) | 0·96 | 232 | 17·77 | 1·27 (1·10, 1·46) | 0·001 |
| Per additional week (trend test) | 0·90 (0·89, 0·91) | <0·001 | 0·90 (0·89, 0·92) | <0·001 | 0·90 (0·88, 0·92) | <0·001 | ||||||
| Attained ages 10–19 years | ||||||||||||
| Preterm (<37 wks) | 390 | 29·40 | 1·48 (1·34, 1·65) | <0·001 | 249 | 34·28 | 1·44 (1·26, 1·64) | <0·001 | 141 | 23·50 | 1·57 (1·32, 1·87) | <0·001 |
| Extremely preterm (<28 wks) | 9 | 33·35 | 1·89 (0·98, 3·63) | 0·06 | 5 | 35·52 | 1·68 (0·70, 4·04) | 0·25 | 4 | 30·99 | 2·25 (0·84, 6·00) | 0·11 |
| Very preterm (28–33 wks) | 99 | 37·64 | 1·91 (1·56, 2·33) | <0·001 | 60 | 41·24 | 1·74 (1·35, 2·25) | <0·001 | 39 | 33·19 | 2·23 (1·62, 3·07) | <0·001 |
| Late preterm (34–36 wks) | 282 | 27·21 | 1·37 (1·21, 1·55) | <0·001 | 184 | 32·46 | 1·35 (1·17, 1·57) | <0·001 | 98 | 20·87 | 1·39 (1·14, 1·71) | 0·001 |
| Early term (37–38 wks) | 1,006 | 21·05 | 1·10 (1·03, 1·18) | 0·007 | 650 | 26·16 | 1·12 (1·03, 1·22) | 0·01 | 356 | 15·52 | 1·06 (0·95, 1·19) | 0·30 |
| Full-term (39–41 wks) | 3,702 | 19·22 | Reference | 2,296 | 23·49 | Reference | 1,406 | 14·83 | Reference | |||
| Post-term (≥42 wks) | 507 | 20·55 | 1·00 (0·91, 1·10) | 0·93 | 315 | 24·06 | 0·98 (0·87, 1·10) | 0·73 | 192 | 16·59 | 1·05 (0·90, 1·22) | 0·51 |
| Per additional week (trend test) | 0·95 (0·94, 0·96) | <0·001 | 0·95 (0·93, 0·96) | <0·001 | 0·95 (0·93, 0·97) | <0·001 | ||||||
| Attained ages 20–29 years | ||||||||||||
| Preterm (<37 wks) | 591 | 69·08 | 1·38 (1·26, 1·50) | <0·001 | 437 | 92·41 | 1·32 (1·20, 1·46) | <0·001 | 154 | 40·25 | 1·54 (1·31, 1·82) | <0·001 |
| Extremely preterm (<28 wks) | 14 | 102·40 | 2·12 (1·26, 3·59) | 0·005 | 7 | 101·48 | 1·45 (0·69, 3·03) | 0·33 | 7 | 103·34 | 4·00 (1·90, 8·41) | <0·001 |
| Very preterm (28–33 wks) | 120 | 72·30 | 1·42 (1·19, 1·70) | <0·001 | 91 | 98·52 | 1·40 (1·13, 1·72) | 0·002 | 29 | 39·41 | 1·50 (1·04, 2·17) | 0·03 |
| Late preterm (34–36 wks) | 457 | 67·62 | 1·35 (1·23, 1·48) | <0·001 | 339 | 90·73 | 1·30 (1·17, 1·46) | <0·001 | 118 | 39·05 | 1·50 (1·24, 1·81) | <0·001 |
| Early term (37–38 wks) | 1,682 | 55·70 | 1·15 (1·09, 1·22) | <0·001 | 1,257 | 78·75 | 1·15 (1·08, 1·22) | <0·001 | 425 | 29·82 | 1·16 (1·04, 1·30) | 0·006 |
| Full-term (39–41 wks) | 5,873 | 47·15 | Reference | 4,315 | 67·93 | Reference | 1,558 | 25·53 | Reference | |||
| Post-term (≥42 wks) | 881 | 50·76 | 1·06 (0·98, 1·14) | 0·12 | 651 | 72·21 | 1·06 (0·97, 1·15) | 0·19 | 230 | 27·58 | 1·06 (0·92, 1·22) | 0·40 |
| Per additional week (trend test) | 0·96 (0·95, 0·97) | <0·001 | 0·97 (0·96, 0·98) | <0·001 | 0·95 (0·93, 0·97) | <0·001 | ||||||
| Attained ages 30–45 years | ||||||||||||
| Preterm (<37 wks) | 319 | 80·90 | 1·28 (1·14, 1·43) | <0·001 | 208 | 94·22 | 1·17 (1·01, 1·34) | 0·03 | 111 | 63·96 | 1·55 (1·28, 1·89) | <0·001 |
| Extremely preterm (<28 wks) | 6 | 128·59 | 2·04 (0·92, 4·55) | 0·08 | 3 | 127·40 | 1·53 (0·49, 4·75) | 0·46 | 3 | 129·81 | 3·11 (1·00, 9·65) | 0·05 |
| Very preterm (28–33 wks) | 71 | 95·17 | 1·48 (1·17, 1·87) | 0·001 | 40 | 94·97 | 1·15 (0·84, 1·57) | 0·38 | 31 | 95·44 | 2·31 (1·61, 3·29) | <0·001 |
| Late preterm (34–36 wks) | 242 | 76·82 | 1·22 (1·07, 1·39) | 0·003 | 165 | 93·60 | 1·17 (1·00, 1·36) | 0·05 | 77 | 55·49 | 1·35 (1·07, 1·70) | 0·01 |
| Early term (37–38 wks) | 925 | 70·39 | 1·16 (1·08, 1·25) | <0·001 | 642 | 89·59 | 1·15 (1·06, 1·26) | 0·001 | 283 | 47·36 | 1·18 (1·04, 1·35) | 0·01 |
| Full-term (39–41 wks) | 3,658 | 58·94 | Reference | 2,435 | 76·81 | Reference | 1,223 | 40·28 | Reference | |||
| Post-term (≥42 wks) | 688 | 62·47 | 1·04 (0·96, 1·13) | 0·34 | 467 | 83·92 | 1·07 (0·97, 1·19) | 0·16 | 221 | 40·56 | 0·98 (0·85, 1·13) | 0·76 |
| Per additional week (trend test) | 0·97 (0·96, 0·98) | <0·001 | 0·98 (0·97, 1·00) | 0·049 | 0·94 (0·92, 0·97) | <0·001 | ||||||
Mortality rate per 100,000 person-years.
Adjusted for birth year, sex, birth order, maternal age, maternal education, and maternal smoking
In analyses of the entire age range (0–45 years), a strong inverse association was observed between gestational age at birth and mortality (adjusted HR per additional week of gestation, 0·78; 95% CI, 0·78–0·78; P<0·001). Those born preterm or early term had 5-fold and 1·3-fold mortality, respectively, relative to those born at full-term (adjusted HR, 5·01; 95% CI, 4·88–5·15; P<0·001; and 1·34; 95% CI, 1·30–1·37; P<0·001). These associations were present among both males and females, although the HRs were slightly higher among females due to a lower baseline mortality rate among girls born at full-term (Table 2).
In analyses of narrower age intervals, both preterm and early term births were strongly associated with increased mortality during infancy (adjusted HRs 17·15 and 1·95, respectively). The HRs weakened but remained significantly elevated in each subsequent age interval (Table 2). At ages 30–45 years, the adjusted HRs for mortality associated with preterm and early term birth were 1·28 (95% CI, 1·14–1·43; P<0·001) and 1·16 (95% CI, 1·08–1·25, P<0·001), respectively, relative to full-term birth. These associations remained significant among both males and females. Figure 1 shows the adjusted HRs (fitted by cubic spline) for all-cause mortality by attained age for different gestational age groups. Compared to all adjusted results, unadjusted HRs were ~5% higher on average (Table S2, Appendix, page 3). Kaplan-Meier survival curves by gestational age group are shown in Figure S2 (Appendix, page 7).
Figure 1.
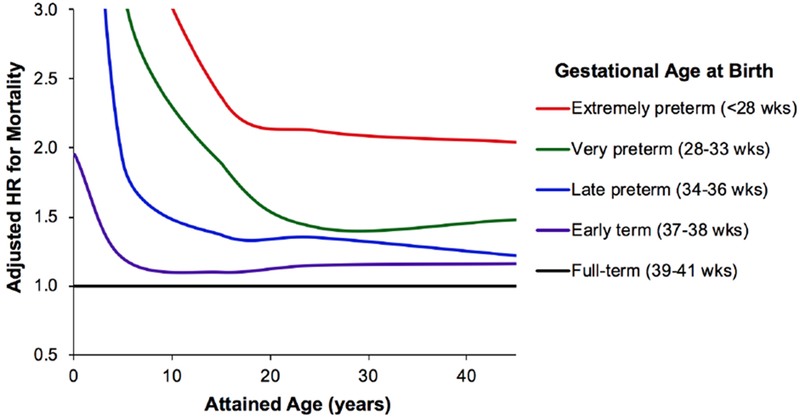
Adjusted hazard ratios for all-cause mortality at ages 0–45 years by gestational age at birth relative to full-term births, Sweden, 1973–2017.
Table 3 shows interactions between preterm or early term birth and sex in relation to all-cause mortality across the entire age range (0–45 years). Preterm-born males had the highest overall mortality rate, which was significantly higher relative to preterm-born females (adjusted HR, 1·22; 95% CI, 1·17–1·28; P<0·001). Furthermore, a positive additive interaction was found between preterm birth and male sex (i.e., the combined effect of these factors on mortality exceeded the sum of their separate effects; P<0·001), indicating that preterm birth accounted for significantly more total deaths among males. In adulthood (ages 20–45 years), there was a modest positive additive interaction between early term (but not preterm) birth and male sex in relation to mortality (P=0·01; Table S3, Appendix, page 4).
Table 3.
Interactions between gestational age at birth and sex in relation to all-cause mortality at ages 0–45 years.
| Gestational age at birth | HRs (95% CI) for early term vs. full-term within sex stratab | HRs (95% CI) for preterm vs. full-term within sex stratab | ||||||
|---|---|---|---|---|---|---|---|---|
| Full-term (39–41 wks) | Early term (37–38 wks) | Preterm (<37 wks) | ||||||
| Ratea | HR (95% CI)b | Ratea | HR (95% CI)b | Ratea | HR (95% CI)b | |||
| Sex | ||||||||
| Female | 24·82 | Reference | 33·73 | 1·40 (1·34, 1·46) | 153·31 | 6·13 (5·88, 6·40) | 1·40 (1·34, 1·46) | 6·13 (5·88, 6·40) |
| Male | 41·79 | 1·68 (1·64, 1·73) | 53·69 | 2·18 (2·10, 2·27) | 188·78 | 7·49 (7·22, 7·77) | 1·30 (1·25, 1·34) | 4·45 (4·30, 4·60) |
| HRs (95% CI) for male vs. female within gestational age stratab | 1·68 (1·64, 1·73) | 1·56 (1·49, 1·64) | 1·22 (1·17, 1·28) | |||||
| Interaction on additive scale: RERI (95% CI) | 0·10 (0·01, 0·19); P=0·03 | 0·67 (0·36, 0·98); P<0·001 | ||||||
| Interaction on multiplicative scale: HR ratio (95% CI) | 0·93 (0·88, 0·98); P=0·008 | 0·73 (0·69, 0·76); P<0·001 | ||||||
Mortality rate per 100,000 person-years.
Adjusted for birth year, birth order, maternal age, maternal education, and maternal smoking. All HRs had P<0·001.
HR = hazard ratio, RERI = relative excess risk due to interaction
Co-sibling analyses were performed to control for shared genetic and environmental factors within families. In these analyses, we would expect the HRs observed in the main analyses to be reduced to 1 if they were completely confounded by shared familial factors. Instead, they were attenuated by an average of ~3%, suggesting that the associations observed in the main analyses were not due to unmeasured familial confounding (Table S4, Appendix, page 5). In analyses of the entire age range (0–45 years), the adjusted HRs were minimally changed. At ages 30–45 years, the risk estimates were no longer significant and confidence intervals were considerably wider than in the main analyses, reflecting lower statistical power in the co-sibling analyses.
In secondary analyses, further adjustment for fetal growth had a negligible effect on all risk estimates. A strong inverse association remained between gestational age at birth and all-cause mortality (e.g., adjusted HR per additional week of gestation, 0·77; 95% CI, 0·77–0·78; P<0·001 at ages 0–45 years; 0·96; 95% CI, 0·95–0·98; P<0·001 at ages 30–45 years), and a strong association for preterm relative to full-term births (e.g., adjusted HR, 5·04; 95% CI, 4·91–5·17; P<0·001 at ages 0–45 years; 1·30; 95% CI, 1·15–1·45; P<0·001 at ages 30–45 years).
Cause-Specific Mortality
Table 4 shows associations between gestational age at birth (per additional week) and cause-specific mortality for ICD categories with ≥500 total deaths. There were too few deaths attributed to kidney disease (n=59) to analyze this outcome separately with sufficient power. In analyses of the entire age range (0–45 years), gestational age at birth was inversely associated with all major causes of death examined in the entire cohort and among males or females. In infancy, the strongest associations were with mortality from respiratory disorders and other conditions specific to the perinatal period (e.g., fetal hematologic disorders or hemorrhage). However, associations with endocrine, cardiovascular, neurological, and cancer mortality also were present in infancy and childhood as well as young adulthood (ages 20–29 years), and a strong association with endocrine mortality (mostly diabetes mellitus) extended further into adulthood (ages 30–45 years, both sexes: adjusted HR per additional week of gestation, 0·85; 95% CI, 0·79–0·92; P<0·001).
Table 4.
Associations between gestational age at birth (per additional week) and cause-specific mortality, Sweden, 1973–2017.
| All | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Ratea | HR (95% CI)b | P | Deaths | Ratea | HR (95% CI)b | P | Deaths | Ratea | HR (95% CI)b | P | |
| Attained ages 0–45 years | ||||||||||||
| Congenital anomalies | 7,922 | 6·75 | 0·75 (0·75, 0·76) | <0·001 | 4,298 | 7·01 | 0·75 (0·75, 0·76) | <0·001 | 3,624 | 6·47 | 0·76 (0·75, 0·76) | <0·001 |
| Other perinatal conditions | 4,886 | 3·22 | 0·60 (0·60, 0·60) | <0·001 | 2,850 | 3·68 | 0·60 (0·60, 0·61) | <0·001 | 2,036 | 2·74 | 0·60 (0·59, 0·60) | <0·001 |
| Respiratory | 2,793 | 2·30 | 0·67 (0·66, 0·67) | <0·001 | 1,674 | 2·67 | 0·67 (0·66, 0·68) | <0·001 | 1,119 | 1·92 | 0·67 (0·66, 0·68) | <0·001 |
| Endocrine | 957 | 1·00 | 0·85 (0·83, 0·87) | <0·001 | 555 | 1·14 | 0·85 (0·83, 0·88) | <0·001 | 402 | 0·87 | 0·85 (0·81, 0·88) | <0·001 |
| Cardiovascular | 1,571 | 1·65 | 0·86 (0·84, 0·88) | <0·001 | 1,014 | 2·08 | 0·86 (0·84, 0·88) | <0·001 | 557 | 1·19 | 0·86 (0·83, 0·89) | <0·001 |
| Neurological | 1,864 | 1·99 | 0·84 (0·83, 0·86) | <0·001 | 1,097 | 2·28 | 0·83 (0·82, 0·85) | <0·001 | 767 | 1·68 | 0·86 (0·83, 0·88) | <0·001 |
| Cancer | 4,043 | 4·33 | 0·96 (0·94, 0·97) | <0·001 | 2,148 | 4·47 | 0·95 (0·93, 0·97) | <0·001 | 1,895 | 4·18 | 0·97 (0·95, 0·99) | 0·01 |
| External causes | 13,039 | 14·00 | 0·98 (0·97, 0·98) | <0·001 | 9,478 | 19·78 | 0·98 (0·97, 0·99) | 0·001 | 3,561 | 7·88 | 0·96 (0·95, 0·98) | <0·001 |
| Other or unknown causes | 6,841 | 7·17 | 0·84 (0·83, 0·84) | <0·001 | 4,246 | 8·68 | 0·85 (0·84, 0·86) | <0·001 | 2,595 | 5·58 | 0·82 (0·81, 0·83) | <0·001 |
| Attained ages 0 to <1 year | ||||||||||||
| Congenital anomalies | 6,542 | 115·10 | 0·74 (0·74, 0·75) | <0·001 | 3,559 | 119·64 | 0·74 (0·73, 0·75) | <0·001 | 2,983 | 110·30 | 0·74 (0·73, 0·75) | <0·001 |
| Other perinatal conditions | 4,814 | 68·76 | 0·60 (0·59, 0·60) | <0·001 | 2,797 | 78·09 | 0·60 (0·59, 0·60) | <0·001 | 2,017 | 58·90 | 0·60 (0·59, 0·60) | <0·001 |
| Respiratory | 2,198 | 36·35 | 0·64 (0·64, 0·65) | <0·001 | 1,316 | 42·01 | 0·64 (0·63, 0·65) | <0·001 | 882 | 30·37 | 0·65 (0·64, 0·65) | <0·001 |
| Endocrine | 289 | 6·27 | 0·78 (0·76, 0·81) | <0·001 | 166 | 7·09 | 0·79 (0·75, 0·82) | <0·001 | 123 | 5·42 | 0·78 (0·74, 0·82) | <0·001 |
| Cardiovascular | 275 | 5·59 | 0·72 (0·70, 0·74) | <0·001 | 149 | 5·94 | 0·71 (0·68, 0·73) | <0·001 | 126 | 5·22 | 0·74 (0·71, 0·77) | <0·001 |
| Neurological | 544 | 12·55 | 0·77 (0·75, 0·79) | <0·001 | 303 | 13·67 | 0·75 (0·73, 0·77) | <0·001 | 241 | 11·36 | 0·80 (0·77, 0·84) | <0·001 |
| Cancer | 198 | 4·25 | 0·77 (0·74, 0·80) | <0·001 | 99 | 4·11 | 0·74 (0·70, 0·77) | <0·001 | 99 | 4·40 | 0·82 (0·76, 0·88) | <0·001 |
| External causes | 261 | 5·95 | 0·86 (0·82, 0·90) | <0·001 | 137 | 6·03 | 0·89 (0·83, 0·96) | 0·002 | 124 | 5·85 | 0·82 (0·77, 0·88) | <0·001 |
| Other or unknown causes | 2,991 | 66·34 | 0·77 (0·76, 0·78) | <0·001 | 1,748 | 75·71 | 0·78 (0·77, 0·79) | <0·001 | 1,243 | 56·43 | 0·77 (0·75, 0·78) | <0·001 |
| Attained ages 1–9 years | ||||||||||||
| Congenital anomalies | 912 | 2·69 | 0·83 (0·81, 0·85) | <0·001 | 485 | 2·79 | 0·84 (0·81, 0·87) | <0·001 | 427 | 2·59 | 0·82 (0·80, 0·85) | <0·001 |
| Other perinatal conditions | 45 | 0·13 | 0·69 (0·65, 0·73) | <0·001 | 29 | 0·17 | 0·69 (0·65, 0·75) | <0·001 | 16 | 0·10 | 0·69 (0·63, 0·76) | <0·001 |
| Respiratory | 281 | 0·83 | 0·81 (0·78, 0·85) | <0·001 | 155 | 0·89 | 0·83 (0·79, 0·88) | <0·001 | 126 | 0·77 | 0·79 (0·75, 0·84) | <0·001 |
| Endocrine | 269 | 0·79 | 0·88 (0·84, 0·92) | <0·001 | 135 | 0·78 | 0·88 (0·82, 0·95) | <0·001 | 134 | 0·81 | 0·88 (0·81, 0·94) | <0·001 |
| Cardiovascular | 177 | 0·52 | 0·88 (0·83, 0·94) | <0·001 | 94 | 0·54 | 0·85 (0·79, 0·92) | <0·001 | 83 | 0·50 | 0·92 (0·83, 1·02) | 0·13 |
| Neurological | 482 | 1·42 | 0·88 (0·85, 0·92) | <0·001 | 267 | 1·53 | 0·87 (0·83, 0·91) | <0·001 | 215 | 1·31 | 0·91 (0·85, 0·97) | 0·003 |
| Cancer | 1,198 | 3·54 | 0·98 (0·95, 1·01) | 0·21 | 662 | 3·80 | 0·98 (0·94, 1·02) | 0·33 | 536 | 3·26 | 0·98 (0·94, 1·03) | 0·47 |
| External causes | 1,509 | 4·46 | 0·99 (0·96, 1·02) | 0·47 | 943 | 5·42 | 0·99 (0·95, 1·02) | 0·46 | 566 | 3·44 | 1·00 (0·95, 1·04) | 0·87 |
| Other or unknown causes | 709 | 2·09 | 0·89 (0·86, 0·92) | <0·001 | 394 | 2·26 | 0·89 (0·85, 0·93) | <0·001 | 315 | 1·91 | 0·89 (0·84, 0·93) | <0·001 |
| Attained ages 10–19 years | ||||||||||||
| Congenital anomalies | 255 | 0·92 | 0·84 (0·80, 0·88) | <0·001 | 146 | 1·02 | 0·84 (0·79, 0·90) | <0·001 | 109 | 0·80 | 0·84 (0·78, 0·90) | <0·001 |
| Other perinatal conditions | 19 | 0·07 | 0·69 (0·62, 0·76) | <0·001 | 18 | 0·13 | 0·68 (0·62, 0·75) | <0·001 | 1 | 0·01 | 1·11 (0·32, 3·91) | 0·87 |
| Respiratory | 108 | 0·39 | 0·86 (0·80, 0·93) | <0·001 | 58 | 0·41 | 0·86 (0·78, 0·96) | 0·006 | 50 | 0·37 | 0·86 (0·76, 0·96) | 0·009 |
| Endocrine | 128 | 0·46 | 0·98 (0·89, 1·07) | 0·59 | 77 | 0·54 | 0·95 (0·85, 1·07) | 0·39 | 51 | 0·38 | 1·01 (0·86, 1·18) | 0·89 |
| Cardiovascular | 291 | 1·04 | 0·93 (0·88, 0·98) | 0·009 | 180 | 1·26 | 0·91 (0·85, 0·98) | 0·009 | 111 | 0·82 | 0·95 (0·87, 1·05) | 0·35 |
| Neurological | 357 | 1·29 | 0·90 (0·85, 0·94) | <0·001 | 209 | 1·46 | 0·91 (0·86, 0·97) | 0·003 | 148 | 1·10 | 0·87 (0·82, 0·94) | <0·001 |
| Cancer | 834 | 2·99 | 0·98 (0·94, 1·01) | 0·18 | 496 | 3·47 | 0·95 (0·91, 0·99) | 0·02 | 338 | 2·50 | 1·03 (0·96, 1·09) | 0·43 |
| External causes | 3,116 | 11·20 | 0·97 (0·96, 0·99) | 0·007 | 2,057 | 14·40 | 0·98 (0·96, 1·00) | 0·05 | 1,059 | 7·83 | 0·97 (0·94, 1·00) | 0·07 |
| Other or unknown causes | 497 | 1·78 | 0·93 (0·89, 0·97) | 0·001 | 269 | 1·88 | 0·94 (0·89, 0·99) | 0·04 | 228 | 1·68 | 0·92 (0·86, 0·98) | 0·01 |
| Attained ages 20–29 years | ||||||||||||
| Congenital anomalies | 143 | 0·79 | 0·86 (0·80, 0·92) | <0·001 | 72 | 0·77 | 0·84 (0·77, 0·92) | <0·001 | 71 | 0·81 | 0·88 (0·79, 0·97) | 0·01 |
| Other perinatal conditions | 5 | 0·03 | 0·75 (0·58, 0·96) | 0·02 | 4 | 0·04 | 0·69 (0·55, 0·86) | 0·001 | 1 | 0·01 | 3·54 (0·92, 13·64) | 0·07 |
| Respiratory | 115 | 0·64 | 0·94 (0·86, 1·03) | 0·16 | 86 | 0·92 | 0·96 (0·86, 1·07) | 0·48 | 29 | 0·33 | 0·88 (0·75, 1·03) | 0·10 |
| Endocrine | 158 | 0·88 | 0·92 (0·86, 0·99) | 0·04 | 97 | 1·04 | 0·94 (0·85, 1·04) | 0·24 | 61 | 0·70 | 0·89 (0·80, 1·00) | 0·06 |
| Cardiovascular | 459 | 2·54 | 0·94 (0·90, 0·98) | 0·005 | 330 | 3·54 | 0·94 (0·89, 0·99) | 0·02 | 129 | 1·48 | 0·93 (0·86, 1·02) | 0·13 |
| Neurological | 327 | 1·81 | 0·88 (0·84, 0·93) | <0·001 | 228 | 2·45 | 0·89 (0·84, 0·95) | <0·001 | 99 | 1·12 | 0·87 (0·79, 0·94) | 0·001 |
| Cancer | 814 | 4·51 | 0·96 (0·92, 0·99) | 0·02 | 448 | 4·81 | 0·96 (0·92, 1·01) | 0·11 | 366 | 4·19 | 0·95 (0·90, 1·00) | 0·07 |
| External causes | 5,805 | 32·13 | 0·98 (0·97, 0·99) | 0·002 | 4,538 | 48·67 | 0·98 (0·97, 0·99) | 0·01 | 1,267 | 14·49 | 0·97 (0·94, 1·00) | 0·07 |
| Other or unknown causes | 1,201 | 6·65 | 0·96 (0·93, 0·99) | 0·01 | 857 | 9·20 | 0·97 (0·94, 1·01) | 0·13 | 344 | 3·94 | 0·94 (0·89, 0·99) | 0·01 |
| Attained ages 30–45 years | ||||||||||||
| Congenital anomalies | 70 | 0·78 | 0·86 (0·78, 0·95) | 0·003 | 36 | 0·77 | 0·89 (0·77, 1·02) | 0·10 | 34 | 0·78 | 0·84 (0·73, 0·96) | 0·01 |
| Other perinatal conditions | 3 | 0·03 | 0·57 (0·47, 0·70) | <0·001 | 2 | 0·04 | 0·61 (0·47, 0·79) | <0·001 | 1 | 0·02 | 0·57 (0·37, 0·88) | 0·01 |
| Respiratory | 91 | 1·01 | 0·87 (0·80, 0·95) | 0·002 | 59 | 1·27 | 0·94 (0·83, 1·06) | 0·30 | 32 | 0·74 | 0·79 (0·70, 0·89) | <0·001 |
| Endocrine | 113 | 1·25 | 0·85 (0·79, 0·92) | <0·001 | 80 | 1·72 | 0·84 (0·77, 0·92) | <0·001 | 33 | 0·76 | 0·88 (0·75, 1·02) | 0·09 |
| Cardiovascular | 369 | 4·09 | 0·97 (0·92, 1·03) | 0·33 | 261 | 5·60 | 0·99 (0·93, 1·06) | 0·77 | 108 | 2·48 | 0·94 (0·85, 1·03) | 0·18 |
| Neurological | 154 | 1·71 | 1·02 (0·93, 1·12) | 0·63 | 90 | 1·93 | 1·05 (0·93, 1·18) | 0·43 | 64 | 1·47 | 0·98 (0·86, 1·13) | 0·82 |
| Cancer | 999 | 11·08 | 0·99 (0·95, 1·02) | 0·43 | 443 | 9·50 | 1·01 (0·96, 1·06) | 0·81 | 556 | 12·78 | 0·97 (0·93, 1·01) | 0·19 |
| External causes | 2,348 | 26·04 | 0·99 (0·97, 1·01) | 0·29 | 1,803 | 38·66 | 1·00 (0·98, 1·03) | 0·91 | 545 | 12·52 | 0·95 (0·91, 0·99) | 0·01 |
| Other or unknown causes | 1,443 | 16·01 | 0·96 (0·93, 0·98) | 0·002 | 978 | 20·97 | 0·96 (0·93, 0·99) | 0·02 | 465 | 10·68 | 0·95 (0·90, 0·99) | 0·03 |
Mortality rate per 100,000 person-years.
Adjusted for birth year, sex, birth order, maternal age, maternal education, and maternal smoking.
Sensitivity Analyses
In the main analyses (reported in Table 2), exclusion of deaths due to congenital anomalies or other perinatal conditions resulted in modest attenuation of the inverse association between gestational age at birth and mortality at ages 0–45 years (adjusted HR per additional week of gestation, 0·86; 95% CI, 0·85–0·86; P<0·001) and no change at ages 30–45 years (0·97; 95% CI, 0·96–0·98; P<0·001). Adjusted HRs comparing preterm to full-term births also remained highly significant (e.g., 2·60; 95% CI, 2·51–2·70; P<0·001 at ages 0–45 years; 1·26; 95% CI, 1·12–1·41; P<0·001 at ages 30–45 years).
Exclusion of deaths due to external causes resulted in slightly stronger associations at all ages. Adjusted HRs per additional week of gestation were 0·74 (95% CI, 0·74–0·74; P<0·001) at ages 0–45 years and 0·96 (95% CI, 0·94–0·97; P<0·001) at ages 30–45 years; and comparing preterm to full-term births were 7·69 (95% CI, 7·46–7·92; P<0·001) at ages 0–45 years and 1·34 (95% CI, 1·15–1·55; P<0·001) at ages 30–45 years.
DISCUSSION
In this large national cohort study, low gestational age at birth was associated with increased mortality from infancy into mid-adulthood. This association appeared independent of sociodemographic factors, fetal growth, as well as shared genetic and environmental factors in families that were controlled for using co-sibling analyses. Late preterm (34–36 weeks) and early term (37–38 weeks) births, which together composed >20% of all births, also were associated with significantly increased mortality in adulthood relative to full-term births.
To our knowledge, this is the first study to examine gestational age at birth in relation to mortality into mid-adulthood. In a smaller cohort, we previously found that preterm and early term births were associated with increased mortality in early childhood (up to age 5 years) and young adulthood (18–36 years), but in contrast to the present study, not in late childhood or adolescence (6–17 years).3,4 This discrepancy in age-specific findings may have been due to insufficient statistical power in certain age intervals in the prior cohort. The present study extends those findings in a cohort that is over 6 times larger, with nearly a decade of additional follow-up, and using co-sibling analyses to explore for potential confounding effects of shared familial factors. Another Swedish study with a slightly smaller, overlapping cohort also reported associations between preterm birth and increased mortality up to age 36 years, as well as increased risks of psychiatric disorders.9 An Australian cohort study of 722,399 births reported that low gestational ages were associated with non-significantly increased mortality at ages 6–30 years, but did not report mortality separately in adulthood.10 Because the earliest preterm survivors due to treatment advances in the 1970s-80s have now reached their mid-40s, the present study includes the longest follow-up currently possible in a large population-based cohort. We found that low gestational age at birth was linked with increased mortality at all attained ages up to 45 years, independent of both measured and unmeasured (familial) confounders. These associations affected both males and females, but accounted for significantly more deaths among males.
We found multiple underlying causes, including congenital anomalies; respiratory, endocrine, cardiovascular, and neurological diseases; cancer; and external causes. Although deaths from congenital anomalies and other perinatal conditions predominated in infancy and childhood, increased mortality from respiratory and endocrine disorders persisted into mid-adulthood. These findings are consistent with associations we previously reported between low gestational age at birth and various morbidities in a smaller cohort, including asthma,11 diabetes,12 thyroid disorders,13 hypertension,14 venous thromboembolism,15 infections,16 epilepsy,17 and psychiatric disorders.18 Other studies also have reported associations between preterm birth and increased risks of asthma and other pulmonary conditions,19 type 2 diabetes,20,21 hypertension,22 metabolic syndrome,23 and neurocognitive and psychiatric disorders24 in adulthood. In the present study, gestational age at birth was inversely associated with mortality from cardiovascular disease or cancer earlier in life but not at ages 30–45 years. Additional follow-up will be needed to assess these outcomes at older ages in this or other large cohorts when such data become available.
Our findings provide further evidence for early-life origins of chronic disease. According to the developmental origins theory, alterations in the intrauterine and early postnatal environment may permanently alter organ structure and metabolism, resulting in early-life programming for chronic disease later in life.25 Developmental programming on the background of preterm birth may be particularly important. Preterm birth abruptly interrupts intrauterine growth and maturation of all fetal organs, with differential effects depending on the specific gestational age and critical growth periods for different organ systems, potentially leading to various chronic disorders and disabilities.2 For example, preterm birth interrupts pulmonary alveolar development which primarily occurs during the third trimester, resulting in morphologically immature lungs, reduced lung function, and increased respiratory symptoms that may persist into adulthood.19 Prematurity may predispose to diabetes mellitus through multiple factors, including impaired function of pancreatic β cells which are formed predominantly in the third trimester, exposure to antenatal corticosteroids, and rapid catch-up growth in infancy which may contribute to visceral adiposity and insulin resistance.20,21,23 Iatrogenic factors from intensive care, including suboptimal nutrition and adverse effects of medications or procedures, may further contribute to adverse long-term outcomes.26
The prevalence of preterm birth is approximately 11% worldwide1 and 10% in the US,27 more than twice that in this Swedish cohort. Most adults who were born preterm remain healthy and report a high level of function and quality of life.2,26,28 However, our findings demonstrate that increased long-term risks of various chronic disorders and mortality may also be expected. Preterm birth should be recognized as a chronic condition that requires long-term follow-up for prevention, screening, and treatment of potential health sequelae across the life course.26,29,30 Physicians currently seldom seek birth histories from adult patients.29,30 Medical records and initial history taking should routinely include gestational age at birth and other perinatal history to provide essential early-life context for understanding patients’ health.29,30 Such information can help trigger preventive actions and anticipatory screening to reduce the risks of cardiometabolic and other chronic disorders among preterm-born persons of all ages.26,29,30
A major strength of the present study was the ability to examine gestational age at birth in relation to mortality in a large national cohort with follow-up into mid-adulthood, using birth and death registry data that are nearly 100% complete. This study design minimizes potential selection or ascertainment biases. The results were controlled for potential confounders, both measured and unmeasured using co-sibling analyses.
Limitations included a lack of information on different types of preterm birth (e.g., spontaneous or medically indicated), which was not systematically collected for earlier birth years and hence for individuals with sufficient follow-up into adulthood. Studies with more complete information on types of preterm birth are needed to further elucidate mechanisms and improve risk stratification for long-term outcomes. More detailed information on lifestyle risk factors (e.g., smoking, poor diet, obesity) or socioeconomic factors later in life would be useful to assess their potential modifying effects on mortality among persons born prematurely. Because of ongoing changes in neonatal and pediatric care, it is unclear to what extent our findings will be generalizable to later cohorts. Studies of later birth cohorts as well as additional follow-up of existing cohorts to older ages will be needed in the future when such data become available. Lastly, the present study was limited to Sweden. Studies in other geographic areas, including low- and middle-income countries, are needed to assess long-term outcomes of preterm birth in other diverse populations.
In summary, we found that gestational age at birth is inversely associated with mortality from infancy to age 45 years in a large population-based cohort. Although mortality was highest for the earliest gestational ages, even late preterm and early term births were associated with significantly increased mortality into mid-adulthood. Preterm and early term birth should be recognized as chronic conditions that require long-term follow-up to facilitate prevention, timely detection, and treatment of adverse health sequelae in adulthood.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Advances in the treatment of preterm birth approximately 40 years ago have enabled a generation of preterm survivors to now reach mid-adulthood. Understanding their health sequelae is essential for guiding their long-term care. We did a PubMed search without date or language restrictions for studies in human beings from 1/1/1985 to 12/31/2018 using the following terms: (“preterm birth” or “gestational age”) and “mortality” and “adulthood” (or related alternatives). Preterm birth has previously been linked with increased mortality in infancy and early childhood, as well as young adulthood (ages 18–36 years). However, no studies to date have examined longer-term mortality into mid-adulthood. Clinicians will increasingly encounter adults of all ages who were born preterm, and will need to understand the long-term health sequelae to enable better prevention, detection, and treatment across the life course.
Added value of this study
This is the first study to examine gestational age at birth in relation to mortality into mid-adulthood. In a national cohort of over 4 million people, preterm birth was associated with increased mortality at all attained ages up to 45 years, the longest follow-up currently possible. These associations affected both males and females, and were independent of other sociodemographic factors, fetal growth, as well as shared genetic and environmental factors within families that were controlled for using co-sibling analyses. Late preterm (34–36 weeks) and early term (37–38 weeks) births also were linked with significantly increased mortality in adulthood relative to full-term births (39–41 weeks). Multiple underlying causes were identified, including congenital anomalies; respiratory, endocrine, cardiovascular, and neurological diseases; cancer; and external causes.
Implications of all the available evidence
Preterm birth should be recognized as a chronic condition that requires long-term follow-up for prevention, screening, and treatment of potential health sequelae into mid-adulthood. Medical records and history taking should routinely include gestational age at birth and other perinatal history to provide essential early-life context for understanding patients’ health. Such information can help trigger preventive actions and anticipatory screening to reduce the risks of cardiometabolic and other chronic disorders among preterm-born persons across the life course.
ACKNOWLEDGMENTS
Funding:
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL139536); the Swedish Research Council; the Swedish Heart-Lung Foundation; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the writing of the manuscript or the decision to submit it for publication.
Footnotes
Conflicts of Interest:
We declare that we have no conflicts of interest.
Contributor Information
Casey Crump, Departments of Family Medicine and Community Health and of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Place, Box 1077, New York, NY 10029, USA
Jan Sundquist, Lund University, Center for Primary Health Care Research, Clinical Research Centre (CRC), building 28, floor 11, Jan Waldenströms gata 35, Skåne University Hospital, SE-205 02 Malmö, Sweden
Marilyn A. Winkleby, Stanford University, Stanford Prevention Research Center, Medical School Office Building, 251 Campus Drive, Room X318, Stanford, California 94305, USA
Kristina Sundquist, Lund University, Center for Primary Health Care Research, Clinical Research Centre (CRC), building 28, floor 11, Jan Waldenströms gata 35, Skåne University Hospital, SE-205 02 Malmö, Sweden
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379(9832): 2162–72. [DOI] [PubMed] [Google Scholar]
- 2.Raju TNK, Pemberton VL, Saigal S, et al. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health. J Pediatr 2017; 181: 309–18 e1. [DOI] [PubMed] [Google Scholar]
- 3.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA 2011; 306(11): 1233–40. [DOI] [PubMed] [Google Scholar]
- 4.Crump C, Sundquist K, Winkleby MA, Sundquist J. Early-term birth (37–38 weeks) and mortality in young adulthood. Epidemiology 2013; 24(2): 270–6. [DOI] [PubMed] [Google Scholar]
- 5.Manley BJ, Doyle LW, Davies MW, Davis PG. Fifty years in neonatology. J Paediatr Child Health 2015; 51(1): 118–21. [DOI] [PubMed] [Google Scholar]
- 6.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 7.Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996; 85(7): 843–8. [DOI] [PubMed] [Google Scholar]
- 8.VanderWeele TJ. Causal interactions in the proportional hazards model. Epidemiology 2011; 22(5): 713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry 2013; 70(11): 1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasjois R, Nembhard W, Wong K, Bourke J, Pereira G, Leonard H. Risk of Mortality into Adulthood According to Gestational Age at Birth. J Pediatr 2017; 190: 185–91 e1. [DOI] [PubMed] [Google Scholar]
- 11.Crump C, Winkleby MA, Sundquist J, Sundquist K. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics 2011; 127(4): e913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of diabetes among young adults born preterm in Sweden. Diabetes Care 2011; 34(5): 1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump C, Winkleby MA, Sundquist J, Sundquist K. Preterm birth and risk of medically treated hypothyroidism in young adulthood. Clin Endocrinol (Oxf) 2011; 75(2): 255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of hypertension among young adults who were born preterm: a Swedish national study of 636,000 births. Am J Epidemiol 2011; 173(7): 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoller B, Li X, Sundquist J, Sundquist K, Crump C. Gestational age and risk of venous thromboembolism from birth through young adulthood. Pediatrics 2014; 134(2): e473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crump C, Sundquist K, Sundquist J. Prematurity and mortality in childhood and early adulthood (reply). JAMA 2012; 307: 32–3. [DOI] [PubMed] [Google Scholar]
- 17.Crump C, Sundquist K, Winkleby MA, Sundquist J. Preterm birth and risk of epilepsy in Swedish adults. Neurology 2011; 77(14): 1376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crump C, Winkleby MA, Sundquist K, Sundquist J. Preterm birth and psychiatric medication prescription in young adulthood: a Swedish national cohort study. Int J Epidemiol 2010; 39(6): 1522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolton CE, Bush A, Hurst JR, Kotecha S, McGarvey L. Lung consequences in adults born prematurely. Thorax 2015; 70(6): 574–80. [DOI] [PubMed] [Google Scholar]
- 20.Kajantie E, Strang-Karlsson S, Hovi P, et al. Insulin sensitivity and secretory response in adults born preterm: the Helsinki Study of Very Low Birth Weight Adults. J Clin Endocrinol Metab 2015; 100(1): 244–50. [DOI] [PubMed] [Google Scholar]
- 21.Hofman PL, Regan F, Jackson WE, et al. Premature birth and later insulin resistance. N Engl J Med 2004; 351(21): 2179–86. [DOI] [PubMed] [Google Scholar]
- 22.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012; 59(2): 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 2013; 131(4): e1240–63. [DOI] [PubMed] [Google Scholar]
- 24.Nosarti C, Reichenberg A, Murray RM, et al. Preterm birth and psychiatric disorders in young adult life. Archives of general psychiatry 2012; 69(6): E1–8. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998; 95(2): 115–28. [PubMed] [Google Scholar]
- 26.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr 2017; 106(9): 1409–37. [DOI] [PubMed] [Google Scholar]
- 27.March of Dimes. PeriStats. http://www.marchofdimes.com/Peristats/ (accessed August 1, 2018.
- 28.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008; 371(9608): 261–9. [DOI] [PubMed] [Google Scholar]
- 29.Crump C Medical history taking in adults should include questions about preterm birth. BMJ 2014; 349: g4860. [DOI] [PubMed] [Google Scholar]
- 30.Crump C Birth history is forever: implications for family medicine. J Am Board Fam Med 2015; 28(1): 121–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


