Abstract
Objective:
Low physical fitness and obesity have been associated with higher risk of developing heart failure (HF), but their interactive effects are unknown. Elucidation of interactions among these common, modifiable factors may help facilitate more effective primary prevention.
Methods:
We conducted a national cohort study to examine interactive effects of aerobic fitness, muscular strength, and body mass index (BMI) among 1,330,610 military conscripts in Sweden during 1969–1997 (97–98% of all 18-year-old males) on risk of HF identified from inpatient and outpatient diagnoses through 2012 (maximum age 62 years).
Results:
There were 11,711 men diagnosed with HF in 37.8 million person-years of follow-up. Low aerobic fitness, low muscular strength, and obesity were independently associated with higher risk of HF, after adjusting for each other, socioeconomic factors, other chronic diseases, and family history of HF. The combination of low aerobic fitness and low muscular strength (lowest vs. highest tertiles) was associated with a 1.7-fold risk of HF (95% CI, 1.6–1.9; P<0.001; incidence rates per 100,000 person-years, 43.2 vs. 10.8). These factors had positive additive and multiplicative interactions (P<0.001), and were associated with increased risk of HF even among men with normal BMI.
Conclusions:
Low aerobic fitness, low muscular strength, and obesity at age 18 were independently associated with higher risk of HF in adulthood, with interactive effects between aerobic fitness and muscular strength. These findings suggest that early-life interventions may help reduce the long-term risk of HF and should include both aerobic fitness and muscular strength, even among persons with normal BMI.
Keywords: body mass index, heart failure, muscle strength, obesity, physical fitness
INTRODUCTION
Heart failure (HF) currently affects more than 5 million US adults and is a leading cause of hospitalization and mortality.[1, 2] Because of aging of the population and improved survival among cardiovascular patients, the prevalence of HF is projected to increase nearly 50% by 2030.[2] Common predisposing conditions for HF include hypertension, ischemic heart disease, valvular heart disease, and diabetes mellitus.[3] A growing body of research has sought to identify other risk factors early in life, which may help facilitate earlier and more effective primary prevention.
Prior studies have reported that obesity,[3, 4, 5, 6, 7, 8, 9] low aerobic fitness,[10, 11, 12, 13] and low muscular strength[13] are associated with higher risk of developing HF. However, most studies have examined these factors in mid-adulthood and not earlier in life. In addition, their potential interactive effects on the long-term risk of HF are unclear. It is unknown, for example, whether early-life physical fitness is protective against HF among non-obese as well as obese persons, or whether there are synergistic effects between different types of physical fitness (aerobic fitness and muscular strength). We previously reported associations between these exposures and hypertension,[14] type 2 diabetes,[15] ischemic heart disease,[16] stroke,[17] and mortality.[18] However, to our knowledge, no studies have examined independent effects of these exposures and potential additive or multiplicative interactive effects in relation to long-term risk of HF. A better understanding of these effects may provide further insights into etiologic pathways for HF and help inform patient counseling for susceptible subgroups.
To address these knowledge gaps, we conducted a large national cohort study to examine the interactive effects of aerobic fitness, muscular strength, and body mass index (BMI) on the long-term risk of HF. These exposures were assessed using standardized protocols in 18-year-old male military conscripts in Sweden who were then followed up for HF in adulthood. Our aims were to examine interactive effects of these factors on the development of HF in a large population-based cohort, which may help inform better primary prevention.
METHODS
Study Population
The study population consisted of all 1,547,478 males (age ~18 years) who underwent a military conscription examination in Sweden during 1969–1997.[14, 15, 16, 17, 18] All ~18-year-old males nationally were conscripted each year except for 2–3% who either were incarcerated or had severe chronic medical conditions or disabilities documented by a physician. In the present study, the primary analyses included all individuals with complete data (N=1,330,610; 86.0%), and sensitivity analyses were performed to assess the influence of missing data (as described below). This study was approved by the Regional Ethics Committee of Lund University in Sweden (No. 2010/476). Participant consent was not required as this study used only registry-based secondary data. To ensure confidentiality, all names and national identification numbers were replaced by anonymous serial numbers in adherence to the Personal Data Act (1998:204) and the Act (1995:606) and Ordinance (1995:1060) on Certain Personal Registers.
Aerobic Fitness, Muscular Strength, and BMI Ascertainment
Aerobic fitness, muscular strength, and BMI measurements were obtained using the Swedish Military Conscription Registry, which contains information from a 2-day standardized physical and psychological examination required for all conscripts starting in 1969.[14, 15, 16, 17, 18] Aerobic fitness was measured as the maximal aerobic workload in Watts, using a well-validated electrically-braked stationary bicycle ergometer test.[19] Maximal aerobic workload is highly correlated with maximal oxygen uptake (VO2 max; correlation ~0.9),[20] and its measurement using this ergometer test is highly reproducible, with a test-retest correlation of 0.95.[21] Muscular strength was calculated as the weighted sum of maximal knee extension (weighted × 1.3), elbow flexion (weighted × 0.8), and hand grip (weighted × 1.7), each measured in Newtons using well-validated isometric dynamometer tests.[22] Details of these protocols have been described previously.[14, 15, 16, 17, 18, 19, 20, 21, 22] In the present study, aerobic fitness and muscular strength were examined alternatively as continuous variables or categorical variables in tertiles (aerobic fitness in Watts: low [<240], medium [240–288], high [≥289]; muscular strength in Newtons: low [<1900], medium [1900–2170], high [≥2171]).
Height and weight were measured using standard protocols and examined alternatively as continuous variables or categorical variables in tertiles (height in cm: low [<175.0], medium [175.0–181.4], high [≥181.5]; weight in kg: low [<64.0], medium [64.0–71.3], high [≥71.4]).[14, 15, 16, 17, 18] Body mass index (BMI) also was examined as an alternative to height and weight. BMI was calculated as weight in kilograms divided by the square of height in meters, and examined alternatively as a continuous or categorical variable using Centers for Disease Control and Prevention (CDC) definitions for children and adolescents aged 2 to 19 years to facilitate comparability with US studies: “overweight” is defined as ≥85th and <95th percentile and “obesity” as ≥95th percentile on the CDC’s 2000 sex-specific BMI-for-age growth charts, which correspond to BMI ≥25.6 and <29.0 and BMI ≥29.0, respectively, for 18-year-old males.[23]
Heart Failure Ascertainment
The study cohort was followed up for HF from the date of the military conscription examination through December 31, 2012. HF was identified based on at least 1 inpatient diagnosis or at least 2 outpatient diagnoses using International Classification of Diseases (ICD) codes in the Swedish Hospital and Outpatient Registries (ICD-8: 427.0–427.1; ICD-9: 402.01, 402.11, 402.91, 428; ICD-10: I11.0, I13.0, I13.2, I50). The Swedish Hospital Registry contains all primary and secondary hospital discharge diagnoses from six populous counties in southern Sweden starting in 1964, and with nationwide coverage starting in 1987; and the Swedish Outpatient Registry contains outpatient diagnoses from all specialty clinics nationwide starting in 2001.[14, 15, 16, 17, 18]
Adjustment Variables
Other variables that may be associated with physical fitness or BMI and risk of HF were obtained from the Swedish Military Conscription Registry and national census data, which were linked using an anonymous personal identification number.[14, 15, 16, 17, 18] The following were used as adjustment variables: year of the military conscription examination (as a continuous variable); highest education level attained during the study period (<12, 12–14, ≥15 years); neighborhood socioeconomic status (SES, included because it has been associated with obesity and physical fitness[24] and with cardiovascular risk factors[25]; composed of an index that includes low education level, low income, unemployment, and social welfare receipt, as previously described,[26] and categorized as low [<−1 SD from the mean], medium [−1 to 1 SD], or high [>1 SD]); and family history of HF in a parent or sibling (yes or no, identified from the Swedish Hospital Registry during 1965–2012 and the Swedish Outpatient Registry during 2001–2012, using the same diagnosis codes noted above). In addition, we adjusted for the following chronic diseases that are known risk factors for HF as time-dependent variables: hypertension (ICD-8: 400–401; ICD-9: 401; ICD-10: I10), ischemic heart disease (ICD-8/9: 410–414; ICD-10: I20-I25), valvular heart disease (ICD-8/9: 394–397, 424; ICD-10: I05-I08, I34-I39), and diabetes mellitus (ICD-8/9: 250; ICD-10: E10-E14).
Statistical Analysis
Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between aerobic fitness, muscular strength, height, weight, or BMI and risk of HF.[14, 15, 16, 17, 18] The Cox model time scale was elapsed time since the military conscription examination, which corresponds to attained age because baseline age was the same (18 years) for all conscripts. Individuals were censored at emigration (n=86,797; 6.5%) or death (n=44,183; 3.3%).
Three adjusted models were performed: the first was adjusted for attained age (as the time scale) and year of the military conscription examination; the second additionally included height, weight, aerobic fitness, muscular strength, education level, neighborhood SES, and family history of HF; and the third was further adjusted for hypertension, ischemic heart disease, valvular heart disease, and diabetes mellitus (as defined above). The proportional hazards assumption was evaluated by graphical assessment of log-log plots, which showed a good fit in all models.
Interactions among aerobic fitness, muscular strength, and/or BMI were examined on both the additive and multiplicative scales in relation to the risk of HF.[14, 15, 16, 17, 18] Additive interactions were assessed using the “relative excess risk due to interaction” (RERI), which is computed for binary variables as: RERIHR = HR11 − HR10 – HR01 + 1.[27] Multiplicative interactions were assessed using the ratio of HRs: HR11 / (HR10 × HR01).[27]
Several sensitivity analyses were performed. First, we assessed the sensitivity of results to missing data by repeating all analyses using the entire dataset after multiple imputation of missing data for each variable based on its relationship with all other covariates and HF.[28] Second, we assessed the influence of other chronic diseases by restricting to men who were never diagnosed with hypertension, ischemic heart disease, valvular heart disease, or diabetes mellitus. Third, we assessed different starting points for the follow-up period, alternatively starting in 1987 (at which time inpatient diagnoses were available nationwide instead of only for the most populous counties) or in 2001 (at which time both inpatient and outpatient diagnoses were available nationwide). Fourth, we performed a competing risks analysis in which we accounted for competing risks due to death from other (non-HF) causes. All statistical tests were 2-sided and used an α-level of 0.05. All analyses were conducted using Stata version 14.1.
RESULTS
Among 1,330,610 males in the study cohort, 11,711 (0.9%) were diagnosed with HF in 37.8 million person-years of follow-up (mean follow-up, 28.2 years). The median age at the end of follow-up was 47.2 years (mean 47.4, SD 7.9, range 19.0 to 62.0). The median age at diagnosis with HF was 49.1 years (mean 48.0, SD 7.4, range 19.1 to 62.0). Subject characteristics and HF incidence rates are shown in Table 1 stratified by aerobic fitness tertile, and in Supplementary Table 1 stratified by muscular strength tertile.
Table 1.
Characteristics of men who were or were not subsequently diagnosed with heart failure, stratified by aerobic fitness tertile.
| High aerobic fitness (≥289 Watts) |
Medium aerobic fitness (240–288 Watts) |
Low aerobic fitness (<240 Watts) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No HF | HF | Ratea | No HF | HF | Ratea | No HF | HF | Ratea | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Overall | 466,223 (100.0) | 1,178 (100.0) | 9.7 | 461,255 (100.0) | 3,440 (100.0) | 22.8 | 391,421 (100.0) | 7,093 (100.0) | 42.3 |
| Muscular strength, tertiles | |||||||||
| High (≥2171 Newtons) | 237,772 (51.0) | 668 (56.7) | 10.8 | 165,349 (35.9) | 1,464 (42.6) | 26.8 | 78,986 (20.2) | 1,039 (14.7) | 36.7 |
| Medium (1900–2170 Newtons) | 133,619 (28.7) | 320 (27.2) | 9.3 | 164,394 (35.6) | 1,171 (34.0) | 22.2 | 128,975 (32.9) | 3,011 (42.4) | 43.7 |
| Low (<1900 Newtons) | 94,832 (30.3) | 190 (16.1) | 7.4 | 131,512 (28.5) | 805 (23.4) | 18.4 | 183,460 (46.9) | 3,043 (42.9) | 43.2 |
| Height, tertiles | |||||||||
| High (≥181.5 cm) | 208,180 (44.7) | 550 (46.7) | 10.2 | 157,832 (34.2) | 1,246 (36.2) | 24.1 | 95,014 (24.3) | 1,186 (16.7) | 34.7 |
| Medium (175.0–181.4 cm) | 163,833 (35.1) | 405 (34.4) | 9.4 | 166,952 (36.2) | 1,175 (34.2) | 21.7 | 133,335 (34.1) | 1,565 (22.1) | 32.8 |
| Low (<175.0 cm) | 94,210 (20.2) | 223 (18.9) | 8.9 | 136,471 (29.6) | 1,019 (29.6) | 22.6 | 163,072 (41.7) | 4,342 (61.2) | 50.8 |
| Weight, tertiles | |||||||||
| High (≥71.4 kg) | 249,624 (53.5) | 842 (71.5) | 13.0 | 160,041 (34.7) | 1,853 (53.9) | 34.9 | 71,090 (18.2) | 1,380 (19.5) | 54.9 |
| Medium (64.0–71.3 kg) | 162,633 (34.9) | 269 (22.8) | 6.1 | 179,966 (39.0) | 1,074 (31.2) | 18.1 | 119,438 (30.5) | 1,363 (19.2) | 31.8 |
| Low (<64.0 kg) | 53,966 (11.6) | 67 (5.7) | 4.9 | 121,248 (26.3) | 513 (14.9) | 13.2 | 200,893 (51.3) | 4,350 (61.3) | 43.6 |
| Body mass index | |||||||||
| Normal | 416,785 (89.4) | 848 (72.0) | 7.8 | 424,175 (92.0) | 2,698 (78.4) | 19.4 | 371,382 (94.9) | 6,516 (91.9) | 40.5 |
| Overweight | 36,133 (7.7) | 193 (16.4) | 20.3 | 26,457 (5.7) | 443 (12.9) | 52.3 | 13,639 (3.5) | 342 (4.8) | 72.8 |
| Obese | 13,305 (2.9) | 137 (11.6) | 38.4 | 10,623 (2.3) | 299 (8.7) | 90.4 | 6,400 (1.6) | 235 (3.3) | 107.9 |
| Education, years | |||||||||
| <12 | 27,569 (5.9) | 157 (13.3) | 19.6 | 62,746 (13.6) | 840 (24.4) | 38.3 | 91,955 (23.5) | 2,510 (35.4) | 59.9 |
| 12–14 | 197,447 (42.4) | 589 (50.0) | 11.5 | 213,585 (46.3) | 1,628 (47.3) | 24.2 | 179,141 (45.8) | 2,962 (41.8) | 41.1 |
| ≥15 | 241,207 (51.7) | 432 (36.7) | 6.9 | 184,924 (40.1) | 972 (28.3) | 15.7 | 120,325 (30.7) | 1,621 (22.8) | 30.2 |
| Neighborhood SES | |||||||||
| High | 59,202 (12.7) | 175 (14.9) | 11.5 | 80,122 (17.4) | 708 (20.6) | 27.7 | 85,270 (21.8) | 1,233 (17.4) | 42.7 |
| Medium | 287,198 (61.6) | 761 (64.6) | 9.9 | 291,340 (63.2) | 2,287 (66.5) | 23.1 | 245,402 (62.7) | 5,233 (73.8) | 44.0 |
| Low | 119,823 (25.7) | 242 (20.5) | 8.1 | 89,793 (19.5) | 445 (12.9) | 16.6 | 60,749 (15.5) | 627 (8.8) | 31.4 |
| Hypertension | |||||||||
| No | 455,629 (97.7) | 687 (58.3) | 5.8 | 434,768 (94.3) | 1,693 (49.2) | 12.1 | 357,764 (91.4) | 3,609 (50.9) | 24.1 |
| Yes | 10,594 (2.3) | 491 (41.7) | 141.2 | 26,487 (5.7) | 1,747 (50.8) | 161.4 | 33,657 (8.6) | 3,484 (49.1) | 196.0 |
| Ischemic heart disease | |||||||||
| No | 463,540 (99.4) | 874 (74.2) | 7.3 | 452,155 (98.0) | 2,133 (62.0) | 14.5 | 377,714 (96.5) | 3,709 (52.3) | 23.3 |
| Yes | 2,683 (0.6) | 304 (25.8) | 309.5 | 9,100 (2.0) | 1,307 (38.0) | 321.0 | 13,707 (3.5) | 3,384 (47.7) | 412.3 |
| Valvular heart disease | |||||||||
| No | 464,949 (99.7) | 998 (84.7) | 8.2 | 459,096 (99.5) | 2,969 (86.3) | 19.8 | 388,876 (99.3) | 9,965 (85.1) | 36.1 |
| Yes | 1,274 (0.3) | 180 (15.3) | 427.6 | 2,159 (0.5) | 471 (13.7) | 492.1 | 2,545 (0.7) | 1,746 (14.9) | 676.0 |
| Diabetes mellitus | |||||||||
| No | 460,526 (98.8) | 963 (81.7) | 8.0 | 448,875 (97.3) | 2,610 (75.9) | 17.9 | 374,757 (95.7) | 4,982 (70.2) | 31.6 |
| Yes | 5,697 (1.2) | 215 (18.3) | 118.7 | 12,380 (2.7) | 830 (24.1) | 167.2 | 16,664 (4.3) | 2,111 (29.8) | 210.9 |
| Family history of HF | |||||||||
| No | 407,702 (87.4) | 816 (69.3) | 7.8 | 359,313 (77.9) | 2,064 (60.0) | 18.1 | 279,914 (71.5) | 4,748 (66.9) | 38.9 |
| Yes | 58,521 (12.6) | 362 (30.7) | 20.9 | 101,942 (22.1) | 3,376 (40.0) | 37.1 | 111,507 (28.5) | 2,345 (33.1) | 51.1 |
HF incidence rate per 100,000 person-years.
HF = heart failure, SES = socioeconomic status.
Main Exposure Effects
Low aerobic fitness at age 18 was independently associated with higher risk of HF in adulthood, after adjusting for muscular strength, height, weight, and other factors (Table 2, lowest vs. highest tertile, fully adjusted HR: 1.45; 95% CI, 1.34–1.56; P<0.001). Low muscular strength also was associated with a modestly increased risk of HF (lowest vs. highest tertile, adjusted HR: 1.17; 95% CI, 1.11–1.24; P<0.001). When examined as continuous variables, both aerobic fitness and muscular strength had highly significant inverse linear trends across their full distribution in relation to HF risk (trend tests, P<0.001; Table 2), although aerobic fitness was the stronger risk factor (Pheterogeneity<0.001).
Table 2.
Associations between aerobic fitness, muscular strength, or other factors and risk of heart failure.
| Model 1a | Model 2b | Model 3c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Aerobic fitness, tertiles | |||||||||
| High (≥289 Watts) | 1.00 | 1.00 | 1.00 | ||||||
| Medium (240–288 Watts) | 1.41 | 1.32, 1.52 | <0.001 | 1.27 | 1.19, 1.37 | <0.001 | 1.20 | 1.11, 1.29 | <0.001 |
| Low (<240 Watts) | 2.18 | 2.03, 2.33 | <0.001 | 1.82 | 1.69, 1.96 | <0.001 | 1.45 | 1.34, 1.56 | <0.001 |
| Per 100 Watts (trend test) | 0.49 | 0.47, 0.52 | <0.001 | 0.51 | 0.48, 0.54 | <0.001 | 0.65 | 0.62, 0.69 | <0.001 |
| Muscular strength, tertiles | |||||||||
| High (≥2171 Newtons) | 1.00 | <0.001 | 1.00 | 1.00 | |||||
| Medium (1900–2170 Newtons) | 1.16 | 1.11, 1.21 | <0.001 | 1.20 | 1.14, 1.26 | <0.001 | 1.13 | 1.08, 1.19 | <0.001 |
| Low (<1900 Newtons) | 1.23 | 1.18, 1.29 | <0.001 | 1.25 | 1.18, 1.32 | <0.001 | 1.17 | 1.11, 1.24 | <0.001 |
| Per 1000 Newtons (trend test) | 0.71 | 0.67, 0.75 | <0.001 | 0.72 | 0.67, 0.77 | <0.001 | 0.80 | 0.75, 0.85 | <0.001 |
| Height, tertiles | |||||||||
| High (≥181.5 cm) | 1.00 | 1.00 | 1.00 | ||||||
| Medium (175.0–181.4 cm) | 0.99 | 0.94, 1.04 | 0.56 | 1.07 | 1.02, 1.13 | 0.009 | 0.96 | 0.91, 1.01 | 0.09 |
| Low (<175.0 cm) | 1.57 | 1.50, 1.64 | <0.001 | 1.79 | 1.70, 1.88 | <0.001 | 1.23 | 1.16, 1.29 | <0.001 |
| Per 5 cm (trend test) | 0.84 | 0.83, 0.85 | <0.001 | 0.81 | 0.81, 0.82 | <0.001 | 0.88 | 0.87, 0.90 | <0.001 |
| Weight, tertiles | |||||||||
| High (≥71.4 kg) | 1.00 | 1.00 | 1.00 | ||||||
| Medium (64.0–71.3 kg) | 0.59 | 0.57, 0.62 | <0.001 | 0.49 | 0.47, 0.52 | <0.001 | 0.64 | 0.61, 0.67 | <0.001 |
| Low (<64.0 kg) | 0.93 | 0.89, 0.97 | 0.001 | 0.46 | 0.43, 0.48 | <0.001 | 0.64 | 0.60, 0.68 | <0.001 |
| Per 5 kg (trend test) | 1.06 | 1.05, 1.07 | <0.001 | 1.24 | 1.23, 1.25 | <0.001 | 1.14 | 1.12, 1.15 | <0.001 |
| BMI | |||||||||
| Normal | 1.00 | 1.00 | 1.00 | ||||||
| Overweight | 2.07 | 1.94, 2.21 | <0.001 | 2.27 | 2.12, 2.43 | <0.001 | 1.48 | 1.38, 1.58 | <0.001 |
| Obese | 3.93 | 3.63, 4.25 | <0.001 | 4.00 | 3.69, 4.34 | <0.001 | 2.06 | 1.90, 2.24 | <0.001 |
| Per 1 BMI unit (trend test) | 1.07 | 1.07, 1.08 | <0.001 | 1.08 | 1.08, 1.09 | <0.001 | 1.06 | 1.06, 1.07 | <0.001 |
| Education, years | |||||||||
| <12 | 2.41 | 2.30, 2.53 | <0.001 | 1.84 | 1.75, 1.94 | <0.001 | 1.58 | 1.51, 1.67 | <0.001 |
| 12–14 | 1.70 | 1.63, 1.78 | <0.001 | 1.46 | 1.39, 1.53 | <0.001 | 1.30 | 1.24, 1.36 | <0.001 |
| ≥15 | 1.00 | 1.00 | 1.00 | ||||||
| Per higher category (trend test) | 0.65 | 0.63, 0.66 | <0.001 | 0.74 | 0.72, 0.76 | <0.001 | 0.80 | 0.78, 0.82 | <0.001 |
| Neighborhood SES | |||||||||
| High | 1.00 | 1.00 | 1.00 | ||||||
| Medium | 1.40 | 1.32, 1.49 | <0.001 | 1.15 | 1.08, 1.22 | <0.001 | 1.04 | 0.98, 1.10 | 0.20 |
| Low | 1.42 | 1.32, 1.52 | <0.001 | 1.13 | 1.05, 1.21 | 0.001 | 1.02 | 0.95, 1.09 | 0.67 |
| Per higher category (trend test) | 0.86 | 0.83, 0.89 | <0.001 | 0.95 | 0.92, 0.99 | 0.006 | 1.00 | 0.96, 1.03 | 0.89 |
| Family history of HF | |||||||||
| No | 1.00 | 1.00 | 1.00 | ||||||
| Yes | 1.33 | 1.28, 1.38 | <0.001 | 1.29 | 1.24, 1.34 | <0.001 | 1.19 | 1.15, 1.24 | <0.001 |
| Hypertension | |||||||||
| No | 1.00 | 1.00 | |||||||
| Yes | 9.53 | 9.17, 9.90 | <0.001 | 2.90 | 2.77, 3.04 | <0.001 | |||
| Ischemic heart disease | |||||||||
| No | 1.00 | 1.00 | |||||||
| Yes | 19.24 | 18.51, 20.00 | <0.001 | 7.21 | 6.90, 7.55 | <0.001 | |||
| Valvular heart disease | |||||||||
| No | 1.00 | 1.00 | |||||||
| Yes | 21.41 | 20.33, 22.54 | <0.001 | 7.43 | 7.05, 7.84 | <0.001 | |||
| Diabetes mellitus | |||||||||
| No | 1.00 | 1.00 | |||||||
| Yes | 7.50 | 7.20, 7.82 | <0.001 | 2.01 | 1.91, 2.10 | <0.001 | |||
Adjusted for age and year of military conscription examination.
Adjusted for age, year of military conscription examination, aerobic fitness, muscular strength, height, weight, education, neighborhood SES, and family history of HF.
Adjusted for age, year of military conscription examination, aerobic fitness, muscular strength, height, weight, education, neighborhood SES, hypertension, ischemic heart disease, valvular heart disease, diabetes mellitus, and family history of HF.
BMI was modeled as an alternative to height and weight in a separate model. The reference category for all variables is indicated by an HR of 1.00.
BMI = body mass index, HF = heart failure, HR = hazard ratio, SES = socioeconomic status.
Height was inversely and weight was positively associated with risk of HF (Table 2). High BMI at age 18 (examined as an alternative to height and weight) was strongly associated with higher risk of HF in adulthood, including a highly significant linear trend (trend test, P<0.001; Table 2). Obesity was associated with a ~2-fold risk, and overweight with nearly a 1.5-fold risk, relative to normal BMI (Table 2). Family history of HF was associated with a modestly increased risk of developing HF. After adjusting for other factors, ischemic and valvular heart disease were each associated with >7-fold risks, hypertension with nearly a 3-fold risk, and diabetes mellitus with a 2-fold risk of HF (Table 2).
Among the adjustment variables, education level accounted for most of the difference in risk estimates in Model 2 compared to Model 1, and IHD and diabetes accounted for most of the difference in Model 3 compared to Model 2. All of the sensitivity analyses yielded similar risk estimates as the main analyses, and the overall conclusions were unchanged. For example, after restricting to men never diagnosed with hypertension, ischemic heart disease, valvular disease, or diabetes (N=1,217,678; 91.5%), adjusted HRs were 1.49 comparing lowest vs. highest tertile of aerobic fitness (95% CI, 1.31–1.69; P<0.001), 1.32 comparing lowest vs. highest tertile of muscular strength (1.19–1.46; P<0.001), and 2.50 comparing obesity vs. normal BMI (2.05–3.06; P<0.001). In the competing risks analysis, the corresponding HRs were 1.46 for aerobic fitness (95% CI, 1.28–1.66; P<0.001), 1.31 for muscular strength (1.18–1.45; P<0.001), and 2.78 for obesity (2.28–3.40; P<0.001).
Interactions Among Aerobic Fitness, Muscular Strength, and BMI
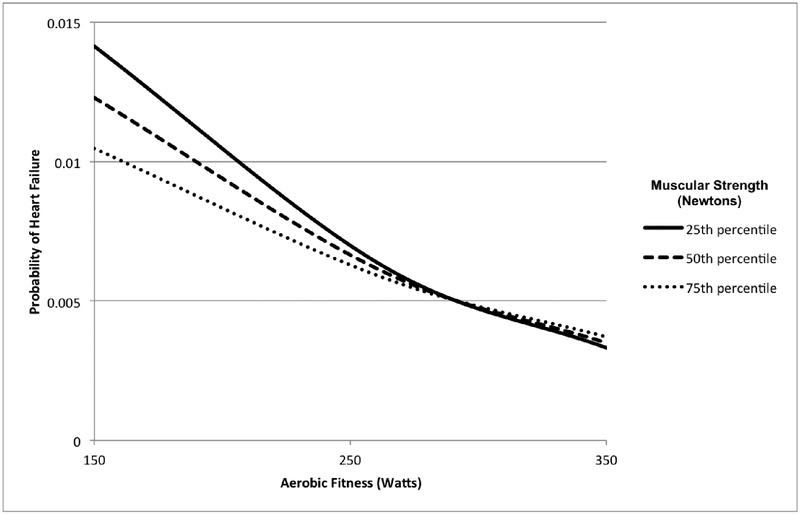
The interactive effects of aerobic fitness and muscular strength on risk of HF are shown in Table 3. The combination of low aerobic fitness and low muscular strength was associated with highest risk (lowest vs. highest tertiles, adjusted HR: 1.72; 95% CI, 1.57–1.88; P<0.001; incidence rates per 100,000 person-years, 43.2 vs. 10.8). Low aerobic fitness was associated with increased risk of HF irrespective of muscular strength level (adjusted HRs, 1.18 to 1.72; Table 3, marginal HRs in right-most column); whereas low muscular strength was associated with increased risks only among men with medium or low aerobic fitness (marginal HRs in lower part of Table 3: “HRs for low muscular strength within strata of aerobic fitness”). There was a highly significant positive interaction between low aerobic fitness and low muscular strength on both the additive and multiplicative scales (i.e., the association between either of these factors and HF varied significantly across levels of the other factor, and their combined effect exceeded the sum or product of their separate effects; P<0.001). Figure 1 shows the probability of HF among men at the 25th, 50th, and 75th percentiles of muscular strength across the full distribution of aerobic fitness, from the fully adjusted model. The non-parallel lines, particularly at the lower range of aerobic fitness, reflect a significant interaction.
Table 3.
Interactions between aerobic fitness and muscular strength in relation to risk of heart failure.a
| Muscular strength tertile | Aerobic fitness tertile | HRs for medium aerobic fitness within strata of muscular strength | HRs for low aerobic fitness within strata of muscular strength | |||||
|---|---|---|---|---|---|---|---|---|
| High (≥289 Watts) | Medium (240–288 Watts) | Low (<240 Watts) | ||||||
| Rate (no. cases)b | HR (95% CI) | Rate (no. cases)b | HR (95% CI) | Rate (no. cases)b | HR (95% CI) | |||
| High (≥2171 Newtons) | 10.8 (671) | 1.00 | 26.8 (1,465) | 1.16 (1.06, 1.26); P=0.001 | 36.7 (1,042) | 1.18 (1.07, 1.30); P=0.001 | 1.16 (1.06, 1.26); P=0.001 | 1.18 (1.07, 1.30); P=0.001 |
| Medium (1900–2170 Newtons) | 9.3 (331) | 0.99 (0.88, 1.12); P=0.91 | 22.2 (1,177) | 1.20 (1.09, 1.31); P<0.001 | 43.7 (2.973) | 1.52 (1.39, 1.66); P<0.001 | 1.21 (1.07, 1.35); P=0.004 | 1.53 (1.36, 1.70); P<0.001 |
| Low (<1900 Newtons) | 7.4 (194) | 1.00 (0.86, 1.16); P=0.99 | 18.4 (809) | 1.28 (1.16, 1.41); P<0.001 | 43.2 (3,049) | 1.72 (1.57, 1.88); P<0.001 | 1.28 (1.08, 1.47); P=0.005 | 1.72 (1.47, 1.97); P<0.001 |
| HRs (95% CI) for medium muscular strength within strata of aerobic fitness | 0.99 (0.88, 1.12); P=0.91 | 1.04 (0.96, 1.11); P=0.34 | 1.29 (1.20, 1.38); P<0.001 | |||||
| HRs (95% CI) for low muscular strength within strata of aerobic fitness | 1.00 (0.86, 1.16); P=0.99 | 1.10 (1.01, 1.20); P=0.03 | 1.45 (1.35, 1.56); P<0.001 | |||||
| Interaction on additive scale, lowest vs. highest tertiles: RERI (95% CI) | 0.54 (0.37, 0.71); P<0.001 | |||||||
| Interaction on multiplicative scale, lowest vs. highest tertiles: Ratio of HRs (95% CI) | 1.45 (1.21, 1.69); P<0.001 | |||||||
HRs are adjusted for age, year of military conscription examination, height, weight, education, neighborhood SES, hypertension, ischemic heart disease, valvular heart disease, diabetes mellitus, and family history of heart failure.
Incidence rate per 100,000 person-years, and total number of cases.
HR = hazard ratio, RERI = relative excess risk due to interaction, SES = socioeconomic status.
Figure 1.
Probability of heart failure by aerobic fitness and muscular strength at age 18 years (median attained age 47 years, maximum 62 years), adjusted for height, weight, and other covariates.
Aerobic fitness and BMI had no additive and a negative multiplicative interaction (i.e., their combined effect was less than the product of their separate effects, P=0.02; Supplementary Table 2). Muscular strength and BMI had no interactions on either the additive (P=0.74) or multiplicative (P=0.75) scale (Supplementary Table 3). Either low aerobic fitness or low muscular strength was associated with increased risk of HF among men with normal BMI (adjusted HR, lowest vs. highest tertile: aerobic fitness, 1.60; 95% CI, 1.48–1.72; P<0.001; muscular strength, 1.14; 95% CI, 1.08–1.20; P<0.001; Supplementary Tables 2 and 3). In a secondary analysis, we also found positive additive and multiplicative interactions between low height and either low aerobic fitness or low muscular strength in relation to HF risk, after adjusting for weight and other covariates (lowest vs. highest tertiles, P<0.01 for each interaction).
DISCUSSION
In this large population-based cohort study, we found that low aerobic fitness, low muscular strength, and obesity at age 18 were independently associated with higher risk of developing HF in adulthood, after adjusting for each other, socioeconomic factors, other chronic diseases, and family history of HF. Low aerobic fitness and (less strongly) low muscular strength were each associated with increased risk even among men with normal BMI. Furthermore, these factors had positive additive and multiplicative interactions, i.e., their combined effect on HF risk exceeded the sum or product of their separate effects.
To our knowledge, this is the first study to examine not only the independent effects of these exposures on risk of HF, but also additive and multiplicative interactions. Additive interactions are seldom examined despite having more implications for public health, by indicating in which subgroup an intervention would prevent the most cases. Previous studies have reported similar main effects of obesity,[3, 4, 5, 6, 7, 8, 9] aerobic fitness,[10, 11, 12, 13] or muscular strength[13] on the risk of developing or being hospitalized with HF. A meta-analysis of 23 studies with a total of 647,388 participants reported that the relative risk for association between each 5-unit increase in BMI and incident HF was 1.41 (95% CI, 1.34–1.47).[7] Other studies have suggested that self-reported physical activity is inversely associated with HF risk,[3, 4] irrespective of BMI.[5]
The present study found not only important independent effects of these factors at age 18 on long-term risk of HF, but highly significant interactions between aerobic fitness and muscular strength. The additive interaction indicates that low aerobic fitness accounted for more HF cases among men with low compared with high muscular strength. If the observed associations are causal, improvements in aerobic fitness would be expected to have the greatest public health impact on HF prevention among persons with low muscular strength (or vice versa, improvements in muscular strength would prevent more HF cases among those with low aerobic fitness).
We also found that low height was independently associated with higher risk of developing HF. Moreover, it had significant interactive effects with low aerobic fitness or muscular strength, indicating that these factors may account for more HF cases among persons of short stature. To our knowledge, these findings have not been previously reported. Further investigations are warranted to confirm them and identify the relevant mediating factors.
The mechanisms by which low aerobic fitness, low muscular strength, and obesity may lead to HF are complex and include common intermediates. These exposures may increase the risk of HF partly through their contribution to other predisposing conditions, such as hypertension, diabetes, and ischemic or valvular heart disease. However, we found that they were associated with HF even after adjusting for or excluding these conditions. Clinical and experimental evidence suggests that they also have direct physiologic effects on hemodynamic load, neurohormonal activation, and oxidative stress,[29] which adversely affect cardiac structure and function,[30] diastolic filling,[30] and left ventricular mass and wall thickness.[31] Both increased adiposity and low aerobic fitness have adverse effects on insulin sensitivity, lipid metabolism, autonomic tone, fibrinolysis, and inflammation,[32, 33] which also are associated with increased development of HF.[34, 35] The present study suggests that early-life aerobic fitness and muscular strength are potentially important predictors of HF risk across the lifespan. Additional development of risk prediction models may be useful to confirm their long-term predictive and prognostic significance, and may help identify high-risk patients in whom to target earlier interventions.
Findings from the present study are consistent with or slightly stronger than associations we previously reported between aerobic fitness, muscular strength, or obesity and other CVD-related outcomes (Table 4).[14, 15, 16, 17, 18] Furthermore, associations between these exposures and HF appeared only partly mediated by hypertension and other common conditions (i.e., risk estimates were only modestly attenuated after adjusting for other conditions), suggesting the possibility of other independent physiologic effects on the development of HF. These findings to date from a large cohort suggest that early interventions to improve physical fitness and obesity may help reduce the long-term risk of HF as well as other CVD outcomes and mortality. Such interventions should include clinical counseling on physical fitness, which has been shown to be efficient, clinically effective, and cost-effective, yet remains underutilized.[36]
Table 4.
Adjusted hazard ratios for associations between aerobic fitness, muscular strength, or BMI and risk of CVD-related outcomes or mortality.
| Aerobic fitness (trend test per 100 Watts) | Muscular strength (trend test per 1000 Newtons) | BMI (trend test per 1 unit) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HRa | 95% CI | P | HRa | 95% CI | P | HRa | 95% CI | P | |
| Hypertension | 0.70 | 0.69, 0.71 | <0.001 | 1.02 | 1.00, 1.04 | 0.02 | 1.08 | 1.07, 1.09 | <0.001 |
| Type 2 diabetes | 0.66 | 0.64, 0.67 | <0.001 | 0.84 | 0.81, 0.87 | <0.001 | 1.06 | 1.06, 1.07 | <0.001 |
| Ischemic heart disease | 0.64 | 0.63, 0.66 | <0.001 | 1.01 | 0.98, 1.05 | 0.37 | 1.07 | 1.06, 1.07 | <0.001 |
| Stroke | 0.61 | 0.59, 0.64 | <0.001 | 0.87 | 0.83, 0.91 | <0.001 | 1.05 | 1.04, 1.06 | <0.001 |
| Heart failure | 0.51 | 0.48, 0.54 | <0.001 | 0.72 | 0.67, 0.77 | <0.001 | 1.08 | 1.08, 1.09 | <0.001 |
| CVD mortality | 0.46 | 0.44, 0.49 | <0.001 | 0.58 | 0.55, 0.62 | <0.001 | 1.08 | 1.07, 1.09 | <0.001 |
| All-cause mortality | 0.55 | 0.54, 0.57 | <0.001 | 0.73 | 0.71, 0.75 | <0.001 | 1.03 | 1.02, 1.04 | <0.001 |
HRs are adjusted for age, year of military conscription examination, aerobic fitness, muscular strength, BMI, education, neighborhood SES, and family history of the respective condition (or family history of CVD in analyses of mortality).
BMI = body mass index, CVD = cardiovascular disease, HR = hazard ratio.
The present study has several strengths, including prospective ascertainment of the study exposures and HF in a large national cohort, which helps prevent self-reporting or selection biases. We examined well-validated measures of aerobic fitness and muscular strength that are more objective and reliable than self-reported physical activity.[14, 15, 16, 17, 18] The results were adjusted for other common risk factors, including individual- and neighborhood-level socioeconomic factors, family history, and chronic diseases that are commonly associated with HF.
Limitations included the lack of clinical data needed to distinguish HF with preserved vs. reduced ejection fraction, and hence we were unable to examine differences in risk factors for these respective outcomes. Our study exposures were measured at only one age (~18 years), and therefore we were unable to examine changes in these factors over time. The study cohort also was all-male and relatively young (maximum attained age, 62 years). Other studies will be needed to examine these relationships in women and with additional follow-up to older ages when HF is more common.[14, 15, 16, 17, 18]
In summary, this large national cohort study found that low aerobic fitness, low muscular strength, and obesity at age 18 were independently associated with higher risk of HF in adulthood, with additive and multiplicative interactions between aerobic fitness and muscular strength. Preventive interventions should begin early in life and include not only weight control but both aerobic fitness and muscular strength, even among non-obese persons.[14, 15, 16, 17, 18]
Supplementary Material
KEY QUESTIONS.
What is already known about this subject?
Low aerobic fitness, low muscular strength, and obesity have been reported to increase the risk of heart failure (HF); however, their interactive effects are unknown. A better understanding of interactions among these common factors may help facilitate more effective primary prevention.
What does this study add?
This large cohort study found that low aerobic fitness, low muscular strength, and obesity at age 18 were independently associated with higher risk of HF in adulthood. Low aerobic fitness and low muscular strength had positive additive and multiplicative interactions, and were associated with higher risk of HF even among men with normal body mass index (BMI).
How might this impact on clinical practice?
These findings suggest that early-life interventions may help reduce the long-term risk of HF and should include both aerobic fitness and muscular strength, even among persons with normal BMI.
ACKNOWLEDGMENTS
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights.
Funding Sources: This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL116381); the Swedish Research Council; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. There were no conflicts of interest.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics−-2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161:996–1002. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation 2009;119:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu G, Jousilahti P, Antikainen R, et al. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation 2010;121:237–44. [DOI] [PubMed] [Google Scholar]
- 6.Rosengren A, Aberg M, Robertson J, et al. Body weight in adolescence and long-term risk of early heart failure in adulthood among men in Sweden. Eur Heart J 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aune D, Sen A, Norat T, et al. Body Mass Index, Abdominal Fatness, and Heart Failure Incidence and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Circulation 2016;133:639–49. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Botker HE, Pedersen L, et al. Young adulthood obesity and risk of acute coronary syndromes, stable angina pectoris, and congestive heart failure: a 36-year cohort study. Ann Epidemiol 2014;24:356–61 e1. [DOI] [PubMed] [Google Scholar]
- 9.Bjorck L, Novak M, Schaufelberger M, et al. Body weight in midlife and long-term risk of developing heart failure-a 35-year follow-up of the primary prevention study in Gothenburg, Sweden. BMC Cardiovasc Disord 2015;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry JD, Pandey A, Gao A, et al. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail 2013;6:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan H, Kunutsor S, Rauramaa R, et al. Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fail 2014;16:180–8. [DOI] [PubMed] [Google Scholar]
- 12.Pandey A, Patel M, Gao A, et al. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: the Cooper Center Longitudinal Study. Am Heart J 2015;169:290–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen K, Rasmussen F, Held C, et al. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young Swedish men: cohort study. BMJ 2015;351:h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crump C, Sundquist J, Winkleby MA, et al. Interactive Effects of Physical Fitness and Body Mass Index on the Risk of Hypertension. JAMA Intern Med 2016;176:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crump C, Sundquist J, Winkleby MA, et al. Physical Fitness Among Swedish Military Conscripts and Long-Term Risk for Type 2 Diabetes Mellitus: A Cohort Study. Ann Intern Med 2016;164:577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crump C, Sundquist J, Winkleby MA, et al. Interactive effects of obesity and physical fitness on risk of ischemic heart disease. Int J Obes (Lond) 2017;41:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crump C, Sundquist J, Winkleby MA, et al. Interactive effects of physical fitness and body mass index on risk of stroke: A national cohort study. Int J Stroke 2016;11:683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crump C, Sundquist J, Winkleby MA, et al. Interactive Effects of Aerobic Fitness, Strength, and Obesity on Mortality in Men. Am J Prev Med 2017;52:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordesjo L, Schele R. Validity of an ergometer cycle test and measures of isometric muscle strength when predicting some aspects of military performance. Swedish J Defence Med 1974;10:11–23. [Google Scholar]
- 20.Patton JF, Vogel JA, Mello RP. Evaluation of a maximal predictive cycle ergometer test of aerobic power. Eur J Appl Physiol Occup Physiol 1982;49:131–40. [DOI] [PubMed] [Google Scholar]
- 21.Andersen LB. A maximal cycle exercise protocol to predict maximal oxygen uptake. Scand J Med Sci Sports 1995;5:143–6. [DOI] [PubMed] [Google Scholar]
- 22.Hook O, Tornvall G. Apparatus and method for determination of isometric muscle strength in man. Scand J Rehabil Med 1969;1:139–42. [PubMed] [Google Scholar]
- 23.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report 2010:1–5. [PubMed] [Google Scholar]
- 24.Hoehner CM, Allen P, Barlow CE, et al. Understanding the independent and joint associations of the home and workplace built environments on cardiorespiratory fitness and body mass index. Am J Epidemiol 2013;178:1094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cubbin C, Sundquist K, Ahlen H, et al. Neighborhood deprivation and cardiovascular disease risk factors: protective and harmful effects. Scand J Public Health 2006;34:228–37. [DOI] [PubMed] [Google Scholar]
- 26.Crump C, Sundquist K, Sundquist J, et al. Neighborhood deprivation and psychiatric medication prescription: a Swedish national multilevel study. Ann Epidemiol 2011;21:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderweele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods 2014;3:33–72. [Google Scholar]
- 28.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 29.Vincent HK, Powers SK, Dirks AJ, et al. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord 2001;25:378–88. [DOI] [PubMed] [Google Scholar]
- 30.Brinker SK, Pandey A, Ayers CR, et al. Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC Heart Fail 2014;2:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation 2010;122:1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–80. [DOI] [PubMed] [Google Scholar]
- 33.Sandvik L, Erikssen J, Thaulow E, et al. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med 1993;328:533–7. [DOI] [PubMed] [Google Scholar]
- 34.Ingelsson E, Sundstrom J, Arnlov J, et al. Insulin resistance and risk of congestive heart failure. JAMA 2005;294:334–41. [DOI] [PubMed] [Google Scholar]
- 35.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 2003;107:1486–91. [DOI] [PubMed] [Google Scholar]
- 36.Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc 2013;88:1446–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.