Abstract
Objectives
Meropenem may be an important drug in the treatment of open tibial fractures and chronic osteomyelitis. Therefore, the objective of this study was to describe meropenem pharmacokinetics in plasma, subcutaneous adipose tissue (SCT), and cancellous bone using microdialysis in a porcine model.
Methods
Six female pigs were assigned to receive 1000 mg of meropenem intravenously over five minutes. Measurements of meropenem were obtained from plasma, SCT, and cancellous bone for eight hours thereafter. Microdialysis was applied for sampling in solid tissues. The meropenem concentrations were determined using ultra-high-performance liquid chromatography.
Results
The penetration of meropenem into cancellous bone, expressed as the ratio of plasma to cancellous bone area under the concentration-curve from zero to the last measured value, was incomplete and delayed. The time with concentration above the minimal inhibitory concentration (T>MIC), for an MIC of 0.5 μg/ml, was shorter for cancellous bone in comparison with both plasma and SCT. For MICs above 0.5 μg/ml, T>MIC in cancellous bone was only shorter than SCT. Considering an MIC of 4 μg/ml, no animals achieved the target of 40% T>MIC in plasma and cancellous bone, while less than 20% achieved it in SCT.
Conclusion
The main finding of this study was short T>MIC in cancellous bone after intravenous administration of 1000 mg meropenem. Consequently, in order to achieve sufficient tissue concentration in the cases of open tibial fractures and chronic osteomyelitis, supplemental application of meropenem may be necessary.
Cite this article: P. Hanberg, A. Lund, K. Søballe, M. Bue. Single-dose pharmacokinetics of meropenem in porcine cancellous bone determined by microdialysis: An animal study. Bone Joint Res 2019;8:342–348. DOI: 10.1302/2046-3758.87.BJR-2018-0308.R1.
Keywords: Microdialysis, Meropenem, Pharmacokinetics, Bone, Penetration
Article focus
This study aimed to describe meropenem pharmacokinetics in plasma, subcutaneous adipose tissue, and cancellous bone.
Key messages
The main finding of this study was short time with concentration above the minimal inhibitory concentration (T>MIC) in all tissues after intravenous administration of 1000 mg meropenem.
In order to achieve sufficient tissue concentration, supplemental application of meropenem may be required.
Strengths and limitations
This is the first study to investigate meropenem cancellous bone pharmacokinetics using microdialysis, which provided us with a high-resolution concentration-time profile.
This is an experimental study using healthy young pigs, which did not suffer from either open tibial fracture or chronic osteomyelitis.
Introduction
Treatment of bone infections is complex and relies on a combination of surgical debridement and prolonged antimicrobial therapy.1 Moreover, treatment failure is common and may partly be explained by an incomplete and heterogeneous tissue distribution of antimicrobials, which has been demonstrated for different combinations of tissue and drug.2-9 In the specific cases of open tibial fractures and chronic osteomyelitis, the literature is inconclusive regarding choice and application of antimicrobial treatment.10-13 Recent studies suggest the use of systemic meropenem in combination with vancomycin as first-line therapy for patients with open tibial fractures or chronic osteomyelitis.10,13
Antimicrobial efficacy relies on its activity against the infectious microorganism and sufficient exposure at the target site. Evaluation of local antimicrobial tissue concentrations following antimicrobial administration is therefore important for improving both the prevention and treatment of infections.14 Particularly for bone, determination of antimicrobial concentrations has been a difficult task.15,16 However, in recent decades, microdialysis has emerged as a promising tool for continuous sampling of the unbound antimicrobial concentrations of the extracellular fluid in the bone.2,3,5-9 Currently, meropenem bone pharmacokinetics is poorly described and has not been assessed by means of microdialysis, whereas vancomycin bone pharmacokinetics has been described under different conditions.2-4 The objective of this study was therefore to describe meropenem pharmacokinetics in plasma, subcutaneous adipose tissue (SCT), and cancellous bone using microdialysis in a porcine model. The primary endpoints were the time with concentration above the minimal inhibitory concentration (T>MIC) and the tissue penetration.
Materials and Methods
This study was conducted at the Institute of Clinical Medicine, Aarhus University Hospital, Denmark. The study was approved by the Danish Animal Experiments Inspectorate and was carried out according to existing laws. Chemical analyses were performed at the Department of Biochemistry, Aarhus University Hospital, Denmark.
Microdialysis
Briefly, microdialysis is a minimally invasive probe-based technique, which is based on diffusion of water-soluble molecules across a semipermeable membrane at the tip of the probe. Due to the continuous perfusion of the microdialysis probe, equilibrium across the semipermeable membrane will never occur. Consequently, the concentration in the dialysate will only represent a fraction of the actual tissue concentration. This fraction is referred to as the relative recovery (RR) and can be determined by various calibration methods. Determination of RR is a prerequisite when evaluating absolute antimicrobial tissue concentrations. In this study, retrodialysis by drug was applied for calibration.17 An in-depth description of microdialysis can be found elsewhere.18
Microdialysis equipment from M Dialysis AB (Stockholm, Sweden) was used. Specifically, the probes used were CMA 63 (membrane length 30 mm with a 20 kilodalton molecule cutoff), and CMA 107 precision pumps produced a flow rate of 2 µl/minute.
The RR was calculated by using the following equation:
where Cout is the concentration in the dialysate and Cin the concentration in the perfusate.
The absolute tissue concentrations, Ctissue, were obtained by correcting for RR using the following equation:
Individual probe calibration was performed for all the probes at location. In the data analysis, the measured concentrations were attributed to the midpoint of the sampling intervals.
Animals, anaesthetic, and surgical procedures
Six female pigs were included in the study (Danish Landrace Breed, 72 to 81 kg). The anaesthetic consisted of a combination of propofol (450 to 500 mg/hour) and fentanyl (0.45 mg/hour). The pH and core temperature of the animals were monitored and regulated throughout the study and kept within the range of 7.40 to 7.48 and 36.9°C to 39.2°C, respectively.
Surgery was initiated immediately after induction of anaesthesia. With the pig in the supine position, the left proximal tibia was exposed via a medial incision. A 2 mm drill hole with a depth of 40 mm was made approximately 10 mm distal to the epiphyseal line in the cancellous bone of the tibial condyle. Next, a microdialysis probe (30 mm) was placed in the drill hole and fixed to the skin with a single suture. In addition, a microdialysis probe (30 mm) was placed in the SCT of the lateral thoracic wall, according to the guidelines of the manufacturer. Correct location of the bone probes was evaluated by necropsy.
Sampling procedures
Immediately after placement of the microdialysis probes, all probes were perfused with 0.9% sodium chloride (NaCl) holding 5 µg/ml meropenem. Initially, a 30-minute tissue equilibration was allowed for. All probes were then calibrated by collecting three 20-minute samples. Following this, the perfusate was changed to blank 0.9% NaCl, and a 130-minute washout was performed. At time zero, a dialysate was collected to evaluate the efficacy of the washout and 1000 mg of meropenem was administered intravenously over five minutes. From time 0 to 60 minutes, dialysates were collected during 15-minute intervals, from time 60 to 120 minutes during 30-minute intervals, and from time 120 to 480 minutes during 60-minute intervals, giving a total of 13 samples over eight hours. Venous blood samples were drawn from a central venous catheter at time 10, 20, 30, 45, 60, 120, 180, 240, and 480 minutes.
Handling of samplings
The dialysates were instantly frozen and stored at -80°C until analysis. Serum blood samples were kept at room temperature for a minimum of 30 minutes and a maximum of 90 minutes before being centrifuged at 3000 g for 10 minutes. Serum aliquots were then frozen and stored at -80°C until analysis.
Ultra-high-performance liquid chromatography analysis of meropenem
Meropenem in dialysates and serum was analyzed using ultra-high-performance liquid chromatography (UHPLC). The UHPLC system consisted of an eluent pump, autosampler, column compartment, and a UV detector (Agilent 1290 Infinity; Agilent Technologies, Santa Clara, California) equipped with a 1.7 μm 100 by 2.1 mm C18 column (Kinetex; Phenomenex, Torrance, California). For analysis of meropenem in dialysates, 15 µl of dialysate was mixed with 20 µl of phosphate buffer at pH 3 (10 nM NaH2PO4, adjusted with hydrogen chloride (HCl)). After mixing, 5 µl was injected into the UHPLC system. Standards of 0.25, 0.5, 1.0, and 2.0 µg/ml meropenem in 15 µl 0.9% NaCl were prepared and analyzed to prepare calibration curves for meropenem. For this purpose, the Chemstation Software (Agilent Technologies) was used. Before analysis of meropenem in the serum, 300 µl of serum was placed in an ultrafilter 96-well plate with a 30 kilodalton molecular mass cutoff (AcroPrep 30K Omega; Pall Corporation, New York, New York) and centrifuged for 30 minutes at 1000 g. A total of 15 µl of the filtrate was prepared for analysis as was done for the dialysates. The UHPLC system (Agilent 1290 Infinity) was equipped with a 1.7 μm 100 by 2.1 mm C18 column (Kinetex), and chromatography was performed with a gradient of acetonitrile in phosphate buffer (10 nM NaH2PO4, adjusted with HCl). The concentration of acetonitrile was increased from 0% to 30% over a time span of 2.5 minutes, and the total analysis time was four minutes with a post-run time of one minute. Meropenem was detected at 304 nm. The lower limits of quantification (LLOQ) was found to be 0.5 µg/ml. Interrun imprecision (percent coefficients of variation (%CV)) was 3.0% at 2.0 µg/ml for quantification of meropenem. The assays showed linearity in the measurement response, which was within the range of 0.5 µg/ml to 105 µg/ml. The accuracy of the quantification of meropenem was found to be between -4.3% and 4.8%.
Pharmacokinetics analysis and statistics
The standard pharmacokinetic parameters: area under the concentration-time curves from zero to the last measured value (AUC0-last), peak drug concentration (Cmax), time to Cmax (Tmax), and half-life (T1/2) were determined separately for each compartment for each animal by non-compartmental analysis using the pharmacokinetic series of commands in Stata v. 14.1 (StataCorp LLC, College Station, Texas). The AUC0-last was calculated using the trapezoidal rule. Cmax was calculated as the maximum of all the recorded concentrations, and Tmax as the time to Cmax. T1/2 was calculated as ln(2)/λeq, where λeq is the terminal elimination rate constant estimated by linear regression of the log concentration on time. All the variables were analyzed using a mixed model taking the variance between pigs into account. Consequently, means and 95% confidence intervals (CIs) of AUC0-last, Cmax, Tmax, and T1/2 are given in Table I. The model assumptions were tested by visual diagnosis of residuals, fitted values, and estimates of random effects. A correction for degrees of freedom due to small sample size was handled using the Kenward–Roger approximation method. Overall, comparisons between the compartments were conducted using the F test and pairwise comparisons with the t-test. A p-value < 0.05 was considered significant. No correction for multiple comparisons was applied. The tissue AUC0-last to plasma AUC0-last ratio (AUCtissue/AUCplasma) was calculated as a measure of the tissue penetration. Statistical analyses were also performed using Stata.
Table I.
Key pharmacokinetic parameters for plasma, subcutaneous adipose tissue, and cancellous bone
| Pharmacokinetic parameter | Plasma | Subcutaneous adipose tissue | Cancellous bone | Overall comparison* |
|---|---|---|---|---|
| Mean AUC0 – last, min μg/ml (95% CI) | 3347 (2689 to 4005) | 4944 (4286 to 5602)† | 1874 (1216 to 2532)† | p < 0.001 |
| Mean Cmax, μg/ml (95% CI) | 74.3 (60.0 to 88.7) | 81.6 (67.2 to 96.0) | 21.4 (7.0 to 35.8)‡ | p < 0.001 |
| Mean Tmax, mins (sd) | 13.3 (8.2) | 22.5 (0.0) | 37.5 (0.0) | N/A |
| Mean T½, mins (95% CI) | 38.2 (32.8 to 43.5) | 43.5 (38.2 to 48.9) | 51.4 (46.0 to 56.7)§ | p < 0.002 |
| Mean AUCtissue/AUCplasma (95% CI) | N/A | 1.50 (1.14 to 1.87) | 0.56 (0.49 to 0.64) | N/A |
Overall comparison using F test for plasma, subcutaneous adipose tissue, and cancellous bone
p < 0.005 for comparison with the corresponding plasma value
p < 0.001 for comparison with both plasma and subcutaneous adipose tissue
p < 0.05 for comparison with both plasma and subcutaneous adipose tissue
AUC0–last, area under the concentration–time curve from 0 to the last measured value; CI, confidence interval; Cmax, peak drug concentration; Tmax, time to Cmax; N/A, not applicable; T1/2, half-life at β-phase; AUCtissue/AUCplasma, tissue penetration expressed as the ratio of AUCtissue/AUCplasma
By use of Microsoft Excel V. 16.16.11 (Microsoft Corporation, Redmond, Washington), the T>MIC was estimated using linear interpolation. The number of animals attaining the target of 40% T>MIC was counted manually. One-way analysis of variance with random animal effect was used for pairwise comparisons of the T>MIC.
Results
All six pigs completed the study and data were obtained from all probes. The mean RRs (sd) were 35.5% (8.5) and 20.9% (5.0) for cancellous bone and SCT, respectively. As the mean concentration of meropenem at time zero was less than 0.1 µg/ml for both compartments, these results were not included in the analysis.
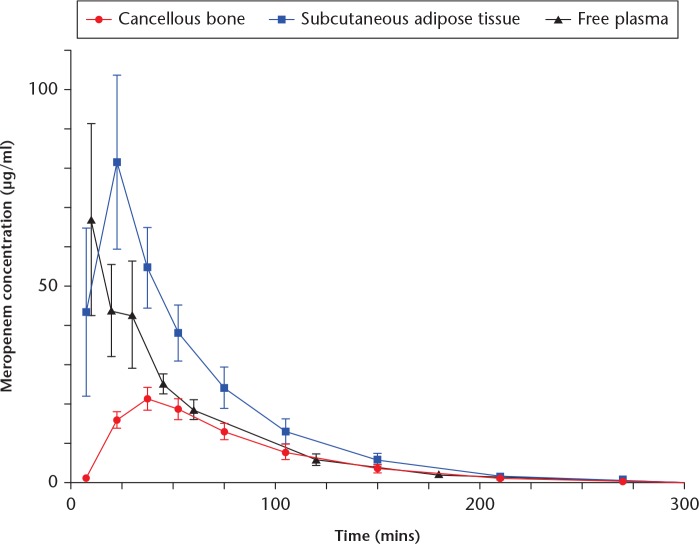
Mean meropenem concentration-time profiles for the different compartments are presented in Figure 1. Corresponding pharmacokinetic parameters are provided in Table I. For cancellous bone, the tissue penetration (95% CI) was incomplete 0.54 (0.47 to 0.61) and delayed. A lower AUC0-last and Cmax was found in cancellous bone in comparison with that of plasma. Furthermore, a prolonged elimination rate was found in cancellous bone compared with plasma and SCT. For SCT, the AUC0-last was higher than that of plasma with a corresponding higher tissue penetration ratio (95% CI) of 1.46 (1.10 to 1.81).
Fig. 1.
Mean concentration-time profiles. Due to low concentrations, the x axis is cut at 300 minutes. Bars represent 95% confidence intervals.
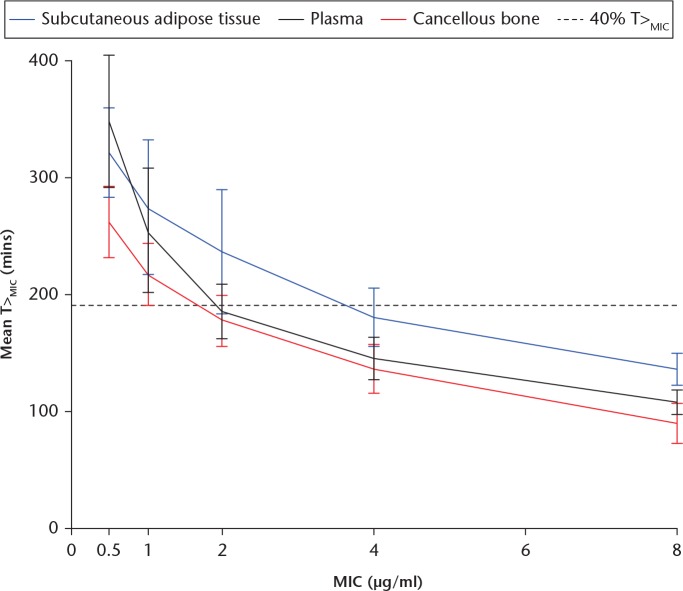
The relationship between the T>MIC and different MICs for all compartments are shown in Figure 2. For MICs up to 0.5 µg/ml, the T>MIC was shorter for cancellous bone compared with both plasma and SCT (p < 0.003). For MICs above 0.5 µg/ml, T>MIC in cancellous bone was only shorter than SCT (p < 0.03).
Fig. 2.
Time with concentration above the minimal inhibitory concentration (T>MIC) versus minimal inhibitory concentrations. Bars represent 95% confidence intervals.
Table II shows the proportion of animals achieving the target of 40% T>MIC for a range of different MICs. For an MIC of 0.5 µg/ml, all animals achieved the target of 40% T>MIC in all compartments. However, for MICs above 0.5 µg/ml, the proportion of animals achieving 40% T>MIC decreased rapidly for both plasma and cancellous bone. Considering the SCT, all animals achieved the target of 40% T>MIC for MIC values up to 2 µg/ml. However, at higher MIC values, none or fewer than 20% of the animals achieved the target of 40% T>MIC.
Table II.
The probability of target attainment (PTA) for different minimal inhibitory concentration (MIC) values in plasma, subcutaneous adipose tissue, and cancellous bone
| MIC, µg/ml | Plasma PTA, % (40% T>MIC) | Subcutaneous adipose tissue PTA, % (40% T>MIC) | Cancellous bone PTA, % (40% T>MIC) |
|---|---|---|---|
| 0.5 | 100 | 100 | 100 |
| 1.0 | 83 | 100 | 83 |
| 2.0 | 33 | 100 | 17 |
| 4.0 | 0 | 17 | 0 |
| 8.0 | 0 | 0 | 0 |
T>MIC, time with concentration above the minimal inhibitory concentration
Discussion
To the best of our knowledge, this is the first study to report meropenem bone pharmacokinetics using microdialysis. The main finding was short T>MIC in cancellous bone. For meropenem, a probability of target attainment of 90% for the target of 40% T>MIC has been associated with bactericidal effect.19 In cancellous bone, this probability of target attainment was only achieved for an MIC of 0.5 µg/ml, while a proportional decrease was found with increasing MICs. Furthermore, we found an incomplete and delayed tissue penetration of meropenem from plasma into the cancellous bone. The most common pathogens in open tibial fractures and chronic osteomyelitis are Staphylococcus aureus, Coagulase-negative staphylococci, and Enterococcus spp. For meropenem, these pathogens exhibit MICs in the range of 0.125 µg/ml to 4 µg/ml.20 For MICs of 4 µg/ml no animals achieved the target of 40% T>MIC in plasma and cancellous bone, and fewer than 20% of the animals achieved it in the SCT. Accordingly, our findings suggest that 1000 mg of meropenem will not provide sufficient bone and tissue concentrations. Recently, higher targets up to 100% T>MIC have been proposed in several clinical studies.21-23 If higher targets are applied to our data, the probability of target attainment will proportionally decrease with an increasing target. Consequently, in order to achieve sufficient tissue concentration in the cases of open tibial fractures and chronic osteomyelitis, supplemental application of meropenem may be necessary.
On a more basic pharmacokinetic level, it is noteworthy that the highest AUC0-last and tissue penetration was found in the SCT. Accordingly, the SCT displayed the best T>MIC results. This may be explained by a fast penetration and high Cmax in the SCT combined with an elimination rate equal to that of plasma. These findings are important, as sufficient antimicrobial exposure should be reached in all relevant target tissues. When evaluating antimicrobial tissue concentrations, it is therefore important to evaluate the pharmacokinetics in all relevant compartments and under different conditions.
Recent studies, with comparable setups, have indicated that the antimicrobial penetration may differ between drugs.2,7 Table III summarizes important antimicrobial cancellous bone pharmacokinetic differences between cefuroxime, vancomycin, and meropenem.2,7 When looking at these results, the cefuroxime cancellous bone penetration seems superior to both meropenem and vancomycin. This may indicate that the selection of antimicrobials for treatment and prevention of bone infections should not only be based on the sensitivity to the pathogen but also on the pharmacokinetic profile in relevant tissues. However, it is important to recognize the differences in the antimicrobial specific targets.
Table III.
Healthy porcine cancellous bone pharmacokinetic parameters of different antimicrobials obtained by microdialysis
| Pharmacokinetic parameter | Meropenem | Cefuroxime7 | Vancomycin2 |
|---|---|---|---|
| Mean AUCtissue/AUCplasma (95% CI) | 0.56 (0.49 to 0.64) | 0.79 (0.67 to 0.91) | 0.59 (0.42 to 0.75) |
| Cmax, μg/ml (95% CI) | Mean: 21.4 (7.0 to 35.8) | Mean: 22.9 (16.3 to 29.5) | Median: 15.7 (10.9 to 20.4) |
| Tmax, mins | Mean: 37.5 (sd 0) | Median: 45 (range: 15 to 45) | Median: 191 (95% CI: 149 to 233) |
| T½, mins (95% CI) | Mean: 51.4 (46.0 to 56.7) | Median: 36.7 (32.0 to 41.3) | Median: 255 (206 to 305) |
AUCtissue/AUCplasma, tissue penetration expressed as the ratio of AUCtissue/AUCplasma; CI, confidence interval; Cmax, peak drug concentration; Tmax, time to Cmax; T1/2, half-life at β-phase
Open tibial fractures and chronic osteomyelitis are conditions that are difficult to treat. Recently, the use of meropenem and vancomycin in combination as first-line therapy has been recommended for these conditions.10,13 Previous studies have found both incomplete and delayed vancomycin tissue penetration into both bone and SCT.2-4 The present study contributes new knowledge regarding meropenem cancellous bone and SCT pharmacokinetics. However, both the vancomycin studies and the present study only evaluate single-dose monotherapy administration and do not assess the potential effects of dual-antimicrobial therapy and achievement of steady state. Therefore, future studies investigating these matters are warranted.
To some extent, pigs resemble humans in terms of physiology and anatomy.24 Nonetheless, the interspecies and age-related differences are important to consider before uncritically extrapolating these results to a clinical setting. Applying the Cierny–Mader classification, these pigs may represent an A-host with both a good immune response and local delivery of the antimicrobial.25 Furthermore, the animals in the present study did not suffer from either open tibial fracture or chronic osteomyelitis. Previous studies have, however, shown that bone infections lower the antimicrobial penetration by inducing intratrabecular suppuration resulting in ischaemic osseous sequestration and reduced vascularization.1,2,7,25,26 Chronic osteomyelitis may therefore lead to shorter T>MIC values and lower penetration ratios in bone, than found in this study. Moreover, the presence of bacterial biofilm will increase the pathogens tolerance towards the antimicrobial due to increased MIC values.27 In contrast, fractures may lead to higher antimicrobial bone concentrations. Fractures are typically associated with an acute decrease in the bone blood flow. However, during the first day, the blood flow in the bone rapidly increases three to six times due to angiogenic mechanisms.28,29 This may, in contrast with bone infections, lead to longer T>MIC and higher bone penetration ratios. Nonetheless, this remains speculative, and future studies investigating antimicrobial bone concentration after open tibial fractures and in chronic osteomyelitis are warranted.
In recent decades, microdialysis has emerged as a promising tool for antimicrobial pharmacokinetic studies in bone. This method is advantaged by serial sampling of the free and thus active fraction of drug from the interstitial space.18 However, when performing microdialysis studies, it is important to realise that the mandatory correction for RR is associated with a magnification of the variations associated with preanalytical sample handling and the chemical assay. Consequently, RR should exceed 20% to minimize the variations, which increases with decreasing RR.30 Our setup resulted in mean RRs in the range of 20.9% to 35.5%, which seems acceptable. Furthermore, the variation of the dialysate concentrations was comparable with those found in plasma. It should also be noted that the LLOQ for meropenem was 0.5 µg/ml. As such, we were not able to assess T>MIC for MICs below 0.5 µg/ml. However, this LLOQ was found to be acceptable, as clinical treatment targets below MICs of 0.5 µg/ml seem insufficient.
In conclusion, microdialysis was successfully applied for the assessment of meropenem concentrations in cancellous bone and SCT. The main finding of this study was short T>MIC in cancellous bone after intravenous administration of 1000 mg meropenem, where no animals achieved the target of 40% T>MIC for MICs of 4 µg/ml. Consequently, in order to achieve sufficient tissue concentration in the cases of open tibial fractures and chronic osteomyelitis, supplemental application of meropenem may be required.
Acknowledgments
We thank the Department of Orthopaedic Surgery, Horsens Regional Hospital and the Orthopaedic Research Unit, Aarhus University Hospital for supporting this study. Also, we would like to thank Anette Baatrup for helping with the chemical analyses.
Footnotes
Author contributions: P. Hanberg: Conceptualized the study, Collected and analyzed the data, Wrote and edited the manuscript.
A. Lund: Conceptualized the study, Collected and analyzed the data, edited the manuscript.
K. Søballe: Conceptualized the study, Analyzed the data, edited the manuscript.
M. Bue: Conceptualized the study, Collected and analyzed the data, edited the manuscript.
Ethical review statement: This study was approved by the Danish Animal Experiments Inspectorate and was carried out according to existing laws (License No. 2017/15-0201-01184).
Follow us @BoneJointRes
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med 1997;336:999-1007. [DOI] [PubMed] [Google Scholar]
- 2. Bue M, Hanberg P, Koch J, et al. Single-dose bone pharmacokinetics of vancomycin in a porcine implant-associated osteomyelitis model. J Orthop Res 2018;36:1093-1098. [DOI] [PubMed] [Google Scholar]
- 3. Bue M, Tøttrup M, Hanberg P, et al. Bone and subcutaneous adipose tissue pharmacokinetics of vancomycin in total knee replacement patients. Acta Orthop 2018;89:95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bue M, Hanberg P, Tøttrup M, et al. Vancomycin concentrations in the cervical spine after intravenous administration: results from an experimental pig study. Acta Orthop. 2018;89:683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanberg P, Bue M, Birke Sørensen H, Søballe K, Tøttrup M. Pharmacokinetics of single-dose cefuroxime in porcine intervertebral disc and vertebral cancellous bone determined by microdialysis. Spine J 2016;16:432-438. [DOI] [PubMed] [Google Scholar]
- 6. Schintler MV, Traunmüller F, Metzler J, et al. High fosfomycin concentrations in bone and peripheral soft tissue in diabetic patients presenting with bacterial foot infection. J Antimicrob Chemother 2009;64:574-578. [DOI] [PubMed] [Google Scholar]
- 7. Tøttrup M, Bue M, Koch J, et al. Effects of implant-associated osteomyelitis on cefuroxime bone pharmacokinetics: assessment in a porcine model. J Bone Joint Surg [Am] 2016;98-A:363-369. [DOI] [PubMed] [Google Scholar]
- 8. Traunmüller F, Schintler MV, Spendel S, et al. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int J Antimicrob Agents 2010;36:84-86. [DOI] [PubMed] [Google Scholar]
- 9. Tøttrup M, Bibby BM, Hardlei TF, et al. Continuous versus short-term infusion of cefuroxime: assessment of concept based on plasma, subcutaneous tissue, and bone pharmacokinetics in an animal model. Antimicrob Agents Chemother 2015;59:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olesen UK, Juul R, Bonde CT, et al. A review of forty five open tibial fractures covered with free flaps. Analysis of complications, microbiology and prognostic factors. Int Orthop 2015;39:1159-1166. [DOI] [PubMed] [Google Scholar]
- 11. Morgenstern M, Vallejo A, McNally MA, et al. The effect of local antibiotic prophylaxis when treating open limb fractures: a systematic review and meta-analysis. Bone Joint Res 2018;7:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou J, Zhou XG, Wang JW, Zhou H, Dong J. Treatment of osteomyelitis defects by a vancomycin-loaded gelatin/β-tricalcium phosphate composite scaffold. Bone Joint Res 2018;7:46-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheehy SH, Atkins BA, Bejon P, et al. The microbiology of chronic osteomyelitis: prevalence of resistance to common empirical anti-microbial regimens. J Infect 2010;60:338-343. [DOI] [PubMed] [Google Scholar]
- 14. No authors listed. European Medicines Agency. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products, 2016. (Committee for Medicinal Products for Human Use). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500210982.pdf (date last accessed 14 May 2019).
- 15. Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet 2009;48:89-124. [DOI] [PubMed] [Google Scholar]
- 16. Mouton JW, Theuretzbacher U, Craig WA, et al. Tissue concentrations: do we ever learn? J Antimicrob Chemother 2008;61:235-237. [DOI] [PubMed] [Google Scholar]
- 17. Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods 1991;40:31-38. [DOI] [PubMed] [Google Scholar]
- 18. Joukhadar C, Müller M. Microdialysis: current applications in clinical pharmacokinetic studies and its potential role in the future. Clin Pharmacokinet 2005;44:895-913. [DOI] [PubMed] [Google Scholar]
- 19. Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat Rev Microbiol 2004;2:289-300. [DOI] [PubMed] [Google Scholar]
- 20. No authors listed. European Committee on Antimicrobial Susceptibility Testing (EUCAST). Clinical breakpoints and dosing of antibiotics, 2018. http://www.eucast.org/clinical_breakpoints/ (date last accessed 14 May 2019).
- 21. Li C, Du X, Kuti JL, Nicolau DP. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 2007;51:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 2008;31:345-351. [DOI] [PubMed] [Google Scholar]
- 23. Kristoffersson AN, David-Pierson P, Parrott NJ, et al. Simulation-based evaluation of PK/PD indices for meropenem across patient groups and experimental designs. Pharm Res 2016;33:1115-1125. [DOI] [PubMed] [Google Scholar]
- 24. Swindle MM, Makin A, Herron AJ, Clubb FJ, Jr, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol 2012;49:344-356. [DOI] [PubMed] [Google Scholar]
- 25. Cierny G, III, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res 2003;414:7-24. [DOI] [PubMed] [Google Scholar]
- 26. Jensen LK, Koch J, Dich-Jorgensen K, et al. Novel porcine model of implant-associated osteomyelitis: a comprehensive analysis of local, regional, and systemic response. J Orthop Res 2017;35:2211-2221. [DOI] [PubMed] [Google Scholar]
- 27. Jensen LK, Bjarnsholt T, Kragh KN, et al. In vivo gentamicin susceptibility test for prevention of bacterial biofilms in bone tissue and on implants. Antimicrob Agents Chemother 2018;63:e01889-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corbett SA, McCarthy ID, Batten J, et al. Nitric oxide mediated vasoreactivity during fracture repair. Clin Orthop Relat Res 1999;365:247-253. [DOI] [PubMed] [Google Scholar]
- 29. Tomlinson RE, Silva MJ. Skeletal blood flow in bone repair and maintenance. Bone Res 2013;1:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chaurasia CS, Müller M, Bashaw ED, et al. AAPS-FDA Workshop White Paper: microdialysis principles, application, and regulatory perspectives. J Clin Pharmacol 2007;47:589-603. [DOI] [PubMed] [Google Scholar]