Abstract
Objectives
The aim of this study was to review the current evidence and future application for the role of diagnostic and therapeutic ultrasound in fracture management.
Methods
A review of relevant literature was undertaken, including articles indexed in PubMed with keywords “ultrasound” or “sonography” combined with “diagnosis”, “fracture healing”, “impaired fracture healing”, “nonunion”, “microbiology”, and “fracture-related infection”.
Results
The use of ultrasound in musculoskeletal medicine has expanded rapidly over the last two decades, but the diagnostic use in fracture management is not routinely practised. Early studies have shown the potential of ultrasound as a valid alternative to radiographs to diagnose common paediatric fractures, to detect occult injuries in adults, and for rapid detection of long bone fractures in the resuscitation setting. Ultrasound has also been shown to be advantageous in the early identification of impaired fracture healing; with the advent of 3D image processing, there is potential for wider adoption. Detection of implant-related infection can be improved by ultrasound mediated sonication of microbiology samples. The use of therapeutic ultrasound to promote union in the management of acute fractures is currently a controversial topic. However, there is strong in vitro evidence that ultrasound can stimulate a biological effect with potential clinical benefit in established nonunions, which supports the need for further investigation.
Conclusion
Modern ultrasound image processing has the potential to replace traditional imaging modalities in several areas of trauma practice, particularly in the early prediction of impaired fracture healing. Further understanding of the therapeutic application of ultrasound is required to understand and identify the use in promoting fracture healing.
Cite this article: J. A. Nicholson, S. T. J. Tsang, T. J. MacGillivray, F. Perks, A. H. R. W. Simpson. What is the role of ultrasound in fracture management? Diagnosis and therapeutic potential for fractures, delayed unions, and fracture-related infection. Bone Joint Res 2019;8:304–312. DOI: 10.1302/2046-3758.87.BJR-2018-0215.R2.
Keywords: Ultrasound, Fractures, Imaging, Microbiology, Impaired fracture healing, Fracture-related infection, Callus prediction, Nonunion, Smoking
Article focus
This article reviews the evidence for the role of ultrasound in fracture management.
Ultrasound is a developing technology and may hold novel advantages in the diagnosis and treatment of impaired fracture healing.
Key messages
The ability to detect the development of callus prior to conventional radiographs, facilitated by emerging 3D imaging advances, may enable the early diagnosis of delayed union or confirmation of union.
Sonication of microbiological samples for culture can improve diagnostic sensitivity.
Despite recent controversy, the experimental evidence for therapeutic ultrasound supports further clinical investigation of its role in the management of impaired fracture healing.
Strengths and limitations
This review of the current literature provides a concise summary of the evidence for ultrasound as a diagnostic and therapeutic modality in fracture management.
There remain gaps in the understanding of ultrasound as a clinical adjunct in fracture management.
Introduction
The application of ultrasound as a clinical adjunct was first explored in 1924, when Wood and Loomis1 described the biological changes related to ultrasonic treatment. Ultrasound refers to pressure waves with a frequency of 20 kHz or more. Ultrasound can be described according to its intensity and frequency: low intensity (< 3 W/cm2) versus high intensity (> 5 W/cm2); and low frequency (< 500 kHz) versus high frequency (> 500 kHz). Medical ultrasound devices utilize frequencies between 20 kHz and 18 MHz, which vary depending on whether it is being used as a therapeutic or diagnostic tool.2,3
The use of ultrasound in musculoskeletal medicine has expanded rapidly over the last two decades. In the field of soft-tissue pathology, diagnostic ultrasound has been widely adopted for the monitoring of synovitis in inflammatory joint disorders, tendinopathy, traumatic tendon ruptures, and the detection of intra-articular effusions.4,5 However, despite this, ultrasound is not in routine use for fracture management.
A structured review of the relevant literature indexed in PubMed was undertaken with keywords “ultrasound” or “sonography” combined with “diagnosis”, “fracture healing”, “impaired fracture healing”, “nonunion”, “microbiology”, and “fracture-related infection”. This review presents the potential diagnostic and therapeutic use of ultrasound in the management of fractures.
Diagnostic application of ultrasound
Fracture diagnosis
Bone has some intrinsic features that make ultrasound examination attractive. The interface between the skin, the soft-tissue envelope, and the bone structure has distinctive planes. The dense nature of bone causes reflection of the ultrasound waves, allowing a clear distinction from the soft-tissue envelope and creating a hyperechoic (bright) reflection from the cortical surface. Fractures can be visualized as a break in the smooth cortical contour and the developing haematoma, and subsequent callus formation can be visualized from an early stage starting as an anechoic (dark) shadow, with a similar appearance to articular cartilage, becoming increasingly hyperechoic with calcification such that the normal appearance of cortical bone is restored (Fig. 1).6 Ultrasound has several appealing features for evaluating fracture healing. It is noninvasive, does not use ionizing radiation, and is delivered in real time. The use of a high frequency transducer (typically between 10 MHz and 18 MHz) allows the study of superficial bones (e.g. tibia shaft) with high resolution but with limited depth penetration. For bones with greater soft-tissue coverage (e.g. femur), a lower frequency transducer (approximately 5 MHz to 10 MHz) will allow a greater depth of penetration beyond 6 cm but image quality decreases proportionately.6 For the majority of fracture diagnostic studies to date, this has been achieved with a high-frequency probe used in 2D in B-mode ( or ‘Brightness mode’). Here a fracture site can be explored in a coronal or sagittal 2D plane. Given the difficulties of depth due to the acoustic shadow of intact bone, several passes of a fracture site with different ‘viewing windows’ for the probe are needed to gain a 3D appreciation of the fracture morphology.
Fig. 1.
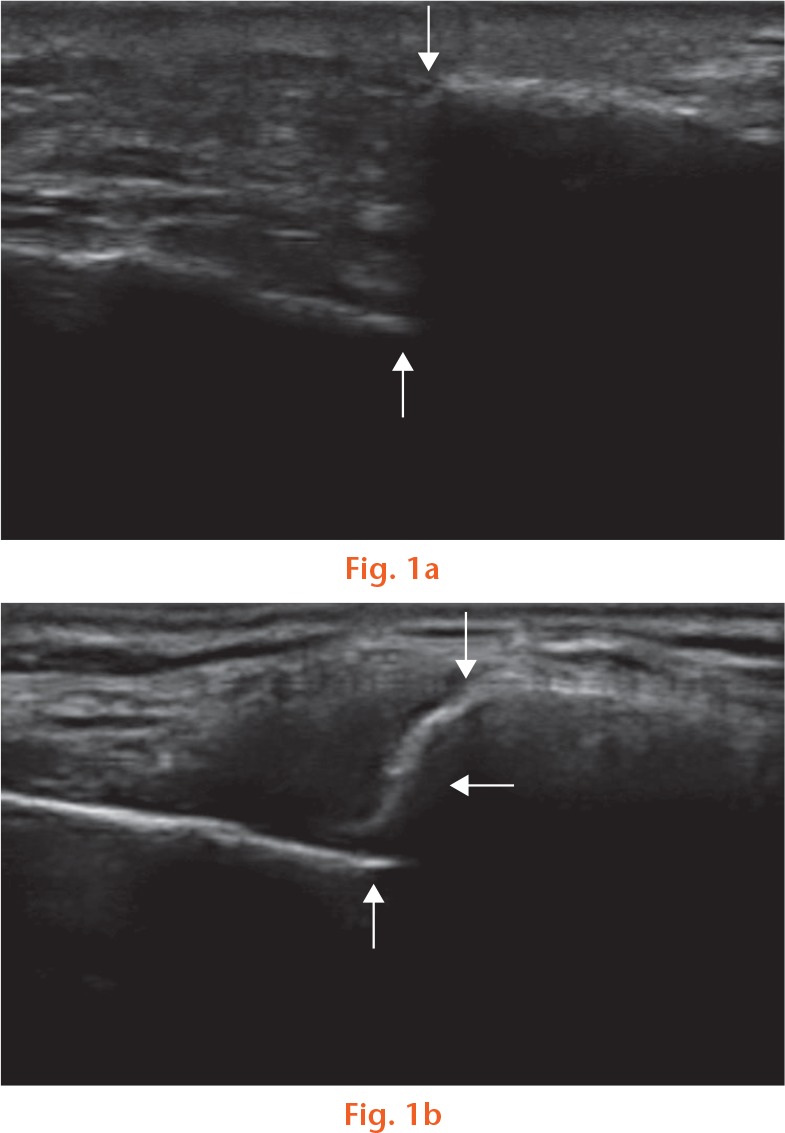
Ultrasound of two patients with a displaced clavicle fracture. a) Nonunion: vertical arrows show end of each fracture fragment with absence of bridging callus at six weeks (symptomatic nonunion confirmed at six months on CT scan). b) Union: vertical arrows show the end of each fracture fragment; the horizontal arrow shows bridging callus at six weeks with a restored cortical bridge.
Paediatrics
The use of ultrasound in paediatric fractures has received attention because of the benefits of removing radiation, and because of the diagnostic challenges for plain x-rays caused by the cartilaginous components of the immature paediatric skeleton.7 The first description of ultrasound for fracture diagnosis was in 1988, in a case series of 41 newborn infants with a suspected clavicle fracture following a traumatic delivery. In all cases, the clavicle fracture was easily identifiable on ultrasound in the first few days following injury, and this was validated with a standard x-ray.8 This finding has been replicated in a larger cohort of 49 newborns with clavicle fractures. This study also found ultrasound advantageous on grounds of the lack of ionizing radiation and equal diagnostic sensitivity.9 The use of ultrasound for costal cartilage injuries in children with suspected nonaccidental injury has been demonstrated.10,11 In a large cohort of 224 paediatric fractures, Hübner et al12 found ultrasound to be an excellent diagnostic tool for diaphyseal humeral, femoral, and forearm fractures. They suggested that it could be used as substitute for radiographs. In undisplaced forearm greenstick and torus fractures, ultrasound has been shown to be comparable to radiographs for diagnosis and decision making.13,14 A recent review of paediatric forearm fractures concluded that ultrasound was a valid alternative to radiographs with a sensitivity and specificity of 0.98.15
Other authors have been more cautious, suggesting that ultrasound can aid physical examination findings and help to determine the need for radiographs rather than replacing them.16 Additional caution has also been advised with open fractures, fractures of the small bones of the hand and foot, and Salter Harris I fractures, as ultrasound is difficult to interpret in these conditions.12
Radiologically occult and stress fractures
Ultrasound has also been used to detect occult adult fractures that might be missed with conventional radiographs. The use of diagnostic ultrasound in foot and ankle trauma was assessed following an initial ‘normal’ radiograph. It was found to detect the missed fractures, which occurred in around 10% of the cohort and was advocated as a potential alternative to MRI for occult fractures.17 The use in sternal fractures has also been shown with equivalent sensitivity to conventional radiographs.18,19 For the diagnosis of adult rib fractures, ultrasound was shown to be better than plain radiographs (78% vs 12%, respectively).20
Other diagnostic uses
In the emergency department, the use of ultrasound to judge reduction of distal radius fractures in real time has been validated.21,22 Additionally, ultrasound has been suggested to aid the diagnosis of long bone fractures in adults during resuscitation of haemodynamically unstable patients.23 With minimal ultrasound training, prehospital care physicians have used ultrasound to detect long bone fractures without the use of radiographs with a sensitivity 93% and specificity 83%.24
Despite these potential advantages, there is resistance to widespread adoption of ultrasound to replace conventional radiographs, perhaps because of the skill and resources needed to perform and interpret the ultrasound images. Furthermore, the ionizing radiation dose of peripheral limb radiographs is considered to be relatively benign.
Monitoring callus progression and fracture union
Conventional radiographs are slow to detect callus formation, which limits their accuracy for the early diagnosis of a delayed union. It is reported to take six to eight weeks for callus to be present and often beyond ten weeks for bridging callus to be evident on plain radiographs.25,26 Furthermore, given the 2D projectional nature of radiographs, the sensitivity of detecting a bridging callus can be challenging. Multiple orthogonal radiographs can aid the objectivity to detect a bridging callus for union, along with scoring systems such as the radiographic union scale in tibial (RUST) scoring system.25 An exciting application of ultrasound is the ability to evaluate the fracture site for evidence of early callus progression and union. The soft bridging callus formed during the early phases of fracture healing can be reliably detected with conventional 2D ultrasound imaging.26 This method was first described in patients undergoing limb lengthening using distraction osteogenesis. The use of 2D ultrasound imaging was shown to be superior to radiographs to observe the quality of bridging callus, which was visible within two weeks. A predictable pattern of anechoic signal was present before evolving into hyperechoic cortical bone, which did not allow transmission of the ultrasound beam. This was consistently detected prior to the appearance of callus on plain radiographs, which was not apparent before eight weeks.26 This has been replicated in similar small cohort studies, which conclude that ultrasound can guide the proposed rate of limb lengthening and detect complications such as cysts or poor callus progression earlier than plain radiographs.27-30 These small case series describe the appearance of early callus formation but do not expand on the rate or quality of callus formation and whether this was predictable in all patients. In a larger study by Ricciardi et al,31 239 diaphyseal fractures treated with external fixators were monitored for fracture healing at regular intervals. The authors found that seven days after surgery, a haematoma could be seen in the fracture gap as a hypoechogenic area with an irregular perimeter.31 Beyond ten to 16 days, a dense, dark anechoic material was visible in keeping with soft callus formation. This became increasingly hyperechogenic, in keeping with calcification of the fracture site seen between days 20 and 35, but with some penetration, which allowed it to be distinguished from the intact cortical bone. Beyond 35 days, the callus was no longer penetrated by the ultrasound and a uniform acoustic shadow was observed. At this point, the early callus could be visualized on conventional radiographs. Although Ricciardi et al31 were the first to describe these early changes in detail, they do not provide details of the individual patients enrolled in their study. Despite the number of patients, there was no reporting of any delayed unions or the inability to detect callus. It is therefore assumed that all patients united with a predictable course. It is possible that there was selection or reporting bias within this study, which limits its applicability to clinical practice.31 Following this, Maffulli and Thornton32 expanded on the evaluation of early callus and potential differences in the presence of fracture nonunion in an observational study of 24 patients. They described the typical haematoma appearance from the first week and the increasing echogenic appearance of the soft callus, initially irregular in morphology, but undergoing linear alignment with time to match the intact cortical bone. They were the first to comment on the appearance of three established humerus fracture nonunions, which appeared to confirm the absence of a bony bridge within maturing soft callus.32 More recently, a small study of 28 conservatively treated paediatric fractures compared radiographs with ultrasound assessment at the time of removal of cast.33 They found that the measurement of callus formation and prediction of union was comparable between radiographs and ultrasound. However, the timing of radiographs and ultrasound was not clearly stated, nor was the reproducibility of ultrasound assessment adequately reported.33 Despite numerous publications over the last decade describing the early detection of callus on ultrasound prior to radiographs, all are limited by incomplete reporting of the reliability and reproducibility of their findings.27-32
Ability to predict delayed fracture union and the clinical application
The most clinically useful study to evaluate the predictive performance of ultrasound was performed by Moed et al.34 In their pilot work, 14 tibial diaphyseal fractures managed with an unreamed statically locked intramedullary nail were prospectively scanned every fortnight until bone union. From three scanning portals, they were able to achieve a 270° view of the tibia fracture site mitigating for the limitations of 2D scanning. They determined the presence or absence of a bridging callus on ultrasound. The progressive loss of visualization of the intramedullary nail at the fracture site was the most reliable predictor of union. They found that bridging callus on ultrasound on at least two views was indicative of bone healing in nine patients, which was detected at a mean of 38 days (17 to 65) versus 127 days (70 to 213) on plain orthogonal radiographs. Of the five patients with delayed union, ultrasound correctly identified the absence of bridging callus and the continued appearance of the nail at the fracture site in all but one patient, giving a sensitivity of 100% (95% confidence interval (CI) 0.6 to 1.0) and specificity of 80% (95% CI 0.3 to 1.0) for the diagnosis of impaired fracture union.34 A subsequent interventional study was undertaken in 47 patients (with 50 tibial diaphyseal fractures), treated with unreamed statically locked intramedullary nails.35 If no signs of healing (as determined by their pilot work) were observed at six weeks, a further nine-week scan was repeated. The authors decided to intervene after two negative ultrasound scans with either dynamization of the fracture or bone grafting for axially unstable patterns. They found in 38 fractures that an ultrasound scan with evidence of healing at six or nine weeks had 97% positive predictive value and 100% sensitivity for fracture union. Typically, they observed fracture healing determined by ultrasound at a mean of 6.5 weeks versus 19 weeks for plain radiographs alone (p < 0.001). The one false positive the authors report was incorrectly described as healing on ultrasound, which on retrospective review was erroneously reported and should have undergone a further nine-week scan. This patient went on to require further intervention for symptomatic nonunion. Of the 12 patients with two negative ultrasound scans, two patients refused surgery and autodynamized when the screws broke and went on to unite. A further ten patients underwent either dynamization or bone grafting following the second scan at nine weeks. Of these, four fractures still went on to nonunion and underwent an exchange nailing. All patients had no evidence of radiological union at the time of their repeat procedure, in keeping with the ultrasound findings. Management of tibial diaphyseal fractures has evolved since this study, with modern nailing techniques tending to use larger reamed nails and early weightbearing.36,37 However, in this well-defined prospective study, the results support the clinical application of ultrasound to predict delayed union.35
Vascular Doppler
Assessment of the microvascular blood flow at the site of early callus formation is of interest for evaluating the progression of vascular invasion. Power Doppler can be used to quantify neovascularization in a defined area of callus. New vessels with a diameter of > 1 mm indicate an increase in vascularity, which can be referenced against healthy periosteum away from the fracture location. It has been suggested that neovascularization can be used to predict callus formation.33,38-40 This relationship was explored by Caruso et al39 with a series of 20 tibia shaft fractures managed using external fixators. New vessel formation within a maturing callus was followed by a decrease that was seen after approximately 100 days, with uneventful union in 18 patients. In two patients with a delayed union, there was a persistently high vascularity beyond three months, in keeping with the absence of bridging callus on ultrasound and radiographs.39 However, there is a concern of scanning error when vascular Doppler is used to assess fracture healing. Low blood flow, image artefacts due to movement, and random 2D slice sampling of a callus can lead to variable interpretation of the clinical picture. It has been suggested that 3D high-frequency power Doppler ultrasound can reduce the variability of multiple 2D slices.41 Sun et al41 evaluated the validity of high-power vascular Doppler using ex vivo microangiography CT as a benchmark in an animal model. They found that the two modalities were strongly correlated and that power Doppler ultrasound was sufficiently reproducible (Fig. 2). The vascularity of the fracture site decreased predictably with time in keeping with the developing callus and bone union.41
Fig. 2.
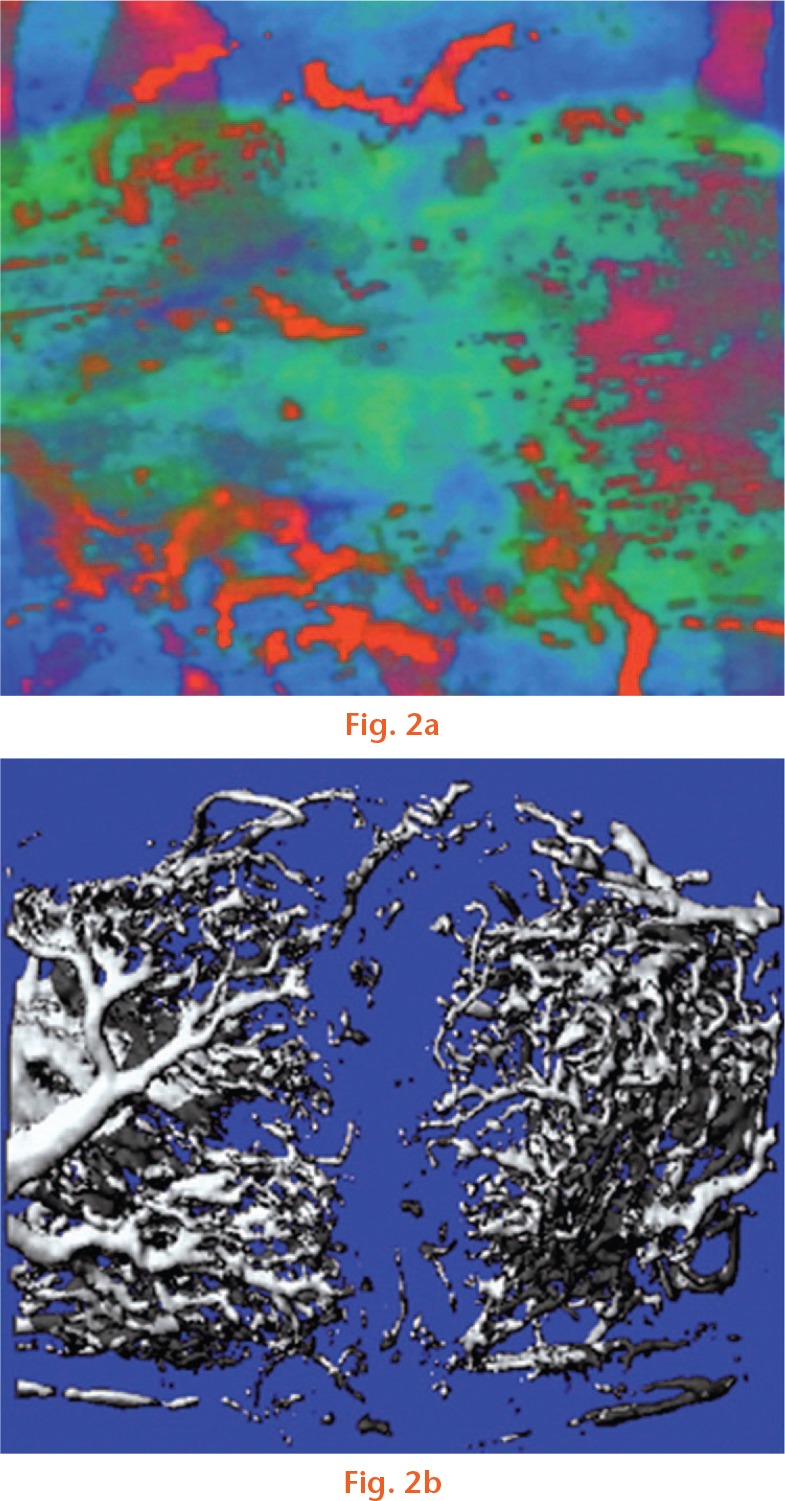
3D reconstruction of a) power Doppler and b) microangiography CT. Reproduced with permission from Sun MH, Leung KS, Zheng YP, et al. Three-dimensional high frequency power Doppler ultrasonography for the assessment of microvasculature during fracture healing in a rat model. J Orthop Res 2012;30:137-143.
The use of contrast-enhanced ultrasound using microbubble agents has been shown to be able to determine vascularity accurately with some increased sensitivity over conventional power Doppler. This has been explored in tendinopathy of the Achilles tendon42 and rotator cuff of the shoulder,43,44 but its application to assess fracture healing has not yet been evaluated. Despite the sound biological principles that underpin vascular inspection of the early fracture site, the clinical use to predict delayed union still requires development. The subtle vascular changes seen by Doppler ultrasound at three months in potential delayed union sites do not offer a significant advantage over traditional radiographs. However, there is potential for further development of this technology, which may enhance the clinical application in this area.
Freehand 3D ultrasound
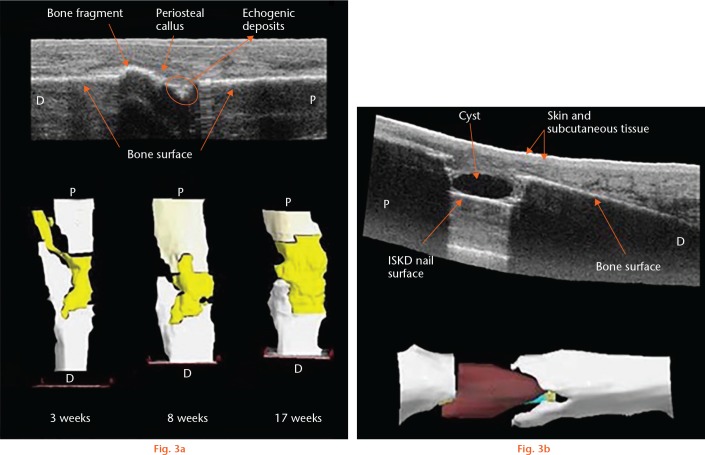
The primary limitation of 2D ultrasound in orthopaedic trauma practice is the difficulty of the interpretation of the images. In addition, there can be temporal and operator variation, making monitoring an area of interest over time for callus progression difficult and potentially error-prone. This can be mitigated by performing multiple passes over the fracture site with predetermined orthogonal views; however, it can still be difficult to ensure correct orientation and repeatability. The use of 3D ultrasound has the ability to overcome these shortcomings. The technology utilizes a tracked ultrasound probe and widely available software (e.g. Stradwin; Cambridge University Engineering Department, Cambridge, United Kingdom). Using a standard diagnostic ultrasound machine with a freehand 2D transducer attached to a positional sensing device, similar to that used in computer-aided navigation, 3D images can be produced. As the normal 2D image is recorded, the position of the transducer is tracked in order to create a series of irregular 2D scans that are then mapped to a 3D lattice, which allows 3D volumetric reconstruction. Optical tracking uses an infrared sensor to capture the transducer position device with a direct ‘line of sight’, capturing images in real time with excellent accuracy.45,46 This has been used to measure muscle volumes in vivo with findings comparable to MRI.47-49 A wide variety of uses have also been advocated such as clubfoot assessment in neonates,50 traumatic muscle injuries,51 and brachial plexus mapping.52 The utilization of 3D ultrasound for the study of fracture healing has distinct advantage over 2D images due to the ability to study a fracture site from multiple angles and compile them into a 3D reconstruction, which facilitates interpretation and allows cross-sectional images to be created. Despite this potential, the use of 3D ultrasound for fracture diagnostics or to evaluate bone healing, particularly in the early ‘forgotten phase’,53 has not been exploited to date. A pilot study showed the feasibility of using 3D ultrasound for early inspection of the fracture site was achievable following intramedullary nailing or distraction osteogenesis of the tibia. In five patients, the early bridging callus was accurately visualized; in one patient, an early cyst at the fracture site was detected prior to radiological detection (Fig. 3).54
Fig. 3.
a) Reconstruction following tibia shaft nailing. 2D reconstruction shows consolidating callus formation and 3D reconstruction shows the bridging callus from an early stage.54 b) Patient with an intramedullary skeletal kinetic distractor (ISKD) nail in situ at eight weeks following distraction osteogenesis. 2D view and 3D view presented with a cyst (red) detected at the distraction site.54 Reproduced with permission from Ross E. Freehand three dimensional ultrasound for imaging components of the musculoskeletal system. Edinburgh Research Archive. 2010. https://www.era.lib.ed.ac.uk/handle/1842/4500.
Microbiology pathogen detection
A recent consensus definition of fracture-related infection included the presence of “phenotypically indistinguishable pathogens identified by culture from at least two separate deep tissue/implant (including sonication-fluid) specimens taken during an operative intervention” as one of four confirmatory criteria.55 The use of ultrasound to liberate organisms from bacterial biofilms56-59 and/or stimulate growth60 from clinical samples has been independently reported by a number of research groups. Trampuz et al56 examined the diagnostic performance of sonication and conventional laboratory culture using explanted orthopaedic implants from patients with aseptic failure and patients with prosthetic joint infections. Sonicated fluid cultures yielded causative organisms in higher numbers than did periprosthetic tissue cultures. Of additional clinical importance was that all six false-negative sonicated fluid cultures occurred with patients taking antimicrobial agents, emphasizing the importance of discontinuation of antimicrobial therapy, ideally for at least two weeks, prior to specimen collection. Interestingly, there was greater improvement in bacterial recovery following sonication compared with standard culturing techniques in patients who had only recently stopped antibiotic therapy (< 14 days) or were still receiving concurrent antibiotic therapy at the time of explantation.56 The reduction of false-negative samples is beneficial for the management of fracture-related infections and antimicrobial stewardship. Targeted antimicrobial therapy is not only a key component to successful curative or suppressive management strategies,61 but can also assist in the struggle against the development of antimicrobial resistance.62
Therapeutic ultrasound
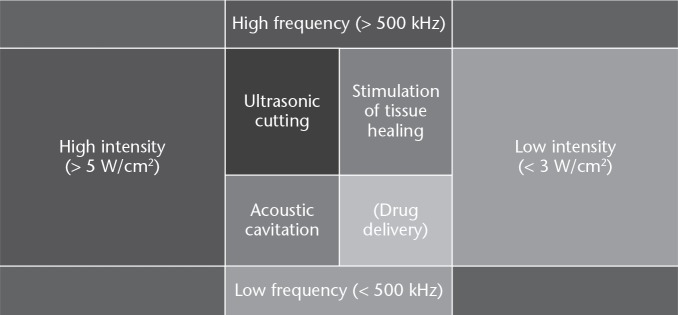
Low-intensity waveforms are aimed at stimulating physiological responses to injury, or accelerating some biological processes, while the purpose of high-intensity treatments is to destroy tissues selectively. In this field, a wide range of ultrasound frequencies have been employed, from approximately 20 kHz up to several MHz; frequencies lower than a few hundred kHz are generally defined as ‘low frequency ultrasound’, and frequencies in the order of 1 MHz and above are generally defined as ‘high frequency ultrasound’. Higher-power ultrasound at lower frequencies (20 kHz to 100 kHz), which is referred to as ‘power ultrasound’, has the ability to cause microbubble formation, also known as cavitation. The resultant interaction between the biological tissues and the waveform can be described as thermal or nonthermal (Fig. 4).63
Fig. 4.
A summary of differing biological effects derived from ultrasound according to frequency and intensity variations.63
Thermal effects
The energy transported by an ultrasonic beam is attenuated as it passes through biological tissue. The energy loss is due to scattering of the ultrasonic beam and absorption by extracellular fluid, resulting in a heating effect. The high absorption coefficients of large protein molecules mean that collagenous tissues, such as cortical bone, myofascial junctions, tendon sheaths, fibrotic muscle, and major nerve trunks, may be heated preferentially. The extent of physiological response to heating is dependent on the peak temperature, rate of temperature rise, time of heating, and heated volume.64
Nonthermal (mechanical) effects
Nonthermal mechanisms that can produce therapeutic changes in biological tissues may be cyclical or noncyclical. Reported macroscopic cyclical effects include local haemodynamic changes65-67 and angiogenesis.68,69 Mechanical effects of ultrasound at a cellular level include stimulation of cell proliferation,70,71 cell membrane depolarization,72 and mast cell degranulation.73
The main noncyclical effect is acoustic cavitation. During the sonication process, pressure waves are created when a sonic wave meets a liquid medium, thereby creating regions of alternating compression and expansion,74 creating unstable microbubbles that fluctuate with the alternating waveform. Eventually the condensed molecules collide violently, creating shock waves, which result in the formation of localized regions of extreme temperature and pressure, up to 5500°C and 50 000 kPa, respectively.
Fracture healing
The use of low-intensity pulsed ultrasound as an adjunct in the management in acceleration of fracture healing in fresh fractures and delayed/nonunions is controversial. The earliest reported trial, performed by Heckman et al,75 found that low-intensity pulsed ultrasound improved the time to clinical and radiological union and overall proportion, achieving union within 150 days in conservatively managed diaphyseal fractures of the tibia. However, the results may have been influenced by one-third of the cohort being lost to follow-up or excluded for deviations in the protocol.75 More recent work investigating the underlying mechanism of low-intensity pulsed ultrasound on fracture healing have proposed multiple cellular effects. It has been reported to induce proliferation of preosteoblast-like bone cells76 and the differentiation of mesenchymal stem cells towards osteogenesis.77 Further in vitro studies have demonstrated that ultrasound stimulation in osteogenesis occurs by mechanotransduction of osteocytes and osteoblasts71,78 via integrin-signalling pathways79 and Piezo ion channels,80 leading to an enhanced local acute inflammatory environment79-81 and subsequent fracture healing.
However, low-intensity ultrasound has not been found to be an effective adjunctive therapy for distraction osteogenesis,82 nor in conjunction with intramedullary nailed acute fractures and osteotomies in a recent, well conducted, multicentre randomized control trial83 and in previous systematic reviews.84,85 There may, however, be a role for ultrasound in the management of impaired fracture healing. A more recent systematic review and meta-analysis, using criteria for defining fracture nonunion as no evidence of radiological union at least three months postinjury, investigated the effects of low-intensity pulsed ultrasound in 1441 fracture nonunions.86 It concluded that ultrasound could be an alternative to surgery for established aseptic nonunions, with > 80% achieving union. Hypertrophic nonunions were found to benefit more than biologically inactive atrophic nonunions. An interval without surgery of more than six months prior to ultrasound treatment was associated with a more favourable result. The role of low-intensity pulsed ultrasound therapy in the management of acute fractures is currently under review by the National Institute of Health and Care Excellence in the United Kingdom,87 but continues to have Food and Drug Administration approval in the United States. With further understanding of the mechanism of ultrasound action and appropriate case selection, a role for therapeutic ultrasound in the management of impaired fracture healing may yet emerge.
In summary, ultrasound is a developing technology in fracture management with potential for both diagnostic and therapeutic use. Emerging evidence has shown the potential of ultrasound as a valid alternative to radiographs in certain clinical situations and, perhaps of greater value, the ability to determine the early presence of callus for the prediction of delayed union. The advent of 3D ultrasound may overcome the previous limitations of 2D ultrasound, producing images that are easier to interpret. The role for ultrasound as a therapeutic adjunct in the management of fracture nonunions remains unclear and under ongoing scrutiny. Further understanding of the acoustic properties of musculoskeletal tissues, as well as the optimization of acoustic parameters, is required to maximize the potential of ultrasound as a diagnostic and therapeutic modality in fracture management.
Footnotes
Author contributions: J. A. Nicholson: Conceptualized the study, Reviewed the literature, Wrote and edited the manuscript.
S. T. J. Tsang: Conceptualized the study, Reviewed the literature, Wrote and edited the manuscript.
T. J. MacGillivray: Reviewed and edited the manuscript.
F. Perks: Reviewed and edited the manuscript.
A. H. R. W. Simpson: Reviewed and edited the manuscript.
Follow us @BoneJointRes
Funding statement
This research was supported by the Royal College of Surgeons of Edinburgh (Joint RCSEd/Cutner Research Fellowship) awarded to S. T. J. Tsang.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Wood RW, Loomis AL. The physical and biological effects of high-frequency sound-waves of great intensity. Lond Edinb Dublin Philos Mag J Sci 1927;4:417-436. [Google Scholar]
- 2. Bejarano F, Feeney A, Wallace R, Simpson H, Lucas M. An ultrasonic orthopaedic surgical device based on a cymbal transducer. Ultrasonics 2016;72:24-33. [DOI] [PubMed] [Google Scholar]
- 3. Hennet P. Piezoelectric bone surgery: a review of the literature and potential applications in veterinary oromaxillofacial surgery. Front Vet Sci 2015;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lento PH, Primack S. Advances and utility of diagnostic ultrasound in musculoskeletal medicine. Curr Rev Musculoskelet Med 2008;1:24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4:341-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho KH, Lee YH, Lee SM, et al. Sonography of bone and bone-related diseases of the extremities. J Clin Ultrasound 2004;32:511-521. [DOI] [PubMed] [Google Scholar]
- 7. Weeks BK, Hirsch R, Nogueira RC, Beck BR. Is calcaneal broadband ultrasound attenuation a valid index of dual-energy x-ray absorptiometry-derived bone mass in children? Bone Joint Res 2016;5:538-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katz R, Landman J, Dulitzky F, Bar-Ziv J. Fracture of the clavicle in the newborn. An ultrasound diagnosis. J Ultrasound Med 1988;7:21-23. [DOI] [PubMed] [Google Scholar]
- 9. Blab E, Geissler W, Rokitansky A. Sonographic management of infantile clavicular fractures. Pediatr Surg Int 1999;15:251-254. [DOI] [PubMed] [Google Scholar]
- 10. Smeets AJ, Robben SGF, Meradji M. Sonographically detected costo-chondral dislocation in an abused child. A new sonographic sign to the radiological spectrum of child abuse. Pediatr Radiol 1990;20:566-567. [DOI] [PubMed] [Google Scholar]
- 11. Battistelli JM, Anselem B. [Echography in injuries of costal cartilages]. J Radiol 1993;74:409-412. [PubMed] [Google Scholar]
- 12. Hübner U, Schlicht W, Outzen S, Barthel M, Halsband H. Ultrasound in the diagnosis of fractures in children. J Bone Joint Surg [Br] 2000;82-B:1170-1173. [DOI] [PubMed] [Google Scholar]
- 13. Williamson D, Watura R, Cobby M. Ultrasound imaging of forearm fractures in children: a viable alternative? J Accid Emerg Med 2000;17:22-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ackermann O, Liedgens P, Eckert K, et al. Sonographische Diagnostik von metaphysären Wulstbrüchen: eine prospektive Multicenterstudie. Unfallchirurg 2009;112:706-711. (Article in German) [DOI] [PubMed] [Google Scholar]
- 15. Friend AJ, Roland D. Distal forearm fractures can be reliably diagnosed using ultrasound. Arch Dis Child Educ Pract Ed 2017;102:275. [DOI] [PubMed] [Google Scholar]
- 16. Galletebeitia Laka I, Samson F, Gorostiza I, Gonzalez A, Gonzalez C. The utility of clinical ultrasonography in identifying distal forearm fractures in the pediatric emergency department. Eur J Emerg Med 2019;26:118-122. [DOI] [PubMed] [Google Scholar]
- 17. Wang CL, Shieh JY, Wang TG, Hsieh FJ. Sonographic detection of occult fractures in the foot and ankle. J Clin Ultrasound 1999;27:421-425. [DOI] [PubMed] [Google Scholar]
- 18. Hendrich C, Finkewitz U, Berner W. Diagnostic value of ultrasonography and conventional radiography for the assessment of sternal fractures. Injury 1995;26:601-604. [DOI] [PubMed] [Google Scholar]
- 19. Jin W, Yang DM, Kim HC, Ryu KN. Diagnostic values of sonography for assessment of sternal fractures compared with conventional radiography and bone scans. J Ultrasound Med 2006;25:1263-1268. [DOI] [PubMed] [Google Scholar]
- 20. Griffith JF, Rainer TH, Ching AS, et al. Sonography compared with radiography in revealing acute rib fracture. AJR Am J Roentgenol 1999;173:1603-1609. [DOI] [PubMed] [Google Scholar]
- 21. Chern TC, Jou IM, Lai KA, et al. Sonography for monitoring closed reduction of displaced extra-articular distal radial fractures. J Bone Joint Surg [Am] 2002;84-A:194-203. [DOI] [PubMed] [Google Scholar]
- 22. McManus JG, Morton MJ, Crystal CS, et al. Use of ultrasound to assess acute fracture reduction in emergency care settings. Am J Disaster Med 2008;3:241-247. [PubMed] [Google Scholar]
- 23. Atkinson P, Lennon R. Use of emergency department ultrasound in the diagnosis and early management of femoral fractures. Emerg Med J 2003;20:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marshburn TH, Legome E, Sargsyan A, et al. Goal-directed ultrasound in the detection of long-bone fractures. J Trauma 2004;57:329-332. [DOI] [PubMed] [Google Scholar]
- 25. Leow JM, Clement ND, Tawonsawatruk T, Simpson CJ, Simpson AHRW. The radiographic union scale in tibial (RUST) fractures: reliability of the outcome measure at an independent centre. Bone Joint Res 2016;5:116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young JW, Kostrubiak IS, Resnik CS, Paley D. Sonographic evaluation of bone production at the distraction site in Ilizarov limb-lengthening procedures. AJR Am J Roentgenol 1990;154:125-128. [DOI] [PubMed] [Google Scholar]
- 27. Derbyshire NDJ, Simpson AHRW. A role for ultrasound in limb lengthening. Br J Radiol 1992;65:576-580. [DOI] [PubMed] [Google Scholar]
- 28. Malde HM, Hemmadi SS, Chadda D, et al. The role of skeletal sonography in limb lengthening procedures. J Postgrad Med 1993;39:127-129. [PubMed] [Google Scholar]
- 29. Eyres KS, Bell MJ, Kanis JA. Methods of assessing new bone formation during limb lengthening. Ultrasonography, dual energy X-ray absorptiometry and radiography compared. J Bone Joint Surg [Br] 1993;75-B:358-364. [DOI] [PubMed] [Google Scholar]
- 30. Craig JG, Jacobson JA, Moed BR. Ultrasound of fracture and bone healing. Radiol Clin North Am 1999;37:737-751. [DOI] [PubMed] [Google Scholar]
- 31. Ricciardi L, Perissinotto A, Dabala M. Mechanical monitoring of fracture healing using ultrasound imaging. Clin Orthop Relat Res 1993;293:71-76. [PubMed] [Google Scholar]
- 32. Maffulli N, Thornton A. Ultrasonographic appearance of external callus in long-bone fractures. Injury 1995;26:5-12. [DOI] [PubMed] [Google Scholar]
- 33. Wawrzyk M, Sokal J, Andrzejewska E, Przewratil P. The role of ultrasound imaging of callus formation in the treatment of long bone fractures in children. Pol J Radiol 2015;80:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moed BR, Watson JT, Goldschmidt P, van Holsbeeck M. Ultrasound for the early diagnosis of fracture healing after interlocking nailing of the tibia without reaming. Clin Orthop Relat Res 1995;310:137-144. [PubMed] [Google Scholar]
- 35. Moed BR, Subramanian S, van Holsbeeck M, et al. Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J Orthop Trauma 1998;12:206-213. [DOI] [PubMed] [Google Scholar]
- 36. Keating JF, O’Brien PJ, Blachut PA, Meek RN, Broekhuyse HM. Locking intramedullary nailing with and without reaming for open fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg [Am] 1997;79-A:334-341. [DOI] [PubMed] [Google Scholar]
- 37. Court-Brown CM. The clinical results of reamed intramedullary. Tech Orthop 1996;11:79-85. [Google Scholar]
- 38. Bottinelli O, Calliada F, Campani R. [Bone callus: possible assessment with color Doppler ultrasonography. Normal bone healing process]. [Article in Italian] Radiol Med 1996;91:537-541. [PubMed] [Google Scholar]
- 39. Caruso G, Lagalla R, Derchi L, Iovane A, Sanfilippo A. Monitoring of fracture calluses with color Doppler sonography. J Clin Ultrasound. 2000;28:20-27. [DOI] [PubMed] [Google Scholar]
- 40. Rawool NM, Goldberg BB, Forsberg F, Winder AA, Hume E. Power Doppler assessment of vascular changes during fracture treatment with low-intensity ultrasound. J Ultrasound Med 2003;22:145-153. [DOI] [PubMed] [Google Scholar]
- 41. Sun MH, Leung KS, Zheng YP, et al. Three-dimensional high frequency power Doppler ultrasonography for the assessment of microvasculature during fracture healing in a rat model. J Orthop Res 2012;30:137-143. [DOI] [PubMed] [Google Scholar]
- 42. Zanetti M, Metzdorf A, Kundert HP, et al. Achilles tendons: clinical relevance of neovascularization diagnosed with power Doppler US. Radiology. 2003;227:556-560. [DOI] [PubMed] [Google Scholar]
- 43. Adler RS, Fealy S, Rudzki JR, et al. Rotator cuff in asymptomatic volunteers: contrast-enhanced US depiction of intratendinous and peritendinous vascularity. Radiology 2008;248:954-961. [DOI] [PubMed] [Google Scholar]
- 44. Cadet ER, Adler RS, Gallo RA, et al. Contrast-enhanced ultrasound characterization of the vascularity of the repaired rotator cuff tendon: short-term and intermediate-term follow-up. J Shoulder Elbow Surg 2012;21:597-603. [DOI] [PubMed] [Google Scholar]
- 45. Treece GM, Gee AH, Prager RW, Cash CJC, Berman LH. High-definition freehand 3-D ultrasound. Ultrasound Med Biol 2003;29:529-546. [DOI] [PubMed] [Google Scholar]
- 46. Cenni F, Monari D, Desloovere K, et al. The reliability and validity of a clinical 3D freehand ultrasound system. Comput Methods Programs Biomed 2016;136:179-187. [DOI] [PubMed] [Google Scholar]
- 47. MacGillivray TJ, Ross E, Simpson HA, Greig CA. 3D freehand ultrasound for in vivo determination of human skeletal muscle volume. Ultrasound Med Biol 2009;35:928-935. [DOI] [PubMed] [Google Scholar]
- 48. Barber L, Barrett R, Lichtwark G. Validation of a freehand 3D ultrasound system for morphological measures of the medial gastrocnemius muscle. J Biomech 2009;42:1313-1319. [DOI] [PubMed] [Google Scholar]
- 49. Rana M, Wakeling JM. In-vivo determination of 3D muscle architecture of human muscle using free hand ultrasound. J Biomech 2011;44:2129-2135. [DOI] [PubMed] [Google Scholar]
- 50. Cash CJC, Treece GM, Berman LH, Gee AH, Prager RW. 3D reconstruction of the skeletal anatomy of the normal neonatal foot using 3D ultrasound. Br J Radiol 2005;78:587-595. [DOI] [PubMed] [Google Scholar]
- 51. Serafin-Król M, Król R, Jedrzejczyk M, et al. Potential value of contrast-enhanced gray-scale ultrasonography in diagnosis of acute muscle injury—preliminary results. Ortop Traumatol Rehabil 2008;10:131-136. [PubMed] [Google Scholar]
- 52. Cash CJC, Sardesai AM, Berman LH, et al. Spatial mapping of the brachial plexus using three-dimensional ultrasound. Br J Radiol 2005;78:1086-1094. [DOI] [PubMed] [Google Scholar]
- 53. Simpson AHRW. The forgotten phase of fracture healing: the need to predict nonunion. Bone Joint Res 2017;6:610-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ross E. Freehand three dimensional ultrasound for imaging components of the musculoskeletal system. Edinburgh Research Archive. 2010. https://www.era.lib.ed.ac.uk/handle/1842/4500 (date last accessed 16 May 2019).
- 55. Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury 2018;49:505-510. [DOI] [PubMed] [Google Scholar]
- 56. Trampuz A, Piper KE, Hanssen AD, et al. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J Clin Microbiol 2006;44:628-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kobayashi H, Oethinger M, Tuohy MJ, Procop GW, Bauer TW. Improved detection of biofilm-formative bacteria by vortexing and sonication: a pilot study. Clin Orthop Relat Res 2009;467:1360-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Portillo ME, Salvadó M, Alier A, et al. Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J Infect 2014;69:35-41. [DOI] [PubMed] [Google Scholar]
- 59. Ensing GTT, Roeder BLL, Nelson JLL, et al. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J Appl Microbiol 2005;99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Monsen T, Lövgren E, Widerström M, Wallinder L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J Clin Microbiol 2009;47:2496-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Metsemakers WJW, Kuehl R, Moriarty TFT, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury 2018;49:511-522. [DOI] [PubMed] [Google Scholar]
- 62. Nathwani D. British Society for Antimicrobial Chemotherapy. Antimicrobial stewardship: from principles to practice, 2018. http://www.bsac.org.uk/antimicrobialstewardshipebook/BSAC-AntimicrobialStewardship-FromPrinciplestoPractice-eBook.pdf. (date last accessed 22 May 2018).
- 63. Erriu M, Blus C, Szmukler-Moncler S, et al. Microbial biofilm modulation by ultrasound: current concepts and controversies. Ultrason Sonochem 2014;21:15-22. [DOI] [PubMed] [Google Scholar]
- 64. ter Haar G. Therapeutic ultrasound. Eur J Ultrasound 1999;9:3-9. [DOI] [PubMed] [Google Scholar]
- 65. Dyson M. Mechanisms involved in therapeutic ultrasound. Physiotherapy 1987;73:116-120. [Google Scholar]
- 66. Hogan RD, Franklin TD, Fry FJ, Avery KA, Burke KM. The effect of ultrasound on microvascular hemodynamics in skeletal muscle: effect on arterioles. Ultrasound Med Biol 1982;8:45-55. [DOI] [PubMed] [Google Scholar]
- 67. Hogan RD, Burke KM, Franklin TD. The effect of ultrasound on microvascular hemodynamics in skeletal muscle: effects during ischemia. Microvasc Res 1982;23:370-379. [DOI] [PubMed] [Google Scholar]
- 68. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353-364. [DOI] [PubMed] [Google Scholar]
- 69. Hansen-Smith FM, Hudlicka O, Egginton S. In vivo angiogenesis in adult rat skeletal muscle: early changes in capillary network architecture and ultrastructure. Cell Tissue Res 1996;286:123-136. [DOI] [PubMed] [Google Scholar]
- 70. Ramirez A, Schwane JA, McFarland C, Starcher B. The effect of ultrasound on collagen synthesis and fibroblast proliferation in vitro. Med Sci Sports Exerc 1997;29:326-332. [DOI] [PubMed] [Google Scholar]
- 71. Bayat M, Virdi A, Rezaei F, Chien S. Comparison of the in vitro effects of low-level laser therapy and low-intensity pulsed ultrasound therapy on bony cells and stem cells. Prog Biophys Mol Biol 2018;133:36-48. [DOI] [PubMed] [Google Scholar]
- 72. Dyson M. Non-thermal cellular effects of ultrasound. Br J Cancer Suppl 1982;5:165-171. [PMC free article] [PubMed] [Google Scholar]
- 73. Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther 2001;81:1351-1358. [PubMed] [Google Scholar]
- 74. Sala FJ, Burgos J, Condón S, Lopez P, Raso J. Effect of heat and ultrasound on microorganisms and enzymes. In: Gould GW, ed. New methods of food preservation. Boston: Springer US, 1995:176-204. [Google Scholar]
- 75. Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg [Am] 1994;76-A:26-34. [DOI] [PubMed] [Google Scholar]
- 76. Katiyar A, Duncan RL, Sarkar K. Ultrasound stimulation increases proliferation of MC3T3-E1 preosteoblast-like cells. J Ther Ultrasound 2014;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kusuyama J, Bandow K, Shamoto M, et al. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem 2014;289:10330-10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fung CH, Cheung WH, Pounder NM, Harrison A, Leung KS. Osteocytes exposed to far field of therapeutic ultrasound promotes osteogenic cellular activities in pre-osteoblasts through soluble factors. Ultrasonics 2014;54:1358-1365. [DOI] [PubMed] [Google Scholar]
- 79. Tang CH, Lu DY, Tan TW, Fu WM, Yang RS. Ultrasound induces hypoxia-inducible factor-1 activation and inducible nitric-oxide synthase expression through the integrin/integrin-linked kinase/Akt/mammalian target of rapamycin pathway in osteoblasts. J Biol Chem 2007;282:25406-25415. [DOI] [PubMed] [Google Scholar]
- 80. Gao Q, Cooper PR, Walmsley AD, Scheven BA. Role of Piezo channels in ultrasound-stimulated dental stem cells. J Endod 2017;43:1130-1136. [DOI] [PubMed] [Google Scholar]
- 81. Li L, Yang Z, Zhang H, et al. Low-intensity pulsed ultrasound regulates proliferation and differentiation of osteoblasts through osteocytes. Biochem Biophys Res Commun 2012;418:296-300. [DOI] [PubMed] [Google Scholar]
- 82. Simpson AH, Keenan G, Nayagam S, et al. Low-intensity pulsed ultrasound does not influence bone healing by distraction osteogenesis: a multicentre double-blind randomised control trial. Bone Joint J 2017;99-B:494-502. [DOI] [PubMed] [Google Scholar]
- 83. TRUST Investigators writing group, Busse JW, Bhandari M, et al. Re-evaluation of low intensity pulsed ultrasound in treatment of tibial fractures (TRUST): randomized clinical trial. BMJ 2016;355:i5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schandelmaier S, Kaushal A, Lytvyn L, et al. Low intensity pulsed ultrasound for bone healing: systematic review of randomized controlled trials. BMJ 2017;356:j656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Poolman RW, Agoritsas T, Siemieniuk RAC, et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: a clinical practice guideline. BMJ 2017;356:j576. [DOI] [PubMed] [Google Scholar]
- 86. Leighton R, Watson JT, Giannoudis P, et al. Healing of fracture nonunions treated with low-intensity pulsed ultrasound (LIPUS): a systematic review and meta-analysis. Injury 2017;48:1339-1347. [DOI] [PubMed] [Google Scholar]
- 87. No authors listed. National Institute for Health and Care Excellence (NICE). Low-intensity pulsed ultrasound to promote healing of fresh fractures at high risk of non-healing: guidance and guidelines, 2018. https://www.nice.org.uk/guidance/indevelopment/gid-ipg10084 (date last accessed 28 May 2018).