Abstract
Ebola virus disease (EVD), a fatal viral hemorrhagic illness, is due to infection with the Ebola virus of the Filoviridae family. The disease has evolved as a global public health menace due to a large immigrant population. Initially, the patients present with nonspecific influenza-like symptoms and eventually terminate into shock and multiorgan failure. There exists no specific treatment protocol for EVD and only supportive and symptomatic therapy is the line of treatment. This review article provides a detailed overview of the Ebola virus; it's clinical and oral manifestations, diagnostic aids, differential diagnosis, preventive aspects, and management protocol.
Keywords: Ebola virus, oral manifestations, public health menace, symptomatic therapy
Introduction
Ebola, earlier termed as Ebola hemorrhagic fever (EHF), is a critically lethal ailment which primarily affects the humans and nonhuman primates. Ebola virus disease (EVD) occurs due to a virus infection which belongs to the family Filoviridae and genus Ebolavirus.[1] EVDs has posed diagnostic challenges and has been a universal public health threat since its discovery. While investigating an alleged yellow fever case, Dr. Peter Piot in the year 1976 first detected the disease in Zaire, Africa (presently the Democratic Republic of Congo).[2] The name “Ebola” was termed as the disease was noticed near the Ebola river in Congo.[3]
Fruit bats of Pteropodidae family, such as Hypsignathus monstrous, Epomops franqueti, and Myonycteris torquata serve as the natural hosts of the EBOV in Africa. Nonhuman primates may develop the infection by eating the partly eaten fruits and may also transmit the infection to humans.[4] Indian population is an impending threat to EVD, as India falls in the home range of Pteropodidae family of fruit bats.[5]
Ebola virus transmission primarily takes place through close bodily contact with the infected patient or their fluids, contaminated tissue surfaces, and clothing from alive, infected or deceased individuals. Unsafe traditional burial practices also play a pivotal role in the disease transmission.[6] There is documented evidence regarding the sexual mode of disease transmission, although transmission through the air is unlikely.[7]
EVD present with bizarre and atypical manifestations mimicking other viral diseases, especially in the initial disease phase. Constitutional symptoms, such as fever, myalgia, headache, vomiting, and diarrhea are the early presenting features. Hemorrhagic rash, internal and external bleeding are usually the warning manifestations in the late stages.[8] Bleeding from the body apertures is a distinguishing EVD manifestation.[9] Gum bleeding, odynophagia, and atypical oral manifestations constitute the oral features of EVD.[10]
Till date, there is no precise antiviral management or vaccination for EVD. The management protocol mainly relies on supportive and symptomatic therapy, along with monitoring coagulopathies and multiorgan dysfunction.[2]
The World Health Organization (WHO) affirmed the EVD outbreak as a “Public Health Emergency of International Concern” on August 8th, 2014.[5]
With the enormous immigrant population, India is estimating the likelihood of a probable EVD outbreak. The Ministry of Health and Family Welfare, Government of India, in collaboration with other agencies has appraised the situation and recommended travel instructions by air, land, and sea and health care professionals.[11]
Taxonomy
The virus belongs to the Ebola virus genus, Filoviridae family, and Mononegavirales order.[12] The genus Ebolavirus includes the following species- Zaire ebolavirus (EBOV), Reston ebolavirus (RESTV), Bundibugyo ebolavirus (BDBV), Taï Forest ebolavirus (TAFV), Sudan ebolavirus (SUDV), and the newly identified Bombali ebolavirus (BOMV).[13] Except for exclusive identification of RESTV in the Philippines, all the other species causes endemic West African EVD.[14]
EBOV responsible for the EHF causes the highest human mortality (57%–90%), followed by SUDV (41%–65%) and Bundibugyo virus (40%). TAFV has caused only two nonlethal human infections to date, whereas RESTV causes asymptomatic human infections.[15]
Figure 1 shows the taxonomy of Ebola virus.
Figure 1.
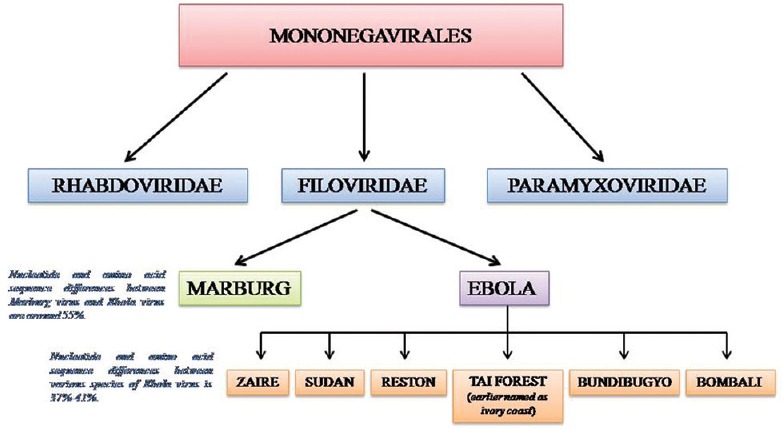
Taxonomy of Ebola virus
Transmission
Based on the Centers for Disease Control and Prevention (CDC) classification, Ebola virus is considered as a biosafety level 4 and category A bioterrorism pathogen with an immense likelihood for massive nationwide transmission.[16]
Source of Infection
Intimate physical contact with the patients in the acute disease stages and contact with the blood/fluids from the dead individuals constitutes the most important modes of transmission.[17]
The long-established funeral ceremonies in the African countries entail direct handling of the dead bodies, thus significantly contributing to the disease dissemination. Unsafe conventional burial procedures accounted for 68% infected cases in 2014 EVD outburst of Guinea.[18]
EBOV RNA may be identified for up to a month in rectal, conjunctival, and vaginal discharges and semen specimens may demonstrate the virus presence up to 3 months, thus signifying the presence of EBOV in recuperating patients.[14] The sexually transmitted case of EVD has been reported between a convalescent patient and close family member. Another study demonstrated a case in a recuperating male patient. The patient's semen specimen tested positive with Ebola viral antigen almost 3 months after the disease onset.[19]
Asymptomatic EBOV carriers are not infectious and do not have a major role play in the EVD outburst, and the field practice in Western Africa supported this assumption.[20] However, this presumption was refuted after the documentation of a pioneer asymptomatic carrier case in North Gabon epidemic (1996).[21]
EBOV has been detected from blood, saliva, semen, and breast milk, while RNA has been isolated from sweat, tears, stool, and on the skin, vaginal, and rectal swabs, thus highlighting that exposure to infected blood and bodily secretions constitute the major means of dissemination.[22]
Eating uncooked infected animal meat such as bats or chimpanzees account significantly to oral EVD transmission, especially in the African countries.[23] The demonstration of the Ebola virus in the Filipino pigs in 2008 triggered the likelihood of an extensive range of possible animal hosts.[24]
EVD dissemination has also been reported with hospital-acquired infections, particularly in areas with poor hygiene conditions. The infected needles usage was responsible for the 1976 EVD outbreak in Sudan and Zaire.[25,26] Improper hygiene and sterilization were the crucial factors for the 1967 Yambuku EVD outburst.[27]
EVD dissemination may also occur through the inanimate materials with infected body secretions (fomites).[19] However, disease transmission through the airborne and droplet infection is ambiguous.[10]
Figure 2 shows the primary and secondary transmission of disease.
Figure 2.
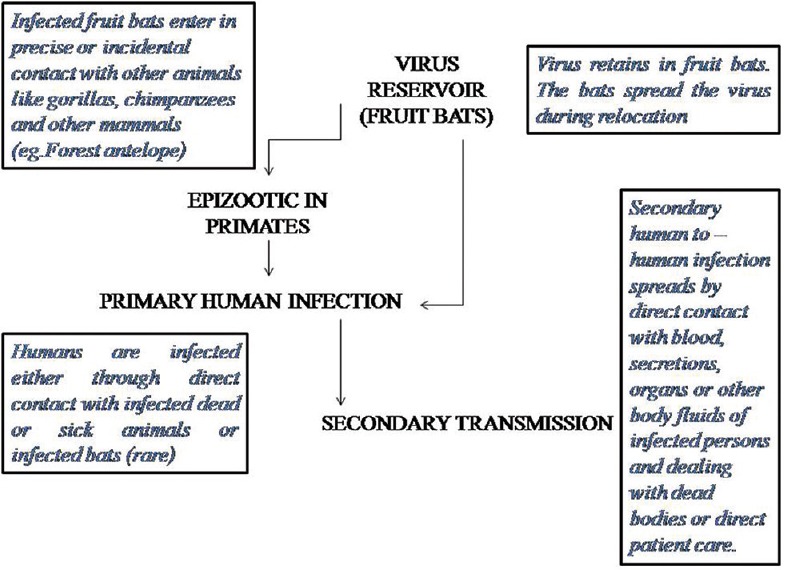
Primary and secondary transmission
Table 1 depicts the possible routes of transmission.
Table 1.
Possible routes of transmission
| Mode of transmission | Consensus likelihood of occurring | Known facts | Unknown facts |
|---|---|---|---|
| Airborne/aerosol (small droplet/droplet nuclei) | Unlikely from epidemiology of disease | EBOV can be aerosolized mechanically and cause lethal disease in nonhuman primates at low concentrations[2,3] | Ability of the virus to become airborne through respiratory tract in humans and animals. |
| Outbreaks contained without airborne precautions in the affected population[4] | Airborne stability of EBOV in tropical climates. | ||
| EBOV detected after 90 min in experimental small aerosols[5] | Whether aerosol generating procedures (AGPs) produce EBOV aerosols that cause transmission | ||
| Fomites | Less likely from environmental sampling | Virus found in dried blood[6] | EBOV stability in tropical climates and on surfaces |
| Persists on glass and in the dark for 5.9 days[7] | |||
| Droplet (large droplet) | Likely from epidemiology and experiments | EBOV found in stool, semen, saliva, breast milk[6] | Whether infectious fluids are formed into droplets by humans |
| Accidental infections in nonhuman primates, possibly from power washing[8,9] | |||
| Range of droplets containing EBOV. | |||
| EBOV infections without direct contact[10] | |||
| Bodily fluids contact | Very likely from epidemiology and experimental data | Sharing needles and handling the deceased or sick are high risk factors[11] | How much virus is shed in different fluids |
| EBOV found in a variety of bodily fluids[6] |
Epidemiology
The vast majority of EVD cases and outbursts have been endemic to African continent ever since the disease detection in 1976,[28] and 36 such outbreaks have occurred in six African countries.[29]
Table 2 shows Ebola epidemiological outbreaks between 1976 and 2014.
Table 2.
Ebola outbreaks between 1976 and 2014 (Adapted from WHO 2014)
| Year | Country/village | Ebola virus subtype | Number of human cases | Number of deaths | Mortality | Source and spread infection |
|---|---|---|---|---|---|---|
| 1976 | Sudan, Nzara and Marida | Sudan virus | 284 | 151 | 53% | Close contact within hospitals, infecting many hospital staff |
| 1976 | Zaire, Yambuku | Ebola virus | 318 | 280 | 88% | Contaminated needles and syringes in hospitals |
| 1976 | England | Sudan virus | 1 | 0 | Laboratory infection; accidental stick of contaminated needles | |
| 1977 | Zaire, Tandala | Sudan virus | 1 | 1 | 100% | Noted retrospectively |
| 1979 | Sudan, Nzara and Marida | Sudan virus | 34 | 22 | 65% | Recurrent outbreak at the same site as 1976 |
| 1989 | USA, Virginia, Pennsylvania | Reston virus | 0 | 0 | Ebola virus was introduced in to quarantine facility by monkeys from the Philippines | |
| 1989-1990 | Philippines | Reston virus | 3 | 0 | Source: Macaques from USA. Three workers (animal facility) developed antibodies, did not get sick. | |
| 1990 | USA, Virginia | 4 | 0 | The same to 1989 | ||
| 1994 | Gabon | Ebola virus | 52 | 31 | 60% | Initially thought to be yellow fever; identified as Ebola in 1995 |
| 1994 | Cote d’Ivoire | Tai forest virus | 1 | 0 | Scientist became ill after autopsy on a wild chimpanzee (Tai Forest) | |
| 1995 | Democratic Republic of Congo (Zaire) | Ebola virus | 315 | 250 | 81% | Case-patient worked in the forest; spread through families and hospitals |
| 1996 | Gabon | Ebola virus | 37 | 21 | 57% | Chimpanzee found dead in the forest was eaten by hunters; spread in families |
| 1996-1997 | Gabon | Ebola virus | Case-patient was a hunter from forest camp; spread by cloth contact | |||
| 1996 | South Africa | Ebola virus | 2 | 1 | 50% | Infected medical professional travelled |
| 1996 | Russia | Ebola virus | 1 | 1 | 100% | Laboratory contamination |
| 2000-2001 | Uganda | Sudan virus | 425 | 223 | 53% | Providing medical care to Ebola case-patient without using adequate personal protection measures |
| 2001-2002 | Gabon | Ebola virus | 65 | 53 | 82% | Outbreak occurred over border of Gabon and Republic of Congo |
| 2001-2002 | Republic of the Congo | Ebola virus | 57 | 43 | 75% | Outbreak occurred over border of Gabon and Republic of Congo |
| 2002-2003 | Republic of the Congo | Ebola virus | 143 | 128 | 89% | Outbreaks in the district of Mboma and Kelle in Cuvette Quest Department |
| 2003 | Republic of the Congo | Ebola virus | 35 | 29 | 83% | Outbreaks in the villages of Mboma district, Cuvette Quest Department |
| 2004 | Sudan, Yambia | Sudan virus | 17 | 7 | 41% | Outbreak concurrent with an outbreak of measles, and several cases were later reclassified as measles |
| 2004 | Russia | Ebola virus | 1 | 1 | 100% | Laboratory infection |
| 2007 | Democratic Republic of the Congo | Ebola virus | 264 | 187 | 71% | The outbreak was declared on November 20. Last death on October 10 |
| 2007-2008 | Uganda | Bundibugyo virus | 149 | 37 | 25% | First reported occurrence of a new strain |
| 2008 | Philippines | Reston virus | 6 | 0 | Six pig farm workers developed antibodies; did not become ill | |
| 2008-2009 | Democratic Republic of the Congo | Ebola virus | 32 | 15 | 47% | Not well identified |
| 2011 | Uganda | Sudan virus | 1 | 1 | 100% | The Uganda Ministry of Health informed the public that a patient with suspected Ebola died on May 6th 2011 |
| 2012 | Uganda, Kibaale | Sudan virus | 11 | 4 | 36% | Laboratory tests of blood samples were conducted by UVRI and CDC |
| 2012 | Democratic Republic of the Congo | Bundibugyo virus | 36 | 13 | 36% | This outbreak has no link to the contemporaneous Ebola outbreak in kibaale, Uganda |
| 2012-2013 | Uganda | Sudan virus | 6 | 3 | 50% | CDC assisted the ministry of Health in the epidemiology and diagnosis of the outbreak |
| 2014 | Democratic Republic of the Congo | Zaire virus | 66 | 49 | 74% | The outbreak was unrelated to the outbreak of West Africa |
UVRI: Uganda Virus Research Institute; CDC: Centers for Disease Control and Prevention
The 2014–2016 EVD started in South East Guinea rural surroundings and eventually became a global public health menace by rapidly disseminating to urban localities and other countries.[28]
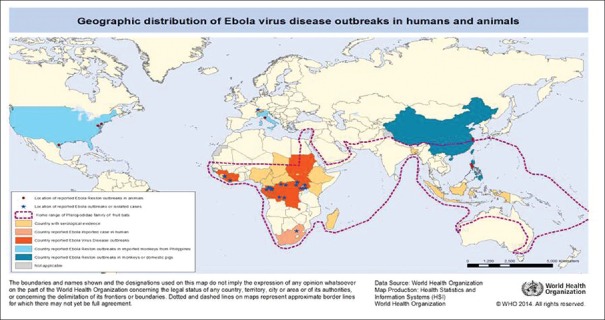
Figure 3 depicts the geographical distribution of Ebola virus disease.
Figure 3.
Geographic distribution of Ebola virus disease outbreaks
The conducive environmental surroundings of the African continent facilitate EVD endemicity. However, intermittent imported Ebola cases have also been noticed in United States, United Kingdom, Canada, Spain, and Thailand.[30,31]
Figure 4 depicts the distribution of Ebola virus disease in West African Countries.
Figure 4.
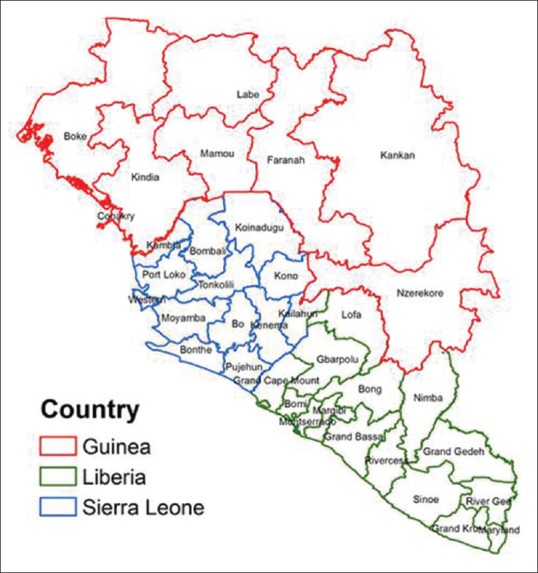
Distribution of Ebola virus disease in West African Countries
Out of the unparalleled globally reported 28,616 cases and 11,310 casualties, Liberia accounted for almost 11,000 cases and over 4,800 deaths.[32]
Table 3 shows the statistics of the 2014–16 West African outbreak.
Table 3.
Statistics of 2014-16 West African outbreak
| WHO report date | Guinea total cases | Guinea total deaths | Liberia total cases | Liberia total deaths | Siera Leone total cases | Sierra Leone total deaths | Total cases | Total deaths |
|---|---|---|---|---|---|---|---|---|
| 13th APRIL 2016 | 3814 | 2544 | 10678 | 4810 | 14124 | 3956 | 28616 | 11310 |
Pathogenesis
Ebola viruses penetrate the human body through mucous membranes, skin lacerations/tear, close contact with infected patients/corpse, or by direct parental dissemination.[33] EBOV has a predilection to infect various cells of immune system (dendritic cells, monocytes, and macrophages), endothelial and epithelial cells, hepatocytes, and fibroblasts where it actively replicates by gene modulation and apoptosis and demonstrate significantly high viremia.[34] The virus reaches the regional lymph nodes causing lymphadenopathy and hematogenous spread to the liver and spleen promote an active inflammatory response.[35] Release of chemical mediators of inflammation (cytokines and chemokines) causes a dysregulated immune response by disrupting the vasculature system harmony, eventually causing disseminated intravascular coagulation and multiple organ dysfunction.[36]
Figure 5 demonstrates the pathogenesis of Ebola virus disease.
Figure 5.
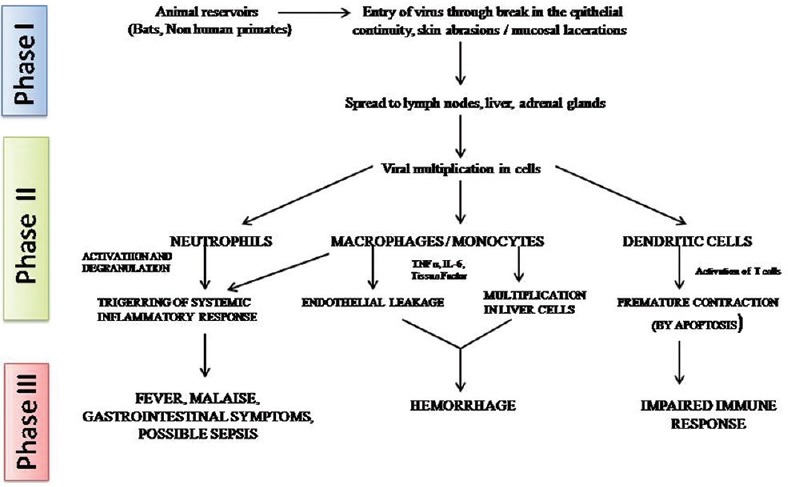
Pathogenesis of Ebola virus disease
Clinical Features
Due to the bizarre and atypical manifestations in the initial phase, mimicking dengue fever, typhoid fever, malaria, meningococcemia, and other bacterial infections, EVD poses diagnostic dilemmas.[37]
The incubation period ranges from 2 to 21 days. However, symptoms usually develop 8–11 days following infection.[38,39]
The initial disease phase is represented by constitutional symptoms.[40] High-grade fever of >38o C is the most frequently reported symptom (85–95%), followed by other vague symptoms such as general malaise (85–95%), headaches (52–74%), dysphagia, sore throat (56–58%), and dry cough.[41,42] The progressively advanced disease is accompanied by abdominal pain (62–68%), myalgia (50–79%), nausea, vomiting, and diarrhea (84–86%).[41]
Variety of hemorrhagic manifestations forms an integral component of the late disease phase.[38] Gastrointestinal tract bleeding manifests as petechiae, hematuria, melena, conjunctival bleeding, contusion, or intraperitoneal bleeding. Mucous membrane and venipuncture site bleeding, along with excess clot formation may also occur. As the features advances with time, the patients experience dehydration, confusion, stupor, hypotension, and multiorgan dysfunction, resulting in fulminant shock and ultimately death.[43,44]
Maculopapular exanthema constitutes a characteristic manifestation of all Filovirus infection, including EVD.[45] The rash usually appears during the 5th to 7th day of disease and occur in 25–52% of patients in the past EVD outbreaks.[46]
Table 4 shows the clinical manifestations of Ebola virus disease.
Table 4.
Clinical manifestations of Ebola virus disease
| Days | Phase | Main features | Other features |
|---|---|---|---|
| O-3 | Early febrile | Fever | Malaise, fatigue, body ache |
| 3-10 | Gastrointestinal | Epigastric pain, nausea, vomiting, diarrhoea | Persistent fever, headache, conjunctival injection, abdominal and chest pain, arthralgia, myalgia, hiccups, delirium |
| 7-12 | Shock or recovery | Shock: diminished consciousness or coma | Recovery |
| Rapid thread pulse, oliguria, anuria, tachypnea | Resolution of gastrointestinal symptoms, increased apetite, increased energy. | ||
| ≥ 10 | Late complications | Gastrointestinal hemorrhage | Secondary infections: oral/esophageal candidiasis, persistent neurocognitive abnormalities |
Although EVD has a number of similar features with other viral hemorrhagic fevers (e.g. dengue), there are differences that set them apart.
Table 5 depicts the differentiating features of the Ebola virus and dengue virus infection.
Table 5.
Differentiating features of Ebola and dengue virus infection
| Differentiating features | Dengue | Ebola |
|---|---|---|
| Incubation period | 3-14 days | 2-21 days |
| Etiology | RNA virus belongs to the genus Flavivirus of family Flaviviridae | RNA virus belongs to the genus Ebola virus of family Filoviridae |
| Mode of transmission | Arthropod borne | Direct contact with infected blood/body fluids and environment contaminated with these secretions |
| Human-human transmission | No | Yes |
| Mortality | 0.04%-0.05% | 50%-90% |
| Typical signs and symptoms | ||
| Fever | Common severely high fever (≥40°) lasts for 4-7 days | Common |
| High fever (≥38°) | ||
| Headache | Common and high intensity (usually retrobulbar) | Common and high intensity |
| Muscle ache and pain | Common and severely intense (known as break bone fever) | Common |
| Nausea and vomiting | Common | Common |
| Ocular involvement | Nonpurulent conjunctivitis | Conjunctival injection; subconjunctival hemorrhage |
| Diarrhea | Uncommon | Common estimated 5 L or more of watery diarrhea per day, lasting for up to 7 days and sometimes longer |
| Bleeding | Unusual | Usual |
| Bleeding from body orifices is a prominent feature | ||
| Rash (maculopapular exanthema) | Moderately elevated; initial rash occurs before or during 1-2 days of fever; 2nd rash is seen 3-5 days later | Elevated; occurs during the 5th-7th day |
| Neurologic complications | Encephalitis | Persistent neurocognitive abnormalities |
| Course of disease | Dengue can be divided into undifferentiated fever, dengue fever, and dengue hemorrhagic fever. | Features can be divided into 4 main phases: Early febrile phase, gastrointestinal phase, shock or recovery phase and late complications |
| Oral manifestations | Erythema, crusting of lips, and tongue and soft palatal vesicles are the prominent oral features. | Gingival bleeding, mucosal lesions, and pain during deglutination (odynophagia) are the most characteristic oral signs and symptoms. |
| Hemorrhagic bullae, petechiae, purpura, ecchymoses, and bleeding gums may also be seen | ||
| Typical blood abnormalities | ||
| Platelets | Low | Low |
| White blood cell count | Low | Low |
| Hematocrit | High | Low |
| Hemoglobin | High | Low |
| Aspartate transferase | Elevated | Elevated |
| INTERVENTIONS TO CONTROL THE SPREAD AND DISSEMINATION | Control of the vectors and their breeding sites | Avoid direct contact with the infected blood/body fluids and adopting universal infection control measures |
| TREATMENT | Supportive | Supportive |
| VACCINE DEVELOPMENT | In progress | In progress |
Orofacial features
Gum bleeding, atypical mucosal lesions, and odynophagia comprise the distinctive oral manifestations. Epistaxis (nasal bleed), bleeding from venipuncture sites, conjunctivitis, and cutaneous exanthema are the other manifestations.[9] Bleeding tendencies and gum bleeding is not seen in asymptomatic or initial EBOV patients reporting to the dental hospital.
EVD dissemination in the field of oral and dental health may appear nonsignificant; although, probable situations which may pose a risk to dental health professional have been appraised by Samaranayake et al.[21] and Galvin et al.[10]
Table 6 depicts the various orofacial manifestations of Ebola virus disease
Table 6.
Orofacial manifestations of Ebola virus disease
| Authors, Year | Oral features | Other features | ||||
|---|---|---|---|---|---|---|
| Oral bleeding | Oral mucosal lesions | Odynophagia | Other bleeding sites | Conjuctivitis | Rash | |
| Anonymous, 1978a | Gingival bleeding (48%) | Dry oral cavity | Painful throat (sensation of dry rope in the throat) (63%) | Epistaxis | Conjunctivae slightly injected but nonicteric | Measles like desquamation (52%) |
| Small aphthous like ulcers | ||||||
| Posterior pharynx slightly injected | ||||||
| Fissures and open sores of the lips and tongue | ||||||
| Anonymous, 1978b | Gingival bleeding (23%) | Herpetiform, grayish exudative patch | Sore throat (32%) | Epistaxis | Conjunctivitis (35%) | Not reported |
| Piot, 1978 | Gingival bleeding (25.6%) | Oral throat lesions (73%) | Sore throat (sensation of “ball” in the throat) (79.2%) | Epistaxis (16.7%) | Conjunctivitis (58.2%) | Skin rash |
| Fissures on the lips | Injection sites (6.6%) | |||||
| Herpetic oral lesions | ||||||
| Grayish exudative patches on soft palate and oropharynx | Dysphagia | |||||
| Sureau PH 1989 | Gingival and oral bleeding | Oropharyngeal bleeding ulcerations in the mouth and in the lips | Sore throat | Epistaxis | Hemorrhagic conjunctivitis | Exanthematous rash on trunk |
| Pharyngitis | Injection sites | |||||
| Dysphagia | ||||||
| Bonnet, 1998 | Diffuse bleeding in the oral cavity (gums & tongue) | Oral thrush like lesions | Not reported | Bruises and bleeding at the injection sites (late stages) | Not reported | Maculopapular rash and petechiae on flanks and limbs (initially); followed by petechiae on the entire body |
| Bleeding cracks on the lips | ||||||
| Bwaka, 1999 | Not reported | Not reported | Odynophagia | Injection sites (5%) | Conjunctival injection (47%) | Maculopapular rash |
| Dysphagia | ||||||
| Sore throat (58%) | ||||||
| Ndanbi, 1999 | Gingival bleeding (30%) | Oral/mucosal redness (30%) | Dysphagia (48%) | Epistaxis (4%) | Conjuctivitis (78%) | Cutaneous eruption (4%) |
| Injection site (30%) | Petechiae (22%) | |||||
| Mupere, 2011 | Gingival bleeding (10%) | Not reported | Sore throat (10%) | Epistaxis (10%) | Conjunctival injection (40%) | Not reported |
| Injection site (10%) | ||||||
| Korepeter, 2011 | Not reported | Pharyngeal Arythema | Sore throat | Bleeding from injection/venepuncture site | Conjuctival | Maculopapular or morbilliform (meseales like) rash/or scar letenoid |
| Hemorrhage | ||||||
| Roddy, 2012 | Gingival bleeding (4%) | Not reported | Dysphagia (58%) | Epistaxis (8%) | Conjuctivitis (50%) | Rash (12%) |
| Injection site (8%) | ||||||
| Chertow, 2014 | Not reported | Oral ulcers and Thrush | Throat pain | Not reported | Conjunctival injection | Not reported |
| Dysphagia | ||||||
| WHO Ebola response team, 2014 | Bleeding gums (2.3%) | Not reported | Dysphagia (32.9%) | Unexplained bleeding (18%) | Conjuctivitis (20.8%) | Rash (5.8%) |
| Sore Throat (21.8%) | Epistaxis (1.9%) | |||||
| Injection site (2.4%) | ||||||
Diagnosis
EVD patients usually demonstrate altered laboratory parameters based on the stage of the disease.
Table 7 shows the laboratory findings in Ebola virus disease.
Table 7.
Laboratory findings in Ebola virus disease
| Timing | Common laboratory findings |
|---|---|
| Early illness | Leukopenia, lymphopenia, and thrombocytopenia |
| Elevated hemoglobin and hematocrit | |
| Elevated aspartate aminotransferase and alanine aminotransferase (ratio≥3:1) | |
| Elevated prothrombin time, activated partial thromboplastin time, and D-dimer | |
| Peak illness | Leukocytosis, neutrophilia, and anemia |
| Hyponatremia, hypo- or hyperkalemia, hypomagnesemia, hypocalcemia, hypoalbuminemia, hypoglycaemia | |
| Elevated creatinine phosphokinase and amylase | |
| Elevated blood urea nitrogen and creatinine | |
| Elevated serum lactate and low serum bicarbonate | |
| Recovery | Thrombocytosis |
The WHO (2014) recommended the sample collection of whole blood or oral swab at suitable centres called Ebola treatment centers.[47] Reverse transcriptase polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) are the most frequently utilized tests for laboratory affirmation of the EVD.[43] RT-PCR is capable of detecting viral RNA in the blood samples of infected patients immediately after the commencement of signs and symptoms,[42,48] has a high sensitivity (up to 100%), and gives results within 1–2 days in cases of epidemics. ELISA detects the immunoglobulins G and M in samples of infected patients, has a low sensitivity (91%) and is not suitable for initial affirmation during an outbreak.[42,49]
Prevention
The most imperative strategy in EVD is to avert the vulnerable population from getting infected and limit the transmission. These preventive strategies entail intensive and rigorous endeavors from the Government, public health amenities, medical units, and personals.[50]
The most essential aspect to curb EVD transmission is to avert direct bodily contact with infected individuals and their body fluids.[51]
Health caregivers are extremely vulnerable and experience an augmented professional threat for EVD.[52] Thus, scrupulous adherence to the universal infection control measures is fundamental in all the hospitals, laboratories, and other health care services.[53] The U.S. CDC has advocated the appropriate use of various personal protective equipment as a mandate for health care professionals.[50]
The risk of rapid importation of Ebola virus into human beings can be prevented by averting the direct bush meat and bats contact.[54]
Unsafe traditional burial procedures, especially in the African continent significantly contributed to the EVD transmission. Hence, it is essential to practice safe and guarded funeral rituals to prevent the disease spread.[55]
WHO recommends the implementation of safe sex practices to combat the sexual transmission of EVD. Strict abstinence or proper and regular condom use in male EVD survivors at least for a period of 12 months of the symptom onset or until their semen has twice tested negative should be followed.[56]
Dental health care personals are extremely susceptible to EVD as they are in regular contact with blood and saliva during the routine diagnostic procedures. There is no documented case of EVD through saliva till date. A study on the identification of EBOV in oral fluids affirmed that patients presenting with demonstrable serum levels of EBOV RNA also exhibit identifiable salivary levels.[57] The incubation period for all body fluids including saliva is 21 days; hence, oral health personals are vulnerable to develop the disease if universal infection control protocol is not followed.[58]
Table 8 demonstrates the various infection control measures to prevent the Ebola virus spread.
Table 8.
Infection control measures to prevent Ebola virus spread
| Personal protective equipments (PPE) | Ebola virus infection may be transmitted through broken skin and mucosa. | Gown, gloves (possibly double gloves), surgical mask, eye visor/goggles, or face shield to protect conjunctival, nasal, and oral mucosae at the same time. use additional personal protective equipment (such as double gloving, leg covers and disposable shoe covers, when there is contact with blood and bodily fluids |
Strength of the evidence High |
| Choose PPE of exact size. | |||
| Gloves or other PPE that becomes contaminated by blood or bodily fluids must be cleaned or changed before touching other instruments or surfaces. | |||
| Gloved/ungloved hand hygiene. Use alcohol-based hand rub or soap and running water. undertake scrupulous hand cleaning before and after glove use | |||
| Sharp instruments | Sharp instruments are extremely dangerous because they become contaminated by blood or bodily fluids and may break skin/mucosae even if protected by PPE. | Use of needles and other sharp instruments must be limited. These instruments must be handled with extreme care and disposed after use in dedicated seal containers. | Strength of the evidence High |
| Nonsharp instruments | Indirect transmission through nonsharp contaminated instruments is not demonstrated | Use of disposable medical equipment is recommended or, alternatively, nondisposable medical equipment must be cleaned and disinfected after use according to manufacturer’s instructions | Strength of the evidence Low |
| Preventive measures are recommended under the | |||
| Precautionary Principle | |||
| Droplets | Airborne transmission is not demonstrated preventive measures are recommended under the precautionary principle | If aerosol generating procedures or events, such as coughing or sputum induction, occur, the use of powered air-purifying respirator or respirator (FFP2 or EN certified equivalent or US NIOSH-certified N95) is recommended | Strength of the evidence Low |
| Environmental surfaces | Environmental surfaces do not pose a risk of infection. However, | Use of standard hospital detergents and disinfectants (e.g., 0.5% chlorine solution or a solution containing 5000 ppm available free chlorine), preceded by cleaning to prevent inactivation of disinfectants by organic matter, is recommended | Strength of the evidence Low |
| Ebola virus is nonenveloped and is able to survive in the environment for long time. | |||
| Preventive measures regarding surfaces visibly contaminated with blood and bodily fluids are recommended under the precautionary principle. | |||
Box 1 shows the travel guidelines to EBOV affected regions.
Box 1.
Shows the UK Travel guidelines to EBV infested regions.
| • Do not handle dead animals or their raw meat |
| • Avoid contact with patients who have symptoms |
| • Avoid unprotected sex with people in risk areas |
| • Wash fruit and vegetables before eating them |
| • Wash hands frequently using soap and water |
Treatment
Till date, there is no precise antiviral management or vaccination for EVD.[51] The management protocol mainly relies on supportive and symptomatic therapy. Public health strategies emphasizing on epidemiological surveillance, contact tracing, and quarantine of the patient have been recommended to combat the dissemination of EVD.[59]
Rehydration, adequate nourishment, analgesics, and blood transfusion form a keystone supportive treatment of EVD patient.[60] Intravenous fluids and oral rehydration solution endow with proper electrolytes substitute and maintain the intravascular volume. Unrelenting vomiting and diarrhea are taken care of by the use of antiemetics and antidiarrheal drugs.[35,60,61] Suspected cases of secondary bacterial infections and septicemia are best managed by the use of prophylactic antibiotic regimen (third generation I.V. cephalosporins).[62] Concurrent parasitic coinfections may also be seen and require prompt investigations and management.[63]
A number of investigative clinical trials emphasizing on the development of vaccine, antibody therapies, and antiviral drugs have been conducted for EVD.[64]
Table 9 shows experimental treatment for Ebola virus disease.
Table 9.
Experimental treatment for Ebola virus disease
| Drug | Drug type | Mechanism of action | Ebola virus clinical trial phase | Result/status | Other clinical trials |
|---|---|---|---|---|---|
| FAVIPIRAVIR (T-705) (Fujifilm Holding Corp) | Nucleotide analogue and viral RNA polymerase inhibitor | Prevents viral replication by RNA chain termination and/or lethal mutaggenesis | Phase II (NCT02329054): JIKI; NCT02662855: Sierra Leone) | Efficacy in patients with low to moderate levels of virus | Administered with ZMapp to a patient who recovered; administered to a patient with convalescent plasma who recovered; retrospective study indicated increased survival and lower viral loads. |
| BCX4430 (BioCryst Pharmaceuticals Inc., Durham, NC) | Synthetic adenosine analogue | Inhibits viral RNA polymerase and results in RNA chain terminaton | Phase I (NCT02319772) | Phase I complete; results not available yet | Not Applicable |
| TKM-Ebola (Tekmira Pharmaceutical Corp.) | Small Interfering (si) RNA agents Lipid nano-particle with si RNA-Ebola virus specific compound | Gene silencing | TKM-100802Phase I (NCT02041715) TKM-130803 Phase II (PACTR201501000997429) |
Terminated Terminated early; did not demonstrate efficacy [77]; development has been suspended |
100802 administered to two patients in combination with convalescent plasma; both survived |
| BrincidofovirCMX001 (Chimerix Durham, NC) | Nucleotide analogue | Inhibits viral replication by inhibiting DNA polymerase | Phase II (NCT02271347) | Terminated due to low enrollment; not currently under further development as EBOV therapeutic agent | Administered to 5 patients during the outbreak, often in combination with other therapies |
| AVI-6002 AVI-7537 (Sarepta Therapeutics Cambridge, MA) |
Small Interfering (si) RNA agents Phosporo-diamidate morpholino oligomer Ebola virus specific compound |
Gene silencing | Phase I AVI-6002: NCT01353027; AVI-7537: NCT01593072 |
AVI-6002: Favorable safety and tolerability AVI-7537: Terminated prior to enrollment; further development has been suspended |
Not Applicable |
| Z-Mapp (Mapp Pharmaceuticals) | Combination of 3 different monoclonal antibodies-Ebola specific compound | Virus neutralisation | Phase II (NCT02363322) | Inconclusive efficacy due to insufficient statistical power | Administered to patients during the outbreak, often in combination with other therapies |
| JK-05 (Sihuan Pharmaceutical Holdings Group Ltd and Academy of Military Medical Sciences (Beijing, China) | Broad spectrum antiviral drug | Inhibits viral RNA polymease | Not Applicable Animal studies completed; now considered for use in emergency situations for Army only |
Not Applicable | Not Applicable |
| Convalescent plasma or blood | Derived from surviving or cured Ebola patients | contains anti Ebola antibodies | Phase I/II: NCT02333578 Phase II/III (NCT02342171; ISRCTN13990511) | Completed; results from one study found no improvement in efficacy in treated group | Whole blood: 1995 Kikwit outbreak—7 out of 8 survivors; administered to patients during the outbreak, often in combination with other therapies |
| GS-5732 | Small molecule monophosphoramidate prodrug of an adenosine analogue | Inhibition of RNA-dependent RNA polymerase | Phase I | Phase I complete; Phase II for efficacy in survivors with viral persistence in semen (NCT02818582) | Administered to a newborn in combination with ZMapp and buffy coat transfusion; patient survived |
| IFN- β | Cytokine family member | Inhibits the viral infection by activating the innate and adaptive immune response | Phase I/II (ISRCTN17414946) | Results not yet released | Not Applicable |
| Amiodarone | Multi-ion channel blocker for treatment of cardiac arrhythmias | Inhibits filovirus entry in vitro by reducing virus binding to target cells | Phase II (NCT02307591) | Terminated early; reduction in case-fatality rate; not statistically significant | - |
| FX-06 | Fibrin derived peptide | Treats hemorrhagic shock by reducing vascular leakage | Not Applicable | Not under current investigation for EBOV indication | 2014 3-day treatment course (400 mg/kg loading dose+200 mg/kg maintenance dose) was administered to a patient in combination with self-administration of amiodarone and intermittent treatment with favipiravir; patient survived |
Various clinical trials in Africa, Europe, and the United States suggest that Ebola vaccines are in various development stages (Phase I–III). A number of candidate vaccines employ diverse platforms, including recombinant viral vectors (most evolved vaccine candidate), DNA vaccines, inactivated viral particles, subunit proteins, recombinant proteins, and virus-like particles. Example of viral vectors expressing ebolavirus glycoproteins include recombinant simian adenovirus (cAd3), recombinant vaccinia virus, recombinant human adenovirus (Ad26), and a live vesicular stomatitis virus used alone or in prime-booster regimens.[65]
However, Ebola virus having the glycosylated surface proteins and preferentially infecting the immune cells impedes the development of an effective vaccine.[66]
Dental Management
Dental health care professionals in Europe have not encountered a case of EVD so far. However, health care personals (including dental surgeons) are more prone to EVD while treating patients in West or sub-Saharan Africa. Dental professionals are more likely to encounter asymptomatic EVD patients or those with early-stage vague symptoms.[27]
Individuals with a travel history to Ebola endemic regions, but with no direct intimate contact with the disease fall in the low-risk category and may undergo any medical/dental health care procedures without restrictions. However, all the nonessential procedures should be postponed for 21 days in individuals with direct exposure to the virus. The regional Health Service Executive Department of Public Health needs to be notified when the exposed patient's treatment cannot be deferred or controlled with pharmacotherapy.[10]
Conclusion
EVD has emerged as a significant global public health menace due to multiple disease outbreaks in the last 25 years. Recent advancements are being carried out in the form of effective Ebola virus vaccine and anti-Ebola virus drugs. However, rapid geographic dissemination, nonspecific clinical presentation, lack of vaccine, and specific diagnostic test are the possible challenges to combat this dreaded public health menace.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Arinola AA, Joel SA, Tubosun OE, Folagbade OA. Ebola virus disease (EVD) information awareness among the people of Ogbomoso Environs. Int J Library Information Sci. 2015;4:55–69. [Google Scholar]
- 2.Rajak H, Jain DK, Singh A, Sharma AK, Dixit A. Ebola virus disease: Past, present and future. Asian Pac J Trop Biomed. 2015;5:337–43. [Google Scholar]
- 3.Rabiah M, Khan A, Fatima M, Ashfaq M, Chaudhry HW, Zafar M. Knowledge and awareness of ebola virus disease among medical students. Pak J Med Health Sci. 2015;9:852–5. [Google Scholar]
- 4.Yobsan D, Walkite F, Nesradin Y. Ebola virus and it's public health significance: A review. J Vet Sci Res. 2018;3:1–10. [Google Scholar]
- 5.Daral S, Singh SK, Khokhar A. Ebola virus: Awareness about the disease and personal protective measures among junior doctors of a tertiary hospital in Delhi, India. Int J Med Public Health. 2015;5:217–21. [Google Scholar]
- 6.Luo D, Zheng R, Wang D, Zhang X, Yin Y, Wang K, et al. Effect of sexual transmission on the West Africa Ebola outbreak in 2014: A mathematical modeling study. Sci Rep. 2019;9:1653. doi: 10.1038/s41598-018-38397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petti S, Messano GA, Vingolo EM, Marsella LT, Scully C. The face of Ebola: Changing frequency of hemorrhage in the West African compared with Eastern-Central African outbreaks. BMC Infect Dis. 2015;15:564. doi: 10.1186/s12879-015-1302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naieni KH, Ahmad A, Raza O, Assan A, Elduma AH, Jammeh A, et al. Assessing the knowledge, attitudes, and practices of students regarding ebola virus disease outbreak. Iran J Public Health. 2015;44:1670–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Samaranayake L, Scully C, Nair RG, Petti S. Viral hemorrhagic fevers with emphasis on Ebola virus disease and oro-dental healthcare. Oral Dis. 2015;21:1–6. doi: 10.1111/odi.12298. [DOI] [PubMed] [Google Scholar]
- 10.Galvin S, Flint SR, Healy CM. Ebola virus disease: Review and implications for dentistry in Ireland. J Ir Dent Assoc. 2015;61:141–3. [PubMed] [Google Scholar]
- 11.Vailaya CGR, Kumar S, Moideen S. Ebola virus disease: Knowledge, attitude, and practices of health care professionals in a tertiary care hospital. J Pub Health Med Res. 2014;2:13–18. [Google Scholar]
- 12.Gebretadik FA, Seifu MF, Gelaw BK. Review on Ebola virus disease: Its outbreak and current status. Epidemiology (Sunnyvale) 2015;5:1–8. [Google Scholar]
- 13.Schindell BG, Webb AL, Kindrachuk J. Persistence and sexual transmission of filoviruses. Viruses. 2018;10:1–22. doi: 10.3390/v10120683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu WB, Li ZX, Du Y, Cao GW. Ebola virus disease: From epidemiology to prophylaxis. Mil Med Res. 2015;2:7. doi: 10.1186/s40779-015-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghadam SRJ, Omidi N, Bayrami S, Moghadam SJ, Alinaghi SAS. Asian Pac J Trop Biomed. 2015;5:260–7. [Google Scholar]
- 16.Lai KY, Ng WY, Cheng FF. Human Ebola virus infection in West Africa: A review of available therapeutic agents that target different steps of the life cycle of the Ebola virus. Infect Dis Poverty. 2014;3:43. doi: 10.1186/2049-9957-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez LL, Roo AD, Guimard Y, Trappier SG, Sanchez A, Bressler D, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, the Democratic Republic of the Congo 1995. J Infect Dis. 1999;179:170–6. doi: 10.1086/514291. [DOI] [PubMed] [Google Scholar]
- 18.Chan M. Ebola virus disease in West Africa–no early end to the outbreak. N Engl J Med. 2014;371:1183–5. doi: 10.1056/NEJMp1409859. [DOI] [PubMed] [Google Scholar]
- 19.Rewar S. Transmission of Ebola virus disease: An overview. Ann Glob Health. 2014;80:444–51. doi: 10.1016/j.aogh.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Drazen JM, Kanapathipillai R, Campion EW, Rubin EJ, Hammer SM, Morrissey S, et al. Ebola and quarantine. N Engl J Med. 2014;371:2029–30. doi: 10.1056/NEJMe1413139. [DOI] [PubMed] [Google Scholar]
- 21.Samaranayake LP, Peiris JS, Scully C. Ebola virus infection: An overview. Br Dent J. 1996;180:264–6. doi: 10.1038/sj.bdj.4809048. [DOI] [PubMed] [Google Scholar]
- 22.Judson S, Prescott J, Munster V. Understanding ebola virus transmission. Viruses. 2015;7:511–21. doi: 10.3390/v7020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–6. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 24.Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, Nichol ST, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325:204–6. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- 25.Ebola hemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull World Health Organ. 1978;56:247–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Ebola hemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 27.Reichart PA, Gelderblom HR, Khongkhunthian P, Westhausen AS. Ebola virus disease: Any risk for oral and maxillofacial surgery? An overview. Oral Maxillofac Surg. 2016;20:111–4. doi: 10.1007/s10006-015-0542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amundsen S. Historical analysis of the Ebola virus: Prospective implications for primary care nursing today. Clin Excell Nurse Pract. 1998;2:343–51. [PubMed] [Google Scholar]
- 29.Aurelie KK, Guy MM, Bona NF, Charles KM, Mawupemor AP, Shixue L, et al. A historical review of Ebola outbreaks. Advances in Ebola control. InTech Open. 2017;2:1–27. [Google Scholar]
- 30.Feldmann H, Geisbert TW. Ebola hemorrhagic fever. Lancet. 2011;377:849–62. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maganga GD, Kapetshi J, Berthet N, Kebela Ilunga B, Kabange F, et al. Ebola virus disease in the Democratic Republic of Congo. N Engl J Med. 2014;371:2083–91. doi: 10.1056/NEJMoa1411099. [DOI] [PubMed] [Google Scholar]
- 32.Raftery P, Condell O, Wasunna C, Kpaka J, Zwizwai R, Nuha M, et al. Establishing Ebola Virus Disease (EVD) diagnostics using GeneXpert technology at a mobile laboratory in Liberia: Impact on outbreak response, case management, and laboratory systems strengthening. PLoS Negl Trop Dis. 2018;12:1–20. doi: 10.1371/journal.pntd.0006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann-Winkler H, Kaup F, Pohlmann S. Host cell factors in filovirus entry: Novel players, new insights. Viruses. 2012;4:3336–62. doi: 10.3390/v4123336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahanty S, Bray M. Pathogenesis of filoviral hemorrhagic fevers. Lancet Infect Dis. 2004;4:487–98. doi: 10.1016/S1473-3099(04)01103-X. [DOI] [PubMed] [Google Scholar]
- 35.Fowler RA, Fletcher T, Fischer WA, 2nd, Lamontagne F, Jacob S, Brett-Major D, et al. Caring for critically ill patients with Ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med. 2014;190:733–7. doi: 10.1164/rccm.201408-1514CP. [DOI] [PubMed] [Google Scholar]
- 36.Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1–9. doi: 10.1016/j.jaut.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Beeching NJ, Fenech M, Houlihan CF. Ebola virus disease. BMJ. 2014;349:7348. doi: 10.1136/bmj.g7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO Ebola Response Team. Ebola virus disease in West Africa-the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–95. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dallatomasina S, Crestani R, Squire JS, Declerk H, Caleo GM, Wolz A, et al. Ebola outbreak in rural West Africa: Epidemiology, clinical features, and outcomes. Trop Med Int Health. 2015;10:448–54. doi: 10.1111/tmi.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gostin LO, Friedman EA. A retrospective and prospective analysis of the West African Ebola virus disease epidemic: Robust national health systems at the foundation and an empowered WHO at the apex. Lancet. 2015;385:1902–9. doi: 10.1016/S0140-6736(15)60644-4. [DOI] [PubMed] [Google Scholar]
- 41.Wong SS-Y, Wong SC-Y. Ebola virus disease in nonendemic countries. J Formos Med Assoc. 2015;114:384–98. doi: 10.1016/j.jfma.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyers L, Frawley T, Goss S, Kang C. Ebola virus outbreak 2014: Clinical review for emergency physicians. Ann Emerg Med. 2015;65:101–8. doi: 10.1016/j.annemergmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Sarwar UN, Sitar S, Ledgerwood JE. Filovirus emergence and vaccine development: A perspective for health in travel medicine. Travel Med Infect Dis. 2011;9:126–34. doi: 10.1016/j.tmaid.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiwanitkit V. Ebola virus infection: Be known? N Am J Med Sci. 2014;6:549–52. doi: 10.4103/1947-2714.145458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bwaka MA, Bonnet MJ, Calain P, Colebunders R, Roo AD, Guimard Y, et al. Ebola hemorrhagic fever in the Kikwit Democratic Republic of the Congo: Clinical observations in 103 patients. J Infect Dis. 1999;179:1–7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 46.Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204:810–6. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- 47.Balami LG, Ismail S, Saliluddin SM, Garba SH. Ebola virus disease: Epidemiology, clinical feature and the way forward. Int J Community Med Public Health. 2017;4:1372–8. [Google Scholar]
- 48.Park SW, Lee YJ, Lee WJ, Jee Y, Choi W. One-step reverse transcription-polymerase chain reaction for Ebola and Marburg viruses. Osong Public Heal Res Perspect. 2016;7:205–9. doi: 10.1016/j.phrp.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.To KK, Chan JF, Tsang AK, Cheng VC, Yuen KY. Ebola virus disease: A highly fatal infectious disease reemerging in West Africa. Microbes Infect. 2015;17:84–97. doi: 10.1016/j.micinf.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omonzejele PF. Ethical challenges posed by the Ebola virus epidemic in West Africa. J Bioeth Inq. 2014;11:417–20. doi: 10.1007/s11673-014-9587-3. [DOI] [PubMed] [Google Scholar]
- 51.Scully C. Ebola: A very dangerous viral hemorrhagic fever. Dent Update. 2015;42:7–12. doi: 10.12968/denu.2015.42.1.7. [DOI] [PubMed] [Google Scholar]
- 52.Matanock A, Arwady MA, Ayscue P, Forrester JD, Gaddis B, Hunter JC, et al. Ebola virus disease cases among health care workers not working in Ebola treatment units-Liberia, June-August, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:1077–81. [PMC free article] [PubMed] [Google Scholar]
- 53.Katz LM, Tobian AA. Ebola virus disease, transmission risk to laboratory personnel, and pre transfusion testing. Transfusion. 2014;54:3247–51. doi: 10.1111/trf.12913. [DOI] [PubMed] [Google Scholar]
- 54.Dixon MG, Schafer IJ. Ebola viral disease outbreak--West Africa 2014. Morb Mortal Wkly Rep. 2014;63:548–51. [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen CF, Kidd S, Sillah AR, Davis E, Mermin J, Kilmarx PH. Improving burial practices and cemetery management during an Ebola virus disease epidemic-Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:20–7. [PMC free article] [PubMed] [Google Scholar]
- 56.Boon SD, Marston BJ, Nyenswah TG, Jambai A, Barry M, Keita S, et al. Ebola virus infection associated with transmission from survivors. Emerg Infect Dis. 2019;25:240–6. doi: 10.3201/eid2502.181011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Formenty P, Leroy EM, Epelboin A, Libama F, Lenzi M, Sudeck H, et al. Detection of Ebola virus in oral fluid specimens during outbreaks of Ebola virus hemorrhagic fever in the Republic of Congo. Clin Infect Dis. 2006;42:1521–6. doi: 10.1086/503836. [DOI] [PubMed] [Google Scholar]
- 58.Samaranayake LP, Scully C, Nair RG, Petti S. The Ebola virus epidemic: A concern for dentistry? Dent Trib News Asia Pac. 2014;15:8–10. [Google Scholar]
- 59.Pandey A, Atkins KE, Medlock J, Wenzel N, Townsend JP, Childs JE, et al. Strategies for containing Ebola in West Africa. Science. 2014;346:991–5. doi: 10.1126/science.1260612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371:2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A, et al. Ebola virus disease in West Africa—Clinical manifestations and management. N Engl J Med. 2014;371:2054–7. doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]
- 62.Plachouras D, Monnet DL, Catchpole M. Severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2015;372:1376–7. doi: 10.1056/NEJMc1500455. [DOI] [PubMed] [Google Scholar]
- 63.O’Shea MK, Clay KA, Craig DG, Matthews SW, Kao RL, Fletcher TE, et al. Diagnosis of febrile illnesses other than Ebola virus disease at an Ebola treatment unit in Sierra Leone. Clin Infect Dis. 2015;61:795–8. doi: 10.1093/cid/civ399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bishop BM. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother. 2015;49:196–206. doi: 10.1177/1060028014561227. [DOI] [PubMed] [Google Scholar]
- 65.Espeland EM, Tsai CW, Larsen J, Disbrow GL. Safeguarding against Ebola: Vaccines and therapeutics to be stockpiled for future outbreaks. PLoS Negl Trop Dis. 2018;12:1–4. doi: 10.1371/journal.pntd.0006275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang ES. Preparedness for the prevention of Ebola virus disease. J Korean Med Sci. 2014;29:1185. doi: 10.3346/jkms.2014.29.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]