Abstract
Introduction:
Myeloproliferative disorders are characterized by proliferation of one or more myeloid lineages cells. In order to assess the burden of these illness for public health planning, it is important to know their frequency.
Objectives:
A study to determine the clinical, hematological, cytogenetic, and molecular profile in chronic myeloid leukemia (CML) patient in and around Eastern UP, India.
Materials and Methods:
Newly diagnosed and follow-up adult and pediatric cases of myeloproliferative disorder were taken into study. Detailed history, physical, and systemic examination was done with informed consent. Investigations like complete blood count including hemoglobin level, platelet count, total and differential leucocyte count, general blood picture, and bone marrow aspiration/biopsy were done. Molecular and cytogenetic studies were also done whenever required.
Results:
In total, 90 patients were enrolled in the study. The median age of presentation of CML was 37 years and the mean age was 38.6 years. M: F ratio of 1.4:1.75 patients (83%) were in CML-chronic phase (CP), 11 patients (12%) in CML-accelerated phase (AP) phase, and 4 patients (5%) were found in CML-blast crisis (BC) phase. The common symptoms of the patients were fullness of the abdomen (66.6%). Among these 69 cases, Philadelphia chromosome was present in 65 (94.2%) cases. Revers transcriptase polymerase chain reaction (RT–PCR) was done in 40 out of 90 cases, breakpoint cluster region (BCR)-Abelson oncogene (ABL) gene came out to be positive in all the 40 cases.
Conclusion:
Most CML patients in eastern UP (India) are relatively young (31–40 years). In addition, males were more commonly affected.
Keywords: BCR-ABL, CML, cytogenetics
Introduction
Myeloproliferative disorders are characterized by proliferation of one or more myeloid lineages cells. These includes chronic myelogenous leukemia, primary myelofibrosis, polycythemia Vera, and essential thrombocythemia.[1,2] According to revised version of the classification, myelo proliferative neoplasms (MPN) includes eight diseases: chronic myeloid leukemia (CML) breakpoint cluster region (BCR)-Abelson oncogene (ABL) 1 positive, chronic neutrophilic leukemia (CNL), polycythemia vera (PV), primary myelofibrosis (PMF), essential thrombocytosis (ET), chronic eosinophilic leukemia (CEL), mastocytosis, unclassifiable MPN.[3,4,5] Chronic myeloid leukemia is originates in an abnormal pluripotent bone marrow stem cell and is characterized by the fusion of the ABL with the BCR.[5] CML is characterized by the fusion of the ABL from chromosome 9q34 with the BCR on chromosome 22q11.2 manifested as a translocation t (9; 22) (q34; q11.2) known as the Philadelphia chromosome (Ph).[6,7,8] Most of the BCR-ABL1-negative MPN are characterized by the presence of an activating mutation in the Janus kinase 2 gene (JAK2 V617F), inducing a high level of proliferation. This mutation is found in >95% of PV, 60% of ET, and 50% of PMF. Chronic myeloid leukemia is the most common leukemia among adults in our country.[9] Epidemiological data regarding the incidence rates for myeloproliferative neoplasm are scarce for Indian population. Most of the Indian patients usually present in the late stage of disease. Although most of the patients are receiving Imatinib as first-line therapy, but there is limited availability of diagnostic facilities for molecular monitoring.[10,11] In order to assess the burden of these illness for public health planning, it is important to know their frequency.
Objectives
The objectives of this study were to determine the clinical, hematological, cytogenetic, and molecular profile in CML patient in and around Eastern UP, India.
Materials and Methods
This study was conducted between June 2016 and June 2018 in the Department of Pathology, in collaboration with Department of Medicine and Department of Pediatrics at a tertiary care hospital. Newly diagnosed and follow-up adult and pediatric cases of myeloproliferative disorder were taken into study. The study was approved by the Ethical Committee of the Institute. Detailed history, physical and systemic examination was done with informed consent. Investigations such as complete blood count including hemoglobin level, platelet count, total and differential leucocyte count, general blood picture, and bone marrow aspiration/biopsy were done. Molecular studies such as RT–PCR for BCR-ABL fusion and cytogenetic studies for Philadelphia chromosome were also done whenever required.
Statistical analysis
Data were analyzed by using SPSS version 20. The data were presented by mean ± standard deviation for continuous variables and frequencies with their respective percentages were given for categorical variables. Spearman correlation coefficient was used to measure the degree of association between two variables. A P value < 0.05 was considered as statistically significant.
Results
In total, 90 patients presented with chronic myeloproliferative disorders in which all the patient were diagnosed with CML. The median age of presentation of CML was 37 years and the mean age was 38.6, the patients were in the age range of 5–72 years. Overall most common age group affected were between 31 and 40 year. About 52 (57.8%) were male and 38 (42.2%) were female with M:F ratio of 1.4:1. Figure 1 is showing the geographical distribution of CML in Eastern Uttar Pradesh and adjoining areas of Bihar State. About 75 patients (83%) were in CML-chronic phase (CP), 11 patients (12%) in CML-accelerated phase (AP) phase, and 4 patients (5%) were found in CML-blast crisis (BC) phase. All patients of BC are of myeloblasts [Figure 2]. All patients were symptomatic at the time of diagnosis. The common symptoms of the patients were fullness of the abdomen (66.6%) followed by weakness (63%), fever (59%), and fatigue (55.5%) [Table 1]. Splenomegaly and anemia was the most common sign [Table 2]. About 13 (17.3%) cases had mild splenomegaly, 20 (26.6%) cases had moderate splenomegaly, and 42 (56%) cases had massive splenomegaly. In AP, 1 (10%) case had mild splenomegaly and 10 (90%) cases had massive splenomegaly whereas in blast crises phase, 100% had massive splenomegaly. Among all patients, 56 (62.2%), 20 (22.2%), and 14 (15.6%) patients presented with massive, moderate, and mild splenomegaly, respectively. In CP, 45 (64.2%) cases presented with moderate anemia, 21 (30%) cases presented with mild anemia, whereas only 4 (5.71%) cases presented with severe anemia. In AP, 54.5% presented with severe anemia and in BC phase 75% presented with moderate anemia. Sixty one (72%) and 24 (28%) patients had hemoglobin concentration less and greater than 10 g/dL. Most of the patients of CP (56%) had leucocyte count in the range (100–250 × 103/dL), whereas leucocyte count >250 × 103/dL seen in AP (63.6%) of CML. About 25% of patients in BC showed leucocyte below 100 × 103/dL. Patients of CP (68%) and AP (72.7%) had normal platelet count (100–450 × 103/dL). Increased platelet count (>450 × 103/dL) was seen more in patients of CP (34.8%). Thrombocytopenia was noted more among patients of CP (53.3%) and BC (25%) as compared with other phases of CML.
Figure 1.
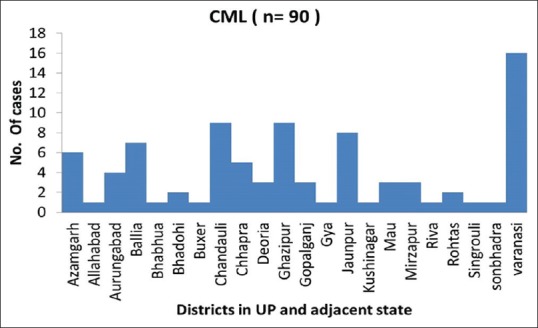
Incidences of chronic myeloid leukemia indifferent districts of eastern UP and adjacent state
Figure 2.
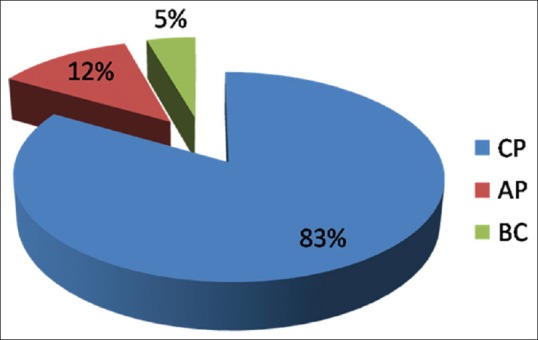
Patients in various phases of diseases in chronic myeloid leukemia
Table 1.
Clinical features of CML patients
| Complaints | CML | Total | |||
|---|---|---|---|---|---|
| CP | AP | BC | n | Percentage | |
| Abdominal fullness | 50 | 8 | 2 | 60 | 66.6 |
| Fever | 43 | 8 | 2 | 53 | 58.8 |
| Weakness | 47 | 7 | 3 | 57 | 62.3 |
| Fatigue | 42 | 6 | 2 | 50 | 55.5 |
| Decreased appetite | 39 | 8 | 1 | 48 | 53.3 |
| Pain abdomen | 35 | 3 | 3 | 41 | 45.5 |
| Weight loss | 37 | 10 | 2 | 49 | 54.4 |
| Body pain | 16 | 3 | 1 | 20 | 22.2 |
| Breathlessness | 8 | 2 | 1 | 11 | 12.2 |
| Cough | 3 | 0 | 1 | 4 | 4.4 |
| Vomiting | 7 | 2 | 1 | 10 | 11.1 |
| Black stool/diarrhea | 6 | 0 | 0 | 6 | 6.6 |
| Easy bruising | 5 | 0 | 0 | 5 | 5.5 |
| Headache | 7 | 1 | 1 | 9 | 10 |
| Chest pain | 5 | 2 | 0 | 7 | 7.7 |
| Joint pain | 3 | 1 | 1 | 5 | 5 |
| Night sweat | 12 | 2 | 0 | 14 | 15.5 |
CML=chronic myeloid leukemia, CP=chronic phase, AP=accelerated phase, BC=blast crisis
Table 2.
Signs at the time of diagnosis
| Signs | CML | Percentage | |
|---|---|---|---|
| Splenomegaly | Massive (≥10 cm) | 56 | 62.2 |
| Moderate (4-9 cm) | 20 | 22.2 | |
| Mild (1-3 cm) | 14 | 15.6 | |
| Anaemia | 85 | 94.4 | |
| Hepatomegaly | 52 | 57.7 | |
| Lymphadenopathy | 5 | 6 |
CML=chronic myeloid leukemia
All the patients (100%) of CP had <10% blast in their peripheral blood and 89.3% of these patients had basophils <10%. About 10 patients (90.9%) had blasts between 10%–19% in peripheral blood. Criteria of 10%–19% blast in AP, 1 patients (9.%) had <10% blasts but basophils >20%; thus, on the criteria of basophils, >20% in peripheral blood in AP. Four patients had >20% blasts fulfilling criteria of BC and 75% of these patients had basophil <10%.
Majority of the patients (58.8%) presented with hemoglobin in the range of 7–10 g/dL and severe anemia (<7 g/dL) was seen in 13% of patients. Total leucocyte were seen in the range (100–250) × 103/dL (52.2%). About 68.9% of patients had platelet count in the range of (100–450) × 103/dL. About 24.4% of the patients had thrombocytosis (>450 × 103/dL) [Table 3], There was a significant direct correlation between spleen size and WBC count (Pearson's correlation: 0.488; P, 0.0001), and inverse correlation between spleen size and hemoglobin levels of patients (Pearson correlation: −0.424; P, 0.001) in CP of the disease. Similarly, WBC counts had significant inverse correlation with hemoglobin levels (Pearson correlation: −0.464; P, 0.001). There was insignificant relation between spleen size and platelet counts of patients [Table 4]. In patients with CP of CML, 47/75 (62.6%) had LAP scores between 0 and 13 (normal: 14–100). In AP of CML, 6 (54.5%) patients had LAP scores <14, 2 (18%) patients had LAP scores >100, whereas the remaining had LAP score within normal limits. In patients with myeloid BC, 1/4 (25%) had LAP score <14, whereas 3/4 (75%) patients had LAP score within normal limits. Among 90 patients, cytogenetic study was done only in 69 patients (76.6%) due to financial constrain. Among these 69 cases, Philadelphia chromosome was present in 65 (94.2%) cases and absent in 4 (5.8%) cases. Among the Philadelphia chromosome positive cases, 53 (81.5%) cases were in CP, 8 (12.3%) cases in AP and 4 (6.15%) cases in BC. All the Philadelphia chromosome negative CML patients (4; 5.8%) were of CP. The four cases, which came negative for Philadelphia chromosome by conventional cytogenetic method, were subjected to FISH analysis and all of them were Philadelphia chromosome positive. RT–PCR was done in 40 out of 90 cases as remaining 50 patients were not affordable. BCR-ABL gene came out to be positive in all the 40 cases. About 78 cases of CML had increased LDH value (normal: 105–248 U/L), among them 65 (86.7%) were in CP, 9 (81.8%) in AP, and all (100%) cases of BC had increased LDH level. None of the patients have reduced LDH level. There is no significant correlation between CP, AP, BC with LDH.
Table 3.
Salient Clinical and Haematological Profile of patients in CML at the time of diagnosis
| Parameters | (n=90) | ||
|---|---|---|---|
| n | % | ||
| Hemoglobin (g/dl) | |||
| < 7 | 11 | 13% | |
| 7-10 | 50 | 58.8% | |
| >10 | 24 | 28.2% | |
| Mean | 9.41±1.75 | ||
| Range | 4.8-12.9 | ||
| Mean±SD | |||
| Total leucocyte count (×103/µl) | |||
| <100 | 20 | 22.2% | |
| 100-250 | 47 | 52.2% | |
| >250 | 23 | 25.6% | |
| MEAN | 182±116 | ||
| RANGE | 15-530 | ||
| Platelet count (×103/µl) | |||
| <100 | 6 | 6.7% | |
| 150-450 | 62 | 68.9% | |
| > 450 | 22 | 24.4% | |
| MEAN | 328±191 | ||
| RANGE | 67-905 | ||
| BLASTS ( in numbers) | |||
| 1-9≥ | 75 | 83.3% | |
| 10-19 | 10 | 11.1% | |
| ≥20 | 5 | 5.5% | |
| MEAN±SD | 6.2±5.4 | ||
| RANGE | 1-26 | ||
| BASOPHILS | |||
| <10 | 76 | 84.4% | |
| 10-19 | 13 | 14.4% | |
| ≥20 | 1 | 1.1% | |
| MEAN±SD | 5.27±4.17 | ||
| RANGE | 1-25 | ||
| SPLEEN (CM) | |||
| 1-3 | 14 | 15.6% | |
| 4-9 | 20 | 22.2% | |
| ≥9 | 56 | 62.2% | |
| MEAN±SD | 11.06±5.15 | ||
| RANGE | 2-18 | ||
Table 4.
Correlation of parameters
| Variables | P | Pearson correlation | |
|---|---|---|---|
| Spleen | TLC | <0.0001 | 0.488 |
| Hb | <0.001 | −0.424 | |
| Platelet count | 0.08 | −0.100 | |
| TLC | Hb | <0.001 | −0.464 |
In our study, 14 (18.7%) patients in CP, 4 (36.4%) patients in AP, and 2 (50%) patients in BC had hyperuricemia. There is no significant finding.
Discussion
The pattern of this disease in India and other Asian countries is still unknown due to paucity of studies reported from these areas. The present study was undertaken to obtain the clinical, hematological, cytogenetic, and molecular profile in CML patient in and around Eastern UP and adjoining district of other state. There were few publications on the clinical and hematological spectrum of patients in CML from the Indian subcontinent.[10] However, comparison with other Indian and International studies was done which enabled us to speculate upon the various differences whatsoever. The median age of the diagnosis of CML is fifth decades (WHO Haemat). Most literature from the Western studies shows median age of presentation is 55 years (European) and 66 years (Americans). The median age of presentation in our study is 38 years. Other Indian studies show age of presentation of Indian population is 42 years. Thus, the age of diagnosis of CML in Indian population is a decade earlier from the Western population.[12] Median age in this study was 37 years, which was comparable with Savage et al. and Deshmukh et al. and is similar with Indian studies.[13,14] The Male: Female ratio of CML patients in our study was 1.4:1. Male preponderance was seen in all the Indian as well as all international studies. Our study is hospital-based study; more male cases were reported as India is male-dominating society and get more attention in our society. The majority of patients (83%) were in CP stage, with age range between 31 and 40 years. For AP, patient's age varies between 41 and 50 years, whereas that of BC patients were between 51 and 60 years of age, which was similar to Motatalib et al.[15]
In this study, the frequency of all three phases of CML at the time of clinical presentation was 83%, 12%, and 5% for CP, AP, and BC, respectively, were observed among 90 patients suffering from CML. Motatalib et al. from Bangladesh reported frequency of all three phases of CML was 81.25%, 14.58%, and 4.17% for CP, AP, and BC, respectively.[15] Ahmed et al. from Pakistan also reported that frequency of all three phases of CML to be 77.8%, 15.5%, and 6.7%, respectively, were observed in among the 45 patients suffering from CML.[16] Tardieu et al. study in France reported the frequencies of CP, AP, and BC as 96.8%, 2.2%, and 0.9%, respectively.[17] More patients in BC phase in our study as well as in neighboring countries but less number of patients in BC in France may be explained as the patients in our region may present at latter stage of the disease or this may be due to ethnic differences. Fever was present in 58% cases in our study which was similar to all Indian studies. Fever was only seen in 6.2% cases in Savage et al.[13] This can probably be explained by the fact that infection rate is very low in western countries as compared with India. Abdominal fullness, weight loss, decreased appetite, and pain abdomen in our study were present in 66%, 54% 53%, and 45% cases, respectively. This was slightly higher when compared with the study by Kumar et al.[18] Joint pain and weakness were seen in 5% and 62% cases, which was similar to the study by Kumar et al.[18] Our study was completely in discordance with the international study done by Savage et al.[13] In this study, most common feature was weight loss (20%). This can be explained by fact that in western countries, symptomatic patients as well as patients selected on screening bases are included in the studies. Fullness of the abdomen was the most common complaint followed by weakness, fever, pain abdomen, and decreased appetite. It is also found in the Asian study of Usman et al.[19] Weakness was the most common symptoms in Kumar et al.[18] study followed by fever and fullness of the abdomen. Weight loss was the most common symptoms in Savage et al. and Singh et al. study.[13,20] Splenomegaly was seen in 100% cases which were same as in Ghalaut et al. study.[21] In other Indian studies, splenomegaly and anemia ranged from 95.2% to100% and 88.5% to 100%, respectively. In Savage et al. study, splenomegaly was seen in 75.8% cases.[13] This study had massive splenomegaly seen in 62.2% cases, moderate splenomegaly seen in 22.2% cases, and mild splenomegaly seen in 15.6% cases. Mean hemoglobin was 9.41 ± 1.75 g/dL with median value 9.5 g/dL. Ahmed et al. found mean hemoglobin was 9.9 g/dL CML.[16] In this study, anemia was seen in 94.4% which was similar to Singh et al., whereas slightly higher than Raghuvansi et al.[20,22] In our study, 94.4% CML patients were anemic and majority (58.8%) were suffering from moderate anemia. Chang et al. also found moderate anemia in 46.9% in CML patients.[23]
Hepatomegaly and lymphadenopathy were seen in 58% and 6% cases, which was similar to as compared in Indian study. In international study, hepatomegaly was seen in 2.2% a case only, which was not comparable to our study. The lymphadenopathy seen in Indian scenario could be due to associated infections; one of them may be tuberculosis. In our study, mean value of total leucocyte count was 182.5 × 103/μL. Minimum TLC was 15.4 × 103/μL and maximum was 546.3 × 103/μL. In Tashfeen et al. study found the mean TLC in the CML group was 184.5 × 109/L. Minimum TLC was 17.9 × 109/L and maximum was 552.3 × 109/L.[24]
Chronic phase of CML
In our study, 83% of the patients were in CP. Among the patients in CP, 43 (57.3%) were males and 32 (42.7%) were females. The male to female ratio was 1.3:1. Maximum patients were within age range of 31–40 years (36%). Most of the patients of CP had anemia (93.3%). Most of the patients had hemoglobin in the range 7–10 g/dL (64.2%) followed by mild anemia (>10 g/dL) seen in 30% of cases. This is similar to Bhatti et al. study with most patients with hemoglobin in the range 7.1–10 g/dL. The mean hemoglobin is 9.4 g/dL.[25] This is lower than findings of Savage et al. (10.3 g/dL).[13] Most of the patients had leucocyte count in the range of (100–250) × 103/μl (52.2%). Most of the patients had platelet count in the range of (100-450) × 103/μl (68.9%). In CP, 26.6% had platelet count >450 × 103/μl. This findings is similar to study by Bhatti et al., who found thrombocytosis in 26% of the cases in CP.[25] All the patients of CP had <10% blast in their peripheral blood and 89.3% of these patients had basophils <10%.
Accelerated phase of CML
In our study, 12.2% of the patients were in AP. Among the patients in AP, 6 (54.5%) were males and 5 (45.5%) were females. In the AP, majority of the patients were between 41 and 50 years (36.3%). All the patients had anemia (100%). Most of the patients had severe anemia (<7 g/dL; 54.5% cases), followed by mild anemia (>10 g/dL; 27.2% cases). These findings are against to Bhatti et al. study.[25]
Most of the patients had leucocyte count >250 × 103/L (63.6%). Majority of the patients had platelet count in the range of (100–450) × 103/μL (72.7.9%). About 18.2% had platelet count >450 × 103/μL. Thrombocytopenia was seen in 9.0% of cases. In this study, thrombocytosis is seen in 18.2% of the patients of AP which is lower as compared with chronic (26.%), but the study of Bhatti et al. showed thrombocytosis seen maximum in patients of AP. One patient (9%) had <10% blasts but basophils >20% in peripheral blood, thus fulfilling criteria of basophils >20% in peripheral blood in AP.[25]
Blast crisis phase
About 4.4% of the patients were in BC. Among the patients in BC, 3 (75%) were males and 1 (25%) were females. Majority of the patients were between 51 and 60 years of age. All the patients had anemia (100%). Most of the patients had hemoglobin in the range 7–10 g/dL (75%). Severe anemia (<7 g/dL) is noted in 25% of cases, which was more than seen in patients of chronic (17.6%). These findings are similar to study by Bhatti et al.[25] Most of the patients had leucocyte count in the range of (100–250) × 103/μl (50%) cases. Majority of the patients had platelet count in the range of (150–450) × 103/μl (75%). About 25% had platelet count <100 × 103/μl. Thrombocytopenia was noted more among patients of BC (35.7%) as compared with other phases of CML. Bhatti et al. study also found thrombocytopenia to be more in patients of the BC.[25] All the four patients had more than 20% blasts, fulfilling criteria of BC, and 75% of these patients had basophil <10%.
Among 90 cases, only 69 cases have undergone conventional cytogenetics for Philadelphia chromosome due to financial constraints. By cytogenetics study, 65 cases were Philadelphia chromosome positive, whereas four cases were Philadelphia chromosome negative. These four cases were further subjected for FISH analysis which confirms Philadelphia chromosome positive. Among the 69 cases, 40 cases are subjected for RT–PCR and all these cases showed p210 kDa BCR-ABL fusion protein positive. De Lemos et al. studied 25 CML patients all cases shows p210 kDa BCR-ABL fusion protein positive.[26] Yaghmmaie et al. studied 75 adult Iranian patients, 83% patients express p210 kDa BCR-ABL fusion protein positive.[27] The results of this study suggest that detection of BCR-ABL fusion gene by RT–PCR is a useful, sensitive, and reliable diagnostic test for the diagnosis of CML.
In our study, molecular testing was used only in certain cases, because karyotyping and molecular testing are not available at this center and many patients are not affordable. Indeed, in our population, majority of patients are of low socioeconomic status. However, according to the WHO, detection of the Philadelphia (Ph) chromosome and/or BCR-ABL1 fusion gene is essential baseline investigation to confirm the diagnosis of CML. Follow-up label of BCR-ABL fusion gene product is essential to see the effect of therapy. Thus, more resources are needed to facilitate access to cytogenetic and molecular diagnosis of all patients in Eastern UP. Lack of conventional cytogenetic study and molecular testing was the lacunae of our study. Liver function test and renal function test parameters were also evaluated. In patients of chronic myeloid leukemia, median value of total bilirubin, LDH, ALT, AST, total protein, albumin, urea, uric acid, creatinine, sodium, potassium, and chloride were 0.6 mg/dL, 903.5 U/L, 26 U/L, 36 U/L, 7.6 g/dL, 4.1 g/dL, 26.7 mg/dL, 5.75 mg/dL, 1.0 mg/dL, 139 mmol/L, 4.42 mmol/L, and 101.3 mmol/L, respectively. In our study, majority of CML patients had normal creatinine level in all phases of disease progression, whereas 14 (18.6%) cases in CP, 2 (18.2%) cases in AP, and 1 (25%) case in BC had increased serum creatinine level. These findings have no significance. About 78 cases of CML had increased LDH value (normal: 105–248 U/L), among them 65 (86.7%) cases in CP, 9 (81.8%) cases in AP, and all (100%) cases of BC. None of the patients have reduced LDH level. There is no significant correlation between CP, AP, and BC with LDH. In our study, 14 (18.7%) patients in CP, 4 (36.4%) patients in AP, 2 (50%) patients in BC had hyperuricemia. This is also a not significant finding. There were limitations in our study. There are overlapping clinical, laboratory, and morphologic findings in different type of myeloproliferative neoplasm. For example, most MPNs result in increased numbers of granulocytes, RBCs, and/or platelets. Despite being common malignancy in India, literature search does not shows any study from this part of country which few studies seen from areas, such as Gujarat, Haryana, Assam, northern Karnataka, Delhi, Mumbai, Calcutta, etc., but not in this geographical area. Therefore, to conclude, most CML patients in eastern UP are relatively young (31–40 years). In addition, males were more commonly affected.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–5. [PubMed] [Google Scholar]
- 2.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–8. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 4.Milosevic JD, Kralovics R. Genetic and epigenetic alterations of myeloproliferative disorders. Int J Hematol. 2013;97:183–97. doi: 10.1007/s12185-012-1235-2. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. Lyon: IARC; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues Fourth edition. [Google Scholar]
- 6.Cortes J, Talpaz M, Kantarjian H. Chronic myeloid leukemia (CML): A review. Am J Med. 1996;100:555–70. doi: 10.1016/s0002-9343(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 7.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018;93:442–59. doi: 10.1002/ajh.25011. [DOI] [PubMed] [Google Scholar]
- 8.Arber DA, Orazi A. Update on the pathologic diagnosis of chronic myelomonocytic leukemia. Mod Pathol. 2019 doi: 10.1038/s41379-019-0215-y. doi: 10.1038/s41379-019-0215-y. [DOI] [PubMed] [Google Scholar]
- 9.Jacob AL, Bapsy PP, Govindbabu K, Lokanatha Imatinib mesylate in newly diagnosed patients of chronic myeloid leukemia. Indian J Med Paediatr Oncol. 2007;28 [Google Scholar]
- 10.Ganesan P, Kumar L. Chronic myeloid leukemia in India. J Global Oncol. 2017;3:64–71. doi: 10.1200/JGO.2015.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra D, Singh J, Deka R, Chauhan R, Sazwal S, Mishra P, et al. The biology of chronic myelogenous leukemia in childhood and young adolescents: An Indian perspective. Indian J Med Paediatr Oncol. 2018;39:142–5. [Google Scholar]
- 12.Singhal MK, Sengar M, Nair R. Summary of the published Indian data on chronic myeloid leukemia. South Asian J Cancer. 2016;5:162–5. doi: 10.4103/2278-330X.187593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage DG, Szydlo RM, Goldman JM. Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br J Haematol. 1997;96:111–6. doi: 10.1046/j.1365-2141.1997.d01-1982.x. [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh C, Saikia T, Bakshi A, Amare-Kadam P, Baisane C, Parikh P. Imatinib mesylate in chronic myeloid leukemia: A prospective, single arm, non-randomized study. J Assoc Physicians India. 2005;53:291–5. [PubMed] [Google Scholar]
- 15.Mottalib MA, Sultana TA, Khalil MI, Gan SH, Islam MS, Choudhury S, et al. Phase distribution of chronic myeloid leukemia in Bangladesh. BMC Res Notes. 2014;7:142. doi: 10.1186/1756-0500-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed R, Naqi N, Hussain I, Khattak BK, Nadeem M, Iqbal J. Presentating phases of chronic myeloid leukaemia. J Coll Physicians Surg Pak. 2009;19:469–47. [PubMed] [Google Scholar]
- 17.Tardieu S, Brun-Strang C, Berthaud P, Michallet M, Guilhot F, Rousselot P, et al. Management of chronic myeloid leukemia in France: A multi-centered cross-sectional study on 538 patients. Pharmacoepidemiol Drug Saf. 2005;14:545–53. doi: 10.1002/pds.1046. [DOI] [PubMed] [Google Scholar]
- 18.Kumar L. Chronic myelogenous leukaemia (CML): An update. Natl Med J India. 2006;19:255–63. [PubMed] [Google Scholar]
- 19.Usman M, Syed NN, Kakepoto GN, Adil SN, Khurshid M. Cronic phase chronic myeloid leukaemia: Response of imatinib myeloid and significance of sokal score, age and disease duration in predicting the haematological and cytogenetic response. J Assoc Physician India. 2007;55:103–7. [PubMed] [Google Scholar]
- 20.Singh G, Parmar P, Kataria SP, Singh S, Sen R. Spectrum of acute and chronic leukemia at a tertiary care hospital, Haryana, India. Int J Res Med Sci. 2016;4:1115–8. [Google Scholar]
- 21.Ghalaut PS, Singh U, Singh V. Imatinib mesylate compared with hydroxyurea in patient with chronic phasechronic myeloid leukaemia. Indian J Haematol Blood Transfusion. 2006;1:18–22. [Google Scholar]
- 22.Raghuwanshi B, Pehlajani NK, Sinha MK, Tripathy S. A retrospective study of transfusion practices in a Tertiary Care Institute. Indian J Anaesth. 2017;61:24–8. doi: 10.4103/0019-5049.198395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang F, Qazi RA, Kan M, Baloch S, Sahito MM, Mir A. Clinico hematological profile and phase distribution of chronic myeloid leukemia. Biol Med (Aligarh) 2015;7:5. [Google Scholar]
- 24.Tashfeen S, Ahmed S, Bhatti FA, Ali N. Real time polymerase chain reaction in diagnosis of chronic myeloid leukemia. J Coll Physicians Sur Pak. 2014;24:1–6. [PubMed] [Google Scholar]
- 25.Bhatti FA, Ahmed S, Ali N. Clinical and hematological features of 335 patients of chronic myelogenous leukemia diagnosed at single centre in northern Pakistan.169 clinical medicine insights. Blood Disord. 2012;5:15–24. [Google Scholar]
- 26.de Lemos JA, de Oliveira CM, Scerni AC, Bentes AQ, Beltrão AC, Bentes IR, et al. Differential molecular response of the transcripts B2A2 and B3A2 to imatinib mesylate in chronic myeloid leukemia. Genet Mol Res. 2005;4:803–11. [PubMed] [Google Scholar]
- 27.Yaghmaie M, Ghaffari SH, Ghavamzadeh A, Alimoghaddam K, Jahani M, Mousavi SA, et al. Frequency of BCR-ABL fusion transcripts in Iranian patients with chronic myeloid leukemia. Arch Iran Med. 2008;11:247–51. [PubMed] [Google Scholar]


