Abstract
Objective:
Systemic lupus erythematosus (SLE) is an autoimmune disease with an unknown etiology that can be life threatening. This study aimed to study the cause of mortality among admitted SLE patients over a period of 5 years at a teaching hospital in India.
Methods:
A 5-year retrospective analysis of mortality in SLE patients admitted under department of medicine of our institute was done. The presenting complaints, treatment history, clinical parameters, laboratory investigations, organ involvement, systemic lupus erythematosus disease activity index (SLEDAI), and cause of mortality were collected from the medical records on a predesigned proforma. A further analysis of two groups based on the cause of mortality was done.
Results:
In total, 53 death records were analyzed. Mortality in 28 SLE patients was due to high disease activity (Group I) and mortality in 25 patients was attributed due to both high disease activity and concomitant infection (Group II). Most of the patients were female (98%) and mean age of patient was 30.6 years. About 19 patients (35.8%) were diagnosed with SLE during hospital admission. Fever was the most common presenting complaint (69.8%) and lupus nephritis was the most common organ dysfunction seen (84.9%). Myocarditis was observed in 11 patients and 9 patients had cerebrovascular accident. The mean hemoglobin was lower in Group II (7.4 vs. 8.7 g/dL, P = 0.02). The median total leukocyte count was significantly higher in Group II (10,200 vs. 6600, P = 0.02). The mean serum urea and creatinine levels were also significantly higher in Group II (141.41 vs. 87.8 mg/dL, P = 0.006 and 4.7 vs. 1.7, P = 0.0001), respectively. The mean SLEDAI in Group I was 20.8 ± 8.9 and in Group II was17.7 ± 7.5. Bacterial pneumonia (17) was the most common infection, followed by tuberculosis (2) and fungal infection (2).
Conclusion:
Mortality among SLE patients could be due to disease flare or concomitant infection. Lung is the most common organ affected by infection in these patients.
Keywords: Anti phospholipid syndrome, infection, lupus nephritis, myocarditis, posterior reversible encephalopathy syndrome
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease, which can have varied manifestations and can affect every organ system in the body. The disease is more prevalent among females and is commonly diagnosed in second to fourth decade of life. Although the outcome of SLE patients has improved in last decade because of improved diagnostic and therapeutic options, still lupus patients have two to five times higher mortality rates as compared to general population.[1,2] The early mortality is caused by disease activity and infection, whereas the late mortality being secondary to cardiovascular complications.[3] Almost all available literature on lupus-related mortality are from American and European countries, the data of which cannot be extrapolated to Indian population. The primary aim of this study was to estimate the cause of mortality in SLE patients and secondary aim was to compare the profile of patients who die either from lupus flare or infection.
Subjects and Methods
This was a retrospective observational study done in the Department of Medicine at All India Institute of Medical Sciences, New Delhi. The study was approved by the ethical committee of the institute. All death records between January 2014 and December 2018 having diagnosis of SLE satisfying either 1997 American College of Rheumatology Modified Classification Criteria or the 2012 Systemic Lupus International Collaborating Clinics Classification Criteria for diagnosis of SLE were included in the study. The records having insufficient information and patients having overlap syndromes were excluded from the study. Out of 70 mortality files having diagnosis of SLE, 53 files were included in the study. About 17 records were excluded as 7 was not satisfying criteria for diagnosis of SLE, 2 had overlap syndrome, and 8 had inadequate information.
Data collection
The demographic, presenting complaints, treatment history, clinical parameters, laboratory investigations, organ involvement, systemic lupus erythematosus disease activity index (SLEDAI) at admission and cause of death were collected from the medical records. An increase in SLEDAI score of >3 was taken as flare. All 53 patients had high disease activity, and depending upon cause of death, they were further classified in two groups—Group I: SLE flare without infection and Group II: SLE flare with infection. Both the groups were then compared on various parameters.
Definitions
Hematological involvement was defined by (1) presence of leucopenia (leukocyte count <4,000/mm3) on two or more occasions in the absence of other causes like drug or infection, or (2) presence of thrombocytopenia (defined by platelet count <100,000/mm3) on two or more occasions in the absence of any other cause, or (3) autoimmune hemolytic anemia, defined by presence of hemolysis with positive direct antiglobulin test. Lupus nephritis was defined by (1) persistent proteinuria >500 mg/day, (2) presence of dysmorphic RBCs and cellular casts, or (3) biopsy-proven lupus nephritis.[4,5]
Neuropsychiatric systemic lupus erythematosus (NPSLE) was defined by as per American College of Rheumatology 1999 case definition. Presence of headache, seizure, cerebrovascular disease, demyelinating disease, myelopathy, movement disorders, aseptic meningitis, cognitive dysfunction, mood disorders, anxiety disorder, psychosis, acute confusion states, mononeuropathy, polyneuropathy, cranial neuropathy, autonomic dysfunction, myasthenia gravis, or acute inflammatory demyelinating polyradiculopathy was considered suggestive of NPSLE.[6] Cardiac disease was said to be present if patient had valvular disease, myocarditis, acute coronary syndrome, or cardiac arrhythmias due to SLE.[7] Patient was labeled to be having serositis if patient had either pleural effusion, pericardial effusion, or ascites of more than 1-day duration. Cutaneous involvement was defined by presence of acute, subacute, or chronic cutaneous lupus erythematosus.[8] Lupus myositis was said to be present if patient satisfied one of the following criteria: (1) proximal muscle weakness on examination with significant elevation of creatine phosphokinase, or lactate dehydrogenase levels (in the absence of rhabdomyolysis, myocardial ischemia, or trauma) and (2) proximal muscle weakness on examination with abnormal electromyography. Pulmonary involvement was considered if patient had radiological evidence of interstitial lung disease, pulmonary hemorrhage, lupus pneumonitis, pulmonary embolism, pulmonary arterial hypertension, and infective pneumonia.[9] Presence of infection was considered based on clinicoradiological assessment, microbiological evidence, and response to antibiotics. A diagnosis of hospital acquired infection was made when infection occurred after ≥48 h of hospital admission and was not present or incubating at time of admission.[10] Steroid use was considered significant if the patient was on daily oral steroid for the last 3 months. Also, use of any other immunosuppressant in last 3 months was obtained from the medical record. The cause of death was ascertained based on the medical records and treating clinician documentation.
Statistical analysis
Data were recorded in a predesigned proforma and managed on an excel sheet. Continuous variables were summarized as mean and standard deviation (SD) or median and range (in presence of outliers). Quantitative variables were analyzed using parametric (Student's t-test) or nonparametric tests (Kruskal–Wallis test), as applicable. All qualitative variables were summarized as frequency (percentage) and were analyzed with Chi-square or Fisher's exact test. The statistical analysis was performed using Stata 12 software. A P value of <0.05 was considered statistically significant.
Results
Mortality in 28 (52.8%) patient was due to high disease activity (Group I) and mortality in 25 (47.2%) patient was attributed due to both high disease activity and concomitant infection (Group II). Most of the patients in the study were females (98%) and mean age of patient was 30.6 ± 10.3 years. The median duration of hospital stay was 7 days and median duration of SLE diagnosis was 2 months. Nineteen patients (35.8%) were diagnosed with SLE during hospital admission. The most common symptom at the time of admission was fever (69.8%), followed by arthralgia (66%), dyspnea (56%), oliguria (50.1%), cough (43.4%), anasarca (37.7%), psychosis (35.7%), seizure (33.9%), oral ulcer (32%), photosensitivity (28%), skin rash (26.4%), myalgia (22.6%), malar rash (20.7%), and alopecia (20.7%). Hypothyroidism was present in 22.6% of all patients and six patients had secondary antiphospholipid syndrome. Almost half of the patients (47.1%) received steroid therapy in the last 3 month [Table 1]. Ten patients received other immunosuppressant in form of [cyclophosphamide (3), mycophenolate (3), and azathioprine (4)] in the last 3 months. The mean SLEDAI of all patients was 19.4 ± 8.3. The most common organ involvement in the study was renal (84.9%) followed by hematological (58.4%), neuropsychiatric (49%), serous membrane (49%), lung (35.8%), cutaneous (32%), cardiovascular (24.5%), and muscular system (9.4%).
Table 1.
Comparison of demographic and clinical profile of SLE patients
| Variables | Total (n=53) | Group I (n=28) | Group II (n=25) | P |
|---|---|---|---|---|
| Female (%) | 52 (98) | 27 (96) | 25 (100) | 0.34 |
| Age (year)* | 30.6±10.3 | 29.1±10.5 | 32.3±10.1 | 0.25 |
| Duration of SLE (month)# | 2 (0-156) | 1 (0-120) | 4 (0-156) | 0.15 |
| Duration of hospital stay (day)# | 7 (1-77) | 6 (1-77) | 8 (1-27) | 0.17 |
| Photosensitivity (%) | 11 (28) | 6 (21.4) | 5 (20) | 0.89 |
| Malar rash (%) | 11 (20.7) | 7 (25) | 4 (16) | 0.42 |
| Arthralgia (%) | 35 (66) | 19 (67.8) | 16 (64) | 0.76 |
| Oral ulcer (%) | 17 (32) | 9 (32.1) | 8 (32) | 0.99 |
| Myalgia (%) | 12 (22.6) | 7 (25) | 5 (20) | 0.66 |
| Fever (%) | 37 (69.8) | 16 (57.1) | 21 (84) | 0.03 |
| Cough (%) | 23 (43.4) | 9 (32.1) | 14 (56) | 0.08 |
| Dyspnea (%) | 30 (56) | 14 (50) | 16 (64) | 0.30 |
| Skin rash (%) | 14 (26.4) | 9 (32) | 5 (20) | 0.31 |
| Seizures (%) | 18 (33.9) | 10 (35.7) | 8 (32) | 0.77 |
| Alopecia (%) | 11 (20.7) | 6 (21.4) | 5 (20) | 0.89 |
| Psychosis (%) | 10 (35.7) | 9 (32.1) | 1 (4) | 0.009 |
| Oliguria (%) | 27 (50.1) | 14 (50) | 13 (52) | 0.88 |
| Anasarca (%) | 20 (37.7) | 10 (35.7) | 10 (40) | 0.74 |
| Hypothyroidism (%) | 12 (22.6) | 9 (32.1) | 3 (12) | 0.08 |
| Steroid in last 3 months (%) | 25 (47.1) | 14 (50) | 11 (44) | 0.66 |
SLE=systemic lupus erythematosus, *Mean±SD. #Median (Min-Max)
Lupus nephritis was present in 45 patients, out to which 15 were diagnosed on renal biopsy. Hematological abnormalities observed were thrombocytopenia (26), leucopenia (10), and autoimmune hemolytic anemia (4). Nine patients had cerebrovascular accident [infarction (4), intracranial bleed (3), subarachnoid hemorrhage (1), and cerebral vein thrombosis (1)]. Posterior reversible encephalopathy syndrome was diagnosed in five patients. Myocarditis was diagnosed in 11 patients, 1 patient had valvular heart disease, and 1 had acute coronary syndrome. Four patients had concomitant myocarditis with cerebrovascular accident. So, overall 17 (32%) patients had mortality because of cardiovascular disease/cerebrovascular accident or both. Four patients had diffuse alveolar hemorrhage and one patient had lupus pneumonitis. One patient had lupus related acute pancreatitis [Table 2].
Table 2.
Comparison of various organ manifestations in two groups
| Organ involved | Total (n=53) | Group I (n=28) | Group II (n=25) | P |
|---|---|---|---|---|
| Renal | 45 (84.9%) | 22 (78.5%) | 23 (92%) | 0.17 |
| Hematological | 31 (58.4%) | 17 (60.7%) | 14 (56%) | 0.72 |
| NPSLE | 26 (49%) | 18 (64.2%) | 8 (32%) | 0.01 |
| Serositis | 26 (49%) | 16 (57.1%) | 10 (40%) | 0.21 |
| Lung | 19 (35.8%) | 4 (14.3%) | 15 (60%) | 0.001 |
| Cutaneous | 17 (32%) | 9 (32.1%) | 8 (32%) | 0.99 |
| Cardiac | 13 (24.5%) | 8 (28.5%) | 5 (20%) | 0.46 |
| Myositis | 5 (9.4%) | 4 (14.2%) | 1 (4%) | 0.21 |
| Pancreatitis | 1 (1.8%) | 0 | 1 (4%) | 0.28 |
NPSLE=neuropsychiatric systemic lupus erythematosus
Steroid was the most common immunosuppressive agent used to treat SLE patients. Methylprednisolone pulse therapy was given to 26 patients and 16 patients received oral prednisolone too after pulse therapy, whereas 12 patients received only oral steroids. In total, 15 patients were not initiated on steroids either because of early mortality or overwhelming infections. Intravenous cyclophosphamide (12), intravenous immunoglobulin (5), mycophenolate (5), plasma exchange (3), and rituximab (1) were other treatment modalities used in these patients [Table 3]. The infections were treated with appropriate antimicrobial agents.
Table 3.
Various in hospital treatment among two groups
| Treatment modality | Total | Group I | Group II | P |
|---|---|---|---|---|
| Oral steroid in hospital (%) | 28 (52.8) | 17 (60.7) | 11 (44) | 0.22 |
| MP pulse in hospital (%) | 26 (49) | 15 (53.5) | 11 (44) | 0.48 |
| MMF use in hospital (%) | 5 (9.4) | 4 (14.2) | 1 (4) | 0.2 |
| PLEX in hospital (%) | 3 (5.6) | 0 | 3 (12) | 0.05 |
| IVIg (%) | 5 (9.4) | 1 (3.5) | 4 (16) | 0.12 |
| Cyclophosphamide in hospital (%) | 12 (22.6) | 6 (21.4) | 6 (24) | 0.8 |
| Rituximab (%) | 1 (1.8) | 0 | 1 (4) | 0.28 |
| Hemodialysis (%) | 21 (39.6) | 5 (17.8) | 16 (64) | 0.001 |
MP=methylprednisolone, MMF=mycophenolate mofetil, PLEX=plasma exchange, IVIg=intravenous immunoglobulin
A further analysis of two groups based on the cause of mortality was done. The median duration of SLE in Group I was 1 month (range: 0–120) and in Group II was 4 months (range: 0–156 months). Fever was present in 84% of patients in Group 2 as compared with 57% in Group 1 (P = 0.03). Psychosis was present in nine patients in Group I and was present in only one patient in Group II (P = 0.009). The mean SLEDAI in Group I was 20.8 ± 8.9 and in Group II was17.7 ± 7.5.
The mean hemoglobin level was significantly lower in Group II as compared with Group I (7.4 ± 1.6 vs. 8.7 ± 2.4 g/dL, P = 0.02). Similarly, median total leukocyte count was significantly higher in second group (10,200 vs. 6600/mm3, P = 0.02). The mean serum urea and creatinine levels were also significantly higher in Group II (141.4 ± 65.1 vs. 87.8 ± 72.5 mg/dL, P = 0.006 and 4.7 ± 3.4 vs. 1.7 ± 1.4, P = 0.0001), respectively. The median serum C-reactive protein (CRP) levels were raised in Group II (88.5 vs. 34.4, P = 0.12) but was not statistically significant [Table 4].
Table 4.
Comparison of laboratory parameter between two groups
| Parameter | Total (n=53) | Group I (n=28) | Group II (n=25) | P |
|---|---|---|---|---|
| Hemoglobin (g/dL)* | 8.1±2.2 | 8.7±2.4 | 7.4±1.6 | 0.02 |
| TLC# | 9,100 (200-36,500) | 6600 (200-19100) | 10,200 (2,200-36,500) | 0.02 |
| Platelet count# | 1,03,000 (9,000-4,12,000) | 1,03,500 (9,000-4,12,000) | 99,000 (11,000-2,00,000) | 0.97 |
| Urea (mg/dL)* | 113.1±73.6 | 87.8±72.5 | 141.4±65.1 | 0.006 |
| Creatinine (mg/dL)* | 3.1±2.9 | 1.7±1.4 | 4.7±3.4 | 0.0001 |
| Bilirubin (mg/dL)* | 1.01±1.65 | 0.88±0.91 | 1.17±2.22 | 0.53 |
| Albumin (mg/dl)* | 2.3±0.56 | 2.4±0.65 | 2.2±0.46 | 0.22 |
| AST (IU/L)* | 115.1±184 | 99.3±182.5 | 131.6±188.1 | 0.54 |
| ALT (IU/L)* | 58.9±88.1 | 43.1±46 | 75.4±116 | 0.20 |
| INR* | 1.51±0.98 (n=43) | 1.36±0.38 (n=19) | 1.629±1.28 (n=24) | 0.38 |
| CPK (U/L)# | 70 (17-1,315) (n=17) | 99 (37-728) (n=10) | 70 (17-1,315) (n=7) | 0.59 |
| LDH (U/L)# | 606 (252-11,319) (n=28) | 677 (275-11,319) (n=15) | 529 (252-3,328) (n=13) | 0.42 |
| ESR (mm/h)* | 63.9±32.1 (n=34) | 60±30.2 (n=15) | 67±34 (n=19) | 0.53 |
| Ferritin# | 888 (155-40,000) (n=19) | 827 (155-40,000) (n=9) | 1194 (237-7,765) (n=10) | 0.96 |
| CRP (mg/L)# | 41.2 (1.3-211) (n=32) | 34.4 (2.5-186) (n=18) | 88.5 (1.3-211) (n=14) | 0.12 |
| SLEDAI* | 19.4±8.3 | 20.8±8.9 | 17.7±7.5 | 0.09 |
TLC=total leukocyte count, AST=aspartate aminotransferase, ALT=alanine aminotransferase, INR=international normalized ratio, CPK=creatine phosphokinase, LDH=lactate dehydrogenase, ESR=erythrocyte sedimentation rate, CRP=C-reactive protein, SLEDAI=systemic lupus erythematosus disease activity index, *Mean±SD, #Median (Min-Max)
Among major organ involvement, NPSLE was significantly more in Group I (64.2% vs. 32%, P = 0.01) and lung involvement was significantly more in Group II (60% vs. 14.3%, P = 0.001). Lupus nephritis was present in 22 (78.5%) patients in Group I and 23 (92%) patients in Group 2 [Table 2]. The number of patients undergoing hemodialysis were significantly more in Group II (16 vs. 5, P = 0.001). The most common infection was bacterial pneumonia (17) followed by urosepsis (2), fungal endocarditis (1), disseminated fungal infection (1), tuberculous empyema (1), and disseminated tuberculosis (1) [Figure 1]. Hospital acquired pneumonia was suspected in seven patients. Acinetobacter baumannii was isolated from seven patients. Pseudomonas, Klebsiella, Candida, and Aspergillus species were isolated from one patient each [Figure 2].
Figure 1.
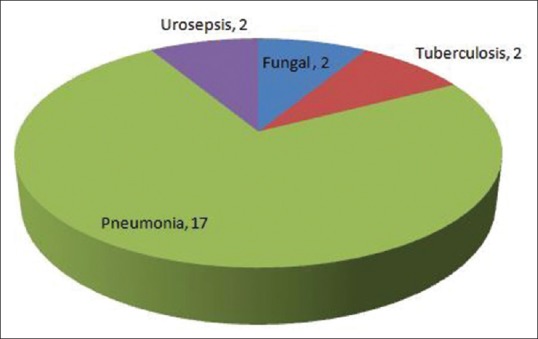
Spectrum of infection in Group II
Figure 2.
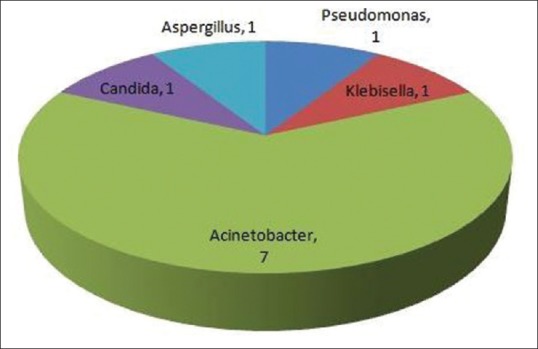
Microbiological profile of infection
Discussion
This study showed that high disease activity was present in all SLE patients who died in the hospital. The number of patients having concomitant disease flare and infection were high. Bacterial pneumonia was the most common infection. Leukocytosis, higher values of serum urea and creatinine were observed in patients with infection. Patient dying only due to disease activity had significantly higher neuropsychiatric manifestations of lupus.
According to a recent meta-analysis, lupus patients have 2.6-fold higher SMR (Standardized mortality ratio) from all causes. Infection was found to increase mortality rate in SLE patients by 4.98-folds, renal disease increased mortality by 4.68-folds, and CVD (cerebrovascular accidents and heart disease) increased mortality by 2.25-folds.[11] We also found disease activity as the most common cause of death, followed by infection. About 85% of patients in this study had renal disease and almost half of them had concomitant infection. About 32% of patients in our study had CVD/heart disease or both. The higher rate of CVD could be due to anti-phospholipid antibody, atherosclerosis, and endothelial dysfunction due to chronic inflammation.[12,13] In a European study, CVD was found to be the most frequent cause of death followed by malignancy and lupus-related disease activity. SLE patients have higher chance of acquiring malignancies, such as non-Hodgkin lymphoma and lung cancer.[14] But, in our study, we could not find any mortality due to malignancy, possibly because cases from oncology department were not included.
The most dreaded complication of SLE remains lupus nephritis, which itself can cause significant increase in mortality rate.[15] A nationwide US billing database found incidence of infection rate per 100 person-years to be 10.8 in SLE cohort, whereas it was 23.9 in LN cohort. They also showed that infection rates were higher in patients on steroids and immunosuppressive drugs.[16] Also, it has been proposed that immune system dysfunction leads to higher risk of acquiring secondary infections.[17] In this study too, 92% of patients with infection had LN.
Lung is the most common organ system affected by infection in patients with SLE.[18] Bacterial pneumonia was the most common infection in this study, comprising 68% of all infections. Fungal infections have been also seen frequently in SLE patients and our study had two mortalities due to this infection. The impaired cell-mediated immunity due to immunosuppression probably increases the risk of acquiring fungal infection.[19] Leukocytosis and raised CRP are known markers of systemic infection in lupus patients, as observed in our study too.[20]
Our study being a retrospective study had certain limitations, such as missing data and small sample size. A significant number of patients were diagnosed with SLE during hospital admission; however, the factors responsible for delay in diagnosis could not be ascertained by this retrospective study. Also, SLE patients from other department, such as nephrology, cardiology, or oncology were not included. Nonetheless, this is the first study on lupus-related mortality from India. A prospective cohort study on SLE patients from India will further help in finding various factors responsible for mortality in these patients.
Conclusion
The data from our study emphasize that mortality occur at a younger age in SLE patients either due to lupus flare or concomitant lupus flare and infection. Renal involvement is the most common organ system affected in these patients and lung is the most common organ affected by infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Manger K, Manger B, Repp R, Geisselbrecht M, Geiger A, Pfahlberg A, et al. Definition of risk factors for death, end stage renal disease, and thromboembolic events in a monocentric cohort of 338 patients with systemic lupus erythematosus. Ann Rheum Diss. 2002;61:1065–70. doi: 10.1136/ard.61.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME. Surviving the butterfly and the wolf: Mortality trends in systemic lupus erythematosus. Autoimmun Rev. 2004;3:423–53. doi: 10.1016/j.autrev.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 4.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 5.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kivity S, Agmon-Levin N, Zandman-Goddard G, Chapman J, Shoenfeld Y. Neuropsychiatric lupus: A mosaic of clinical presentations. BMC Med. 2015;13:43. doi: 10.1186/s12916-015-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miner JJ, Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 2014;40:51–60. doi: 10.1016/j.rdc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Sinha AA. Cutaneous lupus erythematosus: Understanding of clinical features, genetic basis, and pathobiology of disease guides therapeutic strategies. Autoimmunity. 2006;39:433–44. doi: 10.1080/08916930600886851. [DOI] [PubMed] [Google Scholar]
- 9.Keane MP, Lynch JP., 3rd Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax. 2000;55:159–66. doi: 10.1136/thorax.55.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benenson AS. Control of Communicable Diseases Manual. 16th ed. Washington: American Public Health Association; 1995. [Google Scholar]
- 11.Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: An updated meta-analysis. Lupus. 2016;25:727–34. doi: 10.1177/0961203315627202. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal S, Elliott JR, Manzi S. Atherosclerosis risk factors in systemic lupus erythematosus. Curr Rheumatol Rep. 2009;11:241–7. doi: 10.1007/s11926-009-0034-0. [DOI] [PubMed] [Google Scholar]
- 13.Packard RR, Libby P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 14.Ippolito A, Petri M. An update on mortality in systemicerythematosus lupus. Clin Exp Rheumatol. 2008;26(Suppl 51):S72–9. [PubMed] [Google Scholar]
- 15.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 16.Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheum. 2015;67:1577–85. doi: 10.1002/art.39070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JY, Suh CH. Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J Intern Med. 2017;32:429–38. doi: 10.3904/kjim.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladman DD, Hussain F, Ibañez D, Urowitz MB. The nature and outcome of infection in systemic lupus. Erythematosus Lupus. 2002;11:234–9. doi: 10.1191/0961203302lu170oa. [DOI] [PubMed] [Google Scholar]
- 19.Howard DH, Otto V, Gupta RK. Lymphocyte-mediated cellular immunity in histoplasmosis. Infect Immunity. 1971;4:605–10. doi: 10.1128/iai.4.5.605-610.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Serougy E, Zayed HS, Ibrahim NM, Maged LA. Procalcitonin and C-reactive protein as markers of infection in systemic lupus erythematosus: The controversy continues? Lupus. 2018:961203318777101. doi: 10.1177/0961203318777101. doi: 10.1177/0. [DOI] [PubMed] [Google Scholar]


