Abstract
Introduction:
Axillary brachial plexus block is used for anesthesia in hands and forearm surgery. The aim of this study was to compare the hemodynamic changes of magnesium sulfate and dexmedetomidine in axillary block.
Materials and Methods:
This randomized, double-blind clinical trial was conducted on 99 patients undergoing a forearm and hand surgery at the Vali-Asr Hospital. Patients were divided into three groups. Dexmedetomidine group consisted of lidocaine 1.5% plus 0.5 μg/kg dexmedetomidine, magnesium sulfate group included lidocaine 1.5% plus 100 mg magnesium sulfate, and the control group received lidocaine 1.5% with normal saline. The final volume was divided into 35 groups in three groups. Blood pressure, heart rate, and oxygen saturation were measured every 5 minutes during surgery, and data were analyzed by SPSS 23.
Results:
There was a statistically significant difference between the three groups in terms of the mean blood pressure during surgery (P < 0.05). At all times, blood pressure in the dexmedetomidine group was lower as compared to the other two groups. But in the 20th and 25th minutes, there was a relative increase in blood pressure. There was a significant difference between the three groups in terms of heart rate during surgery in minutes 20, 25, 65–100, and 110–120 (P < 0.05).
Conclusion:
The final result showed that the blood pressure and heart rate of the dexmedetomidine group patients at different times were less than the other two groups.
Keywords: Axillary block, brachial plexus, dexmedetomidine, hemodynamic changes, magnesium sulfate
Introduction
Axillary brachial plexusblock is used for anesthesia in hand and forearm surgery. Usually, epinephrine is applied in combination with local anesthetic agents to induce local anesthesia, which has many benefits due to vasoconstrictive effect including increasing the block time and decreasing the maximum plasma level of local anesthetic agent, leading to reducing the side effects of these drugs.[1,2] The basis of this local block is the injection of anesthetic drugs in the vicinity of the root or trunk of the neuron.[3] To improve the severity, quality, and duration of anesthesia in these blocks, other drugs such as opiates, bicarbonate, adrenaline, and dexamethasone were used along with the anesthetic drugs.[4,5,6] Postoperative pain increases the cost of treatment and the duration of hospitalization. Anesthesiologists have performed researches to increase the length of the block with different local anesthetics as increasing the duration of analgesia makes the patient comfortable after surgery. The possibility of opioid receptor has led to the use of various drugs in local blocks to increase the duration of analgesia without increasing side effects. Several studies have used diverse local anesthetics along with narcotics that have completely different outcomes.[7] Magnesium improves the quality of anesthesia both intravenously and intraperitoneally.[8,9] Various studies have revealed that magnesium is effective in reducing the onset time of the block and increasing the quality and duration of anesthesia.[10,11,12] Dexmedetomidine, a selective α2-adrenergic receptor agonist, is considered as an anxiety reducing, sedative, analgesic, and antihypertension drug.[13] Hence, including dexmedetomidine to local anesthetic drugs during the peripheral nervous block can be effective.[14,15] In the year 2018, Aghamohammadi et al. reported that magnesium sulfate had the least effect on the hemodynamics of patients undergoing surgery.[16] Nooraei et al. (2013) indicated that magnesium sulfate has a minor effect on the hemodynamic status of patients and stated that the use of this drug is superior to other drugs for the stability of the hemodynamic state.[17] The results of hemodynamic changes due to the use of magnesium sulfate are evident. On the other hand, most studies have shown that dexmedetomidine is capable of reducing heart rate, and on the other hand, blood pressure first increases and then decreases, as well.[18,19,20]
So far, a study to compare the effect of dexmedetomidine and magnesium sulfate drugs on hemodynamic changes of axillary block patients has not been reported. Magnesium sulfate, being a cheaper drug than dexmedetomidine, If this drug can be replaced, it could be helpful in reducing costs. Therefore, this study was conducted to compare the two drugs dexmedetomidine and magnesium sulfate in the axillary block.
Materials and Methods
This double-blind randomized clinical trial study was performed on and with the informed consent of 99 patients who were referred to Vali-Asr Hospital in Arak, Iran for forearm and hand surgery. Inclusion criteria: ASA I, II, 18–65 years old, both sexes, forearm and hand surgery with axillary block, forearm and hand fracture, absence of more than one fracture in the body or surgery, absence of coagulation disorders and impaired PTT and PT and INR, lack of BMI over 35, lack of psychological problems, no history of allergy to used drugs, lack of pregnancy, absence of chronic pain syndrome, and absence of neurological disorders.
Exclusion criteria: infection at the location of the block and failure of the block.
The surgical time of more than 120 minutes required sedation to be more than stated in the plan and lack of patient's cooperation in performing the block. The patient was transferred to the operating theater and prepared for the axillary block by anesthesia assistant and the block was done by the assistant himself. The drugs were prepared by an anesthetist in each group and were administered for block by an anesthesia assistant blinded to the materials used in the drug. First, the patient was placed in the supine position. The arm was abducted at a 90-degree angle to the trunk, the elbow was subjected to a 90-degree flexion in supine position, and required to lean back on the pillow. Then the location of the axillary pulse was found by touching the region of the axilla from the most proximal area to the distal end. Afterward, the axillary area was disinfected with povidone-iodine (PVP-I). The artery was then pointed toward the humerus and between the left index and the middle fingers of the performer, and the needle was entered from the proximal side to the axillary line. In this study, the exact location of axillary block was determined using a nerve stimulator and a needle block of 5 to 7 (G). After assuring the location of the needle block in the axillary region, the syringe containing the block solution was attached and then the negative aspiration was injected. To increase the success of the axillary block after the needle insertion, injection of the drugs was performed at hours 3, 9, and 12 after receiving neurotic anesthesia.
The patients were randomly divided into three equal groups of dexmedetomidine, magnesium sulfate, and placebo using random number table. For the first group, lidocaine 1.5% plus 0.5 μg/kg of dexmedetomidine in 35 ml volume were used. In the second group, lidocaine 1.5% plus 100 mg of magnesium sulfate in a volume of 35 ml were applied.[17] The control group received lidocaine 1.5% with normal saline in 35cc volume. After ensuring the proper anesthetic, the tourniquet was closed for the patient. It should be noted that side-effects such as bradycardia and reduction of reflexes, hypotension and continuous hypothermia were assessed by continuous patient monitoring during operation. The protocol was acted upon if side effects occurred.
The mean arterial blood pressure, heart rate, and arterial oxygen saturation were measured every 5 minutes until the end of surgery by an anesthetist specialist. Finally, the results of the three groups were compared. Data was collected by a resident anesthetist assistant for double-blinding who was unaware of groupings and the drugs administered to each group. In addition, the axillary block was carried out by an assistant specialist unaware of the medications in each syringe. The collected data was then analyzed using SPSS software version 23.
Results
This double-blind clinical trial was performed on 99 patients who underwent forearm and hand surgery with axillary block in Vali-Asr Hospital in Arak. They were randomly divided into three groups. The failure rate in the dexmedetomidine group was determined to be three cases, followed by three cases in the magnesium sulfate group and two cases in the placebo group, which did not show a significant difference between the different groups (P < 0.05). There was no significant difference in age, sex, and body mass index between the three groups (P < 0.05).
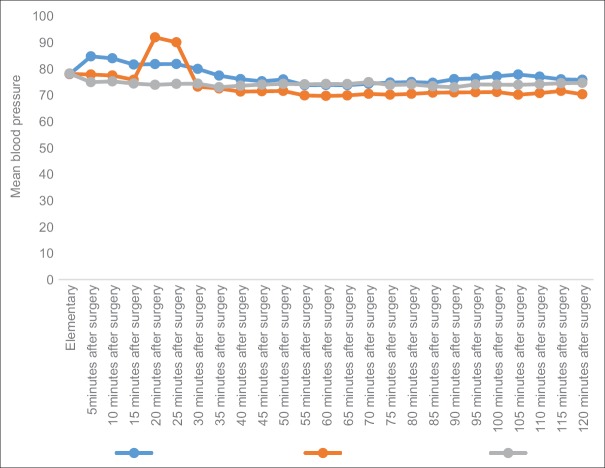
However, there was a significant difference between the three groups in terms of the mean blood pressure during surgery (P < 0.05).
According to Figure 1, the lowest blood pressure was associated with dexmedetomidine and the highest blood pressure was determined for magnesium sulfate group. Only in the 20th and 25th minutes, blood pressure increased in the dexmedetomidine group. The total slope of the graph of both the magnesium sulfate and dexmedetomidine groups were similar, thus indicating a similar reduction ratio. It seems that this decrease in blood pressure and transient rise of blood pressure in minutes 20 and 25 can be due to the vascular accumulation of the drug in the axillary sheath and the systemic absorption of dexmedetomidine. This vascular uptake is likely to result in the systemic effects of dexmedetomidine which is associated with a relative reduction and transient rise in blood pressure [Figure 1].
Figure 1.
Comparison of mean blood pressure in three groups
There was a significant difference between the three groups in terms of heart rate during surgery in minutes 20, 25, 65 to 100, and 110 to 120 (P <0.05). There were no significant differences in heart rate between groups in the early minutes of 20, 30, 65 and 105 (P >0.05). At all times, heart rate was lower in the dexmedetomidine group as compared to the other two groups. Magnesium sulfate group had less heart rate than placebo. The results demonstrated that heart rate was higher in magnesium sulfate group [Table 1].
Table 1.
Comparison of mean and standard deviation of heart rate in the three groups of dexmedetomidine and magnesium sulfate and placebo
| Group heart rate | Magnesium sulfate SD±Mean | Dexmedetomidine SD±Mean | Placebo SD±Mean | P* ANOVA test |
|---|---|---|---|---|
| Elementary | 79.18±8.7 | 77.24±8.75 | 76.81±7.5 | 0.485 |
| 5 min after surgery | 79.42±6.55 | 77.18±8.77 | 76.81±7.5 | 0.331 |
| 10 min after surgery | 78.84±5.46 | 76.66±8.82 | 77.42±7.56 | 0.480 |
| 15 min after surgery | 78.87±6.46 | 75.93±8.76 | 77.48±6.56 | 0.271 |
| 20 min after surgery | 87.27±6.62 | 73.42±7.27 | 77.39±6.33 | 0.010 |
| 25 min after surgery | 78.57±6.72 | 73.96±7.57 | 76.93±7.61 | 0.039 |
| 30 min after surgery | 77.93±7.12 | 75.87±7.46 | 76.96±6.57 | 0.500 |
| 35 min after surgery | 77.67±7.59 | 73.87±7.62 | 76.21±6.29 | 0.105 |
| 40 min after surgery | 75.60±6.24 | 74.39±8.06 | 76.33±6.30 | 0.519 |
| 45 min after surgery | 75.15±6.72 | 73.72±9.42 | 76.24±7.08 | 0.429 |
| 50 min after surgery | 74.54±6.57 | 73.36±8.92 | 76.66±7.05 | 0.206 |
| 55 min after surgery | 74.66±7.02 | 73.54±9.11 | 76.96±7.68 | 0.212 |
| 60 min after surgery | 74.12±7.08 | 73.24±9.47 | 77.51±7.43 | 0.081 |
| 65 min after surgery | 73.09±6.99 | 72.15±8.90 | 78.06±8.69 | 0.009 |
| 70 min after surgery | 74.12±7.74 | 72.03±8.88 | 77.75±8.56 | 0.023 |
| 75 min after surgery | 74.21±7.39 | 71.69±8.87 | 78.03±8.81 | 0.010 |
| 80 min after surgery | 75.27±6.98 | 71.36±8.93 | 77.21±8.09 | 0.013 |
| 85 min after surgery | 74.30±7.17 | 71.33±9.27 | 76.75±6.78 | 0.022 |
| 90 min after surgery | 74.72±6.71 | 71.42±8.77 | 76.15±6.78 | 0.036 |
| 95 min after surgery | 74.84±6.83 | 71.12±9.18 | 76.63±6.67 | 0.014 |
| 100 min after surgery | 74.51±6.91 | 71.27±9.34 | 75.84±6.63 | 0.051 |
| 105 min after surgery | 74.42±6.83 | 70.96±9.38 | 74.81±6.57 | 0.088 |
| 110 min after surgery | 75.12±6.95 | 70.90±10.19 | 75.48±6.43 | 0.014 |
| 115 min after surgery | 74.81±6.61 | 70.96±10.13 | 76.57±6.93 | 0.018 |
| 120 min after surgery | 74.75±6.90 | 70.96±8.71 | 77.36±6.44 | 0.003 |
*ANOVA TEST
On comparison, patients of the dexmedetomidine group showed a lower heart rate than the other groups. This may be due to the vascular absorption of dexmedetomidine which in turn occurred due to the vascular accumulation in the axillary sheath, which led to a decrease in heart rate. It is worth noting that heart rate reduction, based on the average patient base, is not related to the side effects of the drug in this case, and none of the patient groups showed a drug complication leading to management. In addition, no significant difference was found between the three groups in terms of the percentage of saturated oxygen (P > 0.05) [Table 2].
Table 2.
Comparison of mean and standard deviation of oxygen saturation in three groups
| Group saturation Percent of oxygen | Magnesium sulfate Mean±SD | Dexmedetomidine Mean±SD | Placebo Mean±SD | P* ANOVA test |
|---|---|---|---|---|
| Elementary | 98.48±1.30 | 98.51±1.06 | 98.69±1.38 | 0.076 |
| 5 minutes after surgery | 98.36±1.34 | 98.39±1.08 | 98.24±1.27 | 0.871 |
| 10 minutes after surgery | 98.45±1.34 | 98.45±1.09 | 98.30±1.42 | 0.855 |
| 15 minutes after surgery | 98.45±1.30 | 98.45±1.22 | 98.60±1.27 | 0.698 |
| 20 minutes after surgery | 98.39±1.36 | 98.36±1.22 | 98.60±1.19 | 0.636 |
| 25 minutes after surgery | 98.36±1.49 | 98.36±1.27 | 98.63±1.24 | 0.636 |
| 30 minutes after surgery | 98.27±1.48 | 98.54±1.09 | 98.15±1.22 | 0.638 |
| 35 minutes after surgery | 98.18±1.48 | 98.27±1.25 | 98.45±1.50 | 0.803 |
| 40 minutes after surgery | 98.30±1.46 | 98.15±1.22 | 98.09±1.30 | 0.935 |
| 45 minutes after surgery | 98.21±1.47 | 98.27±1.17 | 98.15±1.34 | 0.862 |
| 50 minutes after surgery | 98.24±1.43 | 98.30±1.23 | 98.12±1.45 | 0.493 |
| 55 minutes after surgery | 98.27±1.30 | 98.48±1.20 | 97.63±1.22 | 0.839 |
| 60 minutes after surgery | 98.24±1.29 | 98.36±1.24 | 98.18±1.26 | 0.627 |
| 65 minutes after surgery | 98.39±1.14 | 98.54±1.14 | 98.12±1.36 | 0.470 |
| 70 minutes after surgery | 98.36±1.14 | 98.09±1.30 | 97.81±1.33 | 0.171 |
| 75 minutes after surgery | 98.39±1.17 | 98.09±1.28 | 98.30±1.40 | 0.527 |
| 80 minutes after surgery | 98.39±1.27 | 98.12±1.36 | 98.24±1.39 | 0.323 |
| 85 minutes after surgery | 98.57±1.17 | 9839±1.11 | 98.48±1.17 | 0.106 |
| 90 minutes after surgery | 98.48±1.12 | 98.24±1.06 | 98.30±1.13 | 0.121 |
| 95 minutes after surgery | 98.36±1.31 | 98.24±1.25 | 98.03±1.42 | 0.575 |
| 100 minutes after surgery | 98.33±1.19 | 98.57±1.37 | 98.60±1.27 | 0.202 |
| 105 minutes after surgery | 98.39±1.24 | 98.15±1.39 | 98.03±1.40 | 0.124 |
| 110 minutes after surgery | 98.42±1.27 | 98.27±1.28 | 98.18±1.28 | 0.201 |
| 115 minutes after surgery | 98.54±1.30 | 98.39±1.19 | 98.30±1.33 | 0.252 |
| 120 minutes after surgery | 98.48±1.12 | 98.45±1.30 | 98.45±1.48 | 0.244 |
*ANOVA TEST
Discussion
The aim of this study was to compare the effect of adding magnesium sulfate and dexmedetomidine on the increase in the length of the sensory and motor block of axillary block. This double-blind clinical trial was performed on 99 patients with forearm and hand surgery, candidate for axillary block in Vali-Asr Hospital, Arak, Iran. The subjects were randomly divided into three groups. Aghamohammadi et al. in 2018 investigated the hemodynamic changes after intravenous infusion of magnesium sulfate and the control of postoperative pain after laparotomy. They stated that magnesium sulfate had the least effect on the hemodynamics of patients undergoing surgery.[16] The aforementioned study used intravenous infusion, but an injection has been performed on the axillary block in our study. However, our results also indicated that heart rate and blood pressure were stable in magnesium sulfate group. A study by Zaman et al. in 2017 evaluated the effect of dexmedetomidine in combination with lidocaine on the onset and duration of axillary block. They demonstrated that dexmedetomidine was capable of reducing the heart rate after the block, which was the lowest in 30th and 60th minutes. The mean systolic pressure in the 10th, 15th, 30, and 60th minutes was less in the dexmedetomidine group and the diastolic pressure at 10th, 15th, 30th and 60th and 90th minutes in the dexmedetomidine group have been reported to be decreased in this group. In the dexmedetomidine group, two patients with heart rate <50 were treated with atropine and one patient who also had low blood pressure was injected 5 milligrams of ephedrine.[18]
In our study, at all times, heart rate in the dexmedetomidine group was lower than the other two groups. Although, blood pressure in the dexmedetomidine group was also lower at all times as compared to other two groups, a sudden increase in blood pressure was observed in the 20th and 25th minutes. Our study was also consistent with previous studies. Another study compared the effect of ropivacaine with and without dexmedetomidine for an axillary brachial plexus block.
The aforementioned study indicated that heart rate was lower in the dexmedetomidine group. In addition, no statistically significant difference was found in terms of bradycardia. The systolic and diastolic blood pressures were decreased between 15th minutes and 540th minutes and no significant difference was observed in the oxygen saturation.[19] The results of our study were consisted with these findings.
A study indicated, as in the present study, a small change in the hemodynamic status of the magnesium sulfate group patients, where the use of this drug is prioritized for the stability of hemodynamic status.[17] Albrecht et al. in their review, reported that the effects of magnesium sulfate on hemodynamic status are not low in all patients, and changes in hemodynamics are so high in some patients for unknown reasons that therapeutic measures need to be observed; Therefore, they suggest that this drug be used with caution and it should be used in young patients as well; the partial results of their study (negative effects on hemodynamic status) were consistent with our study. The reason for the difference is that the study by Albrecht et al. is a review of their investigation in which the effect of magnesium sulfate has been focused on in vivo, but in our study magnesium sulfate was assessed for an axillary brachial plexus block.[21] In another study, Kaygusuz et al. examined the dexmedetomidine in combination with levobupivacaine in the axillary block. They revealed that except for the fifth minute, the mean blood pressure and heart rate were lower in the dexmedetomidine group. None of the patients showed hypotension and bradycardia, and oxygen saturation was not different in the two groups.[20] The study results of Kaygusuz et al. are consistent with our study because they have used 0.5% levobupivacaine and we have used lidocaine 1.5%. According to the lower dose and local injection of drugs, systemic and dangerous complications were not observed for therapeutic intervention. Low vascular absorption of dexmedetomidine may have benefits in maintaining stable blood pressure and preventing increased pressure during surgery that indicates the safety of these drugs in mentioned doses.
Conclusion
The findings of the study indicated that the blood pressure and heart rate of the patients under study at different times in dexmedetomidine group were less than the other two groups. No significant side effects were observed in both the dexmedetomidine and magnesium sulfate groups.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kito K, Kato H, Shibata M, Adachi T, Nakao S, Mori K. The effect of varied doses of epinephrine on duration of lidocaine spinal anesthesia in the thoracic and lumbosacral dermatomes. Anesth Analg. 1998;86:1018–22. doi: 10.1097/00000539-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder L, Horlocker T, Schroeder D. The efficacy of axillary block for surgical procedures about the elbow. Anesth Analg. 1996;83:747–51. doi: 10.1097/00000539-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Mehrkens H, Geiger P. Continuous brachial plexus blockade via the vertical infraclavicular approach. Anaesthesia. 1998;53:19–20. doi: 10.1111/j.1365-2044.1998.tb15137.x. [DOI] [PubMed] [Google Scholar]
- 4.Castillo J, Curley J, Hotz J, Uezono M, Tigner J, Chasin M, et al. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996;85:1157–66. doi: 10.1097/00000542-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Droger C, Benziger D, Gao F, Berde C, Feng C. Prolonged intercostals nerve blockade in sheep using controlled-release of bupivacaine and dexamethasone from polymer microspheres. Anesthesiology. 1998;89:969–74. doi: 10.1097/00000542-199810000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg. 2006;102:263–7. doi: 10.1213/01.ane.0000189055.06729.0a. [DOI] [PubMed] [Google Scholar]
- 7.Niazi M, Ansari M, Mortazavi M, Movaseghi G, Shahgoli A, Ghorbanian N. Comparision lidocaeen and lidocaeen-fentanyl in anesthesia in axillary block in upper limb surgery. Armaghan danesh journal. 2014;10:13–20. [Google Scholar]
- 8.Buvanendran A, McCarthy R, Kroin J, Leong W, Perry P, Tuman K. Intrathecal magnesium prolongs fentanil analgesia: A prospective, randomized, controlled trial. Anesth Analg. 2002;95:661–7. doi: 10.1097/00000539-200209000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Koinig H, Wallner T, Marhofer P, Andel H, Hörauf K, Mayer M. Magnesium sulfate reduces intra- and postoperative analgesic requirements. Anesth Analg. 1998;87:206–10. doi: 10.1097/00000539-199807000-00042. [DOI] [PubMed] [Google Scholar]
- 10.Do S. Magnesium: A versatile drug for anesthesiologists. Korean J Anesthesiol. 2013;65:4–8. doi: 10.4097/kjae.2013.65.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malleeswaran S, Panda N, Mathew P, Bagga R. A randomised study of magnesium sulphate as an adjuvant to intrathecal bupivacaine in patients with mild preeclampsia undergoing caesarean section. Int J Obstet Anesth. 2010;19:161–6. doi: 10.1016/j.ijoa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Yousef A, Amr Y. The effect of adding magnesium sulphate to epidural bupivacaine and fentanyl in elective caesarean section using combined spinal-epidural anesthesia: A prospective double blind randomised study. Int J Obstet Anesth. 2010;19:401–4. doi: 10.1016/j.ijoa.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Huang R, Hertz L. Receptor subtype and dose dependence of dexmedetomidine-induced accumulation of [14C] glutamate in astrocytes suggests glial involvement in its hypnotic-sedative and anesthetic-Sparing effects. Brain Res. 2000;873:297–301. doi: 10.1016/s0006-8993(00)02525-7. [DOI] [PubMed] [Google Scholar]
- 14.Biswas S, Das R, Mukherjee G, Ghose T. Dexmedetomidine an adjuvant to levobupivacaine in supraclavicular brachial plexus block: A randomized double blind prospective study. Ethiop J Health Sci. 2014;24:203–8. doi: 10.4314/ejhs.v24i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marhofer D, Kettner S, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 16.Aghamohammadi D, Farzin H, Khanbabayi Gol M, Fooladi SH. Influence of intravenous magnesium sulfate on hemodynamic status and pain control after laparotomy surgery: A double blind clinical trial. J Anesth Pain. 2018;9:66–73. [Google Scholar]
- 17.Nooraei N, Dehkordi ME, Radpay B, Teimoorian H, Mohajerani SA. Effects of intravenous magnesium sulfate and lidocaine on hemodynamic variables following direct laryngoscopy and intubation in elective surgery patients. Tanaffos. 2013;12:57–61. [PMC free article] [PubMed] [Google Scholar]
- 18.Zaman B, Noorizad S, Siamdoust A, Etemadi H. Effects of adding dexmedetomidine to lidocaine on the onset and duration of axillary block for upper extremity surgeries. J Kermanshah Univ Med Sci. 2017;21:87–90. [Google Scholar]
- 19.Bangera A, Manasa M, Krishna P. Comparison of effects of ropivacaine with and without dexmedetomidine in axillary brachial plexus block: A prospective randomized double-blinded clinical trial. Saudi J Anaesth. 2016;10:38–44. doi: 10.4103/1658-354X.169473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaygusuz K, Ozdemir Kol I, Duger C, Gorsoy S. Effects of adding dexmedetomidine to levobupivacaine in axillary brachial plexus block. Curr Ther Res Clin Exp. 2012;73:103–11. doi: 10.1016/j.curtheres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht E, Kirkham KR, Liu SS, Brull R. The analgesic efficacy and safety of neuraxial magnesium sulphate: A quantitative review. Anaesthesia. 2013;68:192–202. doi: 10.1111/j.1365-2044.2012.07337.x. [DOI] [PubMed] [Google Scholar]