Important Compound Classes
Title
ACSS2 Inhibitors and Methods of Use Thereof
Patent Application Number
WO 2019/097515 A1
Publication Date
May 23, 2019
Priority Application
US 62/586,195
Priority Date
November 15, 2017
Inventors
Nakache, P.; Erez, O.; Goutopoulos, A.; Labele, M.
Assignee Company
Metabomed Ltd.
Disease Area
Cancer and neuropsychiatric diseases
Biological Target
Acetyl-CoA Synthetase Short-Chain Family Member 2 (ACSS2)
Summary
Tumor progression and oncogenesis in mammalian fatty acid metabolism dysregulated is a hallmark of cancer and various metabolic disorders, including insulin resistance, obesity, metabolic syndrome, hepatic steatosis, dyslipidemia, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and atherosclerotic vascular diseases. Acetyl-CoA carboxylase (ACC) is a biotin-dependent enzyme that catalyzes the carboxylation of acetyl CoA, which produces malonyl CoA via biotin carboxylase and carboxyltransferase catalytic activities. Two isoforms of mammalian ACC are known, ACC1 and ACC2. ACC1 is a cytosolic enzyme expressed mostly in the liver and adipose tissue that catalyzes fatty acid synthesis. ACC2 is associated with the mitochondrial outer membrane, found mostly in cardiac and skeletal muscle, and is involved in regulating fatty acid oxidation.
Cytosolic acetyl-CoA is the precursor of multiple anabolic reactions, including fatty acids synthesis. Acetyl-CoA carboxylase (ACC) plays a pivotal role in regulating fatty acid metabolism, and ACC inhibitors are under investigation as clinical drug targets in several metabolic diseases.
Acetyl-CoA synthetase Short-Chain Family Member 2 (ACSS2) enzyme catalyzes the formation of acetyl-CoA from acetate, CoA, and ATP. In humans, ACSS2 plays key roles in the activation and utilization of acetate and other small organic acids. The product acetyl-CoA is used for biosynthesis of cholesterol, glucose, and fatty acids. ACSS2 is a target for cancer treatment that plays a key role in cell proliferation, regulation of gene expression, and bioenergetics. Acetate is an important source of acetyl-CoA in hypoxia. ACSS2 supplies a key source of acetyl-CoA for tumors by capturing acetate as a carbon source, and inhibition of acetate metabolism may impair tumor growth.
ACSS2 is expressed in a large proportion of human tumors, and its activity is responsible for the majority of cellular acetate uptake. In addition, ACSS2 has been found to be a critical enzyme in the growth and survival of breast and prostate cancer cells cultured in hypoxia and low serum. ACSS2 has high expression in a number of cancers, including invasive ductal carcinomas of the breast, pancreatic cancer, ovarian cancer, triple-negative breast cancer, glioblastoma, and lung cancer. The expression of ACSS2 in these cancers is often correlated with higher-grade tumors and poorer survival compared to tumors that have low ACSS2 expression. Consequently, ACSS2 is a promising target for the development of pharmaceutical treatments of cancers, obesity, diabetes, cardiovascular disease, and atherosclerosis.
Nonalcoholic steatohepatitis (NASH) and alcoholic steatohepatitis (ASH) are advanced stages of nonalcoholic fatty liver disease (NAFLD) and alcoholic fatty liver disease (AFLD). NAFLD is characterized by excessive fat accumulation in the liver, which may lack other causative evidence in chronic liver diseases. In contrast, AFLD is defined as the presence of steatosis in the liver, which is accompanied by greater alcohol consumption. In acute alcohol hepatitis, inflammation may be enhanced and would cause the conversion of ethanol metabolite acetate to an excess of acetyl-CoA. This is accompanied by an increase in proinflammatory cytokine gene histone acetylation and histone deacetylases (HDAC) inhibition. Consequently, modulation of HDAC or ACSS activity may provide therapeutic value for alcoholic liver injury. Nuclear ACSS2 in certain conditions such as high fat concentration or hypoxia may affect histone acetylation and crotonylation, which then affect the regulation of gene expression. Epigenetic modifications have been implicated in neuropsychiatric diseases such as depression, PTSD, anxiety, and reduction of ACSS2 in the hippocampus leads to memory and neuronal plasticity. Furthermore, nuclear ACSS2 is shown to activate HIF-2alpha and promote lysosomal biogenesis, autophagy, and brain tumorigenesis by affecting histone H3 acetylation.
Inhibition of ACSS2 could directly reduce fatty-acid accumulation in the liver and are expected to have better safety profile compared to ACC inhibitors since they only affect the flux from acetate, which is not a major source for Ac-CoA in normal conditions.
This Patent Highlight consist of compounds that are inhibitors of ACSS2, which may treat, suppress, or reduce the severity of cancer such as breast cancer, ovarian cancer, lung cancer, hepatocellular carcinoma, liver cancer, and prostate cancer. Furthermore, these compounds may treat, suppress, or reduce the severity of neuropsychiatric disease or disorder such as depression, anxiety, autism, and schizophrenia.
Definitions
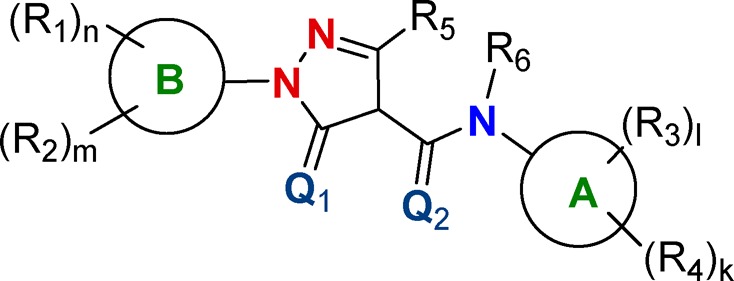
A and B are single or fused aromatic or heteroaromatic rings.
R1 and R2 are H, F, Cl, OH, SH, CF3, CN, NH2, COOH, NHR, −C(O)NHR, and so forth.
R3 and R4 are H, I, OH, SH, NO2, CD3, C1–C5 linear or branched C–(O)–haloalkyl, etc.
R5 is H, C1–C5 linear or branched substituted or unsubstituted alkyl or haloalkyl, OH, CN, etc.
R6 is H, C1–C5 linear or branched alkyl.
m, n, l, and k are integers between 0 and 4.
Q1 and Q2 are S or O.
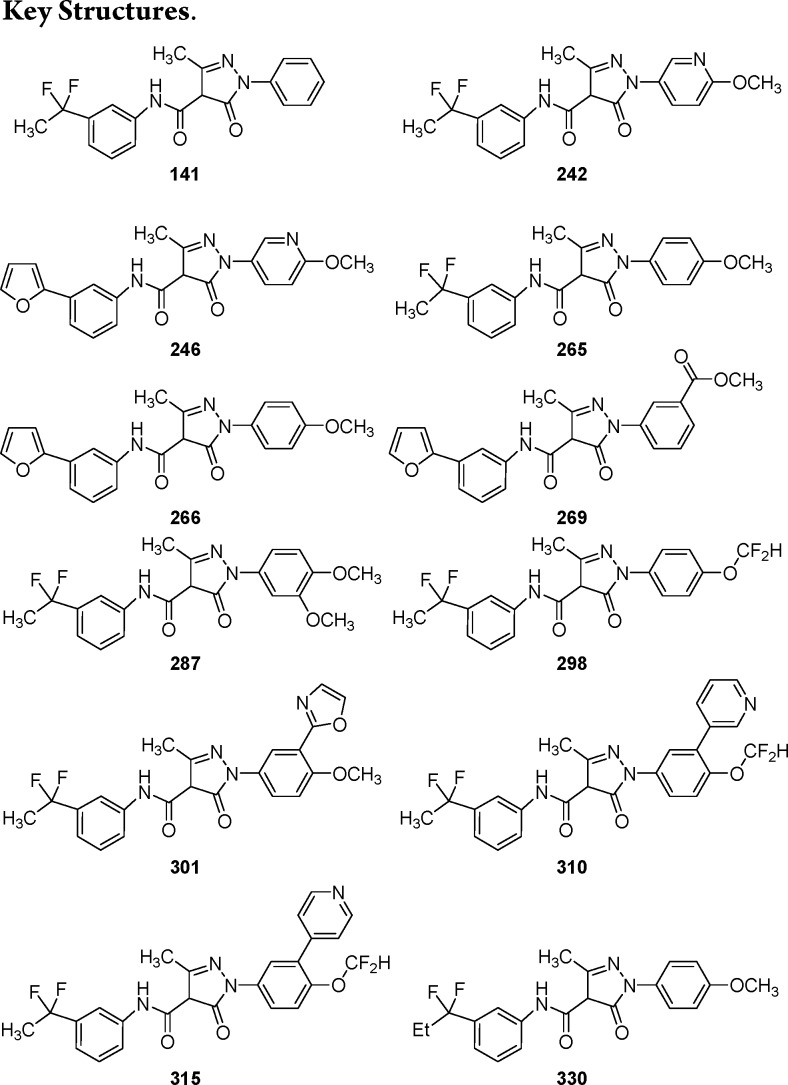
Key Structures
Biological Assay
ACSS2 cell-free and cellular activity assay (cell-free and cellular IC50). In vivo efficacy study was carried out using compound 265, where tumor pieces from breast cancer cell line MDA-MB-468 passaged as subcutaneous xenograft were subcutaneously implanted into female NMRI nude mice.
Biological Data
The compounds in
this Patent Highlight
show inhibitory cellular activity against ACSS2 in fatty-acid assay,
where 13C-acetate was incorporated into the compounds of
interest in MDA-MB-468 cells under hypoxic conditions.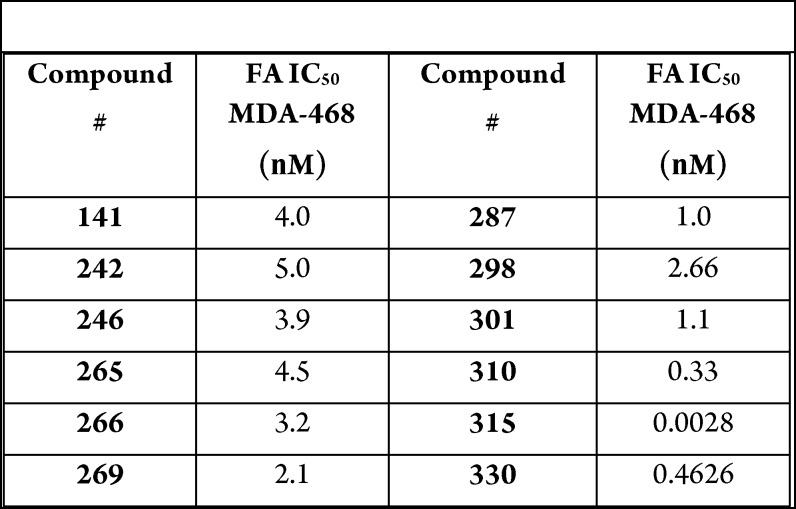
Recent Review Articles
-
1.
Hentchel K. L.; Escalante-Semerena J. C.. Microbiol. Mol. Biol. Rev. 2015, 79, 321.
-
2.
North B. J.; Sinclair D. A.. Trends Biochem. Sci. 2007, 32, 1.
-
3.
Evans D. J.Coor. Chem. Rev. 2005, 249, 1582.
-
4.
Harrop T. C.; Mascharak P. K.. Coor. Chem. Rev. 2005, 249, 3007.
The author declares no competing financial interest.