Abstract
Diabetes is highly prevalent, affecting over 25 million adults in the US, yet it can be effectively prevented through lifestyle interventions, including the well-tested Diabetes Prevention Program (DPP). American Indian/Alaska Native (AIAN) adults, the majority of whom live in urban settings, are more than twice as likely to develop diabetes as non-Hispanic whites. Additionally, prevalent mental health issues and psychosocial stressors may facilitate progression to diabetes and hinder successful implementation of lifestyle interventions for AIAN adults. This 2-phased study first engaged community stakeholders to develop culturally-tailored strategies to address mental health concerns and psychosocial stressors. Pilot testing (completed) refined those strategies that increase engagement in an enhanced DPP for urban AIAN adults. Second, the enhanced DPP will be compared to a standard DPP in a randomized controlled trial (ongoing) with a primary outcome of body mass index (BMI) and a secondary outcome of quality of life (QoL) over 12 months. Obese self-identified AIAN adults residing in an urban setting with one or more components of the metabolic syndrome (excluding waist circumference) will be randomized to the enhanced or standard DPP (n = 204). We hypothesize that addressing psychosocial barriers within a culturally-tailored DPP will result in clinical (BMI) and superior patient-centered (QoL) outcomes as compared to a standard DPP. Exploratory outcomes will include cardiometabolic risk factors (e.g., waist circumference, blood pressure, fasting glucose) and health behaviors (e.g., diet, physical activity). Results of this trial may be applicable to other urban AIAN or minority communities or even diabetes prevention in general.
Keywords: American Indian, Diabetes prevention, Prediabetes, Obesity, Community-based participatory research, Weight loss
1. Introduction
One-third (36%) of American adults are considered obese [1]. Obesity is associated with leading causes of preventable death such as Type 2 diabetes [2]. Type 2 diabetes affects an estimated 25.8 million US adults and an additional 79 million adults have prediabetes [3]. The 5.2 million adults living in the US who identify as American Indian/Alaska Native (AIAN) alone or in combination with some other race are at higher risk of obesity and of developing diabetes compared to non-Hispanic whites [4–7]. Self-reported national data indicate obesity rates to be 46% higher in AIANs (42%) compared to non-Hispanic whites adults (29%) [8]. The prevalence of diabetes in AIANs (18%) is more than double that in non-Hispanic white Americans (8%) [8].
The landmark Diabetes Prevention Program (DPP) trial demonstrated that an intensive lifestyle intervention targeting modest weight loss (7% of baseline weight) and increased physical activity (150 min per week) was effective for preventing diabetes among high-risk adults, although American Indians represented only 5% of the study population [9]. To promote dissemination in American Indian/Alaska Native communities in the US, the Indian Health Service implemented the Special Diabetes Program for Indians Diabetes Prevention (SDPI-DP) [10]. SDPI-DP supported AIAN health care programs to implement and evaluate the 16-session Lifestyle Balance curriculum, a group-based adaptation of the original DPP. The annual incidence of diabetes among participants (n = 2553) was 4.0%, which is similar to the incidence for AIAN participants in the original DPP trial (4.7%) [10]. After one year of follow-up, 22.5% of participants in the SDPI-DP had achieved the weight loss goal of 7% of baseline weight.
As a result of implementing the SDPI-DP, one urban AIAN community was motivated to further examine how to optimize diabetes prevention for urban AIAN adults given stakeholder concern regarding barriers related to mental health issues and psychosocial stressors. Compared to non-Hispanic whites, AIAN adults have higher rates of depression symptoms (e.g., sadness some of the time 14% vs. 8%), any illicit drug use (18% vs. 9%) [6], more than once binge drinking in the last month (32% vs. 17%), reported “not satisfied with life” (10% vs. 5%), 14+ days/month with poor mental health (18% vs. 11 %) [11], and serious psychological distress (5.2% vs. 3.1%). The community established a community-university partnership with the goal of conducting research to elucidate effective strategies that address mental health issues and psychosocial stressors for preventing diabetes among urban AIAN adults.
Support for this approach derives from a recent analysis of the SDPI-DP evaluation showing that several psychosocial factors including depressive symptoms, family support, and psychological distress were related to the degree of weight change [12]. These findings underscore the importance of incorporating strategies to address mental health issues and psychosocial stressors in diabetes prevention for AIAN communities as identified by the community-university partnership. The goal of this study is to develop and test a diabetes prevention program for AIAN adults that incorporate culturally sensitive strategies to address mental health concerns and psychosocial stressors in a comparative effectiveness trial.
2. Methods
A community-university partnership, established in 2011 collaborates to lead this comparative effectiveness trial. The design and implementation of this study prioritizes the community-driven research agenda while balancing the importance of scientific rigor.
2 1. Patient and stakeholder engagement
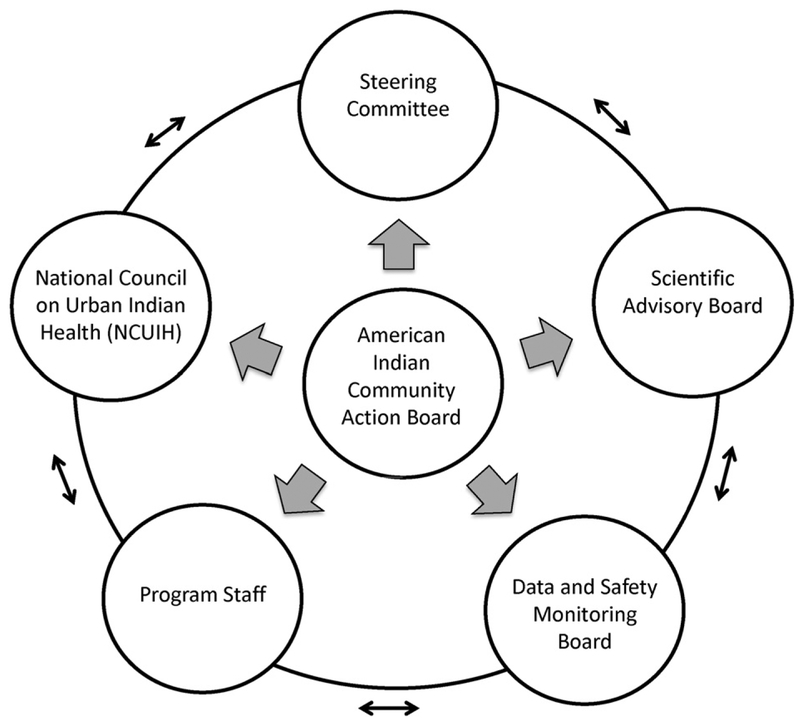
This study is guided by principles of Community-Based Participatory Research: (1) Recognize the community as a unit of identity; (2) Build on strengths and resources of the community; (3) Facilitate collaborative partnerships in all phases of the research; (4) Integrate knowledge and action for mutual benefit of all partners; (5) Promote co-learning and empowering process that attends to social inequalities; (6) Involve a cyclical and iterative process; (7) Address health from both positive and ecological perspectives; and (8) Disseminate findings and knowledge gained to all partners [13]. The community-university partnership developed a community advisory board known as the American Indian Community Action Board (AICAB) in 2011 that is made up of local community members and leaders. The AICAB meets at least monthly and serves as the central governing body of the partnership making key decisions and participating in all phases of the research process (see Fig. 1). In addition, the project includes a steering committee with community and university representatives that manage the day-to-day functions of the study, a scientific advisory board made up of national experts in diabetes prevention, AIAN health, and community-based participatory research, the National Council of Urban Indian Health to facilitate rapid dissemination of results, and a Data and Safety Monitoring Board.
Fig. 1.
Patient-centered organizational structure.
2.2. Study design
This study (05/2014–10/2018) has 2 phases. Phase I (completed) involved pilot testing culturally-tailored strategies to address mental health concerns and psychosocial barriers for incorporation into an enhanced DPP intervention to increase engagement for urban AIANs. Phase II (ongoing) is a comparative effectiveness trial (n = 204) to test the enhanced DPP developed in Phase I. The comparison group will receive a standard DPP program based on the SDPI-DP as recommended by community stakeholders given their assessment of the significant burden of diabetes in this community and the proven success of lifestyle interventions for preventing diabetes in high-risk groups. The entire study protocol was approved by the Institutional Review Boards (IRBs) of the Stanford University and San Jose State University. Additionally, the AICAB was trained to serve as an ethical review board representing community interests.
2.3. Phase I: develop enhanced DPP for urban AIAN adults
The goal of Phase I was to refine and strengthen the existing DPP intervention based on the SDPI-DP to create an enhanced DPP. Early on in the development of an enhanced approach to diabetes prevention, the AICAB identified the issue of historical trauma as a key factor in the AIAN experience that has led to persistently high prevalence of diabetes among AIAN adults. Historical trauma refers to the cross-generational harms created by an experience dominated by attempts to systematically destroy AIAN communities and cultures [14–18]. This concept is closely tied to the personal distress and community cultural displacement that are often labeled mental health disorders in a western medical model. As suggested by the AICAB, confronting historical trauma provides a means of addressing mental health issues, but requires a broader range of services. Thus, the partnership undertook a 12-month formative research phase to pilot test 3 culturally-congruent strategies: (1) Talking Circles; (2) Modified Photovoice; and (3) Digital Storytelling. These strategies were identified by the AICAB as being able to engage urban AIANs in addressing underlying social, historical and psychological stressors within a framework congruent with AIAN culture. Additionally, these strategies were identified as reinforcing AIAN cultural identity, thereby enhancing social support in the group sessions. The aim was to pilot test each strategy to assess feasibility and draw a conclusion regarding their incorporation into an enhanced DPP. In addition to pilot testing these strategies in Phase I, the partnership developed a protocol for providing culturally congruent and accessible mental health support to participants in the enhanced DPP.
2.3 1. Foundation: existing DPP intervention
A group-based adaptation of the original one-on-one intensive lifestyle intervention from the DPP trial [9,19,20,21] based on the SDPI-DP serves as the foundational intervention for this study. The theoretical basis is derived from Social Cognitive Theory [22] and the Transtheoretical Model of Behavior Change [23,24]. The primary goals of the intervention are loss of at least 5% of baseline weight and 150 min of moderate physical activity per week by 6 months. Although the original DPP trial targeted 7% weight loss, 5% weight loss has been found to be sufficient for prevention of chronic disease and is commonly accepted as the goal [25]. The intervention is delivered by a trained lifestyle coach over 16 weekly group sessions covering information on moderate calorie restriction, physical activity, and proven behavioral strategies (see Table 1 for a list of topics). In addition, participants are invited to attend ongoing support sessions for maintenance of lifestyle changes after the completion of the first 16 weeks. The support sessions are offered weekly and participants are encouraged to attend at least once per month.
Table 1.
Intervention schedule and topics.
| Session | Topic |
|---|---|
| 1 | Welcome to the lifestyle balance program |
| 2 | Be a fat detective |
| 3 | Three ways to eat less fat |
| 4 | Healthy eating |
| 5 | Move those muscles |
| 6 | Being active: A way of life |
| 7 | Tip the calorie balance |
| 8 | Take charge of what’s around you |
| 9 | Problem solving |
| 10 | Four keys to healthy eating out |
| 11 | Talk back to negative thoughts |
| 12 | The slippery slope of lifestyle change |
| 13 | Jump start your activity plan |
| 14 | Make social cues work for you |
| 15 | You can manage stress |
| 16 | Ways to stay motivated |
2 3.2. Pilot test potential enhancements
The AICAB members pilot tested each of the three proposed enhancements to assess feasibility for incorporating the strategies into the intervention:
Talking Circles:
A Talking Circle is a traditional method of group communication where AIAN community members come together to share information, provide social support, and solve community issues [26]. Talking Circles have been successfully used as both a qualitative research method as well as an intervention strategy for health issues ranging from cervical cancer screening to diabetes management [26–35]. The partnership pilot tested Talking Circles to determine their potential fit within the DPP as a way to address psychosocial barriers to intervention engagement through fostering self-reflection, social support, and community cohesion among DPP participants. The AICAB conducted 4 Talking Circles with different facilitators and settings with a total of 11 participants who included AICAB members and study staff. Following each Talking Circle the AICAB met to discuss the potential fit within the DPP intervention.
Modified Photovoice:
The AICAB modified the Photovoice methodology and with the support of a facilitator pilot tested it over the course of 10 sessions. Photovoice is a participatory qualitative method using photography and critical dialogue to identify root causes of health problems and elucidate successful intervention strategies [36,37]. The goals of the modified Photovoice were to engage participants to record and reflect on their community’s strengths and weaknesses and to promote dialogue about health issues [36,37]. Photography can be especially useful for engaging underserved minority groups because photographs taken of their own community elicit a collective emotional response. This may uniquely uncover insight into the multi-level factors, such as food scarcity, social influences, and government policies that shape diet and physical activity. In addition, photography can aid in bringing out historical, psychosocial, and mental health connections.
A total of 11 participants who participated in the Talking Circles took part in the modified Photovoice pilot test including 5 females and 6 males ranging in age from 25 to 80 years old. Among the participants, the average number of sessions attended was 6 out of 10, ranging from 3 to 10 sessions attended. Following training in use of the camera and photography, participants took pictures in response to 4 prompts: (1) What does wellness and health look like to you? (2) What does healthy and unhealthy love look like to you? (3) What is a personal challenge you have faced and how have you overcome it? (4) What is your meaning? For each prompt, participants took pictures on their own and then came back to the group to share a selection of their pictures with the other participants.
Digital Storytelling:
Digital stories are short, first-person narratives that can be presented using traditional or social media formats. The participatory process of developing and sharing digital stories can deeply affect both the people who develop their story as well as viewers, “moving them to reflect on their own experiences, modify their behavior, treat others with greater compassion, speak out about injustice, and become involved in civic and political life” [38]. Developing personal digital narratives is a particularly appealing strategy for urban AIANs because it invokes the traditional cultural practice of oral storytelling [39]. A total of 10 AICAB members took part in a digital storytelling workshop to pilot test the strategy for potential incorporation into the DPP. The workshop was delivered over the course of 3 days and each participant created their own digital story.
2.3.3. Mental health support
In addition to developing the three culturally-congruent enhancements, a sub-committee of the AICAB developed AIAN-centric mental health strategies to be offered as part of the enhanced DPP. These strategies included culturally congruent mental health counseling, celebration of AIAN cultural practices, and de-stigmatizing individuals’ signs and symptoms of depression, anxiety and other mental health disorders. Providing these forms of mental health support were identified as a means for reducing barriers that exist for AIANs to engage in the successful behavior change needed to prevent diabetes. Participants in the Standard DPP intervention received referral to other services in a standard manner without specific cultural tailoring. For both arms of the study, it was anticipated that completion of survey questions focused on discrimination and historical trauma might trigger increased participant distress. Protocols were developed and staff were trained to cope with these situations and provide appropriate services and referral as needed.
2.3.4. Patient-centered, participatory intervention adaptation
Following completion of pilot testing the 3 enhancements and developing the mental health support component, the AICAB met 8 times to discuss the findings from the pilot study and finalize the strategies to be included in the enhanced DPP. The AICAB balanced potential for effectiveness with acceptability to participants and feasibility given available resources. In terms of potential for effectiveness and acceptability to participants, the AICAB recognized the important role of tailoring the intervention to each participant’s circumstances and wanted to offer choice and flexibility for engaging in the 3 enhancements. Considering the feasibility of the 3 enhancements, the AICAB noted that the talking circles were easy to implement with few resources while the digital storytelling workshop required the most resources and personnel; the Photovoice project required a moderate level of resources. Balancing these factors, the AICAB decided to incorporate 3 talking circles into the 16-week intervention at the beginning (session 3), middle (session 8) and end (session 15) and the option to engage in a digital storytelling workshop or a Photovoice project. The choice to engage in digital storytelling or Photovoice would be driven by the participant with input from the lifestyle coach.
2.4. Phase II: RCT comparing an enhanced and standard DPP
For the comparative effectiveness trial, participants will be randomized in 4 recruitment cohorts to the enhanced or standard DPP and followed for 12 months.
2.4.1. Trial setting
The trial is conducted in a community-based setting that was selected by the AICAB. All data collection and intervention activities take place within a non-profit recreation facility that is conveniently located near health and human services. The facility provides a wide range of aquatic-based classes, land-based classes, swim lessons, and personal training for individuals of all ages and abilities. Physical and behavioral health services are not available on site.
2.4.2. Eligibility criteria
We will apply permissive inclusion criteria and minimally necessary exclusion criteria to optimize the balance between generalizability, patient safety, intervention adherence, and retention. Men and women will be eligible if they identify their race/ethnicity as indigenous to the US or the Americas (North, Central, and South America) and have a BMI between 30 and 55 kg/m2, are not diagnosed with Type 2 Diabetes, and meet at least one other criterion for metabolic syndrome: (1) Triglycerides: >150 mg/dL; (2) Reduced High-density lipoprotein cholesterol: <40 mg/dL (men); <50 mb/dL (women); (3) Blood pressure: >130/80 mm Hg or current treatment with antihypertensives; (4) Fasting glucose: 100–125 mg/dL (Table 2). This definition is a hybrid of national and international definitions [40,41] whose purpose is to identify a population at substantial risk for progression of dysmetabolism, but who have not yet developed diabetes. Patients with significant psychiatric disorders requiring atypical antipsychotics or multiple medications or medical comorbidities (e.g., uncontrolled metabolic disorders, unstable heart disease, heart failure, and ongoing substance abuse) will be excluded. Additional exclusions are to protect participant safety (e.g., pregnancy) and prevent loss to follow-up (e.g., planned relocation).
Table 2.
Inclusion and exclusion criteria.
| Inclusion criteria: |
| • Age (as of date of enrollment): |
| ○ Lower age limit: 18 years |
| ○ Upper age limit: NONE (only exclude for cause, e.g. disease and functional limitations, as detailed below) |
| • Race/ethnicity: Self-identified as having ancestry from Indigenous peoples of Americas |
| • Gender: Individuals of any gender |
| • Body mass index: 30–55 kg/m2 |
| • Meet at least one other criterion for metabolic syndrome: |
| ■ Triglycerides: 150 mg/dL or higher |
| ■ Reduced High-density lipoprotein cholesterol: <40 mg/dL (men); <50 mb/dL (women) |
| ■ Blood pressure: >130/80 mm Hg or current treatment with antihypertensives |
| ■ Fasting glucose: 100–125 mg/dL |
| Exclusion criteria: |
| • Medical exclusions: |
| ■ Significant medical comorbidities, including uncontrolled metabolic disorders (e.g., thyroid, type 2 diabetes, renal, liver), unstable heart disease, heart failure, and ongoing substance abuse |
| ■ On 10 or more prescription medications |
| ■ Psychiatric disorders requiring atypical antipsychotics or multiple medications |
| ■ Inappropriate for moderate exercise according to the Revised Physical Activity Readiness Questionnaire |
| • Other exclusions: |
| ■ Pregnant, planning to become pregnant, or lactating |
| ■ Family household member already enrolled in the study |
| ■ Already enrolled or planning to enroll in a clinical trial that would limit full participation in the study |
| ■ Resident of a long term care facility or nursing home |
| ■ Lack of spoken English by patient or a household member > 18 years who can serve as interpreter |
| ■ Plans to move during the study period (6 months post-randomization) |
| ■ Investigator discretion for clinical safety or adherence reasons (e.g., unstable housing, chronic pain) |
2.4.3. Recruitment, screening, and baseline visit
The targeted enrollment of 204 participants will be met in 6 recruitment cohorts. Recruitment strategies will include community-based outreach, promotion at AIAN events, hosting community events, and an incentivized referral process. Study staff and AICAB members will conduct outreach at local community-based organizations, community health centers or other healthcare providers (e.g., Veterans Affairs), social service agencies, cultural events (e.g., Pow Wows), and population businesses frequented by AIAN community members. Study staff will also host community events to increase awareness of the study. Additionally, AICAB members, community members, and other stakeholders who refer a participant will receive a $25 incentive for each participant who is successfully randomized.
Potentially eligible individuals will be contacted by phone for initial screening, except for potential participants recruited through direct contact for whom the information normally obtained in the phone screen is already available. The brief phone screen will assess criteria that patients can reliably assess themselves, such as race/ethnicity and willingness to participate in an intensive lifestyle intervention. An in-person screening visit will follow the phone screen where participants answer a brief screening questionnaire (unless already completed during recruitment or phone screening) and are weighed and their height is measured. In addition, measurements will be obtained of their waist circumference and blood pressure and their fasting glucose, triglycerides, and HDL levels are assessed using an Alere Cholestech LDX analyzer (Waltham MA) a point-of-care testing device. If the participant is eligible, their informed consent will be obtained and study staff will conduct an in-person interview to complete the baseline questionnaire.
2.4.4. Randomization and blinding
After completing the baseline visit, eligible participants will be randomized in a 1:1 ratio to the standard or enhanced DPP. Participants will be randomized in blocks to keep the size of the treatment groups similar. The size of each block will be randomly selected to be either 2 or 4. To ensure an equal number of males and females in each intervention arm, we will stratify randomization by gender. Cohorts of participants will be randomized prior to the start of a new session of classes in order to minimize time between randomization and intervention. The unit of randomization will be individual because there is unlikely to be any contamination by primary care provider or neighbourhood, due to diversity of the participants. Treatment will be identifiable to participants and the lifestyle coaches by design, but masking of the investigators, Data and Safety Monitoring Board, outcome assessors, and the statistician performing the data analysis will be enforced.
2.4.5. Fidelity assurance
We will follow recommendations for quality assurance in behavioral interventions [42]. Use of standardized intervention materials, structured staff training and ongoing oversight are fundamental to ensuring high intervention fidelity. Lifestyle coaches will undergo standardized training by a certified master trainer with supplemental training on the enhancements resulting from the formative research. A trained researcher will attend 5% of sessions and grade the session using a session-by-session rating scale adapted from a previous trial [43]. Falling below an a priori performance standard (e.g., 90% adherence to intervention protocol) will trigger more frequent audit and feedback and, if needed, “booster” training for the coach.
Participant engagement and adherence are also essential to intervention fidelity and will be monitored and supported. Participant progress on key intervention tracking parameters (e.g., date, format, duration of contact, most current weight, and physical activity level) will be routinely documented. The coach will review and give feedback on homework and self-monitoring records and document participant progress toward protocol-specific, achievement-based objectives. The lifestyle coach will routinely inquire about barriers to intervention receipt and adherence, recommend personalized, actionable problem-solving strategies, and provide ongoing support via proactive follow-up.
2.4.6. Participant safety
Initial and in-person screening was designed to triage potential participants by their risk of adverse events due to the study’s interventions, including use of the Revised Physical Activity Readiness Questionnaire. Those at highest risk (lack of clinical stability) will be excluded and referred for follow-up as are those with newly diagnosed diabetes. Those of intermediate risk will be reviewed by the principal investigator and required to obtain authorization to participate from their primary care providers. Screening for adverse events will occur formally at each data collection point as well as informally at all study contacts. Monitoring for serious adverse events emphasizes the occurrence of emergency room visits and hospitalizations that might be related to the study. Staff will follow a treatment and referral protocol for a number of other clinically relevant events, including mental health concerns, elevated blood pressure, and out-of-range laboratory values. Additionally, the study protocol is monitored by two IRBs (Stanford and San Jose State University). A Data and Safety Monitoring Board composed of a primary care internist, a biostatistician, and an AIAN clinical psychologist provides additional oversight and concrete advice through their review of recruitment progress, intervention fidelity and serious adverse events.
2.4.7. Retention
We will employ several strategies aimed at participant retention. First, dependence on well-trained, committed staff who practice cultural humility will be critical to retention. This includes members of the AICAB. Second, we will adequately assess participant willingness and desire to participate and carefully screen their eligibility. We will also explain the study protocol in detail to ensure that participants give true informed consent. Third, we will provide incentives that encourage participation but do not coerce participants. Fourth, scheduling group sessions and one-on-one visits in the evenings and on weekends will be especially important for reaching and retaining urban AIAN. Fifth, we will use alternative means to collect data, such as by phone or mail, if needed. We will collect detailed contact information for each participant. Finally, if a participant misses a visit, we will use motivational interviewing strategies to engage participants and problem-solve around barriers to participation. A participant is considered dropped from the study when he/she expresses that desire.
2.4.8. Study measures and data collection schedule
Assessments on described measures in Table 3 will occur at baseline, 6 months, and 12 months. Measures in Table 3 include the following primary, secondary, and tertiary outcomes and potential effect modifiers and mediators.
Table 3.
Measures and data collection.
| Instrument | Source | Baseline | Follow-up | ||
|---|---|---|---|---|---|
| Primary outcome | |||||
| Clinical | |||||
| Body mass index (height, weight) | Scale, stadiometer | Biophysical | X | X | |
| Secondary outcome | |||||
| Patient-centered | |||||
| Quality of life | SF-12 | Interview | X | X | |
| Tertiary outcomes | |||||
| Cardiometabolic risk factors | |||||
| Waist circumference | Measuring tape | Biophysical | X | X | |
| Systolic and diastolic BP | BP cuff | Biophysical | X | X | |
| Total cholesterol | Cholestech | Biophysical | X | X | |
| LDL cholesterol | Cholestech | Biophysical | X | X | |
| HDL cholesterol | Cholestech | Biophysical | X | X | |
| Triglycerides | Cholestech | Biophysical | X | X | |
| Fasting blood glucose | Cholestech | Biophysical | X | X | |
| Hemoglobin Ale | Cholestech | Biophysical | X | X | |
| Health behavior | |||||
| Diet | Special Diabetes Program for Indians Questionnaire | Interview | X | X | |
| Physical activity | Modified BRFSS physical activity | Interview | X | X | |
| Sleep | PROMIS | Interview | X | X | |
| Alcohol | Alcohol-Audit-C | Interview | X | X | |
| Potential effect moderators | |||||
| Sociodemographics | Age, sex, education, employment, occupation, marital status, household size, income, household food insecurity (USDA 6-item food security module) | Interview | X | ||
| Ethnic identity | |||||
| Enculturation | Enculturation Scale (modified Whitbeck) | Interview | X | X | |
| Experience of racism & discrimination | Braveheart Indigenous Peoples Survey | Interview | X | X | |
| Indigenous Experiences | Braveheart Indigenous Peoples Survey | Interview | X | X | |
| Indigenous Identity | Braveheart Indigenous Peoples Survey & Multi-group Ethnic Identity Measure | Interview | X | X | |
| Spirituality | Braveheart Indigenous Peoples Survey | Interview | X | X | |
| Potential effect mediators | |||||
| Depressive symptoms & PTSD | CES-D and PTSD Civilian Checklist | Interview | X | X | |
| Experiences of historical loss | Historical Loss Associated Symptoms Scale (Whitbeck) | Interview | X | X | |
| Community connectedness | Inclusion of Community in Self Scale | Interview | X | X | |
| Empowerment | Scenario and Emotional Empowerment Scales | Interview | X | X | |
2.4.8.1. Outcomes.
Our primary outcome is change from baseline in BMI and our secondary outcome is change from baseline in quality of life (QoL) at 12 months of follow-up. These outcomes encompass both patient-centered and clinical goals. Weight and height will be assessed according to standard protocols [44]. The SF-12 will be used to measure QoL, which has been used in other studies with AIAN adults [45,46]. The SF-12 is a shorter version of the SF-36 that measures functional health and well-being from the patient’s point of view. Tertiary outcomes include cardiometabolic risk factors (e.g., waist circumference, blood pressure, fasting glucose, and lipid levels) and health behaviors (e.g., diet, physical activity, sleep habits and quality, and alcohol consumption). Trained staff will conduct anthropometric and blood pressure measurements [44,47,48]. Measurements of fasting glucose and lipid levels is accomplished through point-of-care testing using the Cholestec to minimize patient burden, maximize access, and provide immediate results. Diet will be measured using an adapted survey from the SDPI-DP evaluation to enable comparability of results [10]. Physical activity will be measured using the BRFSS physical activity questionnaire with modifications to reflect the time for recall [49]. Sleep habits and quality will be assessed using the PROMIS questionnaire [50] and alcohol consumption will be assessed using the AUDIT-C [51].
2.4.8.2. Potential effect modifiers and mediators.
To complement the primary, secondary, and tertiary outcomes, we will examine 2 key domains of effect modifiers: (1) Sociodemographic characteristics and (2) Measures of ethnic identity and experiences of racism/discrimination based on ethnic identity. Sociodemographic characteristics include age, sex, educational attainment, occupational status, marital status, household size, income, and household food insecurity. Constructs of ethnic identity and experiences of racism/discrimination based on ethnic identity are assessed using an adaptation of the Indigenous Peoples Survey developed by Braveheart et al. [52] Putative mediators identified a priori include depressive symptomatology [53], post-traumatic stress disorder, historical losses [54], community connectedness [55], and empowerment [56].
2.4.9. Statistical analysis
The primary model for participant j at time k nested in cohort i
will be used to test the primary hypothesis that BMI will decrease in the enhanced DPP arm relative to the standard DPP arm upon completion of the study. The linear regression model includes a random intercept β0i, to account for within cohort correlation, a random intercept β0ij to account for within participant correlation, and adjusts for gender (Genderj), the stratification factor for randomization. The random intercepts, β0i, and β0ij, and the error term εijk are assumed to be normally distributed. Interventionj indicates that participant j is in the enhanced DPP arm and Month6jk, MonthI2jk, and Monthl8jk indicate whether observation k from subject j is at month 6,12, or 18, respectively. To test whether the change in the primary outcome from baseline at 12 months differs between the standard and enhanced arms, we will test the null hypothesis that β6 = 0 using the Wald test. The outcome will be tested at a two-sided α = 0.05.
The primary analyses will follow the intent-to-treat principle and will use all available data. We will describe any missing data and will conduct sensitivity analyses to evaluate the impact of the missing data on our conclusions. Sensitivity analyses considered to evaluate the robustness of our findings to the presence of missing data will include multiple imputation methods and worst-case imputation where missing values are filled with an extreme value (e.g. 5% greater than value observed at baseline), which can be used to determine how extreme the unobserved missing values would need to be in order to change the conclusion of the trial [57,58]. Secondary and tertiary analyses will replace the primary outcome in the model above with secondary and tertiary outcomes, respectively.
Additional analyses will consider pre-specified moderators and mediators (depressive symptoms, coping skills, and social support/community cohesion) of the primary and secondary outcomes. We will investigate the moderators and mediators using mixed effects linear regression by including an interaction term of treatment and the hypothesized moderator and centering the independent variables [59,60].
2.4.9.1. Sample size and data interpretation.
Our study was designed to provide sufficient statistical power to test the study’s primary hypothesis that the enhanced DPP will result in greater weight loss compared to the standard DPP. In determining the sample size we considered the definition of clinically significant weight loss, the standard deviation of weight change in past clinical trials of lifestyle interventions, and acceptable levels of Type I and Type II errors. Our power estimates are based on simplified assumptions and the actual power may be different because of the correlated errors induced by the cohort effect and the repeated measures over time. In the original DPP trial, the average weight loss in the intensive intervention arm was 6.9% ± 5% after 6 months of follow up and 4.9% ± 7.4% at the end of the trial (mean follow-up of 3.2 years) [61]. This percent weight loss is similar to other studies and greater than that observed in the SDPI evaluation where Jiang et al. reported a percent weight loss of 4.4% following the 16-week program [10]. Based on this literature, we expect a mean percent weight loss of 4.0% in the standard DPP and 6.5% in the enhanced DPP. To be conservative, we powered the study to be able to detect a difference of 2.0%. Dividing a 2.0% difference (6.0%–4.0%) by the DPP SD (4.5%) yields an effect size of approximately 0.45. A sample of 81 participants per arm will be required to compare the standard and enhanced arms for an effect size of 0.45 at a two-sided α = 0.05 with 80% power. We estimate 20% will be missing BMI at follow-up and have therefore inflated the initial sample size to 102 participants per arm. As all other analyses are exploratory, no adjustment for multiple testing will be made for secondary or tertiary analyses. The secondary and tertiary analyses are not intended to produce clinically actionable results, but rather to supplement conclusions based on the primary analysis and to inform future research. They will be interpreted properly within that context, considering the totality of evidence available [62,63].
2.4.10. Data management and quality control
Overall, 2 types of data will be collected: 1) baseline and follow-up questionnaires, and 2) clinical measurements and blood samples. The baseline and follow-up questionnaire data and clinical measures will be collected in a database hosted at Stanford University and created using REDCap, an online data collection and management tool [64]. This data will be encrypted and password-protected. REDCap has been successfully used by the research team in previous community-based trials. All data will only be accessible by password and will only be available to staff that need access for data collection purposes. Visual data will not be accompanied by participant names or identifiers unless consent is received by the participant. Only the Steering Committee will have access to the password-protected folders and database. Upon completion of data collection, the data sets will be cleaned and archived. A copy of the data will be downloaded for statistical analysis in R [65]. The dataset, data dictionary, and code files used in the statistical analysis will be maintained by the study statistician performing the data analysis and stored on a password-protected, encrypted network drive with continual backups.
3. Discussion
Our study will design and evaluate an enhanced DPP intervention for obese urban AIAN adults who are at substantial risk for progression of dysmetabolism to diabetes. Obese urban AIAN adults are a critical group because of their higher risk of developing diabetes [4,5], experience of historically embedded psychosocial stressors, and lack of access to culturally-centered psychosocial supports, particularly native healing practices. Our goal in developing and evaluating a unique enhanced DPP was to provide an intervention that was modified by an active AIAN community group to address key issues underlying the AIAN cultural experience. In particular, the design of the enhanced DPP views the psycho-social stress of historical trauma as a major barrier to participants engaging in the sustained behavior changes needed to prevent diabetes. By addressing these mental health issues through culturally congruent strategies (rather than through mainstream Western medical strategies) we hypothesize that the enhanced DPP will have superior comparative effectiveness.
While providing some promise [43,66–70], the literature on translations of the DPP to real world settings offers several opportunities, particularly in underserved populations such as urban AIAN. First, there have been limited rigorous evaluations of diabetes prevention efforts among AIANs, especially those in urban areas [71,72]. Second, there has been limited engagement of patients and other stakeholders in the design, implementation, and analysis of these programs. Finally, there has been a lack of rigorous research comparing a DPP with and without psychosocial supports. Our study will address the gaps by identifying successful strategies to interrupt the dysmetabolism pathway through engagement with urban AIAN patients and other stakeholders using a rigorous comparative effectiveness design.
This research will also directly respond to community-driven research interests to better understand and address the difficulties that AIAN communities have in thwarting the development and progression of dysmetabolism. Community members have described how the historical and psychosocial realities faced by AIANs promote the progression of dysmetabolism, shape the patient-specific experience of dysmetabolism, and interfere with engagement in evidence-based interventions. The psychosocial stressors experienced by urban AIANs may not meet criteria for referral to behavioral health services (which are also not readily accepted by the AIAN community), but are significant enough to impede successful patient engagement in lifestyle changes. Through this research, community members seek to better understand the impact of historical trauma and potentially related behavioral health conditions on efforts to promote healthy lifestyles and to develop effective support strategies to overcome these challenges. The psychosocial supports, particularly native healing practices, to be identified in this study will fill a critical gap in current services between traditional behavioral health services and diabetes prevention services.
This study design prioritizes the community-driven research agenda while balancing the importance of scientific rigor. As such, the study design has several limitations. The main limitations include limited time for follow-up and anticipated difficulties in recruitment and retention in a hard-to-reach disparity population. Ideally, participants would be followed for 2 or more years to measure maintenance of lifestyle behaviors and weight as well as onset of diabetes as done in the original DPP trial [9]. The partnership also recognizes the challenges of recruitment and retention and actively identifies new strategies at each monthly AICAB meeting. The central governing role of the AICAB in this study promotes a community-driven approach to research with significant potential to identify effective strategies to overcome challenges in this hard-to-reach yet critical disparities population.
In conclusion, if the enhanced DPP is found to be superior to the standard DPP, this model can be disseminated into clinical practice regardless of current psychosocial support services. Because of similar psychosocial stressors, including traumas as a result of exposure to violence, migration, and poverty, approaches designed for AIANs in this study are likely to be relevant for other disparities populations with low socioeconomic status. Offering support through additional visits with a mental health care provider and using traditional healing practices may be feasible within the health care system if the expertise is available in the local community. Engagement with community leaders and coordination with community-based resources can generate feasible and effective strategies that are appealing to patients, and will highly likely address psychosocial barriers that may prevent patients from fully engaging in available programs.
Acknowledgments
The work was primarily supported through a Patient Centered Outcomes Research Institute (PCORI) Project Program Award AD 130602172. Additional support was obtained from UL1 RR025744 and K24 HL086703 from NIH. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. No sponsor or funding source has a role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
The authors extend special thanks to participants, their families, and stakeholders who make this study possible. The authors also thank the American Indian Community Action Board [Al Cross, MSW (Hidatsa/Mandan), Scott Wayne Waters (Oglala Lakota Nation), Ida Sallas (Yaqui, Chiricahua Apache), Robert Garcia (Fort Peck Assiniboine, Sioux Tribe), Craig Pasqua (Cherokee Nation of OK, Pit River), Paul Flores (Apache, Yaqui), Orena Flores (Ft. Yuma Quechan, Maricopa, Mojave), Adrian Kendrick (Oglala Lakota Nation), Matilda Owaleon-Ojeda (Zuni/Navajo), Alberto Ojeda (Zacatecas), Erika Monahan (Ft. Yuma Quechan, Maricopa, Mojave), Shelila Monahan (Ft. Yuma Quechan, Maricopa, Mojave), Manuel Ortega (Apache), Rose Amador Le Beau (Yaqui, Mexica), Dawn Atencio (Navajo/Apache)], the study Data and Safety Monitoring Board members (Virgil Moorehead PsyD, Veronica Yank, MD, Steve Adelsheim, MD, Michael Baiocchi, PhD), the Scientific Advisory Board (Judith McDivitt, PhD, Mary Hoskin, MS, RD, Beverly Calderon, RD, CDE, Donald Warne, MD, MPH, Felicia Hodge, DrPH, Maria Yellow Horse Brave Heart PhD, Meredith Minkler, DrPH, Josephine A. Chase MSW, PhD, Teresa LaFromboise, PhD, Jennifer Ruiz, MBA, Jami Bartgis, PhD, Nina Wallerstein, DrPH, MPH, Michael Yellow Bird, PhD, Darryl Tonemah, PhD), the Stanford Office of Community Health (Rhonda McClinton-Brown, MPH, Jill Evans, MPH), the study team members, and the Program Officers at PCORI (Mira Grieser, MHS) who have made substantial contributions to the conduct of the study (A Patient-Centered Strategy for Improving Diabetes Prevention in Urban American Indians).
References
- [1].Flegal KM, Carroll MD, Kit BK, Ogden CL, Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010, JAMA 307 (5) (2012) 491–497. [DOI] [PubMed] [Google Scholar]
- [2].Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M, Health and economic burden of the projected obesity trends in the USA and the UK, Lancet 378 (9793) (2011) 815–825. [DOI] [PubMed] [Google Scholar]
- [3].Center for Disease Control and Prevention, National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta, GA, 2011. [Google Scholar]
- [4].Liao Y, Bang D, Cosgrove S, et al. , Surveillance of health status in minority communities - racial and ethnic approaches to community health across the U.S. (REACH U.S.) risk factor survey, United States, 2009, Morb. Mortal. Wkly Rep 60 (6) (2011) 1–44 Surveillance summaries. [PubMed] [Google Scholar]
- [5].Kurian AK, Cardarelli KM, Racial and ethnic differences in cardiovascular disease risk factors: a systematic review, Ethn. Dis 17 (1) (2007) 143–152. [PubMed] [Google Scholar]
- [6].Schiller JS, Lucas JW, Ward BW, JA P, Summary health statistics for U.S. adults: National Health Interview Survey, 2010, Vital Health Stat. 10 (252) (2012) 1–207. [PubMed] [Google Scholar]
- [7].Blackwell DL, Lucas JW, TC C, Summary health statistics for US adults: National health interview survey, 2012, Vital Health Stat. 10 (260) (2014) 1–161. [PubMed] [Google Scholar]
- [8].National Health Interview Survey, Tables of Summary Health Statistics, cdc.gov/nchs/nhis/SHS/tables.htm2014.
- [9].Knowler WC, Barrett-Connor E, Fowler SE, et al. , Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin, N. Engl. J. Med 346 (6) (2002) 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang L, Manson SM, Beals J, et al. , Translating the diabetes prevention program into American Indian and Alaska native communities: results from the special diabetes program for Indians diabetes prevention demonstration project, Diabetes Care 36 (7) (2013) 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Centers for Disease Control and Prevention (CDC), Behavioral Risk Factor Surveillance System Survey Data Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (2014). [Google Scholar]
- [12].Dill EJ, Manson SM, Jiang L, et al. , Psychosocial predictors of weight loss among American Indian and Alaska native participants in a diabetes prevention translational project, Journal of diabetes research 2016 (2016) 1546939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Israel BA, Schulz AJ, EA Parker AB Becker, Review of community-based research: assessing partnership approaches to improve public health, Annu. Rev. Public Health 19 (1) (1998) 173–202. [DOI] [PubMed] [Google Scholar]
- [14].Brave Heart MY, DeBruyn LM, The American Indian Holocaust: healing historical unresolved grief, Am. Indian Alsk. Native Ment. Health Res 8 (2) (1998) 56–78. [PubMed] [Google Scholar]
- [15].Whitbeck LB, Adams GW, Hoyt DR, Chen X, Conceptualizing and measuring historical trauma among American Indian people, Am. J. Community Psychol 33 (3–4) (2004) 119–130. [DOI] [PubMed] [Google Scholar]
- [16].Evans-Campbell T, Historical trauma in American Indian/Native Alaska communities a multilevel framework for exploring impacts on individuals, families, and communities, J. Interpers. Violence 23 (3) (2008) 316–338. [DOI] [PubMed] [Google Scholar]
- [17].Walters KL, Mohammed SA, Evans-Campbell T, Beltran RE, Chae DH, Duran B, Bodies don’t just tell stories, they tell histories, Du Bois Review: Social Science Research on Race. 8 (01) (2011) 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gone JP, Redressing First Nations historical trauma: theorizing mechanisms for indigenous culture as mental health treatment, Transl. Psychiatry 50 (5) (2013) 683–706. [DOI] [PubMed] [Google Scholar]
- [19].Knowler WC, Fowler SE, Hamman RF, et al. , 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study, Lancet 374 (9702) (2009) 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Diabetes Prevention Support Center, Group Lifestyle Balance Manual of Operations A Modification of the Diabetes Prevention Program’s Lifestyle Change Program, University of Pittsburgh, Pittsburgh, PA, 2011. http://www.diabetesprevention.pitt.edu/docs/GroupLifestyleBalanceManualofOperations-Complete.pdf (Accessed April 12, 2011). [Google Scholar]
- [21].Kramer MK, Kriska AM, Venditti EM, et al. , Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery, Am. J. Prev. Med 37 (6) (2009) 505–511. [DOI] [PubMed] [Google Scholar]
- [22].Bandura A, Social Foundations of Thought and Action: A Social Cognitive Theory, Englewood Cliffs, N.J., Prentice Hall, 1986. [Google Scholar]
- [23].Prochaska JO, DiClemente CC, Stages and processes of self-change of smoking: toward an integrative model of change, J. Consult. Clin. Psychol 51 (3) (1983) 390–395. [DOI] [PubMed] [Google Scholar]
- [24].Prochaska JO, Velicer WF, The transtheoretical model of health behavior change, Am. J. Health Promot 12 (1) (1997) 38–48. [DOI] [PubMed] [Google Scholar]
- [25].Jensen MD, Ryan DH, Apovian CM, et al. , 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society, J. Am. Coll. Cardiol 63 (25 Pt B) (2014) 2985–3023. [DOI] [PubMed] [Google Scholar]
- [26].Hodge FS, Fredericks L, Rodriguez B, American Indian women’s talking circle. A cervical cancer screening and prevention project, Cancer 78 (7 Suppl) (1996) 1592–1597. [PubMed] [Google Scholar]
- [27].Struthers R, Hodge FS, Geishirt-Cantrell B, De Cora L, Participant experiences of Talking Circles on type 2 diabetes in two Northern Plains American Indian Tribes, Qual. Health Res 13 (8) (2003) 1094–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Struthers R, Hodge FS, De Cora L, Geishirt-Cantrell B, The experience of native peer facilitators in the campaign against type 2 diabetes, J. Rural. Health 19 (2) (2003) 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Strickland CJ, Squeoch MD, Chrisman NJ, Health promotion in cervical cancer prevention among the Yakama Indian women of the Wa’Shat longhouse, J. Transcult. Nurs 10 (3) (1999) 190–196. [DOI] [PubMed] [Google Scholar]
- [30].Johnson RE, Green BL, Anderson-Lewis C, Wynn TA, Community health advisors as research partners: an evaluation of the training and activities, Fam. Community Health 28 (1) (2005) 41–50. [DOI] [PubMed] [Google Scholar]
- [31].Ford CD, Kim MJ, Dancy BL, Perceptions of hypertension and contributing personal and environmental factors among rural Southern African American women, Ethn. Dis 19 (4) (2009) 407–413. [PMC free article] [PubMed] [Google Scholar]
- [32].Fleischhacker S, Vu M, Ries A, McPhail A, Engaging tribal leaders in an American Indian healthy eating project through modified talking circles, Fam. Community Health 34 (3) (2011) 202–210. [DOI] [PubMed] [Google Scholar]
- [33].Cesario SK, Care of the Native American woman: strategies for practice, education, and research, J. Obstet. Gynecol. Neonatal. Nurs 30 (1) (2001) 13–19. [PubMed] [Google Scholar]
- [34].Castro S, O’Toole M, Brownson C, Plessel K, Schauben L, A diabetes selfmanagement program designed for urban American Indians, Prev. Chronic Dis 6 (4) (2009) A131. [PMC free article] [PubMed] [Google Scholar]
- [35].Becker SA, Affonso DD, Beard MB, Talking circles: Northern Plains tribes American Indian women’s views of cancer as a health issue, Public Health Nurs. 23 (1) (2006) 27–36. [DOI] [PubMed] [Google Scholar]
- [36].Dahan R, Dick R, Moll S, et al. , Using Photography to: Help People Share Their Ideas, Improve Our Communities, Give a Voice to Those Not Heard, Hamilton Community Foundation, 2007. [Google Scholar]
- [37].Wang C, Burris MA, Photovoice: concept, methodology, and use for participatory needs assessment, Health Educ. Behav 24 (3) (1997) 369–387. [DOI] [PubMed] [Google Scholar]
- [38].Center for Digital Storytelling, Center for Digital Storytelling, What We Do, http://www.storycenter.org/what-we-offer/2012 (Accessed June 19, 2012). [Google Scholar]
- [39].Moorehead V, LaFromboise T, Suzuki LA, Healing One Story at a Time: American Indian/Alaska Native Social Justice, in: Ponterotto JG, Casas JM, Alexander CM (Eds.), Handbook of Multicultural Counseling, Sage publications, 2009. [Google Scholar]
- [40].Federation ID, The IDF Consensus Worldwide Definition of the Metabolic Syndrome, Brussels, Belgium, International Diabetes Federation, 2006. [Google Scholar]
- [41].Grundy SM, Brewer HB Jr., Cleeman JI, et al. , Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition, Arterioscler. Thromb. Vase. Biol 24 (2) (2004) el3–el8. [DOI] [PubMed] [Google Scholar]
- [42].Bellg AJ, Borrelli B, Resnick B, et al. , Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium, Health Psychol. 23 (5) (2004) 443–451. [DOI] [PubMed] [Google Scholar]
- [43].Ma J, Yank V, Xiao L, et al. , Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial, JAMA Internal Medicine 173 (2) (2013) 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ober C, Hofijan S, Asthma genetics 2006: the long and winding road to gene discovery, Genes Immun. 7 (2) (2006) 95–100. [DOI] [PubMed] [Google Scholar]
- [45].Sawchuk CN, Russo JE, Bogart A, et al. , Barriers and facilitators to walking and physical activity among American Indian elders, Prev. Chronic Dis 8 (3) (2011) A63. [PMC free article] [PubMed] [Google Scholar]
- [46].Ferucci ED, Schumacher MC, Lanier AP, et al. , Arthritis prevalence and associations in American Indian and Alaska Native people, Arthritis Rheum. 59 (8) (2008) 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].NIH and National Heart Lung Blood Institute, Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report, DHHS, Public Health Service, Rockville, MD, October 1998 NIH Publication No. 00–4084. [Google Scholar]
- [48].Pickering TG, Hall JE, Appel LJ, et al. , Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research, Hypertension 45 (1) (2005) 142–161. [DOI] [PubMed] [Google Scholar]
- [49].BRFSS, Questionnaires, http://www.cdc.gov/brfss/questionnaires/2014.
- [50].Yu L, Buysse DJ, Germain A, et al. , Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks, Behav. Sleep Med 10(1) (2011) 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test, Arch. Intern. Med 158 (16) (1998) 1789–1795. [DOI] [PubMed] [Google Scholar]
- [52].Heart MY, Chase J, Elkins J, Altschul DB, Historical trauma among Indigenous Peoples of the Americas: concepts, research, and clinical considerations,]. Psychoactive Drugs 43 (4) (2011) 282–290. [DOI] [PubMed] [Google Scholar]
- [53].Cole JC, Rabin AS, Smith TL, Kaufman AS, Development and validation of a Rasch-derived CES-D short form, Psychol. Assess 16 (4) (2004) 360–372. [DOI] [PubMed] [Google Scholar]
- [54].Whitbeck LB, Adams GW, Hoyt DR, Chen X, Conceptualizing and measuring historical trauma among American Indian people, Am. J. Community Psychol 33 (3–4) (2004) 119–130. [DOI] [PubMed] [Google Scholar]
- [55].Mashek D, Cannaday LW, Tangney JP, Inclusion of community in self scale: a single-item pictorial measure of community connectedness, J. Comp. Psychol 35 (2) (2007) 257–275. [Google Scholar]
- [56].Haswell MR, Kavanagh D, Tsey K, et al. , Psychometric validation of the Growth and Empowerment Measure (GEM) applied with Indigenous Australians, Aust. N. Z. J. Psychiatry 44 (9) (2010) 791–799. [DOI] [PubMed] [Google Scholar]
- [57].White IR, Horton NJ, Carpenter J, Pocock SJ, Strategy for intention to treat analysis in randomised trials with missing outcome data, BMJ 342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hollis S, A graphical sensitivity analysis for clinical trials with non-ignorable missing binary outcome, Stat. Med 21 (24) (2002) 3823–3834. [DOI] [PubMed] [Google Scholar]
- [59].Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D, How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors, Am. J. Psychiatry 158 (6) (2001) 848–856. [DOI] [PubMed] [Google Scholar]
- [60].Chmura Kraemer H, Kiernan M, Essex M, Kupfer DJ, How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches, Health Psychol. 27 (2S) (2008) S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Group DPPR, Achieving weight and activity goals among diabetes prevention program lifestyle participants, Obes. Res 12 (9) (2004) 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lewis JA, Statistical principles for clinical trials (ICH E 9): an introductory note on an international guideline, Stat. Med 18 (15) (1999) 1903–1942. [DOI] [PubMed] [Google Scholar]
- [63].Bender R, Lange S, Adjusting for multiple testing—when and how? J. Clin. Epidemiol 54 (4) (2001) 343–349. [DOI] [PubMed] [Google Scholar]
- [64].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].R: A Language and Environment for Statistical Computing. [Computer Program], R Foundation for Statistical Computing, Vienna, Austria, 2014. [Google Scholar]
- [66].Jackson L, Translating the Diabetes Prevention Program into practice: a review of community interventions, Diabetes Educ. 35 (2) (2009) 309–320. [DOI] [PubMed] [Google Scholar]
- [67].Piatt GA, Seidel MC, Powell RO, Zgibor JC, Comparative effectiveness of lifestyle intervention efforts in the community: results of the Rethinking Eating and ACTivity (REACT) study, Diabetes Care 36 (2) (2013) 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].JA Katula MZ Rosenberger Vitolins, E.L., et al. , One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project, Diabetes Care 34 (7) (2011) 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Saaristo T, Moilanen L, Korpi-Hyovalti E, et al. , Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D), Diabetes Care 33 (10) (2010) 2146–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Piatt GA, Seidel MC, Chen HY, Powell RO, Zgibor JC, Two-year results of translating the diabetes prevention program into an urban, underserved community, Diabetes Educ. 38 (6) (2012) 798–804. [DOI] [PubMed] [Google Scholar]
- [71].Edwards K, Patchell B, State of the science: a cultural view of Native Americans and diabetes prevention, J. Cult. Divers 16 (1) (2009) 32–35. [PMC free article] [PubMed] [Google Scholar]
- [72].McNamara BJ, Sanson-Fisher R, D’Este C, Eades S, Type 2 diabetes in Indigenous populations: quality of intervention research over 20 years, Prev. Med 52 (1) (2011) 3–9. [DOI] [PubMed] [Google Scholar]