Abstract
The human genome encodes thousands of long non-coding RNAs (lncRNAs), the majority of which are poorly conserved and uncharacterized. Here we identify a primate-specific lncRNA (CHROME), elevated in the plasma and atherosclerotic plaques of individuals with coronary artery disease, that regulates cellular and systemic cholesterol homeostasis. LncRNA CHROME expression is influenced by dietary and cellular cholesterol via the sterol-activated liver X receptor transcription factors, which control genes mediating responses to cholesterol overload. Using gain- and loss-of-function approaches, we show that CHROME promotes cholesterol efflux and HDL biogenesis by curbing the actions of a set of functionally related microRNAs that repress genes in those pathways. CHROME knockdown in human hepatocytes and macrophages increases levels of miR-27b, miR-33a, miR-33b and miR-128, thereby reducing expression of their overlapping target gene networks and associated biologic functions. In particular, cells lacking CHROME show reduced expression of ABCA1, which regulates cholesterol efflux and nascent HDL particle formation. Collectively, our findings identify CHROME as a central component of the non-coding RNA circuitry controlling cholesterol homeostasis in humans.
The maintenance of cholesterol homeostasis is essential to human health, and its dysregulation results in disease states, including atherosclerotic cardiovascular disease1. The removal of excess cholesterol from cells and its delivery to the liver via high density lipoproteins (HDL) are central in protecting from pathologic cholesterol deposition in tissues, particularly the artery wall where atherosclerotic plaques form2. Understanding the regulatory circuits that contribute to the dynamic and temporal balancing of cholesterol at the cellular and organismal level are thus of critical importance. At the transcriptional level, the sterol-activated liver-X-receptors (LXR) have been shown to play key roles in the regulation of genes that control the response to cholesterol excess, particularly the cholesterol transporter ABCA1, which mediates cholesterol efflux and HDL biogenesis3. In addition, recent studies indicate that multiple classes of non-coding RNAs, including microRNAs (miRNAs), small nucleolar RNAs, and long non-coding RNAs (lncRNAs) possess regulatory functions that feed into the previously described networks controlling cellular lipid metabolism4–6.
Among the non-coding RNAs, miRNAs have emerged as potent regulators of cholesterol homeostasis through their ability to repress the expression of genes in the integrated pathways of cholesterol and fatty acid biosynthesis, reverse cholesterol transport, and lipid storage6. One of the first such miRNAs to be identified was miR-33 (mmu-miR-33–5p; hsa-miR-33a-5p and hsa-miR-33b-5p), which was shown to reduce cholesterol efflux and reverse cholesterol transport by targeting genes involved in cholesterol export (e.g., ABCA1)7,8, intracellular trafficking (e.g., NPC1, OSBPL6, ATG5)8,9, and bile secretion (e.g., ATP8B1, ABCB11)10. The identification of miR-27b and miR-144 as additional repressors of genes regulating cholesterol efflux (e.g., ABCA1, OSBPL6)9,11–14, and the demonstration that delivery of modified oligonucleotide inhibitors of miR-33 and miR-144 in mice could increase plasma levels of HDL cholesterol, underscored the importance of miRNAs as post-transcriptional regulators of cellular and systemic cholesterol homeostasis. Indeed, the relevance of this mechanism in regulating cholesterol metabolism in humans is supported by genome-wide association studies that have uncovered variants in miRNA loci associated with abnormal lipoprotein levels and cardiometabolic disease risk15. One such example is miR-128, which was found to target ABCA1 and LDLR, and consequently, to regulate plasma levels of HDL and low density lipoprotein (LDL) cholesterol, respectively15. However, given the redundancy in miRNA targeting of cholesterol efflux pathways described above, it remains unclear if such functionally related miRNAs are coordinately controlled or regulated by higher order systems.
LncRNAs contribute to regulatory complexity in higher eukaryotes through diverse mechanisms, including by regulating chromosomal architecture, facilitating the formation of ribonucleoprotein complexes, altering the epigenetic landscape of the genome, and mediating transcriptional and post-transcriptional gene regulation16. LncRNAs are broadly defined as non-protein coding RNA transcripts of greater than 200 nucleotides, and they can execute their functions by forming lncRNA-DNA, lncRNA-protein and lncRNA-RNA interactions in the nucleus and/or cytoplasm. While it is estimated that the human genome contains >10,000 lncRNAs, fewer than 5% of human lncRNAs have been functionally characterized, in part, because poor conservation among species has hindered their investigation17,18. Functional roles for lncRNAs in regulating cholesterol metabolism and cardiovascular disease are beginning to emerge5,19. For example, several lncRNAs have been identified that promote or inhibit the function of the sterol response element binding protein (SREBP) transcription factors that regulate genes involved in cholesterol and fatty acid synthesis, including MALAT120, H1921, lncHR122, and LeXis23. Other lncRNAs, such as MeXis24, have been shown to confer cell type selective expression of genes (e.g., ABCA1) by regulating the chromosomal architecture. Notably, the majority of cardiovascular disease-associated sequence variants identified from human genome wide association studies are found in the non-coding landscape of the genome, as is the case for the locus on chromosome 9p21 containing lncRNA ANRIL25,26, which has the highest association with atherosclerotic risk and cardiovascular events. Understanding the functional significance of non-coding RNAs contributing to disease remains a major challenge.
Here we describe a primate-specific lncRNA, increased in the setting of human atherosclerotic vascular disease, that contributes to the maintenance of cholesterol homeostasis. Studies in non-human primates show that expression of lncRNA CHROME is upregulated in response to a cholesterol-enriched diet and by activation of the LXR transcription factor that coordinates the response to cholesterol excess. Using loss- and gain-of-function, we establish that CHROME increases the efflux of excess cholesterol from cells and promotes hepatic HDL biogenesis by curbing the actions of a set of miRNAs, which share the ability to repress genes involved in cholesterol efflux and reverse cholesterol transport. Consistent with these mechanistic studies, we find that hepatic levels of CHROME are inversely correlated with those of its miRNA targets, and positively correlated with plasma levels of HDL-C in a human cohort. In summary, our identification of CHROME unveils the regulatory cross-talk between non-coding RNAs that sustains cholesterol homeostasis in humans in health and disease.
Results
CHROME is a primate-specific lncRNA increased in atherosclerotic vascular disease
LncRNA AC009948.5 (ENSG00000223960), which we have named CHROME (Cholesterol Homeostasis Regulator of MiRNA Expression), is proximal to a locus on human chromosome 2 linked to premature coronary artery disease27 and plasma HDL-C levels28. The CHROME locus contains primate-specific Alu transposable repeat elements29, and CHROME exons are conserved in primates, but are largely absent in other placental mammals or vertebrates (Fig. 1a, Supplementary Fig. 1a-c). We identified 7 splice variants of CHROME (Fig. 1a), which were broadly expressed in human tissues and cell types (Supplementary Fig. 2a-c). To test whether CHROME expression is altered in the setting of atherosclerotic vascular disease, we measured its expression in the plasma and arteries of individuals with coronary artery disease and healthy control subjects. Plasma levels of CHROME, measured by quantitative PCR (qPCR) using primers directed to a common region in all variants, were found to be markedly elevated in individuals with coronary artery disease compared to healthy individuals (Fig. 1b), whereas LIPCAR and TapSAKI (other known circulating lncRNAs) were unchanged (Supplementary Fig. 3a). Furthermore, analysis of CHROME expression in the Biobank of Karolinska Endarterectomies (BiKE) Affymetrix microarray datasets30 showed that CHROME RNA levels were increased in atherosclerotic plaques from patients with symptomatic and asymptomatic carotid stenosis compared to control arterial samples (Fig. 1c). This was confirmed by qPCR for individual CHROME variants using exon-spanning primers complementary to unique sequences present in each of the CHROME isoforms (Supplementary Fig. 3b). To understand the cellular distribution of CHROME in the healthy and diseased artery wall, we performed RNA in situ hybridization. We found that in carotid artery plaques CHROME RNA (shown in blue) was localized to infiltrating inflammatory cells in both the neointima and areas of plaque rupture (Fig. 1d, boxed regions, Supplementary Fig. 3c). By contrast, there was little detectable CHROME RNA in the normal artery wall (Fig. 1e, Supplementary Fig. 3d). Together, these data suggest a potential function for CHROME in the setting of atherosclerotic vascular disease.
Figure 1. Levels of lncRNA CHROME are increased in atherosclerotic cardiovascular disease.

a, Schematic representation of the human CHROME locus, which encodes seven splice variants (CHROME1–7). PhastCons analysis showing the conservation of CHROME in primates but not in other mammals or vertebrates. Red boxes indicate exonic sequences. b, Box and whiskers plot depicting plasma levels of CHROME RNA in individuals with coronary artery disease (CAD, n=14) or healthy control subjects (n=33). Box represents 25th to 75th percentiles, with line in middle indicating the median. c, CHROME expression in normal artery (n=10), or carotid endarterectomy plaques from individuals with asymptomatic (n=40) or symptomatic (n=77) carotid stenosis. Data are the mean ± SEM. d-e, Detection of CHROME RNA (purple) by in situ hybridization of human (d) carotid plaque and (e) control iliac artery. Sections were counterstained with nuclear fast red to visualize nuclei (pink). Boxed regions in (d) indicate CHROME expression in areas of inflammatory cell infiltrate (top) and in the neointima (bottom, arrowheads). Lumen (L). Data are representative of staining from 8 independent plaques. P-values were calculated using (b) a Mann-Whitney test, and (c) two-way ANOVA, *P≤0.05, ****P≤0.0001.
CHROME is upregulated in response to excess dietary or cellular cholesterol via LXR
To test whether CHROME expression is responsive to dietary and cellular cholesterol levels, we measured its expression in the livers of non-human primates prior to and after feeding of a diet enriched in cholesterol for 8 weeks. We observed that increasing dietary cholesterol levels increased the expression of CHROME-1, CHROME-3 and CHROME-7 in the liver - a tissue central to the regulation of systemic cholesterol homeostasis - compared to low fat chow diet feeding (Fig. 2a). We found that CHROME was abundantly expressed in primary human hepatocytes (102 copies/cell; Supplementary Fig 4a) and levels of CHROME variants increased with cyclodextrin-cholesterol treatment, compared to vehicle treatment (Fig. 2b). In addition to hepatocytes, macrophages play key roles in cholesterol homeostasis, particularly in the atherosclerotic plaque where they are recruited to clear accumulated LDL31. In human THP-1 monocytic cells differentiated into macrophages in vitro, CHROME is present at more than 102 copies/cell (Supplementary Fig. 4a), and its expression levels increased upon cholesterol loading with acetylated LDL (Fig. 2c). Using prediction algorithms to uncover potential transcriptional regulators of CHROME, we identified putative binding sites for the sterol-activated LXR transcription factors (Supplementary Fig. 4b), which coordinate the expression of genes involved in cellular and systemic responses to cholesterol excess3. We performed chromatin immunoprecipitation in THP-1 macrophages treated with LXR agonist and observed LXR occupancy at the CHROME locus, at sites upstream of the start site of transcription and within the first intron (Fig. 2d). The levels of LXR enrichment over IgG control at these two sites in the CHROME locus were similar in magnitude to that found for the known LXR-responsive gene OSBPL6, which served as a positive control (Fig. 2d). Consistent with a role for LXR in regulating CHROME transcription, treatment of non-human primates with the LXR agonist GW3965 increased levels of CHROME-1, CHROME-3, CHROME-4 and CHROME-7 in the liver, as compared to control vehicle treatment (Fig. 2e). Furthermore, treatment of primary human hepatocytes, HepG2 cells and THP-1 macrophages with the LXR agonist T0901317 increased expression of CHROME variants compared to vehicle treatment (Fig. 2f, Supplementary Fig. 4c). Notably, treatment with T0901317 or acetylated LDL failed to increase CHROME or the control gene ABCA1 in THP-1 macrophages when LXRα/β were silenced using siRNAs (Fig. 2g, Supplementary Fig. 4d). Together, these data identify CHROME as part of the network of genes regulated by LXR in primates in the settings of excess dietary or cellular cholesterol.
Figure 2. LncRNA CHROME is regulated by dietary and cellular cholesterol via the LXR- transcription factor.

a, qPCR analysis of CHROME variant expression in (a) livers of African green monkeys (n=5/group) before fed a high fat moderate cholesterol diet (8 weeks) compared to baseline chow diet. Box represents 25th to 75th percentiles, with line in middle indicating the median. b-c, qPCR analysis of CHROME variant expression in (b) human HepG2 cells treated with cholesterol-cyclodextrin (10 μg/mL; 72 h) compared to vehicle, and (c) human THP-1 macrophages treated with acetylated LDL (37.5 μg/mL, 24 h) compared to untreated. Data are the mean ± SEM of 5 (b) and 4 (c) independent experiments. d, Schematic diagram of predicted LXR response elements (LXRE) in the CHROME locus (top) and relative enrichment of CHROME in LXRα/β chromatin immunoprecipitates from THP-1 cells treated with LXR agonist (T0901317, 10 μM) at each of the predicted sites. Enrichment of LXR at OSBPL6 locus is shown as positive control. Data are normalized to IgG control. e, qPCR analysis of CHROME variant expression in livers of male cynomolgus monkeys treated with LXR agonist (GW3965) or vehicle for 2 days. Data are the mean ± SEM of 5 monkeys/group. f, qPCR analysis of CHROME variant expression in primary human hepatocytes and THP-1 macrophages treated with LXR agonist (10 μM T0901317) relative to vehicle control. Data are the mean ± SEM of 3–5 independent experiments. g, qPCR quantification of CHROME (all variants) and ABCA1 mRNA in THP-1 macrophages transfected with an LXRα/β-targeting or control siRNA for 24 h and then treated with LXR agonist (10 μM T0901317). Data are the mean ± SEM of 3 biological replicates from a single experiment that is representative of 3 experiments. P-values were calculated using (b, c, f, g) two-tailed student’s t-test and (a, e) two-way ANOVA. *P≤0.05, **P≤0.01, ***P≤0.001.
CHROME knockdown in hepatocytes reveals roles in cholesterol metabolism
To determine CHROME’s function, we first examined its subcellular distribution pattern as this can provide insight into the potential biological role of a lncRNA32. Using fluorescence in situ hybridization (FISH), we found that CHROME transcripts were distributed both in the nucleus and the cytoplasm in HEK293T cells (Fig. 3a), THP-1 macrophages (Supplementary Fig. 4e) and HepG2 cells (not shown). CHROME’s localization to the cytoplasm, the site of RNA translation, raised the possibility that it may contain an actively translated open reading frame. However, coding potential and Kozak sequence prediction algorithms indicated low coding potential of CHROME (Supplementary Fig. 5a-b, Supplementary Table 1), and this was confirmed by polysome fractionation in THP-1 macrophages (Supplementary Fig. 5c, d). To test the effect of CHROME loss-of-function, we used shRNAs, which preferentially deplete cytoplasmic lncRNAs33, targeting a region of exon 1 common to all CHROME variants (Supplementary Fig. 6a). Using lentiviral delivery of control and CHROME-targeting shRNAs, we generated human hepatic HepG2 cell lines in which CHROME variants were constitutively knocked down (Fig. 3b), and performed RNA sequencing (RNA-Seq). Ingenuity Pathway Analysis of the RNA-Seq datasets identified lipid metabolism functions as among the top canonical pathways altered upon CHROME knockdown in HepG2 cells (Fig. 3c), most notably, the LXR pathway that regulates cellular responses to excess cholesterol, the farnesoid X receptor (FXR) pathway that regulates bile acid metabolism and cholesterol excretion, and the fatty acid β-oxidation pathway (Fig. 3c, Supplementary Fig. 6b, c).
Figure 3. CHROME depletion in hepatocytes reduces cholesterol efflux and formation of nascent HDL particles.
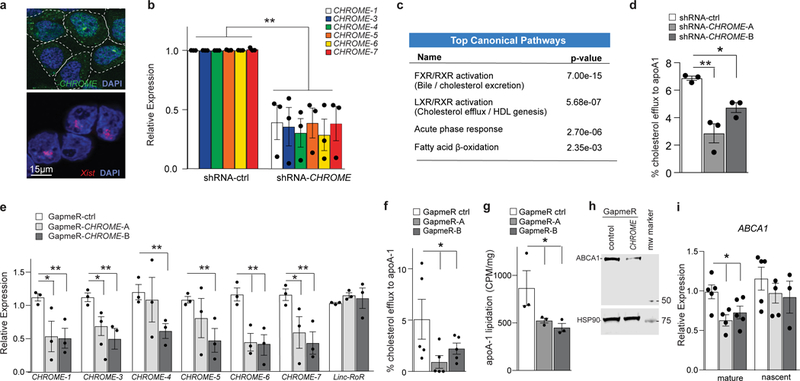
a, Fluorescence in situ hybridization for CHROME (green) and Xist (red) RNA in HEK293T cells stained with DAPI to visualize nuclear DNA (blue). b, qPCR analysis of CHROME variants in HepG2 cells stably expressing an shRNA targeting all CHROME variants (shRNA-CHROME) or control shRNA (shRNA-ctrl). c, Ingenuity pathway analysis of RNA-Seq data from shRNA-CHROME and shRNA-ctrl expressing HepG2 cells showing the top canonical pathways altered upon CHROME knockdown. d, Measurement of cholesterol efflux to exogenous apoA-1 in HepG2 cells expressing shRNA-CHROME or shRNA-ctrl. e, qPCR quantification of CHROME variants in primary human hepatocytes transfected with control (GapmeR-ctrl) or CHROME-targeting GapmeRs (GapmeR-CHROME-A or GapmeR-CHROME-B). f, Measurement of cholesterol efflux to exogenous apoA-1 in primary human hepatocytes transfected with control or CHROME-targeting GapmeRs. g, Measurement of phospholipid efflux to apoA1 secreted from primary human hepatocytes labelled with C14-choline chloride and transfected with control or CHROME-targeting GapmeRs. h, Western blot analysis of ABCA1 and HSP90 (control) in hepatocytes transfected with control or CHROME-targeting GapmeRs. Molecular weight marker is shown at right. i, qPCR analysis of mature and nascent ABCA1 mRNA transcripts in primary human hepatocytes transfected with control or CHROME-targeting GapmeRs. b, d-i, Data are the mean ± SEM of 3–5 independent experiments. P-values were calculated using a two-tailed student’s t-test. *P≤0.05, **P≤0.01.
CHROME post-transcriptionally regulates ABCA1 expression and cholesterol efflux
Activation of LXRs promotes cholesterol homeostasis through induction of a cadre of genes involved in cellular cholesterol efflux and reverse cholesterol transport. To investigate the role of CHROME in LXR-mediated responses, we measured the ability of HepG2 cells expressing control and CHROME-targeting shRNAs to efflux cholesterol to apolipoprotein A1 (apoA-1), a process central to HDL biogenesis in the liver. Cholesterol efflux to exogenously supplied apoA1 was 50% lower in HepG2 cells expressing CHROME shRNAs compared to control shRNAs (Fig. 3d). To confirm our findings, we used a second method of CHROME depletion in primary human hepatocytes, in which we acutely knocked-down CHROME by transfecting GapmeR antisense oligonucleotides targeting a region of exon 1 common to all variants (Fig. 3e). Consistent with our findings using shRNAs, CHROME depletion in primary human hepatocytes using GapmeRs lowered cholesterol efflux to exogenous apoA-1 by approximately 50%, compared to control GapmeR treatment (Fig. 3f). We next measured the effects of CHROME on nascent HDL formation by primary human hepatocytes using 14C-choline to label de novo synthesized phospholipids. Immunoprecipitation of apoA-1 secreted by primary human hepatocytes revealed 50% lower levels of apoA-1 lipidation in GapmeR-CHROME compared to GapmeR-control treated cells (Fig. 3g). By contrast, CHROME knockdown or overexpression in HepG2 cells did not alter the expression of genes involved in LDL uptake or cholesterol synthesis, nor were these processes altered upon manipulation of CHROME levels (Supplementary Fig 7a-e). As the ABCA1 transporter regulates cholesterol and phospholipid efflux to apoA-1, we next measured the effect of CHROME knockdown on ABCA1 protein and mRNA levels. We observed lower ABCA1 protein expression in GapmeR-CHROME compared to GapmeR-control treated hepatocytes (Fig. 3h, Supplementary Fig 7f). Using PCR primers to differentiate mature and unspliced mRNA transcripts34 of ABCA1, we found that CHROME depletion reduced levels of mature, but not nascent ABCA1 mRNA (Fig. 3i), suggesting a post-transcriptional mechanism.
CHROME localizes to P-bodies and associates with microRNAs
The cytoplasmic localization of CHROME raised the possibility that it may interact with cytosolic miRNAs known to post-transcriptionally regulate ABCA1 expression and cholesterol efflux. To explore this potential mechanism, we used the miRTarget2 algorithm, which identified miR-33a, miR-33b, miR-27b and miR-128 as among the top miRNAs predicted to interact with CHROME (Supplementary Table 2). Using RNA hybrid analysis and manual curation, we found that CHROME variants harbor numerous putative binding sites for each of these miRNAs experimentally validated to regulate cholesterol efflux and/or HDL metabolism (Fig. 4a, Supplementary Fig. 8)8,15,35,36. Using RNAcofold, which takes into account the length and secondary structure of the transcripts, we calculated the free energy changes associated with the formation of CHROME-miRNA complexes and found that it predicted stable interactions of CHROME with miR-27b, miR-33a, miR-33b and miR-128, but not the control miRNA miR-16 (Fig. 4b). To first assess whether CHROME associates with miRNA complexes, we immunoprecipitated AGO2 from primary human hepatocytes and used qPCR to detect associated RNA, focusing on CHROME-1, CHROME-3 and CHROME-7 as these were the variants most regulated by cholesterol in monkeys. We found that CHROME-1, CHROME-3 and CHROME-7 transcripts were enriched in AGO2-immunoprecipitates compared to control IgG-precipitates (Fig. 4c). Similar association of CHROME variants with AGO2 was observed in THP-1 macrophages (Fig. 4c). To test whether CHROME binds specifically to miR-27b, miR-33a, miR-33b and miR-128, we used MS2-tagged RNA affinity purification (MS2-TRAP)37. We cloned CHROME-1, −3, and −7 upstream of MS2-binding sequences, and co-expressed these constructs in HEK293T cells with a YFP-tagged MS2-binding protein, which was used as bait to isolate MS2-tagged RNAs. qPCR analysis of RNAs co-precipitated with CHROME-MS2-binding protein complexes using anti-YFP antibody showed specific enrichment of miR-27b, miR-33a, miR-33b and miR-128 relative to control IgG, whereas no enrichment of control miRNAs (e.g., miR-16, miR-224 and miR-155) was detected (Fig. 4d).
Figure 4. CHROME interacts with a set of miRNAs known to repress cholesterol efflux.
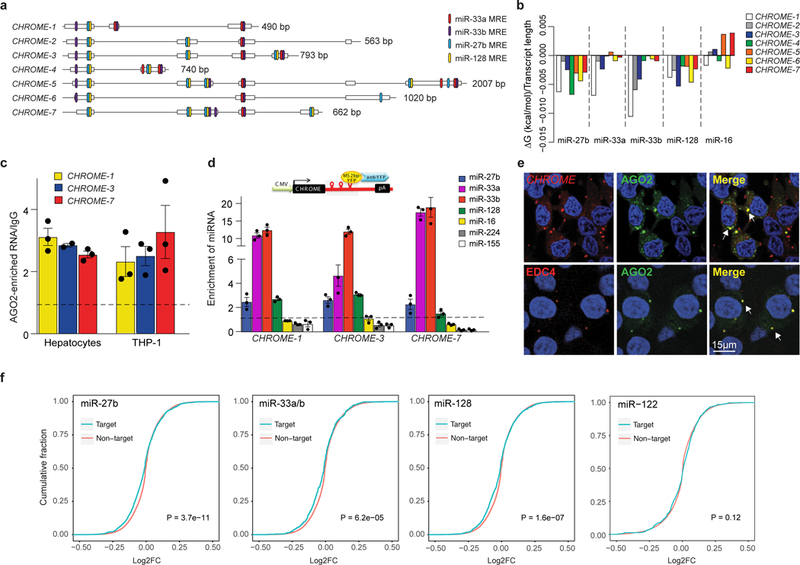
a,Schematic diagram showing multiple miRNA response elements (MRE) for miR-27b, miR-33a, miR-33b and miR-128 in CHROME variants. b, RNAcofold-predicted changes in Gibbs free energy (ΔG) upon binding of CHROME variants to miR-27b, miR-33a, miR-33b, miR-128 or miR-16 (control). c, Enrichment of CHROME RNA in AGO2-immunoprecipitates from primary human hepatocytes and THP-1 macrophages relative to IgG control. d, qPCR detection of miRNAs in CHROME-1, CHROME-3 and CHROME-7 ribonucleoprotein complexes precipitated using MS2-tagged RNA affinity purification (MS2-TRAP). Data are normalized to U6 and control MS2-bs-vector. c-d) Data are the mean ± SEM of a single experiment and are representative of 3 independent experiments. e, Fluorescence in situ hybridization for CHROME (red: top) and immunostaining for the P-body marker EDC4 (red: bottom) in HEK293T cells expressing AGO2-GFP (green) and stained with DAPI nuclear stain (blue). Arrows in merged image indicate signal colocalization (yellow) in punctate structures. Staining is representative of 2 independent experiments. f, Cumulative distribution plots of log2-transformed gene expression fold changes from RNA-Seq of CHROME-shRNA vs control-shRNA HepG2 cells (3 biological replicates each), showing genes containing miR-27b, miR-33a/miR-33b or miR-128 target sites predicted by Targetscan (blue) and all other expressed genes (red). As a control, similar analyses were performed for genes containing target sites of miR-122, a hepatic miRNA with known roles in lipid metabolism. The increased prevalence of negative log2-fold changes among predicted targets of miR-27b, miR-33 and miR-128 (left shift of blue line compared to red), but not miR-122, suggests increased inhibitory effects of those miRNAs upon CHROME knockdown. P-values were calculated a two-sample Kolmogorov-Smirnov test.
To further investigate the association of CHROME with the RNA-Induced Silencing Complex (RISC), we performed RNA-FISH for CHROME in HEK293T cells that stably express AGO2-GFP. We observed colocalization of CHROME and AGO2 in puncta present in the cytoplasm (Fig. 4e). Notably, these puncta stained positive for enhancer of mRNA-decapping protein 4 (EDC4) (Fig. 4e), a marker of RNA processing bodies (P-bodies) that mediate miRNA sequestration or decay38. The presence of CHROME in P-bodies was confirmed by querying publicly available RNA-Seq data (ArrayExpress: E-MTAB-5477) of transcripts isolated from P-bodies purified from HEK293T cells using fluorescence-activated particle sorting39. CHROME was found to be significantly overrepresented in the P-body fraction when compared to the non-P-body fraction (adjusted P-value = 0.03; see Table S2 of 39). Together, these data suggest that CHROME is localized to P-bodies, where it may sequester or degrade its miRNA binding partners. To investigate this possibility, we performed miRNA competition experiments with either wild type CHROME-7 (CHROMEWT) or CHROME-7 with mutated its miRNA binding sites mutated (CHROMEMUT). We transfected HEK293T cells with either CHROMEWT or CHROMEMUT and increasing amounts of control, miR-33a, miR-27b or miR-128 mimic. We found that cells expressing CHROMEWT, but not CHROMEMUT, had reduced levels of miR-33a, miR-27b and miR-128 compared to vector transfected cells. Because we controlled the concentration of miRNA molecules in this experiment by transfection, this suggests that CHROME can induce the decay of miR-33, miR-27b and miR-128 (Supplementary Fig. 9a).
CHROME modulates the actions of microRNAs known to repress cholesterol efflux and HDL biogenesis
To further investigate whether endogenous CHROME binding of miR-27b, miR-33a, miR-33b and miR-128 reduces the biological activities of these miRNAs, we assessed the effects of CHROME knockdown on those miRNAs’ target gene networks using two unbiased approaches. First, we analyzed our RNA-Seq dataset of genes differentially expressed in shRNA-CHROME and shRNA-control expressing HepG2 cells by cumulative distribution function analysis to identify whether mRNAs targeted by miR-33a, miR-33b, miR-27b and miR-128 were selectively downregulated compared to non-target mRNAs. Indeed, we found significant overrepresentation of gene expression inhibition among miR-33a, miR-33b, miR-27b and miR-128 targets predicted by Targetscan, compared to non-target mRNAs (Fig. 4f). This is indicated in the cumulative distribution plot by a shift toward negative log2-fold changes in expression of targets (blue line) compared to non-targets (red line) (Fig. 4f). By contrast, mRNAs predicted to be targeted by miR-122, a control hepatic miRNA with known roles in lipid metabolism, showed no shift in log2-fold changes in expression from non-target mRNAs, among genes differentially expressed in shRNA-CHROME and shRNA-control expressing HepG2 cells (Fig. 4f). To validate these findings, we used the bioinformatics tool miRHub to identify miRNAs that may act as regulatory hubs to control the genes differentially expressed in shRNA-CHROME and shRNA-control expressing HepG2 cells. miRHub predicts miRNA target sites in the 3’UTRs of differentially expressed genes and then determines by Monte Carlo simulation, for each miRNA, whether the number and strength of predicted targets is significantly greater than expected by chance. Using this unbiased approach, miR-27b and miR-128 were predicted to act as master regulators of genes significantly downregulated upon CHROME knockdown (Supplementary Fig. 9b), suggesting that these miRNAs’ activities are increased in the absence of CHROME.
To experimentally test whether CHROME could alter cellular levels of its miRNA binding partners (miR-27b, miR-33a, miR-33b, miR-128) and their target mRNAs, we performed knockdown and overexpression experiments in hepatocytes. In primary human hepatocytes and HepG2 cells in which CHROME was depleted, using GapmeRs (Fig. 5a) or shRNA (Supplementary Fig. 9c), respectively, levels of miR-27b, miR-33a, miR-33b and miR-128 were higher than in cells treated with control GapmerRs or shRNAs. By contrast, no difference in levels of the control miRNA, miR-16, was observed in cells treated with control and CHROME-targeting GapmeRs or shRNAs (Fig. 5a, Supplementary Fig. 9c). Consistent with the increased levels of miR-27b, miR-33a, miR-33b and miR-128, we observed reduced levels of their common mRNA targets upon CHROME depletion by GapmeRs in primary human hepatocytes (Fig. 5b) or shRNA in HepG2 cells (Supplementary Fig. 9d), compared to control treatments. By contrast, we observed no difference in the mRNA levels of the control gene ACTB (Fig. 5b), or PRKRA, the gene adjacent to CHROME (Supplementary Data Fig. 9e). Conversely, overexpression of CHROME-1, CHROME-3, or CHROME-7 by retroviral transduction of HepG2 cells reduced levels of miR-27b, miR-33a, miR-33b and miR-128 (Fig. 5c), and increased mRNA levels of their common target genes (Fig. 5d), compared to control vector-expressing HepG2 cells. Consistent with the shared ability of CHROME-targeted miRNAs to repress cholesterol efflux, we observed an increase in key genes in this pathway, including ABCA1 and OSBPL6 (Fig. 5d), and 2-fold higher levels of cholesterol efflux to apoA-1 (Fig. 5e),> when CHROME was overexpressed in HepG2 cells compared to control vector. No change in expression of PRKRA, the gene adjacent to CHROME, was observed when CHROME was overexpressed (Supplementary Fig. 9f).
Figure 5. Gain or loss of CHROME in hepatocytes alters levels of its interacting miRNAs and their common target mRNAs involved in cholesterol metabolism.
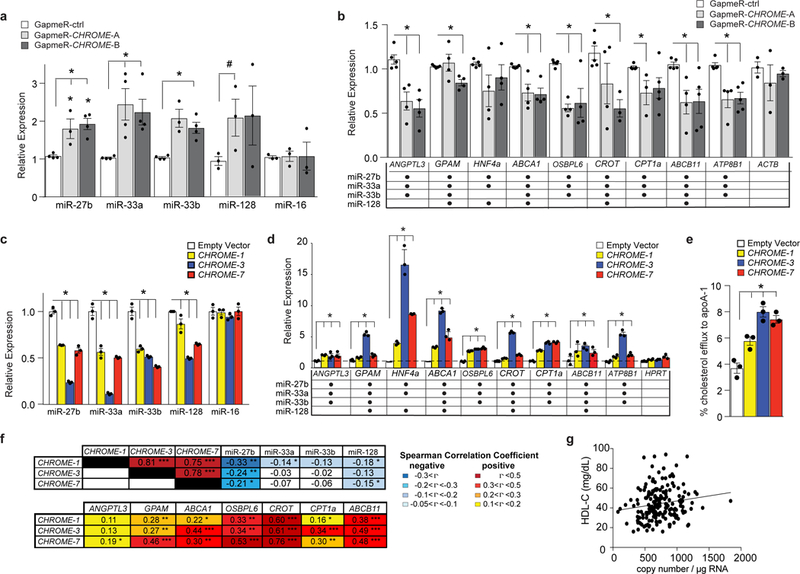
a-d, qPCR analysis of levels of (a, c) miRNAs and (b, d) their common target mRNAs in (a-b) primary human hepatocytes transfected with control (GapmeR-ctrl) or CHROME-targeting GapmeRs (GapmeR-CHROME-A or GapmeR-CHROME-B) or (c-d) HepG2 cells stably expressing CHROME-1, CHROME-3, CHROME-7 or empty vector. Table in (b) and (d) indicates validated targets of miR-27b, miR-33a, miR-33b and miR-128. e, Measurement of cholesterol efflux to exogenous apoA-1 in THP-1 macrophages stably expressing empty vector, CHROME-1, CHROME-3 or CHROME-7. f, Spearman rank correlation coefficients (rho) of CHROME RNA copy number with its interacting miRNAs and their target mRNAs in human liver (n=200). g, Scatter plot showing relationship between hepatic CHROME-7 copy number and plasma levels of HDL cholesterol (n=200). Spearman correlation coefficients between CHROME-7 copy number and plasma levels of HDL cholesterol (n=200, rho=0.1722, P=0.0019) were calculated using the SAS statistic software. Data in (a-e) are the mean ± SEM of 3 independent experiments and P-values were calculated using a two-tailed student’s t-test. #P≤0.1, *P≤0.05, **P≤0.001, ***P≤0.000001.
To further investigate the relationship of CHROME with its miRNA binding partners and their target genes in humans, we obtained healthy hepatic tissue from 200 patients undergoing liver surgery and measured RNA levels by qPCR. Consistent with our in vitro findings, we found that levels of CHROME in the liver were inversely correlated with levels of miR-27b, miR-33a, miR-33b and miR-128, and positively correlated with levels of their target mRNAs (Fig. 5f). Furthermore, consistent with a role for CHROME in regulating hepatic cholesterol efflux to apoA-1, we found that hepatic expression of CHROME-7 was positively and significantly correlated with plasma levels of HDL-C (Fig. 5g) and apoA-1 (Supplementary Fig. 9g).
CHROME regulates macrophage cholesterol efflux through non-coding RNA crosstalk
In addition to regulating hepatic HDL production, the efflux of excess cellular cholesterol is essential for maintaining homeostasis in peripheral tissues, particularly the artery wall, where macrophage cholesterol efflux protects from atherosclerotic plaque formation2. Given that CHROME was increased in cholesterol-loaded macrophages in vitro and in atherosclerotic plaques, we next tested the effects of CHROME loss-of-function in THP-1 macrophages. As we observed in hepatic cells, CHROME-GapmeR treated THP-1 macrophages showed lower ABCA1 mRNA and protein levels compared to control GapmeR treated THP-1 macrophages (Fig. 6a, b, Supplementary Fig 10a), and this was associated with lower levels of cholesterol efflux to apoA-1 (Fig. 6c). Furthermore, GapmeR-depletion of CHROME in THP-1 macrophages increased levels of miR-27b, miR-33a, miR-33b, and miR-128, while decreasing levels of their target mRNAs (Supplementary Fig. 10b-d) relative to control GapmeR treatment, suggesting that CHROME acts via a similar mechanism in macrophages and hepatocytes to control cholesterol efflux. To directly test whether CHROME’s interactions with miRNAs mediate its effects on ABCA1 expression, we assessed the ability of CHROME-7WT and CHROME-7MUT, in which the miRNA binding sites are mutated, to relieve miRNA-mediated repression of ABCA1. To do this, HEK293T cells were co-transfected with a human ABCA1-3’ UTR luciferase reporter construct and either empty vector, CHROME-7WT or CHROME-7MUT, and then subsequently treated with miR-27b, miR-33a or control mimic. In control empty vector expressing cells, overexpression of miR-27b or miR-33a reduced ABCA1-3’UTR luciferase activity compared to control mimic, as expected (Fig. 6d). By contrast, miR-27b and miR-33a failed to repress ABCA1-3’UTR luciferase activity in CHROME-7WT expressing cells (Fig. 6d). This protection required the miRNA response elements in CHROME, as miR-27b and miR-33a reduced ABCA1-3’UTR luciferase activity in CHROME-7MUT-expressing cells to levels comparable to those observed in EV-transfected cells (Fig. 6d). Moreover, similar results were obtained using a 3’UTR-luciferase reporter construct for OSBPL6 (Fig. 6e), which is another shared target of miR-27b and miR-33a/b that regulates cholesterol efflux. We then generated THP-1 cell lines expressing comparable levels of CHROME-7WT or CHROME-7MUT, or empty vector by retroviral transduction (Supplementary Fig. 10e). Consistent with the ability of CHROME to protect the ABCA1- and OSBPL6-3’UTRs from miRNA-mediated repression, THP-1-CHROME-7WT macrophages showed higher levels of ABCA1, OSBPL6 and NPC1 mRNA (Fig. 6f), greater cholesterol efflux to apoA-1 (Fig. 6g) and reduced accumulation of cytoplasmic lipid droplets (Fig. 6h), as compared to THP-1EV macrophages. These differences were associated with lower levels of miR-27b, miR-33a, miR-33b and miR-128 in THP-1-CHROME-7WT than THP-1EV macrophages (Fig. 6i), consistent with the ability of CHROME to functionally deplete these miRNAs. Notably, no differences in miR-27b, miR-33a, miR-33b or miR-128 levels, or ABCA1, OSBPL6, and NPC1 expression, or cholesterol efflux and lipid droplet burden were observed between THP-1-CHROME-7MUT and THP-1EV macrophages (Fig. 6f-i). Collectively, these data suggest that CHROME’s capacity to regulate cellular cholesterol efflux and lipid droplet burden depend on interactions at its miRNA response elements.
Figure 6. CHROME regulates cholesterol efflux in macrophages via its interaction with miRNAs.
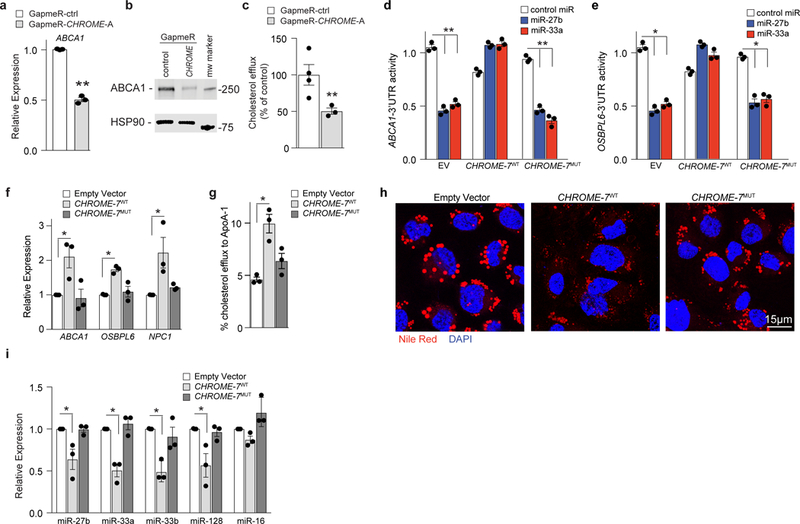
a) qPCR analysis of ABCA1 mRNA in THP-1 macrophages transfected with control (ctrl) or CHROME-targeting GapmeRs. Data are the mean ± SEM of 3 independent experiments. (b) Western blot of ABCA1 and HSP90 (internal control) protein in THP-1 macrophages transfected with control (ctrl) or CHROME-targeting GapmeRs. Molecular weight marker is shown at right. Data are representative of 3 independent experiments. c, Measurement of cholesterol efflux to exogenous apoA-1 in THP-1 macrophages transfected control (ctrl) or CHROME-targeting GapmeRs. Data are the mean ± SEM of 4 independent experiments. d-e, Activity of (d) ABCA1-3’UTR and (e) OSBPL6-3’UTR luciferase reporter genes in HEK293T cells expressing empty vector (EV), wild type CHROME-7 (CHROME-7WT), or CHROME-7 with its miR-33, miR-27b and miR-128 binding sites mutated (CHROME-7MUT) following treatment with control miR, miR-27b or miR-33 mimics. Data are the mean ± SEM of 3 biological replicates from a single experiment. Data are representative of 3 independent experiments. f, qPCR analysis of miR-27b, miR-33 and miR-128 target genes regulating cholesterol efflux in THP-1 macrophages stably expressing empty vector, CHROME-7WT or CHROME-7MUT. Data are the mean ± SEM of 3 experiments. g, Measurement of cholesterol efflux to apoA-1 in THP-1 macrophages stably expressing empty vector, CHROME-7WT or CHROME-7MUT. Data are the mean ± SEM of 3 experiments. h, Representative images of Nile Red stained lipid droplet accumulation in acetylated LDL treated THP-1 macrophages stably expressing empty vector, CHROME-7WT or CHROME-7MUT. Cells were stained with DAPI (blue) to visualize nuclear DNA. i, qPCR analysis of miRNA levels in THP-1 cells stably expressing empty vector, CHROME-7WT or CHROME-7MUT. miR-16 is included as a negative control. Data are the mean ± SEM of 3 experiments. P-values were calculated using a two-tailed student’s t-test. *P≤0.05, **P≤0.01, ***P≤0.001.
Discussion
Our work identifies a novel primate-specific lncRNA, CHROME, that is increased in the setting of human atherosclerotic vascular disease and contributes to the regulation of cholesterol homeostasis. We find that CHROME levels are elevated in the plasma and arterial plaques of individuals with cardiovascular disease compared to healthy controls, and its expression is regulated in response to dietary and cellular cholesterol challenge. Mechanistic studies reveal a role for CHROME in the response of hepatocytes and macrophages to cholesterol overload, through the upregulation of cholesterol efflux and HDL biogenesis. CHROME’s expression is upregulated in vitro and in vivo by activation of the sterol-activated transcription factor LXR, which controls the expression of a cadre of genes involved in the response to cholesterol excess. Using gain- and loss-of-function studies, we establish that CHROME curbs the actions of the functionally related miRNAs, miR-27b, miR-33a, miR-33b and miR-128, to derepress their collective target genes, most notably those involved in cholesterol efflux and reverse cholesterol transport. This LXR-regulated mechanism enhances the cell’s ability to respond to the changing environment by removing existing post-transcriptional “brakes” on cholesterol efflux, coincident with LXR-induced transcription of genes involved in the response to cholesterol excess. Together, our findings uncover an intricate interplay among non-coding RNA species in the fine tuning of cholesterol metabolism gene networks, and expand our understanding of the regulatory mechanisms that govern human cholesterol homeostasis in health and disease.
Many human lncRNAs exhibit low evolutionary conservation40, as is the case for CHROME. Phylogenetic analyses suggest that CHROME emerged in the primate lineage (Supplementary Fig. 1b), and we did not detect shared synteny in other species (Supplementary Fig. 1c). Our studies in non-human primates show that CHROME’s expression is upregulated in the liver upon feeding a cholesterol-enriched diet or short-term treatment with an LXR agonist. Using primary human hepatocytes, we identify an important role for CHROME in the efflux of cholesterol and phospholipid to apoA1, processes that are critical to the production of nascent HDL particles. In a related process, we show that CHROME also regulates efflux of excess cholesterol from macrophages, which mediate the clearance of cholesterol-rich lipoproteins that can accumulate in tissues, such as the arterial wall. The primate-specificity of CHROME precludes in vivo studies of its effects on HDL metabolism and cholesterol efflux in typical preclinical animal models, such as mice. Nevertheless, loss-of-function studies in primary human hepatocytes reveal CHROME’s role in regulating HDL biogenesis, which support our observational data in a patient cohort showing a positive correlation of CHROME and plasma levels of HDL-C. Further testing of CHROME’s ability to regulate reverse cholesterol transport in vivo will require loss-of-function studies in non-human primates.
Cholesterol levels in the cell, tissue and organism are dynamically controlled by a complex network of feedback mechanisms that maintain cholesterol homeostasis. In the last decade, studies have revealed the critical contributions of miRNAs in the post-transcriptional regulation of genes directing cholesterol homeostasis6, and particularly, the cholesterol efflux pathway41. The ABCA1 mRNA interacts with multiple miRNAs, including miR-2042, miR-27b11,14, miR-33a and miR-33b7,8, miR-12815, miR-14412,13, miR-14815, and which collectively impart post-transcriptional repression of the ABCA1 transporter. Inhibition of those individual miRNAs in vitro using anti-sense oligonucleotides can increase ABCA1 protein and cholesterol efflux up to two-fold 7,8,11–15,42, and delivery of miR-33a/b7,8,43–45, miR-12815, or miR-14412,13 inhibitors to mice or monkeys can raise plasma levels of HDL cholesterol by up to 50%. Thus, CHROME’s ability to simultaneously inhibit miR-27b, miR-33a, miR-33b and miR-128 provides a coordinated mechanism to relieve this potent post-transcriptional repression of the cholesterol efflux pathway. Furthermore, each variant of CHROME contains multiple binding sites for its interacting-miRNAs, which each repress multiple common target genes in lipid metabolism pathways, providing a mechanism for substantial regulatory crosstalk by CHROME. As levels of distinct miRNAs may vary by cell type and condition, CHROME’s ability to restrain the actions of a set of miRNAs with shared biological function allows for greater flexibility in the dynamic adjustment of cellular cholesterol efflux in discrete cell types, such as macrophages, enterocytes, and hepatocytes, which each express ABCA1 and play distinct roles in reverse cholesterol transport and protection from atherosclerosis46,47.
We show that CHROME’s interactions with miR-27b, miR-33a, miR-33b and miR-128 are central to its function in maintaining cholesterol homeostasis, as its ability to regulate the expression of ABCA1, cholesterol efflux and lipid droplet burden depend directly on the integrity of its binding sites for those miRNAs. Although the exact mechanisms by which CHROME titrates the availability of its miRNA binding partners remains unclear, our finding of CHROME in P-bodies opens the possibility that it traffics bound miRNAs to this granule for destabilization or degradation. miRNA-RISC complexes can have half-lives of days or more48, thus CHROME-mediated compartmentalization of miRNAs in P-bodies could accelerate their turnover, or alternatively compromise their function through sequestration. Further studies will be needed to understand the spacio-temporal dynamics of CHROME’s associations with miRNAs, as well as other factors that may influence these interactions. For example, interactions of cytoplasmic lncRNAs and miRNAs are likely to be influenced by subcellular microenvironments that may increase local concentrations of RNAs and RNA binding proteins, as has been observed in neurons49. Furthermore, lncRNAs may interact with RNA binding proteins that may modify lncRNA stability, localization, and chemical (e.g., methylation) or secondary structure50. The identification of CHROME, and other lncRNAs of similar function, provides a gateway to begin to investigate the mechanisms underlying such interactions.
Our findings also reveal the expanding roles for non-coding RNAs in mediating crosstalk between the LXR and SREBP-2 transcription factors, which regulate opposing gene programs in the cholesterol overloaded and depleted states. Recent studies established that activation of LXR inhibits the expression of genes involved in cholesterol biosynthesis, including SREBF2, by upregulating the lncRNA LeXis, which sequesters the transcriptional co-factor RALY away from the promoters of cholesterol biosynthetic genes23. Conversely, we and others have shown that SREBP-2 represses the expression of genes involved in cholesterol efflux and reverse cholesterol transport by upregulating expression of miR-33a7,8, which potently represses ABCA1 and other genes contributing to cholesterol efflux and excretion (e.g., OSBPL69, NPC17,8, ATG551, ABCB1110, ATP8B110). The identification of CHROME as an LXR-regulated lncRNA that represses the actions of miR-33a, expands this paradigm of reciprocal cross-talk between LXR and SREBP-2 mediated by non-coding RNAs. In addition, our RNA-Seq analyses suggest a role for CHROME in the regulation of fatty acid oxidation and the acute phase response in hepatocytes. As miR-33, miR-27b and miR-128 have been shown to repress genes regulating β-oxidation of fatty acids (e.g., CROT, CPT1A), CHROME’s interaction with these miRNAs is likely to underlie its effects on fatty acid oxidation. The mechanisms by which CHROME alters the acute phase response are less clear, and further studies will be needed to investigate CHROME’s role in the inflammatory response in hepatocytes and macrophages. Collectively, our data identify CHROME as a novel component of the multi-layered regulatory circuitry that is activated in response to cellular and systemic cholesterol overload, and unveil the regulatory crosstalk between non-coding RNAs that sustains cholesterol homeostasis in humans.
Methods
Human Studies.
All human studies complied with relevant ethical regulations. To assess the levels of CHROME RNA in individuals with coronary artery disease, we identified patient with established vascular disease in ongoing studies (NCT02106429 and NCT01897103)52. Plasma samples (coronary artery disease (CAD, n=14) or healthy control subjects (n=33)) were collected in accordance with the policies of New York University Langone Medical Center. All studies were approved by the NYULMC ethics committee. RNA was isolated from the plasma as described below. To measure CHROME expression in human healthy arterial and plaque atherosclerotic plaques, tissues and clinical data were obtained from patients undergoing surgery for stable or unstable carotid stenosis at the Department of Vascular Surgery, Karolinska University Hospital, Stockholm, Sweden, in collaboration with the Biobank of Karolinska Endarterectomies (BiKE) at the Centre for Molecular Medicine, Karolinska Institute. Gene expression analyses were performed in material obtained from individuals prospectively enrolled in the BiKE study between 2005 and 2008, total n=127 patient plaques and n=10 normal arteries (undiseased macroscopically atherosclerosis free-arteries, iliac and one aorta) were obtained from organ donors without any current or history of cardiovascular disease. No power calculation or selection of patients based on any other clinical criteria than symptomatic or asymptomatic carotid disease was done for this purpose; instead tissues were arrayed based on sample availability and RNA quality. All samples were collected with informed consent from patients, organ donors or their guardians. The BiKE study is approved by the Ethical Committee of Northern Stockholm with following ethical permits: EPN DNr 95– 276/277; DNr 02–146; DNr 02–147, DNr 2005/83–31; DNR 2009/512–31/2; 2012/619–32). The project is performed under the Swedish biobank regulations and prospective sampling is approved with informed consent procedure (DNr 2009/512–31/2). BiKE is registered at Socialstyrelsen (The National Board of Health and Welfare) and Biobank of Karolinska and approved by the Swedish Data Inspection Agency (approval date/number 2002–09-30 DNr 916–2002). The BiKE study cohort demographics, details of sample collection, processing and analyses have been previously described53. Gene expression profiles were analyzed by Affymetrix HG-U133 plus 2.0 Genechip microarrays. Robust multiarray average (RMA) normalization and correction for batch effect was performed and processed gene expression data was returned in log2-scale. The microarray dataset is available from Gene Expression Omnibus (GSE21545). In situ hybridization of CHROME was performed on formalin-fixed samples of human carotid endarterectomy samples or iliac arteries as control tissues with LNA Detection Probes for In Situ Hybridization® (Exiqon) recognizing a sequence common to all CHROME variants (Supplementary Table 3) or control scramble-ISH custom LNA Detection Probe (Exiqon) at a final concentration 25 nM and labeled with DIG. To measure hepatic expression of CHROME and its miRNA targets, healthy liver tissue and serum samples were obtained from individuals undergoing liver surgery and experimental procedures were performed within the framework of the non-profit foundation HTCR Stiftung, including the informed patient’s consent54. No power calculation or selection of patients based on any other clinical criteria other than undergoing liver surgery was done for this purpose; instead tissues were arrayed based on sample availability and RNA quality. This study was approved by the Ethics Committee of the Bavarian Medical Association (Bayerische Landesärztekammer) and the Ethics Committee of the Ludwig-Maximilians-University Munich. RNA (n=200) was isolated, and reverse transcribed (2 μg for mRNA; 1 μg for miRNA), and analyzed by qPCR as described below. Serum levels of apoA-1 and HDL cholesterol were determined using an immunoturbidimetric assay (Beckman Coulter, Krefeld, Germany) and an enzymatic assay (Beckman Coulter), respectively, and run on an AU5800 automated analyzer (Beckman Coulter).
Animal Studies.
Monkeys were housed in an AAALAC-accredited facility under the direct care of the Wake Forest University Health Sciences (WFUHS) Animal Resources Program. All experiments were approved by the WFUHS Institutional Animal Care and Use Committee (IACUC) and complied with relevant ethical regulations. Monkeys were singly housed in climate-controlled conditions with a 12 h light and dark cycle. Male African green (Chlorocebus aethiops) monkeys (n=5/group; age 4–9 years) maintained on a chow diet (0.002 mg/kcal cholesterol) were fed a high fat, moderate cholesterol (0.15 mg/kcal) diet for 8 weeks, and liver biopsies were obtained prior to and after diet feeding. For treatment with LXR agonist, adult male cynomolgus (Macaca fascicularis) monkeys (mean age 2.7 years) were fasted, anesthetized, and gavaged with either vehicle or 10 mg/kg GW3965 (n=5/group) daily for 2 days. Exactly 6 hr after dosing on day 2, fasted monkeys were euthanized and tissues were collected.
Cell Culture.
HEK293T, HepG2 and THP-1 cell lines were obtained from American Type Tissue Collection, authenticated using standard American Type Tissue Collection methods (morphology check by microscope, growth curve analysis) and tested monthly for mycoplasma contamination. HEK293T were maintained in high-glucose DMEM (Corning) supplemented with 10% fetal bovine serum (FBS, Life Technologies) and 1% penicillin/streptomycin (P/S, Life Technologies). HepG2 were maintained in EMEM (Corning) with 10% non-heat inactivated FBS and 1% P/S. THP-1 cells were maintained in RPMI 1640 (Corning) with 10% FBS and 1% P/S, and were differentiated into macrophages in the presence of 100 nM phorbol-12-myristate acetate (PMA, Sigma) for 48–72 h. Primary human hepatocytes were obtained from Yecuris (Tualatin, OR), seeded on Coating Matrix (Life Technologies, #R011K) and maintained in HBM medium supplemented with HCM bullet kit (Lonza). To assess response to cholesterol loading and LXR activation, cells were stimulated with 37.5 μg/mL acetylated LDL (Biomedical Technologies) for 24 h, 10 μM T0901317 (Cayman Chemical Company) for 8 or 24 h, or 10 μg/mL cholesterol-cyclodextrin (Sigma) for 72 h. For transient knockdown of LXR in THP-1 cells, SMARTpool: ON-Targetplus siRNA against LXRα and LXRβ (Dharmacon, 25 nM each) was used for transfection. Non-targeting siRNA was used as control. For siLXR-studies, 48 h after transfection, cells were treated with 50 μg/mL acetylated LDL (acLDL) for 24 h.
RNA Isolation and qPCR.
Total RNA was isolated using TRIzol reagent (Invitrogen) and Direct-zol RNA MiniPrep columns (Zymo Research). RNA was reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories), and quantitative PCR (qPCR) analysis was conducted using KAPA SYBR green Supermix (KAPA Biosystems) according to the manufacturer’s instructions and quantified on a Mastercycler Realplex (Eppendorf). miRNA quantification was performed in quadruplicate with the miScript SYBR Green PCR Kit (Qiagen) using Qiagen miScript Primer Assays listed in Supplementary Table 3. Fold change in mRNA and miRNA expression was calculated using the comparative cycle method (2−ΔΔCt) normalized to the housekeeping genes (GAPDH for mRNA; U6 or miR-16 for miRNA), or μg of RNA (for tissue profiling). For plasma samples, RNA was isolated using TRIzol reagent (4:1 vol) followed by the RNA Clean & Concentrator-5 Plasma/Serum kit (Zymo Research). 5 μL of total RNA was reverse transcribed and used for qPCR. Copy numbers were determined using standard curves of CHROME variants.
Chromatin Immunoprecipitation Assay.
THP-1 cells differentiated with PMA were treated with 10 μM T0901317 for 8 h, and cells were treated with 1% formaldehyde to cross-link. The reaction was stopped with 0.125 M glycine, followed by washing in ice cold PBS, and the cells were pelleted. Nuclear lysates were collected and passed through a 25-gauge needle and sonicated until chromatin was sheared to 500–2000 bp fragments as previously described. For immunoprecipitation of LXR, 30 μg of chromatin was incubated with a cocktail of 3 μg LXRα and 3 μg LXRβ antibody (Abcam, #ab41902 and #ab56237) or isotype matched IgG (Abcam, #ab171870). Bound chromatin was immunoprecipitated using Dynabeads Protein G (Life Technologies) and bead/chromatin complexes were washed and dissociated at 65°C. DNA was purified using PrepEase DNA Clean-Up Kit (Affymetrix) and 1 μL of DNA was used for quantitative PCR.
RNA Fluorescence In Situ Hybridization
RNA fluorescence in situ hybridization and simultaneous RNA/DNA detection were performed as described55,56. Briefly, cells were fixed in 4% paraformaldehyde (PFA), permeabilized in CSK buffer, 5% triton X-100, and vanadyl ribonucleoside complex (VRC), and subsequently stored in PBS or 70% ethanol. DNA probes (1 μg/reaction) were nick-translated using biotin-11-dUTP or digoxigenin-16-dUTP (Roche Diagnostics). RNA-specific hybridization was carried out under non-denaturing conditions where the DNA was not accessible. For colocalization with AGO2, HEK293T cells were transfected with AGO2-GFP plasmid (Addgene, #21981) for 24 h, and fluorescence in situ hybridization was performed for CHROME as described above, or cells were fixed in 4% PFA blocked/permeabilized in 2.5% BSA/0.1% Triton X-100, and stained for EDC4, cells using a rabbit anti-human EDC4 (Cell Signaling Technology, #2548) followed by fluorescent goat anti-rabbit secondary antibodies (Life Technologies, #A-11011).
Gain- and Loss-of-Function Studies.
For RNA interference studies, short hairpin RNAs (shRNA) targeting a region common to all CHROME variants or a non-targeting control shRNA (Supplementary Table 3) were cloned into the pLKO vector (Addgene, #8453) and transfected into HEK293T cells with packaging vectors psPAX2 (Addgene, #12260) and pMD2.G (Addgene, #12259). After 48 h, the culture media was isolated and used to transduce HepG2 cells. Cells stably expressing the shRNAs were selected using 5 μg/mL puromycin (Thermo Fisher Scientific). For transient knockdown of CHROME, primary human hepatocytes, HepG2 cells or PMA-differentiated THP-1 cells were transfected with 62.5 nM locked nucleic acid (LNA) GapmeRs targeting a common regions of all CHROME variants or Negative Control A (Exiqon) using Lipofectamine RNAiMax (Life Technologies). To create CHROME-overexpressing cell lines, CHROME cDNAs were obtained from Genscript and cloned into pMSCV-PIG vector (Addgene, #21654) and transfected into HEK293T cells with packaging vectors pCMV-VSV-G (Addgene, #8454) and pCMV-Gag-Pol (Cell Biolabs, #RV-111). After 48 h, the culture media was isolated and used to transduce THP-1 or HepG2 cells, and cells were selected using puromycin (5 μg/mL, Thermo Fisher Scientific). To mutate the predicted miR-27b, miR-33a, miR-33b and miR-128 binding sites within CHROME-7, we deleted the seed match sequence for those miRNAs with the Quickchange XL kit (Stratagene) using the primers indicated in Supplementary Table 3. CHROME-7 mutant expressing cell lines were generated as described above. All constructs were confirmed by sequencing.
RNA-sequencing
RNA was isolated in triplicate from HepG2 cells expressing shRNA against all CHROME variants or control shRNA. RNA was used to generate barcoded cDNA libraries using the TruSeq RNA Sample Preparation kit (Illumina). Indexed libraries were pooled and sequenced (paired-end 50 or 100 bp reads) on the Illumina HiSEQ platform. RNA-seq reads were aligned using the STAR Aligner against hg19 annotations. Gene counting was done using featureCounts. Raw counts were normalized and DE-analysis was performed using DESeq2. RNA-Seq data are deposited in the Gene Expression Omnibus under the accession number GSE97469.
Bioinformatics.
The coding Potential of CHROME variants was predicted using the Coding Potential Calculator (CPC, http://cpc.cbi.pku.edu.cn/programs/cpc.do) and the Coding Potential Tool Assessment (CPAT, http://lilab.research.bcm.edu/cpat/calculator_sub.php) algorithms. Kozak strength was determined using the NCBI ORFFinder (http://atgpr.dbcls.jp/). LXRE sequence elements in the regulatory regions of CHROME were defined using JASPAR (http://jaspar.genereg.net/). For calculations of the mean free energy changes associated with the formation of CHROME-miRNA complexes were calculated using RNAcofold (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAcofold.cgi), which predicts secondary structures of single stranded RNA sequences upon dimer formation This algorithm takes into account the length of the transcript and secondary structure. One thousand randomly selected sections of the human genome of length 21–22 nucleotides long were run through RNAcofold, along with annotated miRNA sequences including miR-27b, miR-128, miR-33a, miR-33b and miR-16 and aligned pairwise with CHROME transcripts. For RNA-Seq analyses, Ingenuity Pathway Analysis (Qiagen) was used to evaluate the most significantly altered genes and pathways. Cumulative distribution function analysis was performed using the statistical software package R 2.8.0 (www.r-project.org)57 for predicted targets of miRNA-27b, miR-33a/b, miR-128, and miR-122 versus non-targets (defined using Targetscan; http://www.targetscan.org/vert_72/). Statistical enrichment of predicted miR-33a/b, miR-27b and miR-128 target sites among differentially regulated genes in HepG2 RNA-Seq datasets was performed using miRhub as described58.
Western Blots
Protein was extracted in RIPA buffer (Cell Signaling) with protease and phosphatase inhibitors (Roche) and subsequently normalized by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Samples (30 μg/well) were electrophoresed on 4–20% TGX-gradient gels (Bio-Rad Laboratories) and transferred to nitrocellulose membranes at 125 V for 2 h. Membranes were incubated overnight with specified antibodies directed against ABCA1 (Novus Biologicals, NB400–105) and HSP90 (BD Biosciences, #610419). Proteins were visualized using appropriate secondary antibodies and scanned using the Odyssey Imaging System (Li-Cor Biosciences). Quantification was performed using Image Studio software (Li-Cor Biosciences).
Cholesterol Efflux Assay and Neutral Lipid Staining.
For cholesterol efflux assays, HepG2 or THP-1 cells stably expressing CHROME variants were seeded in 24-well plates at a density of 1 × 106 cells, and THP-1 cells were treated with PMA as described above. Primary hepatocytes were seeded at 2 × 105 in 48-wells plates and transfected with control or CHROME-targeting GapmeRs. Cells were labeled with 0.5 μCi/mL of 3H-cholesterol (PerkinElmer) for 24 h, washed twice with PBS and then incubated with 2 mg/mL fatty-acid free BSA (FAFA, Sigma-Aldrich) in media for 24 h. To induce efflux, 50 μg/mL human apoA-1 in FAFA media was added and supernatants were collected after 8 or 24 h. ApoA-1 dependent efflux was expressed as a percentage of total cell 3H-cholesterol content. To visualize neutral lipids, THP-1 cells stably expressing CHROME variants were treated with PMA as described above and then incubated with 37.5 μg/mL acetylated LDL for 24 h. Cells were then stained using Nile Red (Thermo Fisher Scientific) as previously described59.
Endogenous apoA-1 Lipidation.
To assess phospholipid efflux to endogenous apoA-1 in primary human hepatocytes, cells were seeded at 2 × 105 in 48-wells plates and transfected with GapmeRs as described above. Hepatocytes were labeled with 5 μCi/mL of 14C-choline chloride (PerkinElmer) for 48 h, washed twice and incubated for 24 h in serum-free HCM supplemented with 10 μM T0901317 (Cayman Chemical Company). ApoA-1 secreted into the cell culture supernatant was immunoprecipitated from the culture medium with polyclonal anti-human apoA-1 (EMD Millipore, #178422) and protein G-sepharose (Sigma-Aldrich), washed in PBS, and scintillation counting was performed to assess amount of phospholipid efflux.
Immunoprecipitation of AGO2.
Argonaute-2 (AGO2) was immunoprecipitated from primary human hepatocytes or PMA differentiated THP-1 macrophages using the MagnaRIP RNA-Binding Protein Immunoprecipitation Kit (EMD Millipore) according to the manufacturers’ instructions. Briefly, an antibody targeting human AGO2 (Millipore, RIPAb+ #03–110) or an isotype matched control antibody (EMD Millipore, #CS200621) were bound to magnetic beads and incubated with lysed cells at 4°C for 24 h. Beads were isolated and coprecipitated RNA was purified, cleaved from AGO2 by proteinase K, and purified using TRIzol reagent. PCR analysis of total RNA was performed to detect CHROME, and enrichment of CHROME variants in the AGO2 fraction was determined by correcting for both GAPDH levels and the fraction that coprecipitated with IgG antibodies.
MS2-tagged RNA Affinity Purification.
MS2-tagged RNA affinity purification (MS2-TRAP) was performed as previously described37. Briefly, HEK293T cells were transfected with plasmids expressing CHROME-1, CHROME-3, or CHROME-7 in frame with an MS2 binding sequence, together with a plasmid expressing YFP-tagged MS2 binding protein for 24 h. MS2 binding protein was immunoprecipitation using an antibody against GFP (Roche Diagnostics, #118144600001) or isotype matched IgG control antibody (EMD Millipore, #CS200621), and coprecipitated RNA was isolated using Trizol. Total RNA was analyzed by qPCR for miR-16, miR-27b, miR-33a/b, miR-128, miR-155, miR-224 and U6. Enrichment of miRNAs bound to CHROME was determined by correcting for U6 levels, the fraction that coprecipitated with IgG antibodies and the expression levels of miRNAs for the empty control.
Cholesterol Biosynthesis and LDL Uptake.
HepG2 cells were seeded and incubated with 5 μCi/mL of 3H-acetate (Amersham) in EMEM supplemented with 10% LPDS. After 24 h, cells were lysed with 0.25N NaOH and lipid extraction was performed as described60. The organic layer was dried under N2 gas and samples were resuspended in hexane. Lipids were separated by thin layer chromatography in the solvent system hexane:diethyl ether:acetic acid (105:45:1). The bands corresponding to cholesterol fractions were collected and radioactivity was determined by liquid scintillation counting. Data were normalized to cellular protein levels obtained by Pierce BCA Protein Assay Kit. To measure LDL uptake, HepG2 cells were seeded in poly-D-lysine coated Labtek slides and incubated for 4 h with Dil-LDL (10 μg/mL, AlfaAesar) in EMEM supplemented with 2 mg/mL BSA. Intracellular LDL was detected by microscopy (Leica SP5) and fluorescence intensity was quantified using Fiji software (http://www.fiji.com) from 6 fields-of-view.
microRNA Degradation.
HEK293T cells were co-transfected with wild-type or mutant CHROME-7 plasmid and miRNA mimics at various concentration (25 nm, 50 nm, 100 nm) with Lipofectamine 2000 (Invitrogen) overnight. miRNA expression was quantified by qPCR. Control non-targeting mimic and empty vector (EV) were used for normalization.
3’UTR Luciferase Experiments.
Human ABCA1- and human OSBPL6-3’UTR luciferase/renilla reporter constructs were obtained from GeneCopoeia. HEK293T cells were cotransfected with 1 μg of 3’UTR luciferase reporter vector and wild-type or mutant CHROME plasmid. Cells were then treated with 50 nM miRNA mimic or control mimic using Lipofectamine 2000 (Invitrogen) in a 96-well plate. 3’UTR activity was assessed by measuring luciferase using the Dual Glo Luciferase Assay System (Promega). Firefly luciferase activity was normalized to renilla luciferase activity.
Statistics.
The statistical significance of differences of 3 or more independent biological replicates was evaluated with the Student’s t test or two-way analysis of variance (ANOVA) for multiple group comparisons. The Tukey and Dunnett tests were used as follow-up tests to the ANOVAs, where the Tukey test was used to compare every mean with every other mean, and the Dunnett test was used when comparing every mean to a control mean. For human liver gene expression studies, data were quantile normalized using the statistical software package R 2.8.057. Normality of distribution was tested using the Kolmogorov-Smirnov test implemented in the PRISM statistical software (GraphPad). No significant effects of age and sex on gene expression levels were detected. Spearman correlation coefficients between gene expression data and serum apoA-1 and HDL cholesterol levels and levels of significance were calculated using the SAS statistic software (SAS Version 9.3, SAS Institute, Inc.).
Supplementary Material
Acknowledgements:
This work was supported by grants from the NIH [R01HL119047 (KJM), R35HL135799 (KJM), R01HL117226 (MJG), T32HL098129 (EJH, CvS), R01HL114978 (JSB), R00HL088528 (RET), R01HL111932 (RET), R01HL128996 (KCV), P01HL116263 (KCV), R01DK105965 (PS)], American Heart Association [14POST20180018 (CvS), 13CRP14410042 (JSB)], FINOVI (EPR), the Swedish Society for Medical Research (LPM) and the Heart and Lung Foundation (LPM), the German Research Foundation CRC 1123 Project B1 (DT, LMH), German Biobank Alliance BMBF 01EY1711C (German Ministry of Education and Research to DT and LMH) and Leducq Foundation CAD genomics (DT, LMH). The BiKE study was supported by the Swedish Heart and Lung Foundation, the Swedish Research Council (K2009–65X-2233–01-3, K2013–65X-06816–30-4, 349–2007-8703), Uppdrag Besegra Stroke (P581/2011–123), the Strategic Cardiovascular Programs of Karolinska Institutet and Stockholm County Council, the Foundation for Strategic Research and the European Commission (CarTarDis, AtheroRemo, VIA, AtheroFlux projects). We would like to thank E.A. Fisher (New York University) for helpful discussions, S. Zhao and Q. Sheng (Vanderbilt University) for their efforts in Seq-data analysis.
Footnotes
Data Availabilty
The Biobank of Karolinska Endarterectomies (BiKE) microarray dataset is deposited in the Gene Expression Omnibus (GEO) and available under accession GSE21545. HepG2 RNA-sequencing datasets are deposited in GEO under accession GSE97469. Data that supports the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Competing Interests: KJM and New York University hold a patent (US 9241950, Status: issued 1/26/2016) on the use of miR-33 inhibitors to treat inflammation. All other authors have no competing interests.
REFERENCES
- 1.Goedeke L & Fernandez-Hernando C Regulation of cholesterol homeostasis. Cell Mol Life Sci 69, 915–930 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqi HK, Kiss D & Rader D HDL-cholesterol and cardiovascular disease: rethinking our approach. Curr Opin Cardiol 30, 536–542 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Lee SD & Tontonoz P Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis 242, 29–36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scacalossi KR, van Solingen C & Moore KJ Long non-coding RNAs regulating macrophage functions in homeostasis and disease. Vascul Pharmacol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Solingen C, Scacalossi KR & Moore KJ Long noncoding RNAs in lipid metabolism. Curr Opin Lipidol 29, 224–232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinberg MW & Moore KJ MicroRNA Regulation of Atherosclerosis. Circ Res 118, 703–720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najafi-Shoushtari SH, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328, 1566–1569 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayner KJ, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328, 1570–1573 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouimet M, et al. miRNA Targeting of Oxysterol-Binding Protein-Like 6 Regulates Cholesterol Trafficking and Efflux. Arterioscler Thromb Vasc Biol 36, 942–951 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen RM, et al. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med 4, 882–895 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goedeke L, et al. miR-27b inhibits LDLR and ABCA1 expression but does not influence plasma and hepatic lipid levels in mice. Atherosclerosis 243, 499–509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Aguiar Vallim TQ, et al. MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor farnesoid X receptor. Circ Res 112, 1602–1612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez CM, et al. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ Res 112, 1592–1601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu XH, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331–3p in gastric cancer. Mol Cancer 13, 92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagschal A, et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat Med 21, 1290–1297 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn JJ & Chang HY Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17, 47–62 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Palazzo AF & Lee ES Non-coding RNA: what is functional and what is junk? Front Genet 6, 2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz SU, Grote P & Herrmann BG Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci 73, 2491–2509 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman JE, Miano JM, National Heart L & Blood Institute Workshop P Challenges and Opportunities in Linking Long Noncoding RNAs to Cardiovascular, Lung, and Blood Diseases. Arterioscler Thromb Vasc Biol 37, 21–25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan C, Chen J & Chen N Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep 6, 22640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, et al. Long noncoding RNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis. Hepatology 67, 1768–1783 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, et al. Identification of a novel human long non-coding RNA that regulates hepatic lipid metabolism by inhibiting SREBP-1c. Int J Biol Sci 13, 349–357 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallam T, et al. Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding RNA LeXis. Nature 534, 124–128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallam T, et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat Med 24, 304–312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasmant E, Sabbagh A, Vidaud M & Bieche I ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J 25, 444–448 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Holdt LM & Teupser D Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations. Arterioscler Thromb Vasc Biol 32, 196–206 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Nsengimana J, et al. Enhanced linkage of a locus on chromosome 2 to premature coronary artery disease in the absence of hypercholesterolemia. European journal of human genetics : EJHG 15, 313–319 (2007). [DOI] [PubMed] [Google Scholar]
- 28.North KE, Martin LJ, Dyer T, Comuzzie AG & Williams JT HDL cholesterol in females in the Framingham Heart Study is linked to a region of chromosome 2q. BMC genetics 4 Suppl 1, S98 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapusta A, et al. Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet 9, e1003470 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perisic L, et al. Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis. Arterioscler Thromb Vasc Biol 33, 2432–2443 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Moore KJ & Tabas I Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabili MN, et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol 16, 20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennox KA & Behlke MA Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res 44, 863–877 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.John S, et al. Kinetic complexity of the global response to glucocorticoid receptor action. Endocrinology 150, 1766–1774 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davalos A, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proceedings of the National Academy of Sciences of the United States of America 108, 9232–9237 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vickers KC, et al. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology 57, 533–542 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon JH, Srikantan S & Gorospe M MS2-TRAP (MS2-tagged RNA affinity purification): tagging RNA to identify associated miRNAs. Methods 58, 81–87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Valencia-Sanchez MA, Hannon GJ & Parker R MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 7, 719–723 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubstenberger A, et al. P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol Cell 68, 144–157 e145 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Ulitsky I Evolution to the rescue: using comparative genomics to understand long non-coding RNAs. Nat Rev Genet 17, 601–614 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Rayner KJ & Moore KJ MicroRNA control of high-density lipoprotein metabolism and function. Circ Res 114, 183–192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang B, et al. MicroRNA-20a/b regulates cholesterol efflux through post-transcriptional repression of ATP-binding cassette transporter A1. Biochim Biophys Acta 1862, 929–938 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Marquart TJ, Allen RM, Ory DS & Baldan A miR-33 links SREBP-2 induction to repression of sterol transporters. Proceedings of the National Academy of Sciences of the United States of America 107, 12228–12232 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rayner KJ, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 478, 404–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayner KJ, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest 121, 2921–2931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abumrad NA & Davidson NO Role of the gut in lipid homeostasis. Physiol Rev 92, 1061–1085 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westerterp M, et al. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res 114, 157–170 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Ameres SL, et al. Target RNA-directed trimming and tailing of small silencing RNAs. Science 328, 1534–1539 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cajigas IJ, et al. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 74, 453–466 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jens M & Rajewsky N Competition between target sites of regulators shapes post-transcriptional gene regulation. Nat Rev Genet 16, 113–126 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Ouimet M, et al. microRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. Arterioscler Thromb Vasc Biol 37, 1058–1067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
METHODS ONLY REFERENCES
- 52.Montenont E, et al. Platelet WDR1 suppresses platelet activity and associates with cardiovascular disease. Blood (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perisic L, et al. Gene expression signatures, pathways and networks in carotid atherosclerosis. J Intern Med 279, 293–308 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Thasler WE, et al. Charitable State-Controlled Foundation Human Tissue and Cell Research: Ethic and Legal Aspects in the Supply of Surgically Removed Human Tissue For Research in the Academic and Commercial Sector in Germany. Cell and tissue banking 4, 49–56 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Johnson CV, Singer RH & Lawrence JB Fluorescent detection of nuclear RNA and DNA: implications for genome organization. Methods in cell biology 35, 73–99 (1991). [PubMed] [Google Scholar]
- 56.Tam R, Smith KP & Lawrence JB The 4q subtelomere harboring the FSHD locus is specifically anchored with peripheral heterochromatin unlike most human telomeres. The Journal of cell biology 167, 269–279 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ihaka R.a.G.R A Language for Data Analysis and Graphics. Journal of Computational and Graphical Statistics 5, 299–314 (1996). [Google Scholar]
- 58.Baran-Gale J, Fannin EE, Kurtz CL & Sethupathy P Beta cell 5’-shifted isomiRs are candidate regulatory hubs in type 2 diabetes. PLoS One 8, e73240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Listenberger LL & Brown DA Fluorescent detection of lipid droplets and associated proteins. Curr Protoc Cell Biol Chapter 24, Unit 24 22 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Folch J, Lees M & Sloane Stanley GH A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226, 497–509 (1957). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


