Abstract
Objective
In MOBILITY (NCT01061736), sarilumab significantly reduced disease activity, improved physical function and inhibited radiographic progression at week 52 versus placebo in patients with rheumatoid arthritis (RA) and an inadequate response to methotrexate. We report 5-year safety, efficacy and radiographic outcomes of sarilumab from NCT01061736 and the open-label extension (EXTEND; NCT01146652), in which patients received sarilumab 200 mg every 2 weeks (q2w) + methotrexate.
Methods
Patients (n=1197) with moderately to severely active RA were initially randomised to placebo, sarilumab 150 mg or sarilumab 200 mg subcutaneously q2w plus weekly methotrexate for 52 weeks. Completers were eligible to enrol in the open-label extension and receive sarilumab 200 mg q2w + methotrexate.
Results
Overall, 901 patients entered the open-label extension. The safety profile remained stable over 5-year follow-up and consistent with interleukin-6 receptor blockade. Absolute neutrophil count <1000 cells/mm3 was observed but not associated with increased infection rate. Initial treatment with sarilumab 200 mg + methotrexate was associated with reduced radiographic progression over 5 years versus sarilumab 150 mg + methotrexate or placebo + methotrexate (mean±SE change from baseline in van der Heijde-modified Total Sharp Score: 1.46±0.27, 2.35±0.28 and 3.68±0.27, respectively (p<0.001 for each sarilumab dose versus placebo)). Clinical efficacy was sustained through 5 years according to Disease Activity Score (28-joint count) using C reactive protein, Clinical Disease Activity Index (CDAI) and Health Assessment Questionnaire-Disability Index. The number of patients achieving CDAI ≤2.8 at 5 years was similar among initial randomisation groups (placebo, 76/398 (19%); sarilumab 150 mg, 68/400 (17%); sarilumab 200 mg, 84/399 (21%)).
Conclusion
Clinical efficacy, including inhibition of radiographic progression, reduction in disease activity and improvement in physical function, was sustained with sarilumab + methotrexate over 5 years. Safety appeared stable over the 5-year period.
Keywords: rheumatoid arthritis, DMARDs (biological), treatment
Video abstract.
Key messages.
What is already known about this subject?
In the phase III MOBILITY trial (part B cohort 2; NCT01061736), sarilumab (150 and 200 mg every 2 weeks plus weekly methotrexate (MTX)) was shown to significantly reduce disease activity, improve physical function and inhibit radiographic progression at week 52 versus placebo (plus MTX) in adult patients with rheumatoid arthritis (RA) and an inadequate response to MTX.
What does this study add?
In this open-label extension study, the safety profile of sarilumab remained stable over 5-year follow-up and was consistent with interleukin 6 blockade, with most common adverse events being injection-site erythema, neutropenia, and upper respiratory tract infection.
The clinical efficacy of sarilumab, including inhibition of radiographic progression, reduction in disease activity and improvement in physical function (according to DAS28-CRP, Clinical Disease Activity Index and Health Assessment Questionnaire-Disability Index), was sustained over 5 years. While initial treatment with either dose of sarilumab was associated with significantly better radiographic outcomes versus placebo over 5 years of follow-up, the best outcomes were observed in patients initially randomised to sarilumab 200 mg in the double-blind study.
How might this impact on clinical practice or future developments?
These results support the long-term use of sarilumab for adult patients with RA and an inadequate response to MTX.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease characterised by persistent joint inflammation, which leads to bone and cartilage destruction, deformation and disability.1 In addition to progressive joint damage, RA is associated with a range of extra-articular manifestations, including cardiovascular disease, fatigue, pain and depression.2 The articular and systemic manifestations of RA are mediated, in part, by cytokines such as interleukin 6 (IL-6).3 4 IL-6 is a pleiotropic cytokine that plays a role in homeostasis, metabolism and regenerative processes.5 IL-6 levels increase locally in response to infection or injury, promoting proinflammatory activities. In autoimmune conditions, such as RA, persistently elevated IL-6 levels can contribute to chronic inflammation and disease progression.6
Sarilumab is a human monoclonal antibody that binds membrane-bound and soluble IL-6 receptor-α to inhibit IL-6-mediated cis- and trans-signalling, and is approved for the treatment of adults with moderately to severely active RA.7 The safety and efficacy of sarilumab administered subcutaneously as monotherapy and in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) have been demonstrated in active-comparator and placebo-controlled phase III trials in adults with RA.8–10
The phase III randomised controlled trial MOBILITY (part B cohort 2; NCT01061736) demonstrated superiority of subcutaneous sarilumab administered at doses of 200 mg and 150 mg every 2 weeks (q2w) plus methotrexate (MTX) compared with placebo plus MTX in reducing the signs and symptoms of RA, improving physical function and inhibiting progression of radiographic structural damage in patients with RA and an inadequate response to methotrexate (MTX-IR).8
Although the benefit–risk profile of sarilumab has been demonstrated in the short to medium term, the use of therapies in chronic disease necessitates long-term evaluation. Consequently, there is a need to evaluate safety and efficacy associated with long-term sarilumab use.11 This analysis assessed the safety and efficacy of sarilumab over 5 years of treatment in patients who completed the double-blind study and entered the open-label extension (EXTEND; NCT01146652).
Methods
The multicentre, randomised, double-blind, placebo-controlled study has previously been described.8 The study was an operationally seamless phase II/III study. Part A was the phase II dose-ranging portion, and part B was the phase III portion of the study. Two cohorts of patients were enrolled in part B: those who were randomised before dose selection (cohort 1) and those who were randomised after dose selection (cohort 2). In part B cohort 2, MTX-IR adult patients with moderately to severely active RA were randomised (1:1:1) to placebo, sarilumab 150 mg or sarilumab 200 mg subcutaneously q2w in combination with weekly MTX for 52 weeks. After 16 weeks, patients with <20% improvement from baseline in either swollen joint count or tender joint count at two consecutive visits were offered rescue therapy with open-label sarilumab 200 mg q2w for the remainder of the study. Patients who completed the study were eligible for enrolment in the open-label extension in which they received sarilumab 200 mg q2w plus MTX. All patients continued to receive MTX background therapy, which could be reduced, discontinued or switched to an alternative approved non-biological DMARD for safety or tolerability reasons. Reasons for MTX discontinuation were not recorded. Patients were excluded from the open-label extension if they had adverse events (AEs) or other abnormalities that would adversely affect participation in the study, determined according to investigator judgement or protocol. The studies were conducted in accordance with Good Clinical Practice and with the principles stipulated in the Declaration of Helsinki; all protocols and patient information materials were approved by appropriate ethical review boards and all patients provided written informed consent.
Dose reduction to sarilumab 150 mg q2w was permitted for patients who recovered following a laboratory event, which included absolute neutrophil count (ANC) ≥500–<1000 cells/mm3 (once ANC had returned to ≥1000 cells/mm3), platelet count ≥50–<100×109 cells/L (once platelet count had returned to ≥100×109 cells/L), alanine aminotransferase (ALT) 3–5× the upper limit of normal (ULN; once ALT had returned to <3 ULN) or per investigator judgement. Sarilumab was to be permanently discontinued in cases of significant laboratory abnormalities (ALT >5× ULN or ALT >3× ULN with concomitant total bilirubin >2× ULN; neutrophil count <500 cells/mm3 or neutrophil count <1000 cells/mm3 with evidence of infection; platelet count <50×109 cells/L or platelet count <100×109 cells/L with evidence of bleeding), opportunistic infection, active tuberculosis, positive culture for non-tuberculosis mycobacteria, hypersensitivity or anaphylactic reactions, severe neurological disease, HIV positive status, acute renal failure, pregnancy, use of other biological agents or any AEs deemed by the investigator to jeopardise patient safety.
The last visit of the double-blind study was the first visit in the open-label extension. The primary objective of the open-label extension was to evaluate the long-term safety of sarilumab in patients with RA. The secondary objective was to assess the long-term efficacy of sarilumab. Safety and efficacy assessments and laboratory tests were conducted every 4 weeks up to week 12, every 12 weeks up to week 96 and then at 24-week intervals. Additional laboratory testing, including haematology and liver function tests, were performed at week 2, week 6 and week 10. AEs were collected throughout the study.
The safety population comprised all patients who received at least one dose of sarilumab. Safety assessments included incidences of treatment-emergent AEs, serious treatment-emergent AEs, adverse events of special interest (AESIs) and changes in specific laboratory parameters. Serious infections were defined as infections requiring hospitalisation and/or intravenous antibiotics. Major adverse cardiovascular events (MACE) were reviewed by an independent cardiovascular adjudication committee and suspected gastrointestinal perforations were confirmed by medical review. Leucopenia was included as an AESI to capture investigator-reported AEs of neutropenia. Thromboembolic events were not prespecified as an AESI in the study protocols but are reported here post hoc based on the Medical Dictionary for Regulatory Activities high-level group term ‘Embolism and thrombosis’. Incidence rates by 6-month interval were analysed for serious adverse events (SAEs), serious infections, malignancies, MACE, injection-site reactions, ANC <1000 cells/mm3, ALT >3× ULN and platelet count <100×109 cells/L. The exact method was used to calculate 95% CIs for proportions. Incidences of infection and serious infection were calculated by maximum neutropenia grade recorded at any time during the study. For infections that occurred within 12 weeks after an ANC assessment, incidence of infection or serious infection was calculated by the last ANC assessment before the onset of the infection.
Clinical efficacy assessments included Disease Activity Score (28 joints) using C reactive protein (DAS28-CRP) and proportion of patients achieving DAS28-CRP <3.2 and <2.6; Clinical Disease Activity Index (CDAI) and proportion of patients achieving CDAI ≤10 (CDAI low disease activity) and ≤2.8 (CDAI remission); and Health Assessment Questionnaire-Disability Index (HAQ-DI) and proportion of HAQ-DI responders (change from baseline of ≥0.22). Efficacy results are presented as observed, including patients who received rescue medication with sarilumab 200 mg q2w but excluding patients who discontinued study medication, without imputation for missing data. Responder rates are presented as a percentage of the intention-to-treat (ITT) population and as a percentage of the number of patients assessed (observed case approach). There was no confirmatory analysis for the efficacy variables. The baseline value for efficacy parameters was the original baseline from the double-blind study.
Radiographic progression was assessed by change from baseline in van der Heijde-modified Total Sharp Score (mTSS) recorded in four reading campaigns (one campaign in the double-blind phase and three campaigns in the open-label extension). In a reading campaign, independent readers were presented with a predefined set of images comprising radiographs of hands and feet from specific timepoints during the study. Readers were blinded to the chronological order, patient identity and treatment group. A post hoc integrated analysis was conducted to analyse radiographic progression based on all available campaign data. Repeated measurements by visit were analysed using a linear mixed-effects model with baseline mTSS, region and prior biological use as covariates and visit, treatment and the interaction of visit and treatment in the model and nested subjects in each campaign (campaign (reader) was a random effect as conducted previously12). Compound symmetry was chosen as a variance correlation structure. The change from baseline in mTSS at each timepoint was estimated by least-squares means. The percentage of patients with no mTSS progression at the end of the open-label extension (change from baseline ≤0 and ≤0.5) was analysed in the last reading campaign. For this analysis, data collected after treatment discontinuation or starting rescue medication were used as observed. The linear extrapolation method was used to impute missing mTSS. Patients with still missing mTSS after the imputation step were considered as progressors. All analyses were performed using SAS version 9.2 or above (SAS Institute, Cary, North Carolina, USA).
Results
Patient characteristics and disposition
Of the 1197 adult patients who entered the double-blind trial (placebo, n=398; sarilumab 150 mg, n=400; sarilumab 200 mg, n=399), more patients in the placebo group (n=156) than in the sarilumab 150 mg group (n=55) or sarilumab 200 mg group (n=46) received rescue therapy. A total of 901 patients completed the double-blind trial and enrolled in the open-label extension: 307 from the placebo group, 300 from the sarilumab 150 mg group and 294 from the sarilumab 200 mg group. Two patients were not treated; therefore, 899 received sarilumab 200 mg (online supplementary figure 1). During the open-label extension, discontinuation rates were similar across original treatment groups (figure 1). Overall in the double-blind study and the open-label extension, discontinuations due to safety reasons were reported in 340 patients (28%); discontinuations due to non-safety reasons were reported in 222 patients (19%), including 52 patients (4%) due to lack of efficacy and 29 (2%) due to poor compliance with the protocol. At baseline of the double-blind study, demographics and disease characteristics of patients who enrolled in the open-label extension were similar both between treatment groups and to those of the total population who were randomised in the double-blind study (table 1). Demographics and patient characteristics were also similar across original randomisation groups at baseline of the open-label extension (online supplementary table 1).
Figure 1.
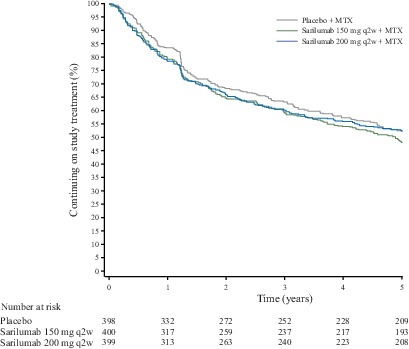
Kaplan-Meier estimate of continuation in the double-blind study and the open-label extension by original randomised treatment group. MTX, methotrexate; q2w, every 2 weeks.
Table 1.
Demographics and disease activity at entry into the double-blind study by original randomisation group for the double-blind (n=1197) and open-label extension (n=901) populations
| Parameter at double-blind study baseline | Double-blind study | Open-label extension | ||||
| Placebo + MTX (n=398) | Sarilumab q2w + MTX | Placebo + MTX (n=307) | Sarilumab q2w + MTX | |||
| 150 mg (n=400) | 200 mg (n=399) | 150 mg (n=300) | 200 mg (n=294) | |||
| Female, n (%) | 321 (81) | 319 (80) | 337 (84) | 246 (80) | 241 (80) | 246 (84) |
| Age, mean (SD), years | 50.9 (11.2) | 50.1 (11.9) | 50.8 (11.8) | 50.8 (10.7) | 50.3 (11.8) | 50.2 (11.6) |
| Prior biological DMARD use, n (%) | 86 (22) | 87 (22) | 84 (21) | 71 (23) | 75 (25) | 64 (22) |
| Duration of RA, mean (range), years | 9 (0–44) | 10 (0–45) | 9 (0–34) | 9 (0–44) | 10 (0–45) | 9 (0–34) |
| Seropositive for RF, n (%) | 336 (84) | 345 (87)* | 328 (83)* | 260 (85) | 261 (88)† | 250 (85)† |
| Seropositive for anti-CCP autoantibody, n (%) | 340 (85) | 359 (90)‡ | 337 (85)‡ | 264 (86) | 273 (91) | 255 (87)§ |
| Tender joint count (0–68), mean (SD) | 26.8 (13.7) | 27.2 (14.1) | 26.5 (14.5) | 26.8 (13.6) | 27.4 (14.4) | 26.9 (14.4) |
| Swollen joint count (0–66), mean (SD) | 16.7 (9.3) | 16.6 (9.0) | 16.8 (9.7) | 17.1 (9.4) | 16.7 (9.2) | 17.0 (9.5) |
| CRP, mean (SD), mg/L | 20.5 (23.0) | 22.5 (23.1) | 22.2 (23.8) | 20.1 (22.1) | 22.8 (24.0) | 21.5 (20.6) |
| DAS28-CRP, mean (SD) | 5.9 (0.9) | 6.0 (0.9) | 6.0 (0.9) | 5.9 (0.9) | 6.0 (0.9) | 6.0 (0.9) |
| HAQ-DI, mean (SD) | 1.6 (0.7) | 1.6 (0.6) | 1.7 (0.6) | 1.6 (0.7) | 1.6 (0.6) | 1.7 (0.6) |
*For RF, n=396 for sarilumab 150 mg and n=397 for sarilumab 200 mg.
†For RF, n=298 for sarilumab 150 mg and n=293 for sarilumab 200 mg.
‡For anti-CCP, n=398 for sarilumab 150 mg and n=293 for sarilumab 200 mg.
§n=293.
CCP, cyclic citrullinated peptide; CRP, C reactive protein; DAS28-CRP, Disease Activity Score (28 joints) using C reactive protein; DMARD, disease-modifying antirheumatic drug; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate; q2w, every 2 weeks; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation.
rmdopen-2018-000887supp001.pdf (369.5KB, pdf)
Adverse events
Across the double-blind study and the open-label extension, mean exposure to either dose of sarilumab was 3.3 years (median exposure: 4.1 years), maximum exposure was 5.9 years, cumulative exposure was 3767 patient-years (PY), and 476 patients (42%) were treated for ≥240 weeks (approximately 5 years; online supplementary table 2). The exposure-adjusted incidence rates of AEs and SAEs were 137.7 and 9.1 per 100 PY, respectively, for patients receiving either dose of sarilumab (table 2). The most common AEs (with any dose of sarilumab) were injection-site erythema (incidence rate 13.5 per 100 PY), neutropenia (12.8 per 100 PY) and upper respiratory tract infection (7.6 per 100 PY; table 2). The most common AESIs were infections (incidence rate 55.1 per 100 PY), injection-site reactions (21.6 per 100 PY) and leucopenia (17.7 per 100 PY; online supplementary table 3). The incidence rate of AEs was generally stable over >5 years of treatment and there was no signal for an increased rate over time for any of the AEs (including SAEs and serious infections) when analysed by 6-month interval (online supplementary figure 2).
Table 2.
Investigator-reported treatment-emergent AEs in the double-blind study and the open-label extension combined
| AE | nE (nE/100 PY) | ||
| Sarilumab 150 mg initial dose + MTX | Sarilumab 200 mg initial dose*+ MTX | Any sarilumab dose†+ MTX | |
| Summary ‡ | |||
| Any AE | 299 (182.3) | 668 (142.0) | 1041 (137.7) |
| SAE | 36 (10.3) | 183 (9.9) | 313 (9.1) |
| AE leading to discontinuation | 56 (16.1) | 172 (8.4) | 318 (8.4) |
| AE leading to death | 2 (0.6) | 7 (0.3) | 16 (0.4) |
| AEs with incidence rate ≥ 5 per 100 PY in any dose group § | |||
| Cumulative total AE observation period, PY | 355.5 | 2082.5 | 3826.0 |
| Injection-site erythema | 77 (21.7) | 267 (12.8) | 518 (13.5) |
| Neutropenia | 64 (18.0) | 235 (11.3) | 491 (12.8) |
| Upper respiratory tract infection | 47 (13.2) | 145 (7.0) | 289 (7.6) |
| Accidental overdose¶ | 30 (8.4) | 137 (6.6) | 220 (5.8) |
| Urinary tract infection | 24 (6.8) | 124 (6.0) | 213 (5.6) |
| ALT increased | 43 (12.1) | 108 (5.2) | 211 (5.5) |
| Viral upper respiratory tract infection | 32 (9.0) | 84 (4.0) | 172 (4.5) |
| Bronchitis | 19 (5.3) | 104 (5.0) | 173 (4.5) |
| Injection-site pruritus | 28 (7.9) | 75 (3.6) | 132 (3.5) |
| Influenza | 19 (5.3) | 53 (2.5) | 111 (2.9) |
| Headache | 22 (6.2) | 41 (2.0) | 83 (2.2) |
*Including placebo patients from the double-blind phase who switched to sarilumab 200 mg in the open-label extension.
†Any dose includes exposure on all sarilumab doses.
‡Incidence rate (nE/100 PY) for summary is over time to first event.
§Incidence rate (nE/100 PY) is over cumulative total AE observation period.
¶Administration of two or more doses of study drug during an interval <11 days.
AE, adverse event; ALT, alanine aminotransferase; MTX, methotrexate; nE, number of events; nE/100 PY, number of events per 100 PY; PY, patient-years; SAE, serious adverse event.
Laboratory abnormalities
Elevations of ALT to >3× ULN occurred in 158 patients (14%) receiving either dose of sarilumab and normalised on treatment in 84 (53%) of these patients (online supplementary table 4). ANC <1000 cells/mm3 occurred in 143 patients (13%) receiving either dose of sarilumab and normalised on treatment in 104 (73%) of these patients. Platelet counts <100×109 cells/L were observed in 33 patients (3%) receiving either dose of sarilumab and normalised on treatment in 20 (61%) of these patients.
Infections
Serious infections occurred at a rate of 3.9 events per 100 PY in patients treated with either dose of sarilumab (online supplementary table 3). The incidence of infections and serious infections was similar between patients with and without a recorded event of neutropenia at any time during the study (online supplementary table 5). Moreover, the incidence of infection was similar between patients with a lowest on-study ANC of ≥1500 cells/mm3—lower limit of normal (grade 1 neutropenia), ≥1000–<1500 cells/mm3 (grade 2) and ≥500–<1000 cells/mm3 (grade 3). Of the total 2109 infections observed, 1879 (49.1 events per 100 PY) occurred within 12 weeks after an ANC assessment. ANC values were normal at the last ANC assessment before infection for the majority of infections occurring within 12 weeks after an ANC assessment (1652/1879 (88%); online supplementary table 6). Similar results were observed for serious infection: ANC values were normal at the last ANC assessment before serious infection for 125/130 (96%) serious infections occurring within 12 weeks of an ANC assessment. Herpes zoster infection was reported in 19 patients (1.6%; 0.5 events per 100 PY); all cases were non-disseminated.
Other safety findings
Malignancies occurred in 22 patients (1.9%; 0.6 events per 100 PY). The most common malignancies (occurring in >1 patient) were basal cell carcinoma (n=4; 0.4%), malignant melanoma (n=3; 0.3%) and breast cancer (n=2; 0.2%). There were five confirmed cases of gastrointestinal perforation (0.1 events per 100 PY): two upper and three lower (online supplementary table 3). Lipid elevations were reported in 149 patients treated with any dose of sarilumab (13.1%; 5.6 events per 100 PY) and included increased levels of total cholesterol (n=15; 1.3%; 0.5 events per 100 PY), low-density lipoprotein cholesterol (n=12; 1%; 0.4 events per 100 PY) and high-density lipoprotein cholesterol (n=2; 0.2%; <0.1 events per 100 PY). There were 13 MACE (1.1%; 0.3 events per 100 PY), which consisted of cardiovascular death (n=5), myocardial infarction (n=4) and stroke (n=4). Thromboembolic events (as reported by the investigators and evaluated post hoc; not a prespecified AESI) occurred at a rate of 0.9 per 100 PY.
Radiographic outcomes
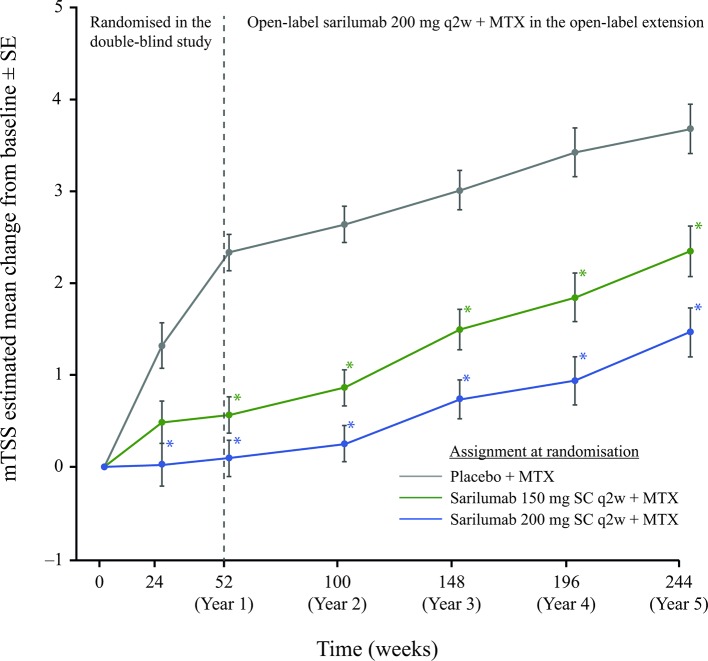
This integrated analysis incorporates radiographic data from four different reading campaigns that assessed data at years 5, 4, 3 and baseline (n=660 patients; campaign 4), years 3, 2 and baseline (n=717; campaign 3), years 2, 1 and baseline (n=810; campaign 2) and year 1, month 6 and baseline (n=1158; campaign 1). At 5 years, mean (SE) changes from baseline in mTSS score were 1.46 (0.27), 2.35 (0.28) and 3.68 (0.27) in the sarilumab 200 mg, sarilumab 150 mg and placebo initial randomisation groups, respectively (p<0.001 for each sarilumab dose vs placebo). In the groups initially assigned to sarilumab 150 or 200 mg, mTSS progression was reduced compared with the group initially assigned to placebo throughout the open-label extension with sarilumab 200 mg (figure 2). The trajectory of mean mTSS progression in the group initially assigned to placebo showed a marked inflection at 1 year, coinciding with the switch to open-label sarilumab 200 mg. Notably, the different trajectories of radiographic progression observed between the three treatment groups from year 1 were sustained over the succeeding years of follow-up; patients initially treated with sarilumab 200 mg sustained a lower mean mTSS score at 5 years than patients initially treated with either sarilumab 150 mg or placebo.
Figure 2.
Estimated mean change from baseline in mTSS. *Nominal p<0.001 versus placebo. mTSS, van der Heijde-modified Total Sharp Score; MTX, methotrexate; q2w, every 2 weeks; SC, subcutaneously.
At 5 years, the proportion of patients with no mTSS progression (change from baseline ≤0) was 47.1% (107/221), 42.2% (94/223) and 37.2% (87/234) in the sarilumab 200 mg, sarilumab 150 mg and placebo initial randomisation groups, respectively. Results were the same for mTSS non-progression defined as change from baseline ≤0.5.
Clinical efficacy
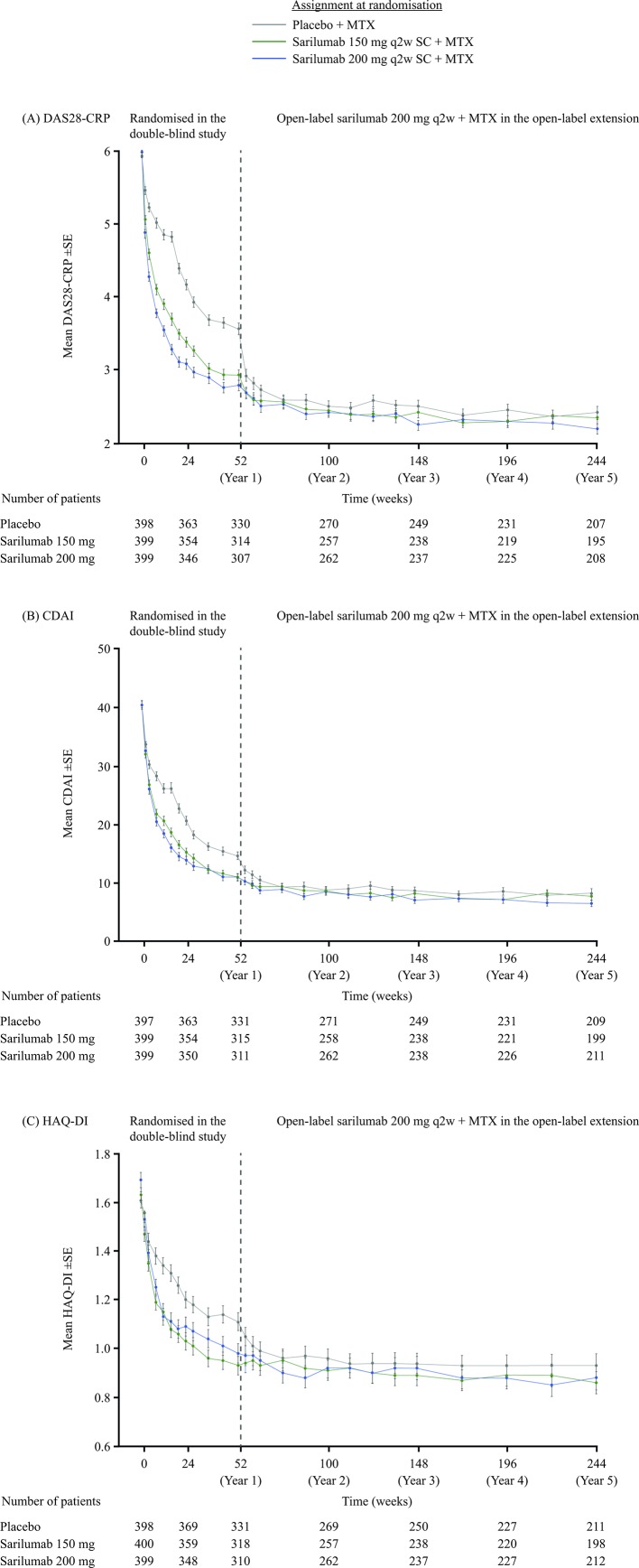
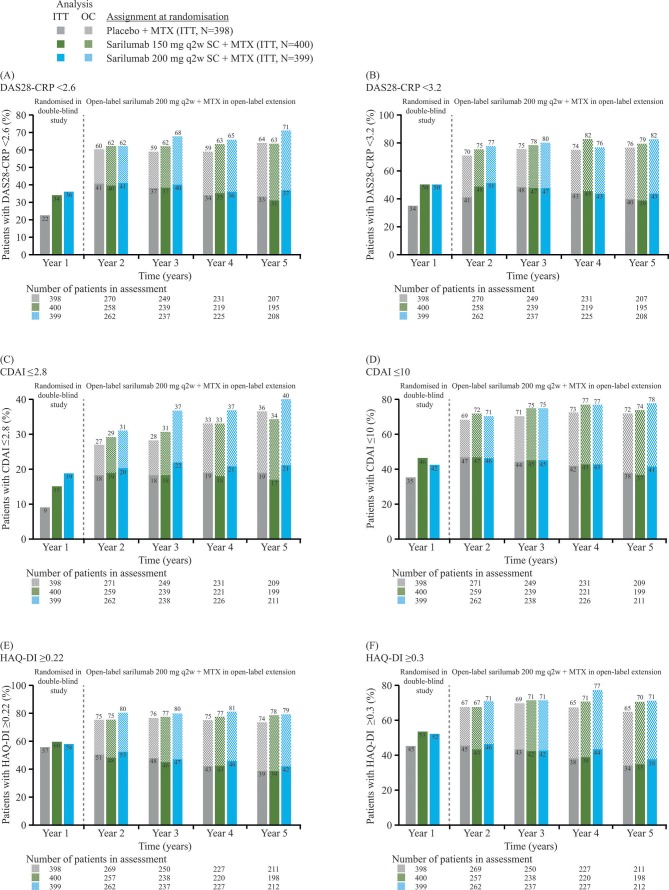
Clinical efficacy according to DAS28-CRP, CDAI and HAQ-DI was sustained through 5 years of follow-up. At year 5, mean (SE) DAS28-CRP scores were similar among initial randomisation groups: sarilumab 200 mg, 2.21 (0.07); sarilumab 150 mg, 2.36 (0.08) and placebo, 2.43 (0.09) (figure 3A), having decreased by 62% with sarilumab 200 mg from 5.97 (0.04) at baseline (based on 208/399 patients with data at year 5). Likewise, the proportions of patients achieving DAS28-CRP <2.6 and <3.2 were similar among initial randomisation groups after 1 year of open-label sarilumab treatment in the extension study (ie, at 2 years of follow-up) and remained generally similar thereafter to 5 years of follow-up (figure 4A, B). Results for CDAI were similar to those for DAS28-CRP. At year 5, mean (SE) CDAI was 6.56 (0.49), 7.76 (0.63) and 8.38 (0.68) in the sarilumab 200 mg, sarilumab 150 mg and placebo initial randomisation groups, respectively (figure 3B), having decreased by 83% with sarilumab 200 mg from 40.4 (0.62) at baseline (based on 211/399 patients with data at year 5). The proportions of patients achieving CDAI ≤2.8 and ≤10 were similar among initial randomisation groups after 1 year of open-label sarilumab treatment in the extension study (ie, at 2 years of follow-up) and remained generally similar thereafter through 5 years of follow-up (figure 4C, D). Results for physical function, assessed by HAQ-DI, were similar to those observed for DAS28-CRP and CDAI; mean (SE) HAQ-DI scores at 5 years were 0.88 (0.049), 0.86 (0.046) and 0.93 (0.049) in the sarilumab 200 mg, sarilumab 150 mg and placebo initial randomisation groups, respectively (figure 3C), having decreased by 47% with sarilumab 200 mg from 1.69 (0.03) at baseline (based on 211/399 patients with data at year 5). The proportions of patients achieving HAQ-DI ≥0.22 and ≥0.30 were similar among initial randomisation groups after 1 year of open-label sarilumab treatment in the extension study (ie, at 2 years of follow-up) and remained generally similar thereafter through 5 years of follow-up (figure 4E, F).
Figure 3.
Mean clinical efficacy scores over time, as observed, without imputation for missing patients: (A) DAS28-CRP, (B) CDAI and (C) HAQ-DI. CDAI, Clinical Disease Activity Index; DAS28-CRP, Disease Activity Score (28 joints) using C reactive protein; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate; q2w, every 2 weeks; SC, subcutaneously.
Figure 4.
Proportions of patients at each year achieving: (A) DAS28-CRP <2.6, (B) DAS28-CRP <3.2, (C) CDAI≤2.8, (D) CDAI≤10, (E) HAQ-DI ≥0.22 and (F) HAQ-DI ≥0.30. In the ITT analysis, the denominator for percentages at all timepoints is the ITT population. In OC analysis, the denominator for percentages is the number of patients in the assessment at that timepoint. CDAI, Clinical Disease Activity Index; DAS28-CRP, Disease Activity Score (28 joints) using C reactive protein; HAQ-DI, Health Assessment Questionnaire-Disability Index; ITT, intention to treat; MTX, methotrexate; OC, observed cases; q2w, every 2 weeks; SC, subcutaneously.
Dose reduction and MTX discontinuation
Dose reduction from sarilumab 200 to 150 mg occurred in 177 patients (20% of 899 patients treated with sarilumab 200 mg). The most common reasons for dose reduction were ANC decrease (n=108, 61% of patients who reduced their dose) and ALT elevation (n=44, 25%). Following temporary discontinuation, sarilumab was reinitiated at the reduced dose of 150 mg once ANC levels returned to ≥1000 cells/mm3 or ALT levels returned to <3× ULN. ANC reductions and ALT elevations started to improve within 1 month of dose reduction and improvements continued thereafter (online supplementary table 7). Clinical efficacy was sustained after dose reduction (online supplementary figure 3).
In total, 92 patients discontinued MTX during the open-label extension: 10 patients switched to an alternative approved csDMARD and 82 patients permanently discontinued MTX and received sarilumab monotherapy. Clinical efficacy was sustained in those patients remaining in the study after MTX discontinuation.
Discussion
The safety and efficacy of sarilumab 200 mg and 150 mg plus MTX were previously demonstrated in MTX-IR patients with moderately to severely active RA in the 52-week double-blind study.8 Results from this 5-year analysis of the open-label extension study confirm the long-term safety and efficacy of sarilumab in this group of patients.
The safety profile of sarilumab was stable over 5 years of follow-up and observations were generally consistent, not only with those in the randomised portion of the study but also with other studies in the sarilumab RA clinical programme.8–10 13 In addition, the safety findings are consistent with the anticipated safety profile of IL-6 blockade.14 No new safety signals emerged and the most common AEs were injection-site erythema, neutropenia, upper respiratory tract infection, urinary tract infections and increased ALT. Occurrence of injection-site reactions was common (21.6 events per 100 PY) but consistent with long-term data for subcutaneous tocilizumab (26.1 events per 100 PY), another IL-6 receptor antagonist.15 Neutropenia was also common with sarilumab treatment, which is consistent with the phase III clinical trials of sarilumab and with this class of therapy.8–10 14 However, despite neutropenia occurring at a rate of 12.8 events per 100 PY at any sarilumab dose, the infection rate reported in these patients was low, which suggests that neutropenia related to sarilumab treatment is not associated with an increased risk of infection. Furthermore, the last ANC recorded before onset of infection or serious infection was normal in the majority of cases. These results are consistent with a post hoc analysis of three sarilumab phase III trials and patients entering the open-label extensions of two of these trials.16 The post hoc analysis also found that the majority of patients in the randomised controlled trials and open-label extensions who temporarily discontinued treatment with sarilumab due to low ANC were able to continue or reinitiate sarilumab with no apparent clinically meaningful impact on long-term efficacy or safety.16 Published data suggest that patients with RA are at a twofold higher risk of herpes zoster infection than individuals without RA,17 but the reported rate was low in the open-label extension (0.5 events per 100 PY) and comparable to the general population (0.3–0.5 events per 100 PY).18 While the absolute incidence of AEs was lower in the patients initially randomised to sarilumab 150 mg than those receiving the 200 mg dose, the exposure-adjusted incidence rates were higher. This result should be interpreted with caution, due to the limited duration of exposure in the sarilumab 150 mg group and the relatively small number of events. Furthermore, the absolute incidence of AEs in the double-blind phase was lower in patients randomised to sarilumab 150 mg than those randomised to sarilumab 200 mg.8 No increases in rates of AEs or SAEs were observed with increasing exposure duration and the incidence rates of AEs and laboratory abnormalities generally remained stable when analysed over time by 6-month interval. Furthermore, the incidences of injection-site reactions and ALT >3× ULN declined over time.
It has previously been reported that patients with RA are at an increased risk of gastrointestinal perforation, which is a rare but SAE.19 20 Although gastrointestinal perforations were reported infrequently over long-term sarilumab use in the extension study (two upper and three lower gastrointestinal perforations), the protocol exclusion of patients with a history of diverticulitis—another recognised risk factor for gastrointestinal perforation19—may have mitigated against the risk of gastrointestinal perforation in this population. Investigation of potential gastrointestinal perforation should take into consideration the C reactive protein-lowering effect of IL-6 signalling inhibition.
Evidence suggests that patients with active, untreated RA have reduced total, low-density lipoprotein and high-density lipoprotein cholesterol levels, despite a 50% higher cardiovascular risk than the general population.21 22 This effect is known as the lipid paradox. Although increases in low-density lipoprotein cholesterol were observed in this open-label extension, MACE were reported infrequently. This supports previous findings with IL-6 receptor inhibitors suggesting that increases in serum lipids associated with effective RA immunosuppression do not increase cardiovascular event risk.23 Other analyses across phase III clinical trials of sarilumab observed that sarilumab treatment was associated with an increase in lipid levels compared with placebo and adalimumab; however, levels of lipoprotein (a) (a cardiovascular risk marker) were reduced and lipid elevations were largely associated with a reduction in inflammatory markers (serum amyloid A and C reactive protein).24 25 These results suggest that inhibition of IL-6 signalling does not affect cardiovascular risk, despite the effects on lipid levels.
Preventing joint damage progression is an important therapeutic goal in RA and joint damage can progress in patients despite clinically quiescent disease.26 Initial treatment with either dose of sarilumab was associated with significantly better radiographic outcomes versus placebo over 5 years of follow-up, with the best outcomes observed in patients initially randomised to sarilumab 200 mg in the double-blind study. These treatment differences persisted through year 5 in the open-label extension, at which time all patients were receiving sarilumab 200 mg. This suggests that the excess structural damage observed in patients who had received placebo or sarilumab 150 mg during the double-blind study likely occurred during that first year of study treatment. These results are consistent with other studies that have demonstrated improvements in long-term outcomes with early intensive treatment in patients with RA.27–31 Furthermore, these results support an analysis demonstrating suppression of circulating biomarkers of bone resorption and synovial damage with sarilumab plus MTX versus placebo plus MTX in MTX-IR patients.32
Clinical efficacy, according to DAS28-CRP, CDAI and HAQ-DI, was sustained through 5 years of follow-up. Similar changes from baseline in DAS28-CRP, CDAI and HAQ-DI were observed regardless of the initial randomisation group in the randomised portion of the study. Improvements in the proportions of patients achieving DAS28-CRP <3.2 and <2.6 and CDAI ≤10 and ≤2.8 continued over time. These results are consistent with—and build on—the 2 year observations.13
Among the main limitations associated with long-term extension studies is enrichment of the cohort for patients who respond well to treatment and tolerate any AEs they encounter. As a result, the measurement of continuous variables can be influenced by the progressively smaller number of patients who remain in the study. To help minimise this bias, the absolute numbers of patients are presented at each relevant timepoint in addition to percentage response rates. Furthermore, ITT analyses are presented alongside completer analyses for dichotomous variables.11
In conclusion, the durable efficacy of sarilumab was demonstrated through 5 years of follow-up in an open-label extension study, and reductions in disease activity, inhibition of radiographic progression and improvements in physical function were observed. No new safety signals were identified, and the safety profile remained stable and consistent with IL-6 blockade.
rmdopen-2018-000887supp002.pdf (1.8MB, pdf)
Acknowledgments
The authors thank the patients and their families, as well as the investigators and other study staff involved in the studies. The authors would also acknowledge Chunfu Qiu for providing statistical support for safety-related data. These data were previously presented in part at the 21st Asia Pacific League of Associations for Rheumatology Congress (APLAR) in conjunction with the Australian Rheumatology Association, 8–11 April 2019, Brisbane, Queensland, Australia: International Journal of Rheumatic Diseases 2019; 22 (Suppl 3): 206–207 (Abstract 2–100).
Footnotes
MCG and DvdH contributed equally.
Contributors: MCG, DvdH and HvH contributed to the design of the study; MCG, AK, JAM-C and MS contributed to data acquisition; YL contributed to data review and addressed data-related queries; and all authors contributed to data analysis and interpretation. All authors were involved in revising the manuscript critically for important intellectual content and approved the final version to be published.
Funding: This study was supported by Sanofi and Regeneron Pharmaceuticals, Inc., which funded medical writing support by Natalie Roberts, PhD, Adelphi Communications.
Competing interests: MCG has received research grants or consulting fees from R-Pharm, Roche/Genentech and Sanofi Genzyme. DvdH has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB. YL, SW and HvH are employees of Sanofi Genzyme and may hold stock and/or stock options in the company. GSJ is an employee of Regeneron and may hold stock and/or stock options in the company. JJG-R has received research support and/or consulting fees from Biogen, Gilead, Eli Lilly, Merck Sharp & Dohme, Pfizer and Roche. AK has received consulting fees and/or participated in speakers’ bureaus for AbbVie, Pfizer, Genentech, UCB, Sanofi/Regeneron, Celgene, Horizon and Merck. JAM-C has received consulting fees and/or participated in speakers’ bureaus for Pfizer, Merck Sharp & Dohme, Sanofi Aventis, Novartis, Bristol-Myers Squibb, Roche, Boehringer Ingelheim, Schering-Plough, Abbott, UCB, Eli Lilly and Gilead. MS has received consulting fees from R-Pharm. GRB has received research support and/or consulting fees from AbbVie, Lilly, Merck Sharp & Dohme, Pfizer, Roche, Sanofi and UCB. BS has nothing to disclose.
Patient consent for publication: All patients provided written informed consent.
Ethics approval: The MOBILITY and EXTEND protocols were approved by the appropriate ethics committees/institutional review boards.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Qualified researchers may request access to patient level data and related study documents including clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
References
- 1. Picerno V, Ferro F, Adinolfi A, et al. . One year in review: the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 2015;33:551–8. [PubMed] [Google Scholar]
- 2. Choy EHS, Calabrese LH. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology 2018;57:1885–95. 10.1093/rheumatology/kex391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity 2017;46:183–96. 10.1016/j.immuni.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 2012;8:656–64. 10.1038/nrrheum.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schett G. Physiological effects of modulating the interleukin-6 axis. Rheumatology 2018;57(suppl 2):ii43–50. 10.1093/rheumatology/kex513 [DOI] [PubMed] [Google Scholar]
- 6. Tanaka T, Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int J Biol Sci 2012;8:1227–36. 10.7150/ijbs.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kevzara Prescribing Information , 2017. Available: https://wwwaccessdatafdagov/drugsatfda_docs/label/2017/761037s000lblpdf
- 8. Genovese MC, Fleischmann R, Kivitz AJ, et al. . Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol 2015;67:1424–37. 10.1002/art.39093 [DOI] [PubMed] [Google Scholar]
- 9. Fleischmann R, van Adelsberg J, Lin Y, et al. . Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol 2017;69:277–90. 10.1002/art.39944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burmester GR, Lin Y, Patel R, et al. . Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis 2017;76:840–7. 10.1136/annrheumdis-2016-210310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buch MH, Silva-Fernandez L, Carmona L, et al. . Development of EULAR recommendations for the reporting of clinical trial extension studies in rheumatology. Ann Rheum Dis 2015;74:963–9. 10.1136/annrheumdis-2013-204948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landewé R, Østergaard M, Keystone EC, et al. . Analysis of integrated radiographic data from two long-term, open-label extension studies of adalimumab for the treatment of rheumatoid arthritis. Arthritis Care Res 2015;67:180–6. 10.1002/acr.22426 [DOI] [PubMed] [Google Scholar]
- 13. Genovese MC, van Adelsberg J, Fan C, et al. . Two years of sarilumab in patients with rheumatoid arthritis and an inadequate response to MTX: safety, efficacy and radiographic outcomes. Rheumatology 2018;57:1423–31. 10.1093/rheumatology/key121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim GW, Lee NR, Pi RH, et al. . IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res 2015;38:575–84. 10.1007/s12272-015-0569-8 [DOI] [PubMed] [Google Scholar]
- 15. Burmester GR, Rubbert-Roth A, Cantagrel A, et al. . Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis 2016;75:68–74. 10.1136/annrheumdis-2015-207281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curtis JR, St. John G, Pannucci M, et al. Reductions in Absolute Neutrophil Count (ANC) with Sarilumab Resulting in Dose Delays or Dose Decreases: Effects on Efficacy and Safety [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 10). https://acrabstracts.org/abstract/reductions-in-absolute-neutrophil-count-anc-with-sarilumab-resulting-in-dose-delays-or-dose-decreases-effects-on-efficacy-and-safety/. Accessed November 22, 2018.
- 17. Smitten AL, Choi HK, Hochberg MC, et al. . The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Care Res 2007;57:1431–8. 10.1002/art.23112 [DOI] [PubMed] [Google Scholar]
- 18. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014;4:e004833 10.1136/bmjopen-2014-004833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curtis JR, Lanas A, John A, et al. . Factors associated with gastrointestinal perforation in a cohort of patients with rheumatoid arthritis. Arthritis Care Res 2012;64:1819–28. 10.1002/acr.21764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie F, Yun H, Bernatsky S, et al. . Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol 2016;68:2612–7. 10.1002/art.39761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters MJL, Symmons DPM, McCarey D, et al. . EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325–31. 10.1136/ard.2009.113696 [DOI] [PubMed] [Google Scholar]
- 22. Aviña-Zubieta JA, Choi HK, Sadatsafavi M, et al. . Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7. 10.1002/art.24092 [DOI] [PubMed] [Google Scholar]
- 23. Giollo A, Bissell L-A, Buch MH. Cardiovascular outcomes of patients with rheumatoid arthritis prescribed disease modifying anti-rheumatic drugs: a review. Expert Opin Drug Saf 2018;17:697–708. 10.1080/14740338.2018.1483331 [DOI] [PubMed] [Google Scholar]
- 24. Charles-Schoeman C, St John G, Leher H, et al. . The relationship between lipid profile changes and inflammation across the phase 3 sarilumab rheumatoid arthritis (RA) developmental program [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 10). Available: https://acrabstracts.org/abstract/the-relationship-between-lipid-profile-changes-and-inflammation-across-the-phase-3-sarilumab-rheumatoid-arthritis-ra-developmental-program/ [Accessed November 22, 2018].
- 25. Gabay C, Msihid J, Paccard C, et al. . Sarilumab significantly suppresses circulating biomarkers of bone resorption and cardiovascular risk compared with adalimumab: biomarker analysis from the phase 3 monarch study. Ann Rheum Dis 2017;76:570 10.1136/annrheumdis-2017-eular.4534 [DOI] [Google Scholar]
- 26. Steunebrink LMM, Versteeg LGA, Vonkeman HE, et al. . Radiographic progression in early rheumatoid arthritis patients following initial combination versus step-up treat-to-target therapy in daily clinical practice: results from the DREAM registry. BMC Rheumatol 2018;2 10.1186/s41927-018-0009-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lard LR, Visser H, Speyer I, et al. . Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. The American Journal of Medicine 2001;111:446–51. 10.1016/S0002-9343(01)00872-5 [DOI] [PubMed] [Google Scholar]
- 28. Nell VPK, Machold KP, Eberl G, et al. . Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology 2004;43:906–14. 10.1093/rheumatology/keh199 [DOI] [PubMed] [Google Scholar]
- 29. Quinn MA, Emery P. Window of opportunity in early rheumatoid arthritis: possibility of altering the disease process with early intervention. Clin Exp Rheumatol 2003;21:S154–7. [PubMed] [Google Scholar]
- 30. Steunebrink LMM, Versteeg GA, Vonkeman HE, et al. . Initial combination therapy versus step-up therapy in treatment to the target of remission in daily clinical practice in early rheumatoid arthritis patients: results from the DREAM registry. Arthritis Res Ther 2016;18:60 10.1186/s13075-016-0962-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steunebrink LMM, Vonkeman HE, ten Klooster PM, et al. . Recently diagnosed rheumatoid arthritis patients benefit from a treat-to-target strategy: results from the DREAM registry. Clin Rheumatol 2016;35:609–15. 10.1007/s10067-016-3191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boyapati A, Msihid J, Fiore S, et al. . Sarilumab plus methotrexate suppresses circulating biomarkers of bone resorption and synovial damage in patients with rheumatoid arthritis and inadequate response to methotrexate: a biomarker study of mobility. Arthritis Res Ther 2016;18:225 10.1186/s13075-016-1132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2018-000887supp001.pdf (369.5KB, pdf)
rmdopen-2018-000887supp002.pdf (1.8MB, pdf)