Abstract
Objectives
In year 2016, Danish national guidelines included a mandatory switch of patients with inflammatory rheumatic diseases treated with originator etanercept (ETA) to biosimilar SB4 in routine care. We aimed to explore if switching lead to increased healthcare utilisation and costs.
Methods
Observational cohort study. Adult patients who switched from ETA to SB4 were identified in the Danish nationwide DANBIO registry. In the National Patient Registry, we identified health utilisation (hospital admissions/hospital days/outpatient visits/prescription medication use) and comorbidities. Estimation of health utilisation included average use and costs 1 year before/after switch, changes after the switch, and whether patient characteristics affected changes. Analyses were by adjusted two-step gamma distributed regression models, and for changes over time a generalized estimation equations (GEE) model was applied. Impact of comorbidities was explored as interaction terms in the model. Medication costs of ETA and SB4 were not included in model.
Results
1620 patients were included (mean age 55 years (SD 14.7), 40% male). Costs before and after switching were mainly driven by outpatient visits (67%/72% of all costs). Monthly fluctuations of costs were similar before/after switch. After switching, use (8%) and costs (7%) of outpatient services increased, whereas costs of admissions (55%) and medication (5%) decreased. Patients with longer ETA treatment duration had an increase in use and costs of healthcare resources, whereas gender and comorbidities had no impact. Higher age was associated with an increase in costs of inpatient services.
Conclusion
We demonstrated no obvious changes in overall use and costs of healthcare services following switch from originator to biosimilar etanercept.
Keywords: biological DMARDs, outcomes research, rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, anti-TNF
Key messages.
What is already known about this subject?
Mandatory switching from originator to a corresponding cheaper biosimilar biological agent (=non-medical switch) has been suggested in inflammatory rheumatic diseases.
The economic benefit might potentially be outweighed by extra costs (eg, patient education, closer monitoring).
What does this study add?
We found no obvious changes in healthcare utilisation and costs in 1620 Danish patients who were switched from originator to biosimilar etanercept in routine care.
How might this impact on clinical practice?
Non-medical etanercept switching of long-term treated patients with inflammatory rheumatic disease has no short-term negative economic consequences.
Introduction
The biological disease modifying antirheumatic treatments (bDMARDs) have transformed the treatment options among patients with inflammatory rheumatic diseases otherwise refractory to conventional synthetic (cs-)DMARDs. In recent years, patents on widely prescribed bDMARDs have expired and consequently cheaper biosimilar products are emerging.1
Randomised studies of patients on stable treatment with originator etanercept (ETA) who switched to the corresponding biosimilar have demonstrated no negative impact of such a non-medical switch on safety and treatment efficacy.2 3 This has created a financial incentive for healthcare payers to stimulate switching with the potential of reduced medication costs. This would potentially allow more patients to be treated.4 The economic benefit from switching may potentially be partly outweighed by extra costs if the number of outpatient visits and contacts to healthcare providers rises due to for example, patient education or closer monitoring.5–8 However, existing pharmaco-economic and health-economic studies have focused mainly on drug prices and the direct cost of treatment,9 whereas overall utilisation of healthcare and associated costs have not been addressed.
Denmark has had a rapid uptake of biosimilar products reflecting strong adherence to the national guidelines.10 The switches have been prospectively registered in the nationwide DANBIO registry11 12 and linkage to other national registries allows for detailed study of any impact of treatment changes.11 13 14 Thus, in year 2017 Danish national guidelines recommended switch of all patients with inflammatory rheumatic diseases treated with ETA to the corresponding biosimilar SB4.10 The switch was mandatory, that is, neither the physician nor the patient had any say in the matter—– only very specific individual patient considerations should prevail switching.10 The recommendation was due to economic reasons as SB4 upon marketing cost 49% less than ETA. A similar switch occurred in 2016 for infliximab,15 where we found no major changes in the number of healthcare services and the visit rate provided in rheumatology outpatient care 6 months following the switch among 769 patients with inflammatory arthritis.16
Infliximab and etanercept have different routes of administration (intravenous vs subcutaneous) and may thus potentially be used by patients with different demographic and disease characteristics. Furthermore, the perception of biosimilars among healthcare professionals and patients may change over time.6 17 Thus, experience regarding switch outcomes from infliximab cannot readily be extrapolated to etanercept. Thus, the aim of this study was to explore healthcare utilisation and costs 1 year before and after the Danish nationwide mandatory switch from ETA to biosimilar SB4 etanercept among patients with inflammatory rheumatic diseases followed in routine care. Furthermore, we wanted to explore if selected patient and disease characteristics affected outcomes. Switch patterns and the impact of the switch on disease activity and retention to SB4-treatment have been published.12
Methods and materials
Study design
A register-based before-after switching health cost study of Danish adult patients with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis followed in the DANBIO registry who switched from ETA to SB4 in 2016 (=switchers). Patients served as their own controls in the statistical analyses of use before and after switch.
The analysis of health utilisation and costs was divided in three parts: (1) an estimation of average use before and after the switch, (2) an analysis of change in use after the switch and (3) investigation of how patient characteristics affected change in use.
Study population and study period
Switchers during the time period 1 April 2016 to 1 January 2017 were identified in the Danish nationwide DANBIO registry as previously described.12 DANBIO prospectively collects information from Danish departments of rheumatology regarding treatment and outcomes in patients with inflammatory arthritis.11 The index date was the date of the switch. The preperiods and postperiods were defined as 1 year (=52 weeks) prior to and 1 year following the index date, respectively.
Study variables
Health utilisation and health costs 1 year before versus 1 year after the switch (including the index date) were identified and compared.
Health utilisation
Health utilisation was measured as inpatient services (hospital admissions, hospital days), outpatient visits and use of medication other than etanercept (measured as defined daily dose (DDD)).18 By use of unique civil registration numbers (CPR), these data were obtained through linkage to national registries: information on hospital contacts was obtained from The National Patient Registry (NPR), which has virtually complete data on inpatient and outpatient contacts in specialised care.14 Information about the total sale of prescribed medicinal products in Denmark was obtained from The Danish Prescription Drug Register.19
Health costs
The cost analysis was divided into costs for inpatient, outpatient and primary sector contacts as well as medication other than etanercept. Costs for inpatient and outpatient hospital treatment were calculated according to the activity-based fees (DRG and DAGS tariffs).20 Inpatient and outpatient costs were both divided into costs related to (1) the musculoskeletal system and connective tissue (action diagnoses, ICD10 codes M00-M99) and (2) other diseases.
The primary sector includes general practitioners, other specialist doctors, physiotherapists, chiropractors and foot specialists. The Health Insurance Register contains information on the settlement of health insurance services between the hospital owners (Danish Regions) and providers covered by the health insurance that is, general practitioners, practicing specialists, dentists, psychologists and so on which was used to estimate the costs of health utilisation.21
Medication was grouped into pain medication (non-steroid anti-inflammatory drugs, NSAIDs (ATC-codes M01A, N02B) and opioids (N02A)) and other medication. We looked at cost of the medication and the DDD of medication total costs.18 Only medication handled by prescription was included. Thus, ETA and SB4 were not included because biological treatments in Denmark are provided free of charge directly to the patients by the outpatient rheumatology clinics.
Changes in utilisation and costs
To detect changes in utilisation and costs over time, all contacts and costs were identified in the 52 weeks preindex and postindex date. The post period included the index date.
Patient characteristics
Information regarding age, gender, death and emigration was identified in the Danish civil registration system. For each patient, previous comorbidities were identified through healthcare utilisation (in-hospital and outpatient) in the time-window from 12 to 24 months prior to the index-date. Comorbidities were coded according to the WHO-system in 21 chapters/groups.22
Variables from DANBIO included whether treatment with SB4 was withdrawn within 0–180 days from index date (yes/no), and duration of ETA treatment before index date (years, divided in 0–1/2/3/4/5/>5 years).
Ethics and dissemination
The study was approved by the Danish data protection agency. According to Danish law, approval from ethics committees is not necessary for registry research.
Statistical analyses
All analyses were performed in SAS V.9.3. A p value <0.05 was considered statistically significant.
Health utilisation and costs before and after switch
To analyse the yearly health utilisation and costs and we used a two-step gamma distributed regression model23 where we controlled for age, gender, death in the post period, SB4 withdrawal within 180 days, duration of ETA before index date and previous comorbidities (ie, each of the 21 comorbidity groups excluding musculoskeletal were included as dummies; a patient could have more than one comorbidity dummy). From these regressions, costs were predicted before and after switch for a patient characterised by: being female, 55 years old, not dead in the postperiod, not stopped within 180 days, previous duration of ETA treatment >5 years and with no comorbidities (excluding musculoskeletal) 12–24 months before index date (=adjusted, predicted).
Analysis of change in costs after switch
To analyse whether there were changes in costs over time, we included a dummy variable (after=1) in the model. The estimate for this dummy showed whether there were changes over time (before-after). Since the costs before and after included repeated measures in the same patients, the costs at the two time-points were correlated. Thus, to ensure robust standard errors, we applied a GEE model.24
Analysis of any influence of population characteristics on change in health utilisation and costs after switch
To analyse if population characteristics had any influence on the changes in health utilisation and costs over time, we extended the statistical model to include interactions terms. The regression model adjusted for the following main effects: gender, age, dead in post period, duration of ETA before index date, withdrawn SB4 treatment within 180 days, and previous comorbidities. The following interaction terms were included in the model: after*gender, after*age, after*duration of ETA before index date, and after*stopped SB4 within 180 days.
To estimate whether previous comorbidities had any influence on health utilisation and costs after the switch, the regression (controlled for after, gender, age, dead in post period, stopped SB4 within 180 days) we performed a regression for each of the 21 WHO chapters one at a time. Furthermore, an interaction term for each of the 21 WHO chapters were included one by one in the model (after*WHO_chapter) to see if having a diagnosis from each particular WHO chapter influenced change in costs.
Results
Patient cohort
Of the 1623 patients in DANBIO who switched from ETA to SB4, three patients were excluded due to data errors (double registration and missing demographic data). Thus, 1620 patients were included in the analyses (table 1). A total of 848 patients (52.3%) had been treated with ETN >5 years before index date.
Table 1.
Baseline characteristics of included patients (n=1620)
| Age, years, mean (SD) | 55 (14.7) | |
| Gender, male, n (%) | 655 (40.4) | |
| Duration of ETA, n (%) | 1 year | 78 (4.8) |
| 2 years | 106 (6.5) | |
| 3 years | 176 (10.9) | |
| 4 years | 207 (12.8) | |
| 5 years | 205 (12.7) | |
| >5 years | 848 (52.3) | |
| Comorbidities*, n (%) | 1 Certain infectious and parasitic diseases | 42 (2.6) |
| 2 Neoplasms | 78 (4.8) | |
| 3 Diseases of blood and blood forming organs | 26 (1.6) | |
| 4 Endocrine, nutritional, metabolic diseases | 134 (8.3) | |
| 5 Mental and behavioural disorders | 17 (1.0) | |
| 6 Diseases of the nervous system | 67 (4.1) | |
| 7 Diseases of eye and adnexa | 72 (4.4) | |
| 8 Diseases of ear and mastoid | 35 (2.2) | |
| 9 Diseases of circulatory system | 175 (10.8) | |
| 10 Diseases of respiratory system | 109 (6.7) | |
| 11 Diseases of digestive system | 157 (9.7) | |
| 12 Diseases of skin and subcutaneous system | 143 (8.8) | |
| 13 Diseases of musculoskeletal system and connective tissue | 1598 (98.6) | |
| 14 Diseases of genitourinary system | 113 (7.0) | |
| 15 Pregnancy, childbirth and puerperium | 15 (0.9) | |
| 16 Conditions in perinatal period | – | |
| 17 Congenital malformations, deformations, chromosomal abnormalities | 16 (1.0) | |
| 18 Symptoms, signs and abnormal clinical etc | 199 (12.3) | |
| 19 Injury, poisoning, consequences of external causes | 185 (11.4) | |
| 20 External causes of morbidity and mortality | – | |
| 21 Factors influencing health status and contact with health services | 863 (53.3) |
*WHO diagnosis chapters 12–24 months previously.
ETA, originator etanercept.
Within 180 days after the switch, 199 patients (12.3%) stopped SB4. Within 1 year after the switch, 11 patients died (0.7%).
Average health costs and health utilisation before and after the switch
Outpatient services represented the largest share of the total health costs before and after switching (mean number per patient 12.3 visits and 13.3 visits, respectively, table 2). Unadjusted, the outpatient costs represented nearly 70% of total costs whereas inpatient costs was 17%, primary sector costs 10% and medication 6%. The distribution was similar before/after switch and in the unadjusted/adjusted analyses.
Table 2.
(A): Health utilisation, and (B) health costs per patient 12 months before and after switch, unadjusted and adjusted (=predicted)* n=1620 patients
| (A) Total health utilisation per patient | Unadjusted number, mean (SD) | Adjusted number, mean (95% CI)* | |||
| Preswitch | Post-switch | Preswitch | Postswitch | ||
| Outpatient | Number of outpatient visits | 12.30 (9.40) | 13.31 (9.75) | 9.42 (7.91 to 11.22) | 11.09 (9.34 to 13.16) |
| Inpatient services | Number of inpatient admissions | 0.25 (0.64) | 0.11 (0.40) | 0.15 (0.03 to 0.67) | 0.06 (0.01 to 0.69) |
| Number of days in hospital | 1.19 (5.26) | 0.49 (2.73) | 0.66 (0.06 to 7.25) | 0.20 (0.004 to 10.47) | |
| Medication† | Pain medication | 171 (254) | 170 (250) | 168 (88 to 320) | 165 (86 to 315) |
| Other medication | 937 (1119) | 955 (1135) | 659 (355 to 1223) | 678 (364 to 1262) | |
| Total | 1108 (1225) | 1124 (1241) | 832 (447 to 1549) | 848 (455 to 1584) | |
| (B) Total health costs per patient | Unadjusted average cost, mean (SD) | Adjusted average cost, mean (95% CI)* | |||
| Preswitch, € | Postswitch, € | Preswitch, € | Postswitch, € | ||
| Inpatient services | Diseases of the musculoskeletal system and connective tissue | 315 (1645) | 306 (2330) | 122 (58 to 258) | 385 (144 to 1030) |
| Other diseases | 1014 (4326) | 1121 (4607) | 491 (268 to 902) | 297 (150 to 587) | |
| Total | 1330 (4720) | 1428 (5501) | 660 (355 to 1227) | 500 (255 to 977) | |
| Outpatient services | Diseases of the musculoskeletal system and connective tissue | 4445 (6898) | 4700 (7381) | 4051 (2171 to 7559) | 4040 (2150 to 7589) |
| Other diseases | 860 (2623) | 1069 (3498) | 368 (197 to 688) | 484 (253 to 928) | |
| Total | 5305 (7369) | 5769 (8107) | 4436 (2529 to 7779) | 4593 (2618 to 8059) | |
| Primary sector† | General practitioner | 201 (208) | 208 (218) | 171 (93 to 314) | 174 (95 to 318) |
| Other practicing specialist | 105 (229) | 103 (211) | 98 (53 to 180) | 89 (48 to 164) | |
| Physiotherapist, chiropractor, foot specialist | 397 (807) | 384 (801) | 423 (222 to 807) | 388 (200 to 751) | |
| Other | 75 (112) | 70 (97) | 66 (35 to 124) | 63 (33 to 118) | |
| Total | 778 (936) | 765 (921) | 756 (408 to 1404) | 709 (381 to 1321) | |
| Medication‡ | Pain medication | 75 (239) | 74 (231) | 63 (33 to 119) | 62 (32 to 118) |
| Other medication | 391 (638) | 372 (595) | 255 (136 to 478) | 250 (134 to 466) | |
| Total | 466 (721) | 446 (682) | 321 (172 to 601) | 312 (167 to 584) | |
| Total costs | 7880 (9427) | 8408 (10489) | 6176 (3685 to 10353) | 6264 (3741 to 10491) | |
*Predicted costs are for female, 55 years old, not dead in the postswitch period, not withdrawn SB4 within 0–180 days, previous duration of ETA treatment >5 years and with no previous comorbidities.
†For primary sector, no specific data are available for health utilisation thus only costs are shown.
‡Defined daily dose of medication total costs.
The majority (≈80%) of outpatient costs were related to diseases of the musculoskeletal system and connective tissue whereas it for inpatient costs was the minority (21%–24% of costs). In general, the adjusted health costs were lower than the unadjusted costs, for example, number of inpatient admissions, hospital days and inpatient costs (table 2) due to the adjustment for no previous comorbidities.
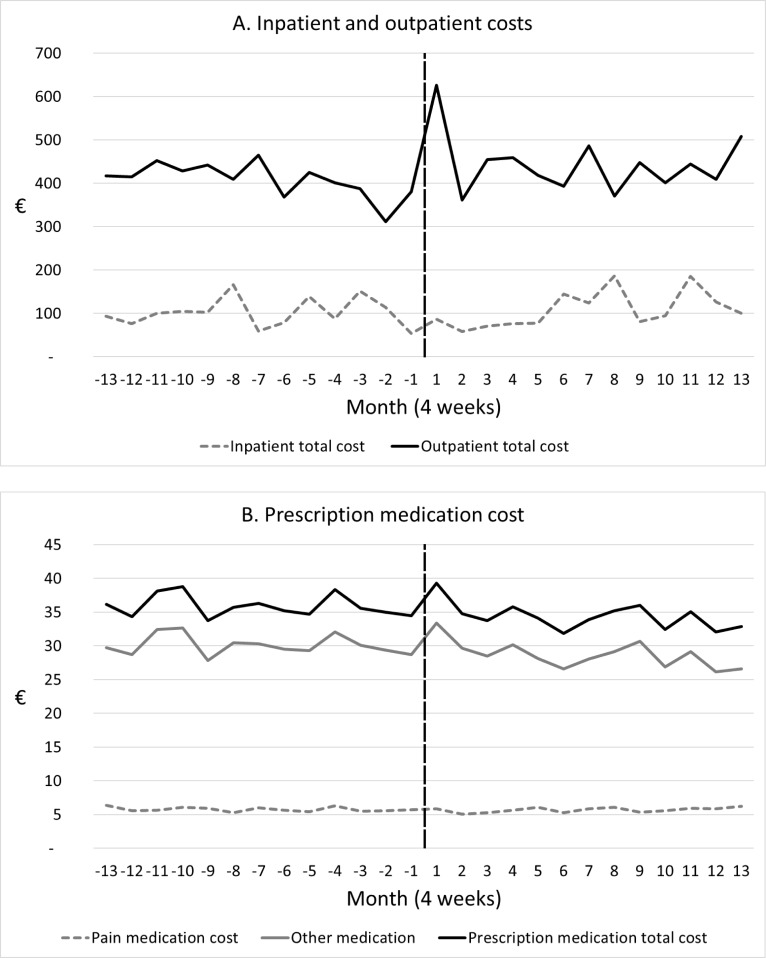
There was in increase in outpatient health costs during the first month after switch (figure 1A). This time-period included date of switch (=index date) and illustrated that patients often received a service in outpatient care in relation to switching. Health costs (inpatient and outpatient) and costs of medication fluctuated throughout the 2-year interval with no clear pattern (figure 1A,B). Similar results were seen for primary sector (not shown).
Figure 1.
Monthly average costs 12 months before and after switch from originator to biosimilar etanercept (n=1620 patients). Black dotted vertical line illustrates time of switch. (A) Inpatient and outpatient costs, (B) prescription medication costs.
Changes in health utilisation and costs after the switch
There was an 8% increase in number of outpatient visits after the switch (estimate 1.08 (95% CI 1.05 to 1.11)). On the other hand, number of inpatient days and number of days in hospital decreased (table 3) keeping in mind that the total number of patients with inpatient services was low (table 2).
Table 3.
(A) Changes in health utilisation, and (B) changes in costs per patient 12 months before and after switch including adjusting variables*
| After* | |||
| Exp (estimate), 95% CI | P value | ||
| (A) Changes in health utilisation per patient | |||
| Inpatient services | Number of inpatient admissions | 0.45 (0.37 to 0.54) | <0.0001 |
| Number of days in hospital | 0.36 (0.25 to 0.51) | <0.0001 | |
| Outpatient | Number of outpatient visits | 1.08 (1.05 to 1.11) | <0.0001 |
| Medication† | Pain medication, DDD | 0.99 (0.96 to 1.03) | 0.702 |
| Other medication | 1.03 (1.00 to 1.05) | 0.051 | |
| Total | 1.02 (1.00 to 1.04) | 0.100 | |
| (B) Changes in health costs per patient | |||
| Inpatient services | Diseases of the musculoskeletal system and connective tissue | 0.70 (0.44 to 1.10) | 0.119 |
| Other diseases | 0.81 (0.62 to 1.05) | 0.114 | |
| Total | 0.82 (0.65 to 1.04) | 0.100 | |
| Outpatient services | Diseases of the musculoskeletal system and connective tissue | 1.05 (1.00 to 1.10) | 0.057 |
| Other diseases | 1.24 (1.09 to 1.41) | 0.001 | |
| Total | 1.07 (1.03 to 1.12) | 0.001 | |
| Primary sector | General practitioner | 1.03 (0.99 to 1.08) | 0.130 |
| Other practicing specialist | 0.97 (0.85 to 1.10) | 0.603 | |
| Physiotherapist, chiropractor, foot specialist | 0.97 (0.92 to 1.02) | 0.258 | |
| Other | 0.94 (0.88 to 1.02) | 0.140 | |
| Total | 0.99 (0.95 to 1.02) | 0.426 | |
| Medication | Pain medication | 0.98 (0.93 to 1.03) | 0.331 |
| Other medication | 0.95 (0.91 to 1.00) | 0.061 | |
| Total | 0.95 (0.92 to 0.99) | 0.019 | |
| Total costs | 1.04 (0.99 to 1.09) | 0.138 | |
Statistically significant results are marked with bold types.
*Adjusted for gender, age, dead in post period, duration of ETA before index date, stopped SB4 treatment within 180 days and comorbidities (WHO chapters) 12–24 months before index date.
†Defined daily dose of medication total costs (DDD).
There was a 7% increase in costs of outpatient services after the switch due to a 24% increase in costs unrelated to diseases of the musculoskeletal system and connective tissue (table 3). Prescription medication total costs decreased 5% after switch.
Population characteristics influencing change in health utilisation and costs after switch
Gender had no influence on changes in health utilisation or costs after switching (table 4). Higher age (3% per year increase) was associated with higher inpatient total costs related to diseases of the musculoskeletal system and connective tissue diseases (table 4).
Table 4.
Regression model estimating the influence of patient characteristics on (A) changes in health utilisation after switch, and (B) changes in costs after switch. Models include interaction term ‘after’ and adjusting variables†
| Interaction term | |||||||
| After*gender (Male=1) | After*age/year | After*ETA duration‡ | |||||
| Exp (estimate) | P value | Exp (estimate) | P value | Exp (estimate) | P value | ||
| (A) Changes in health utilisation per patient | |||||||
| Inpatient services | Number of inpatient admissions | 0.98 (0.64–1.48) | 0.907 | 1.01 (0.99–1.03) | 0.208 | 1.11 (0.97–1.27) | 0.136 |
| Number of days in hospital | 0.83 (0.40–1.73) | 0.622 | 1.02 (0.99–1.04) | 0.200 | 1.12 (0.87–1.43) | 0.392 | |
| Outpatient | Number of outpatient visits | 1.01 (0.95–1.07) | 0.826 | 1.00 (1.00–1.00) | 0.841 | 1.07 (1.05– 1.19) | <0.0001 |
| Medication | Pain medication, DDD | 1.01 (0.94–1.09) | 0.777 | 1.00 (0.99–1.00) | 0.763 | 1.01 (0.99–1.04) | 0.293 |
| Other medication | 0.98 (0.93–1.03) | 0.431 | 1.00 (0.99–1.00) | 0.383 | 1.00 (0.98–1.02) | 0.767 | |
| Total | 0.98 (0.94–1.03) | 0.477 | 1.00 (0.99–1.00) | 0.344 | 1.01 (0.99–1.02) | 0.571 | |
| (B) Changes in health costs per patient | |||||||
| Inpatient services | Diseases of the musculoskeletal system and connective tissue | 0.61 (0.23–1.61) | 0.321 | 1.03 (1.00–1.05) | 0.066 | 1.19 (0.88–1.66) | 0.251 |
| Other diseases | 0.99 (0.58–1.72) | 0.984 | 1.03 (1.01– 1.05) | 0.002 | 1.00 (0.82–1.21) | 0.966 | |
| Total | 0.97 (0.60–1.58) | 0.903 | 1.03 (1.01– 1.04) | 0.002 | 1.02 (0.87–1.21) | 0.784 | |
| Outpatient services | Diseases of the musculoskeletal system and connective tissue | 1.08 (0.98–1.19) | 0.126 | 1.00 (0.99–1.00) | 0.976 | 1.04 (1.00–1.08) | 0.072 |
| Other diseases | 1.15 (0.89–1.48) | 0.284 | 1.00 (0.99–1.01) | 0.304 | 1.10 (1.01– 1.19) | 0.028 | |
| Total | 1.08 (0.99–1.18) | 0.093 | 1.00 (1.00–1.00) | 0.521 | 1.04 (1.00– 1.08) | 0.036 | |
| Primary sector | General practitioner | 1.07 (0.98–1.16) | 0.153 | 1.00 (1.00–1.00) | 0.682 | 1.04 (1.01– 1.08) | 0.007 |
| Other practicing specialist | 1.04 (0.80–1.36) | 0.756 | 1.00 (0.99–1.01) | 0.668 | 0.99 (0.89–1.09) | 0.810 | |
| Physiotherapist, chiropractor, foot specialist | 1.02 (0.91–1.15) | 0.705 | 1.00 (1.00–1.00) | 0.256 | 0.99 (0.95–1.04) | 0.754 | |
| Other | 1.06 (0.90–1.24) | 0.484 | 1.00 (0.99–1.00) | 0.719 | 0.98 (0.92–1.04) | 0.465 | |
| Total | 1.05 (0.98–1.13) | 0.176 | 1.00 (1.00–1.00) | 0.423 | 1.00 (0.98–1.03) | 0.769 | |
| Medication§ | Pain medication | 0.99 (0.88–1.10) | 0.823 | 1.00 (1.00–1.00) | 0.957 | 1.03 (0.99–1.07) | 0.119 |
| Other medication | 0.94 (0.85–1.04) | 0.214 | 1.00 (0.99–1.00) | 0.375 | 1.00 (0.97–1.04) | 0.835 | |
| Total | 0.96 (0.88–1.04) | 0.287 | 1.00 (1.00–1.00) | 0.404 | 1.01 (0.98–1.03) | 0.513 | |
| Total costs | 1.03 (0.94–1.14) | 0.516 | 1.00 (1.00–1.01) | 0.085 | 1.03 (0.99–1.07) | 0.146 | |
Statistically significant results are marked with bold types.
†Adjusted for gender, age, dead in post period, duration of ETA before index date, stopped SB4 treatment within 180 days and comorbidities 12–24 months before index date.
‡ETA duration as categories.
§Medication handled by prescription included (ETA, SB4 not included).
Longer duration of ETA treatment before switch was related to increased number of outpatient visits after switch (1.07 (1.05–1.19)), higher outpatient total costs (1.04, p=0.036) and costs related to general practitioners (1.04 (1.01–1.08)).
We also tested whether previous comorbidities were associated with changes in health utilisation and health costs (see online supplementary table 1A,B). Thus, comorbidities with estimates below one was associated with a decrease whereas estimates above one was associated with increase. Overall, there was no signal that any comorbidity was associated with changes in both health utilisation and costs.
rmdopen-2019-001016supp001.pdf (23.2KB, pdf)
Discussion
In this observational study of 1620 patients who switched from originator to biosimilar etanercept following a mandatory nationwide guideline, we detected no negative impact of the switch on the use and costs of a range of healthcare services during the first year. The fluctuations in the monthly use of services were similar during 1 year before and after the switch, and although there was a minor increase in the use and costs of outpatient services, there were on the other hand decreases in the use of inpatient services and (other than biological) medication costs.
The efficacy and safety of biosimilars have been demonstrated in randomised clinical trials on marketing.3 Recent consensus-based recommendations from the European League Against Rheumatism stated that biosimilars should be considered equal to their bio-originators.25 However, performance of biosimilars when extrapolating across indications or especially when switching long-term treated patients in remission has been a subject of debate.26–28 Thus, there has been a great interest in exploring these matters in observational studies of patients in routine care as these would allow investigation of large scale switch-outcomes in patients with inflammatory arthritis.29 30
We have previously demonstrated that the nationwide Danish mandatory switch from originator to biosimilar etanercept had no negative impact on disease activity and flare rates within 6 months after the switch.12 However, patient related factors and non-specific drug effects seemed to affect retention to biosimilar—as previously demonstrated in other switch cohorts.15 31 The existence of a nocebo effect that is, poor performance of a treatment due to negative expectations has been widely discussed when it comes to the biosimilar biological treatments.7 26 28 32 Thus, it has been demonstrated that patients and physicians may be reluctant to use the biosimilars.6 It might be speculated if existence of a nocebo effect might cause a higher need for consultations in inpatient care following the switch due to higher need for guidance and education. In the current study, there was a slight (7%) increase in costs of inpatient services following the switch which was mainly associated with non-musculoskeletal diseases. Bearing in mind that services provided on the switch-date was attributed to the post-switch period, there seemed to be was no strong evidence of a higher need of consultations following the switch. The optimum strategy for performing switch to biosimilar in routine care has been discussed and switching has been feared to have a negative impact on the patient–physician relationship.17 Shared decision making and non-mandatory switch procedures in order to include the patients’ perspectives in the switch process might potentially improve switch outcomes.17 33 Notably, no extra resources or specific education were allocated to the healthcare personnel in Denmark in relation to performing the switch from originator to biosimilar etanercept. It was beyond the scope of our study to explore these aspects of the switch process further.
This study adds important new knowledge to the emerging pool of outcome studies of biosimilar switch by investigating the impact on healthcare use and costs. We investigated whether we could identify any patient population that experienced changes in use and costs of healthcare services following the switch. We found an increasing use in patients that had previously been treated with ETA for a longer time-period—however, this only applied to outpatient, not to other services. The impact of higher age was less clear, whereas gender and comorbidities were unassociated. This might illustrate that patients that have been treated with ETA for several years deserve extra attention during the switch process. Results must however be interpreted with caution due to few in-hospital services during the study period.
We have previously investigated the impact of non-medical switch on the use of healthcare resources among patients with inflammatory arthritis treated with infliximab—and concluded that no major changes could be observed.16 Those previous data focused on services provided in outpatient rheumatology care. In the present study we expanded the health-economic investigation to include inpatient care, use of medications other that the bio-similar and services provided in the primary sector.
The study has strengths and limitations to consider. DANBIO is virtually complete (>95%) when it comes to the registration and monitoring of patients treated with biological DMARDs. All Danish departments of rheumatology were invited to specifically validate the treatment and the switch date. By use of social security numbers, linkage to other national registries was possible. The strength of these registries are high completeness and unbiased prospective collection of data in the Danish population. In the calculation of average healthcare costs and resources we defined a ‘standard person’ as a 55 years old woman, not dead in the post-switch period, not withdrawn from SB4 within 0–180 days, with previous duration of ETA treatment >5 years and with no previous comorbidities. The adjusted averages and costs were considerably lower than the corresponding unadjusted numbers—probably because many patients had comorbidities and these are associated with higher use of resources. Patients served as their own controls in the statistical analyses of use before and after the switch. Thus, confounding by indication (ie, the decision to switch to treatment with SB4) is expected to be minimal. The medication costs of ETA and SB4 were not included in the calculation of healthcare costs since biological treatments were not provided by prescription but by the hospital. In addition, the drug prices were not publicly available.
In conclusion, following a nationwide mandatory non-medical switch from originator to biosimilar etanercept we demonstrated no obvious changes in use and costs of healthcare services during the first year after the switch.
Acknowledgments
We thank all the Danish departments of rheumatology, which report to the DANBIO registry. Especially we would like to thank the colleagues, who validated the switch dates in DANBIO: Anne Gitte Loft, Emina Omerovic, Oliver Hendricks, Asta Linauskas, Jakob Espesen, Kamilla Danebod, Dorte Vendelbo Jensen, Henrik Nordin, Emil Barner Dalgaard, Stavros Chrysidis, Salome Kristensen, Johnny Lillelund Raun, Hanne Lindegaard, Natalia Manilo, Susanne Højmark Jakobsen, Inger Marie Jensen Hansen, Dorte Dalsgaard Pedersen, Inge Juul Sørensen, Lis Smedegaard Andersen, Jolanta Grydehøj. The work of IT consultant Niels Steen Krogh, Zitelab Aps, who extracted data from DANBIO is acknowledged.
Footnotes
Contributors: RI and JK performed analysis of raw data. BG contributed interpretation of data from DANBIO. All authors contributed to study design, interpretation of results and the preparation of manuscript.
Funding: The study was partly funded by Pfizer, who had no access to raw data and had no influence on the preparation of this manuscript or on the decision to publish these data.
Competing interests: BG: Abbvie, Biogen, Pfizer. MH: Abbvie, Biogen, BMS, CellTrion, MSD, Novartis, Orion, Pfizer, Samsung, UCB. JK: Novo Nordisk, Pfizer, Roche, Celgene.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Dörner T, Strand V, Cornes P, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis 2016;75:974–82. 10.1136/annrheumdis-2016-209166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdes S, Thaçi D, Griffiths CEM, et al. Multiple switches between GP2015, an etanercept biosimilar, with originator product do not impact efficacy, safety and immunogenicity in patients with chronic plaque-type psoriasis: 30-week results from the phase 3, confirmatory EGALITY study. J Eur Acad Dermatol Venereol 2018;32:420–7. 10.1111/jdv.14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emery P, Vencovský J, Sylwestrzak A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2017;76:51–7. 10.1136/annrheumdis-2015-207588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulácsi L, Brodszky V, Baji P, et al. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol 2015;11:43–52. 10.1586/1744666X.2015.1090313 [DOI] [PubMed] [Google Scholar]
- 5.Kristensen LE, Alten R, Puig L, et al. Non-Pharmacological effects in switching medication: the nocebo effect in switching from Originator to Biosimilar agent. BioDrugs 2018;32:397–404. 10.1007/s40259-018-0306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waller J, Sullivan E, Piercy J, et al. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence 2017;11:519–30. 10.2147/PPA.S129333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odinet JS, Day CE, Cruz JL, et al. The Biosimilar nocebo effect? A systematic review of double-blinded versus open-label studies. J Manag Care Spec Pharm 2018;24:952–9. 10.18553/jmcp.2018.24.10.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezk MF, Pieper B. Treatment outcomes with biosimilars: be aware of the nocebo effect. Rheumatol Ther 2017;4:209–18. 10.1007/s40744-017-0085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araújo FC, Gonçalves J, Fonseca JE. Pharmacoeconomics of biosimilars: what is there to gain from them? Curr Rheumatol Rep 2016;18:50 10.1007/s11926-016-0601-0 [DOI] [PubMed] [Google Scholar]
- 10.The Danish regions, rads, guidelines for use of biosimilar infliximab and etanercept. Available: http://www.regioner.dk/media/3488/rads-notat-om-anvendelsen-af-biosimilaere-juni-2016.pdf [Accessed May 2019].
- 11.Ibfelt EH, Jensen DV, Hetland ML. The Danish nationwide clinical register for patients with rheumatoid arthritis: DANBIO. Clin Epidemiol 2016;8:737–42. 10.2147/CLEP.S99490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glintborg B, Loft AG, Omerovic E, et al. To switch or not to switch: results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. one-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann Rheum Dis 2019;78:192–200. 10.1136/annrheumdis-2018-213474 [DOI] [PubMed] [Google Scholar]
- 13.Hetland ML. DANBIO-powerful research database and electronic patient record. Rheumatology 2011;50:69–77. 10.1093/rheumatology/keq309 [DOI] [PubMed] [Google Scholar]
- 14.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39(7 Suppl):30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 15.Glintborg B, Sørensen IJ, Loft AG, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis 2017;76:1426–31. 10.1136/annrheumdis-2016-210742 [DOI] [PubMed] [Google Scholar]
- 16.Glintborg B, Sørensen J, Hetland ML. Does a mandatory non-medical switch from originator to biosimilar infliximab lead to increased use of outpatient healthcare resources? A register-based study in patients with inflammatory arthritis. RMD Open 2018;4:e000710 10.1136/rmdopen-2018-000710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherlinger M, Langlois E, Germain V, et al. Acceptance rate and sociological factors involved in the switch from originator to biosimilar etanercept (SB4). Semin Arthritis Rheum 2019;48:927–32. 10.1016/j.semarthrit.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 18.http://www.esundhed.dk/dokumentation/Registre/Sider/Variabel.aspx?rp:A_Register=14&rp:B_Tabel=63&rp:C_Variabel=399& [Accessed May 2019].
- 19.Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, et al. Existing data sources for clinical epidemiology: the Danish national database of Reimbursed prescriptions. Clin Epidemiol 2012;4:303–13. 10.2147/CLEP.S37587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webpage, Sundhedsdatastyrelsen. Available: https://sundhedsdatastyrelsen.dk/da/afregning-og-finansiering/takster-drg/takster-2016 [Accessed May 2019].
- 21.https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/sundhedsoekonomi-og-finansiering/sygesikringsregisteret [Accessed May 2019].
- 22.https://en.wikipedia.org/wiki/Health_21 [Accessed May 2019].
- 23.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J Health Econ 2004;23:525–42. 10.1016/j.jhealeco.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 24.Wang M. Generalized estimating equations in longitudinal data analysis: a review and recent developments. Adv Stat 2014;2014:1–11. 10.1155/2014/303728 [DOI] [Google Scholar]
- 25.Kay J, Schoels MM, Dörner T, et al. Consensus-Based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis 2018;77:165–74. 10.1136/annrheumdis-2017-211937 [DOI] [PubMed] [Google Scholar]
- 26.Cantini F, Benucci M. Switching from the bio-originators to biosimilar: is it premature to recommend this procedure? Ann Rheum Dis 2019;78:e23 10.1136/annrheumdis-2017-212820 [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann R. Editorial: The American College of Rheumatology white paper on biosimilars: it isn't all white-there is some gray and black. Arthritis Rheumatol 2018;70:323–5. 10.1002/art.40402 [DOI] [PubMed] [Google Scholar]
- 28.Cantini F, Benucci M. Mandatory, cost-driven switching from originator etanercept to its biosimilar SB4: possible fallout on non-medical switching. Ann Rheum Dis 2018. doi: 10.1136/annrheumdis-2018-214757 [Epub ahead of print 28 Nov 2018]. 10.1136/annrheumdis-2018-214757 [DOI] [PubMed] [Google Scholar]
- 29.Uhlig T, Goll GL. Reviewing the evidence for biosimilars: key insights, lessons learned and future horizons. Rheumatology 2017;56(suppl_4):iv49–62. 10.1093/rheumatology/kex276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kay J, Dörner T, Emery P, et al. Clinical trial and ‘real-world’ data support switching from a bio-originator to its biosimilar. Ann Rheum Dis 2019. doi: 10.1136/annrheumdis-2018-214994 [Epub ahead of print 18 Jan 2019]. 10.1136/annrheumdis-2018-214994 [DOI] [PubMed] [Google Scholar]
- 31.Tweehuysen L, van den Bemt BJF, van Ingen IL, et al. Subjective complaints as the main reason for Biosimilar discontinuation after open-label transition from reference infliximab to Biosimilar infliximab. Arthritis Rheumatol 2018;70:60–8. 10.1002/art.40324 [DOI] [PubMed] [Google Scholar]
- 32.Scherlinger M, Germain V, Labadie C, et al. Switching from originator infliximab to biosimilar CT-P13 in real-life: the weight of patient acceptance. Joint Bone Spine 2018;85:561–7. 10.1016/j.jbspin.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 33.Scherlinger M, Schaeverbeke T. 'To switch or not to switch': the missing piece in the puzzle of biosimilar literature? Ann Rheum Dis 2019. doi: 10.1136/annrheumdis-2018-214908 [Epub ahead of print 4 Jan 2019]. 10.1136/annrheumdis-2018-214908 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2019-001016supp001.pdf (23.2KB, pdf)