Abstract
Objective
To evaluate the demographics, aetiologies, complications, treatments and visual prognoses of chronic and recurrent non-infectious paediatric-onset uveitis in France.
Methods
Descriptive, retrospective and bicentric study in patients whose disease started before 17 and who were followed up in two centres from January 2010 to May 2017.
Results
We included 147 patients with 268 affected eyes. Eighty-two had juvenile idiopathic arthritis-associated chronic uveitis, 58 were antinuclear antibody (ANA) positive and 24 were ANA negative, 36 had idiopathic uveitis, 9 had enthesitis-related arthritis-associated uveitis, 9 had sarcoidosis-associated uveitis and 11 had other inflammatory aetiologies. These patients cumulated 161 complications: ocular hypertension, cataract, band keratopathy, macular oedema, optic disk oedema and decreased visual acuity, including permanent visual loss for 31 patients. The most used treatments were corticosteroid (CS) eye drops (82%), systemic CSs (34%), methotrexate (58%) and biologics (38%). At the latest follow-up, 45 patients had achieved remission of uveitis without any treatment, 56 had inactive uveitis on topical steroids and 48 still had active uveitis.
Conclusion
Paediatric-onset uveitis are associated with a high rate of complications. However, following the introduction of biologics and particularly antitumour necrosis factor alpha antibodies, a significant proportion of uveitis became inactive on or even off treatment.
Keywords: anti-TNF, juvenile idiopathic arthritis, treatment
Key messages.
What is already known about this subject?
Uveitis is known to be a severe complication of inflammatory diseases.
What does this study add?
This retrospective study establishes an overview of medical practices with a large cohort of patients with non-infectious paediatric-onset chronic or recurrent uveitis.
How might this impact on clinical practice?
Joint care between paediatric rheumatologists and ophthalmologists is essential to prevent uveitis-related complications.
Biotherapies show promising results with a good efficacy and tolerance in uveitis management.
Introduction
Paediatric uveitis is a rare but severe disease, leading to ocular complications in 30%–60% of patients1 2 and legal blindness in 7%–23% of patients.3 4 Paediatric uveitis must be distinguished from adult uveitis with regard to its manifestations, aetiology and management. The overall incidence and prevalence of paediatric uveitis are estimated to be 4.9 and 30.0 per 100 000 children in Europe and America5–8
In most cases, pediatric-onset non-infectious chronic uveitis (pNICU) is associated with early-onset, antinuclear antibody (ANA)-positive juvenile idiopathic arthritis (JIA). Both the aetiological diagnosis and treatment of pNICU are a challenge. Corticosteroids (CSs) and immunomodulatory drugs are the most common treatments.5 9–11 Topical CSs are indicated for non-infectious, anterior paediatric uveitis as first line, regardless of the aetiology. However, long-term CS therapy is associated with side effects, including ocular hypertension (OHT) and cataract. Methotrexate (MTX) has been widely used as second-line therapy.12 Biologics and in particular antitumour necrosis factor (TNF) alpha treatments demonstrated, their efficacy, particularly in JIA-associated or idiopathic anterior pNICU in children13–16 and intermediate, posterior and panuveitis in adults.17 However, there are still few data on patients’ medium-term to long-term outcomes at the era of biological therapy. Here, we analysed the aetiologies, characteristics and outcome of pNICU or pediatric-onset recurrent uveitis in patients followed up in two French tertiary care centres.
Materials and methods
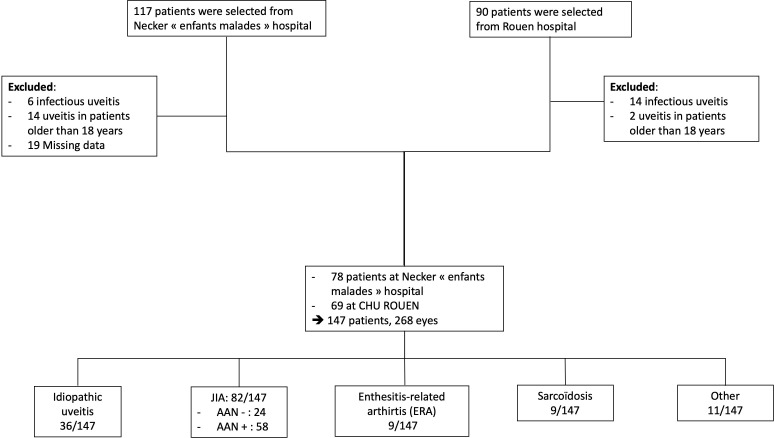
This was a retrospective, observational, descriptive study of patients with pNICU or paediatric-onset non-infectious recurrent uveitis followed between 2010 and 2017 in two French tertiary care centres: Necker Enfants-Malades Hospital in Paris and the University Hospital of Rouen. The exclusion criteria were infectious uveitis, age over 16 at uveitis onset or incomplete data. Medical reports were examined by a paediatrician experienced in paediatric rheumatology and an opthalmologist (figure 1).
Figure 1.
Flowchart. JIA, juvenile idiopathic arthritis.
Patients’ files were collected using the French database for rare diseases, CEMARA,18 with an agreement of the French Commission Nationale Informatique et Libertés.
Data retrieval and analysis included ocular and systemic symptoms, personal and family medical history, current age, age at diagnosis of uveitis, age at first visit, gender, laterality of the uveitis, duration and course of the disease, and follow-up period. We recorded any inflammatory syndrome (elevated erythrocyte sedimentation rate or C reactive protein) only at the uveitis diagnosis. We also recorded auto-antibody (including ANA), elevated ACE, positivity of HLA B27, visual acuity (VA) at presentation and at the last visit, use of local and/or systemic medical therapies, ocular surgeries and complications occurring at any time point.
We collected six different types of aetiologies: ANA+JIA, ANA−JIA, enthesitis-related arthritis (defined by the occurrence of arthritis and enthesitis and at least two of the following criteria: sacroiliac pain and/or inflammatory rachialgia, acute anterior uveitis, presence of HLA B27), sarcoidosis, idiopathic uveitis and other inflammatory diseases.
The recorded complications included band keratopathy, posterior synechiae, OHT, which was defined by an intraocular pressure greater than 21 mm Hg, glaucoma (hypertension associated with lesions of the optic nerve), cataract, macular oedema (detected by optical coherence tomography), papilloedema, posterior synechiae, band keratopathy and decrease VA. All data were collected at diagnosis of uveitis; at 6, 12 and 24 months; and at the latest follow-up.
The diagnosis of uveitis was established based on the recommendations of the International Standardisation of Uveitis Nomenclature. The uveitis was classified as anterior, intermediate, posterior or panuveitis, based on its level of inflammation, which was established by the grading of the cells and flare in the anterior chamber and haze in the vitreous. The course and duration of uveitis were also based on the SUN guidelines.19 20
The SUN criteria were applied to determine if the uveitis was recurrent (repeated episodes separated by periods of inactivity without treatment >3 months after discontinuing treatment) or chronic (chronic persistent uveitis with a relapse within 3 months after discontinuing treatment). The severity of inflammation was graded on a scale of 0–4. Improvement was defined by a decrease of at least 2 points and aggravation by an increase of at least 2 points or worsening from 3 to 4 according to the SUN. Patients with inactive disease for at least 6 months after discontinuation of all treatments for eye disease (including CS eye drops) were considered in remission. The active uveitis was characterised according to Standardization of Uveitis Nomenclature (SUN) criteria (grade cells >1) as for inactive uveitis (grade 0 cells applies to the anterior chamber).
Results
Patient characteristics
Patient characteristics are summarised in table 1.
Table 1.
Patient characteristics
| Cohort n=147 |
ANA+JIA, n=58 (40%) |
ANA−JIA, n=24 (16%) | Idiopathic uveitis, n=36 (25%) | ERA, n=9 (6%) |
Sarcoidosis, n=9 (6%) | Others uveitis, n=11 (7%) | |
| Age at inflammatory disease diagnostic (years) Mean±SD (n–N) |
6.5±4 (1.5–17) | 4±3 (1–13) | 4.4±2 (1.5–10) | 9.1±4.2 (1–17) | 11±4.1 (3–16) | 7.7±4.8 (5–12) | 9.9±4.8 (2.5–15) |
| Age at uveitis diagnostic (years) |
7.4±4.2 (1.5–17) | 5±3 (1.5–15) | 6±2.5 (3–13) | 9.1±4.2 (1–17) | 11.8±4.2 (4–16) | 10.5±2.4 (7–14) | 10.9±4.4 (2–17) |
| Sex, n (%) Female |
107 (74) | 51 (87) | 22 (91) | 17 (48) | 5 (55) | 5 (55) | 7 (63) |
| Follow-up (months) | 52±37.8 (3–168) | 62.5±39 (3–168) | 56±40.5 (3–156) | 40±32 (6–168) | 28±25.5 (3–84) | 52±27.6 (20–84) | 43±40 (5–132) |
| Laboratory analysis, n (%) | |||||||
| ANA | 76 (52) | 58 | 0 | 14 (39) | 2 (22) | 0 | 2 (18) |
| HLA B27 | 12 (8) | 1 | 2 | 0 | 9 | 0 | 0 |
| High ACE | 9 (6) | 0 | 0 | 0 | 0 | 9 | 0 |
| ESR, mean±SD (n – N) | 24±26.6 (1–120) | 36.4±33 (1–120) | 21±20 (1–63) | 14.4±17 (1–65) | 13±8 (1–28) | 12±14 (2–40) | – |
| CRP mean±SD (n – N) | 14.5±26 (1–197) | 16±23.8 (1–197) | 22±32 (4–100) | 5±5.5 (5–9) | 14±25 (5–78) | 5±4.6 (1–15) | – |
| Uveitis location, n (%) | |||||||
| Anterior | 137 (93) | 58 | 24 | 32 (94) | 9 | 7 (77) | 9 (81) |
| Intermediate | 2 (1) | 0 | 0 | 1 (3) | 0 | 0 | 0 |
| Posterior | 1 | 0 | 0 | 0 | 0 | 0 | 1 (10) |
| Panuveitis | 7 (6) | 0 | 0 | 3 (8) | 0 | 2 (23) | 2 (18) |
| Unilateral, n (%) Bilateral, n (%) |
36 (21) 116 (79) |
8 (14) 50 (86) |
6 (25) 18 (75) |
10 (27) 26 (73) |
7 (77) 2 (23) |
0 9 (100) |
2 (18) 9 (82) |
| Granulomatous uveitis, n (%) | 18 (12) | 2 (3) | 4 (16) | 9 (31) | 0 | 2 (23) | 1 (10) |
| Signs at onset, n (%) | |||||||
| Synechiae | 40 (27) | 16 (27) | 11 (45) | 8 (22) | 0 | 3 (33) | 2 (18) |
| Vision loss | 40 (27) | 10 (17) | 7 (30) | 12 (33) | 1 (11) | 4 (44) | 6 (54) |
| SUN classification, cells, at diagnostic, n (%) | |||||||
| SUN 0.5+ | 22 (15) | 14 (25) | 3 (12) | 3 (8) | 1 (11) | 0 | 1 (10) |
| SUN 1+ | 50 (34) | 13 (23) | 12 (50) | 16 (45) | 5 (55) | 2 (22) | 2 (18) |
| SUN 2+ | 60 (41) | 24 (42) | 9 (38) | 15 (41) | 2 (22) | 6 (66) | 4 (36) |
| SUN 3+ | 15 (10) | 6 (10) | 1 (4) | 2 (5) | 1 (11) | 1 (11) | 4 (36) |
ANA, antinuclear antibody; CRP, C reactive protein; ERA, enthesitis-related arthritis; ESR, erythrocyte sedimentation rate; JIA, juvenile idiopathic arthritis; SUN, Standardization of Uveitis Nomenclature.
One hundred forty-seven paediatric patients with uveitis were identified and 107 (74 %) were female. Uveitis was discovered during a systematic consultation in 50 patients (either during the initial consultation or during ophthalmological follow-up) and before the manifestation of any arthritic or systemic symptom in nine patients. The most common diagnoses were JIA-associated uveitis (82/147, 56%) and idiopathic uveitis (36/147, 25%). Other baseline characteristics are summarised in table 1.
Treatments
One hundred twenty-one patients were treated in first intention with topical CS alone or in combination. Oral CSs were used in 51 out of 147 patients (34%) and intravenous CSs were used in 12 (8%). Twenty-eight patients with JIA had CS (34% of JIA-related uveitis) and 23 were not JIA related (36%)
Disease-modifying antirheumatic drugs (DMARDs) were used as second-line therapy in 88 patients (62%), MTX was used in 84 patients and azathioprine in 4.
Sixty patients (41%) underwent biological treatment alone or in combination with MTX: adalimumab in 44 patients (73 %), etanercept in 7, 4 of whom failed to respond and had to switch to adalimumab, tocilizumab in 1 patient, and anakinra and abatacept in 2 patients each.
If we analyse the differences in management before and after 2010, we have two groups of uveitis: before 2010 (n=42) and after 2010 (n=105).
The delay between the diagnosis and the introduction of DMARDs is 20.8 months (n–N: 0–84) before 2010 and 8.7 months (n–N: 0–36) after 2010.
The delay between the DMARDs and the biologic agents is 25 months (n–N: 0–84) before 2010 and 11.5 months (n–N: 0–29) after 2010.
At the last consultation, 46 patients had active uveitis, 56 had inactive uveitis still undergoing treatment and 45 had uveitis in complete remission off treatment. We identified three situations at the last follow-up: uveitis in remissions according to SUN criteria in patients with an inactive disease for at least 6 months after discontinuing topical CS, active uveitis according to SUN (grade cells >0) and inactive uveitis (grade 0 cells in the anterior chamber according to the SUN) under treatment.
Patients with active uveitis or inactive uveitis on topical CS
Among 104 patients still receiving topical CS at the latest follow-up, 56 had inactive uveitis; the distribution by aetiology is described in table 2. Additionally, out of 16 patients under oral CS treatment during the follow-up, 8 still had oral CS it at the last visit. Thirty-six patients had received MTX, as a sole immunomodulatory drug in 7 patients or in 29 patients who had failed to respond to MTX monotherapy, combined to a biologic agent. The biologics used were adalimumab for 23 patients (20 JIA, 2 idiopathic uveitis and 1 Behçet disease) and etanercept for 2 patients, 1 of whom had to switch to adalimumab for non-response.
Table 2.
Uveitis follow-up
| All uveitis (n=147) | ANA+JIA (n=58) | ANA−JIA (n=24) |
ERA (n=9) |
Idiopathic (n=36) | Sarcoidosis (n=9) | Others uveitis* (n=11) | |
| Uveitis in remission off steroids† | |||||||
| Number of cases, n (%) | 45 (30) | 18 (31) | 5 (20) | 5 (55) | 11 (30) | 3 (33) | 3 (27) |
| Follow-up (month), mean±SD (median) | 48±32 (48) | 58±36 (51.5) | 46±37 (60) | 31±31 (13) | 56±43 (48) | 51±29 (48) | 45±39 (48) |
| Age (years) at last consultation, mean (median) | 11.4 (11) | 10 (10) | 10 (9) | 13.8 (15) | 14.1 (14) | 17 | 13.2 (11) |
| DMARDs | 13 (27) | 10 (55) | 0 | 0 | 1 (9) | 1 (33) | 1 (10) |
| Biological therapy | 13 (29) | 5 (27) | 1 (25) | 2 (33) | 1 (9) | 0 | 2 (20) |
| Adalimumab | 8 | 3 | 1 | 2 | 1 | 0 | 1 |
| Etanercept | 3 | 2 | 1 | 0 | 0 | 0 | 0 |
| Abatacept | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anakinra | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Infliximab | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Duration of remission (month) | 24±20 (6 – 76) | 22±19 (12) | 38±29(24) | 15±8 (12) | 25±2124 | 12 (12) | 30±36 (6) |
| Inactive uveitis on topical steroids‡ | |||||||
| Number of cases, n (%) | 56 (39) | 21 (35) | 13 (54) | 2 (22) | 13 (36) | 2 (22) | 5 (45) |
| Follow-up (month), mean±SD (median) | 51.4±39 (42) | 67±43 (72) | 51±38.5 (42) | 22±10 (22.5) | 35±2630 | 69.5±20 (69.5) | 40.6±5218 |
| Age at last consultation (years), mean (n–N) | 12 (2–21) | 11.4 (2–16) | 10.7 (5–18) | 15(14–15) | 12.6 (2.5–17) | 16.25 (11.5–21) | 14.4 (5–21) |
| Topical corticosteroids | 56 | 21 | 13 | 2 | 13 | 2 | 5 |
| Systemic corticosteroids | 10 (21) | 1 (5) | 1 (8) | 0 | 2 (17) | 2 | 4 (44) |
| DMARDs | 37 (66) | 18 (86) | 11(85) | 0 | 6 (50) | 1 (50) | 1 (11) |
| Biological therapy | 26 (45) | 13 (62) | 9 (70) | 0 | 2 (17) | 0 | 2 (11) |
| Adalimumab | 23 | 13 | 7 | 0 | 2 | 0 | 1 |
| Etanercept | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| Anakinra | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Infliximab | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Duration of remission/inactivity (month), mean±SD (median) | 13.8±11 (4 – 48) | 14.5±11 (12) | 19.5±16 (12) | 9±4.2 (9) | 9.6±8.6 (3) | 9±4.2 (9) | 9.6±3.2 (2) |
| Active uveitis§ | |||||||
| Number of cases, n (%) | 46 (31) | 19 (33) | 6 (26) | 2 (11) | 12 (34) | 4 (44) | 3 (27) |
| Follow-up (month), mean±SD (median) | 54±41 (6 –168) | 64±40 (66) | 73.5±46 (60) | 3 | 30.7±22 (27) | 45.6±31 (24) | 45.6±33.8 (33) |
| Age at last consultation (years), mean (n–N) | 11.4 (4–18) | 10 (4–17) | 11.5 (6.5–17) | 14.5 | 10.8 (6.5–17) | 13.7 (9.5–8) | 14.6 (12.5–18) |
| Topical corticosteroids | 48 | 19 | 6 | 1 | 12 | 4 | 5 |
| Systemic corticosteroids | 9 (19) | 4 (21) | 1 (17) | 0 | 4 (33) | 2 (50) | 0 |
| DMARDs | 31 (73) | 15 (79) | 6 (100) | 0 | 6 (50) | 3 (75) | 4 (40) |
| Biological therapy | 25 (52) | 15 (79) | 4 (67) | 0 | 4 (33) | 2 (50) | 1 (10) |
| Adalimumab | 24 | 15 | 4 | – | 3 | 1 | 1 |
| Etanercept | 2 | 1 | 1 | – | 0 | 0 | 0 |
| Abatacept | 2 | 0 | 1 | – | 0 | 1 | 0 |
| Tocilizumab | 0 | 0 | 0 | – | 1 | 0 | 0 |
| Infliximab | 5 | 2 | 1 | – | 1 | 1 | 0 |
| SUN classification, cells, n (%) | |||||||
| 0+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5+ | 27 (58) | 13 (68) | 4 (67) | 1 | 7 (58) | 1 (75) | 1 (33) |
| 1+¶ | 19 (42) | 6 (32) | 2 (33) | 1 | 5 (42) | 3 (25) | 2 (67) |
*Other aetiologies: 4 Behçet disease, 1 Muckle-Wells syndrome, 1 Cogan syndrome, 2 TINU syndrome, 2 Vogt-Koyanagi-Harada syndrome, 1 systemic-onset JIA.
†According to SUN criteria: patients with inactive disease for at least 6 months after discontinuing all topical steroids.
‡According to SUN criteria: grade >0.5 cells apply to the anterior chamber.
§According to SUN criteria: grade 0 cells apply to the anterior chamber.
¶No patient had a score of >1 at the latest follow-up.
ANA, antinuclear antibody; DMARD, disease-modifying antirheumatic drug; ERA, enthesitis-related arthritis; JIA, juvenile idiopathic arthritis.
Among 104 patients still on topical CS at the latest follow-up, 48 still had active uveitis, 26 were classified as SUN 0.5 and 21 were classified as SUN 1. No case of uveitis with an inflammation level higher than SUN 1+ was observed.
In addition to topical CS, among 16 patients under oral steroids during the follow-up, 9 were still on oral steroids at the last visit. Thirty patients had MTX, 7 patients had MTX as monotherapy and 23 patients had MTX associated with biologics (14 ANA+JIA and ANA−JIA, 4 JIA, 3 idiopathic uveitis and 2 sarcoidosis). The biologic used for 24 patients was adalimumab. Two patients had received etanercept but had switched to adalimumab after 1 year, and one patient was on tocilizumab. In most cases, the use of biological treatment was associated with improvement of uveitis. Regarding side effects under biological treatment, one patient had an anaphylactic reaction to adalimumab and was switched to abatacept, and one patient had psychiatric disorders on adalimumab. There were three infections requiring hospitalisation, no opportunistic infection and no death.
Patients with inactive uveitis off steroids at the latest follow-up
Patients with uveitis in remissions are described in table 2. All patients had first been treated with topical CS, and 23 patients had CS as monotherapy. Eighteen had been treated with MTX, 2 of whom stopped for hepatic cytolysis. Eleven patients were still on MTX at the last visit, and MTX was associated with biologics in four cases. They had all stopped steroid treatment (topical and systemic steroids). Thirteen patients received biologics (adalimumab in 8, etanercept in 3, abatacept in 1 and anakinra), 3 patients were able to stop adalimumab and 1 patient received etanercept.
Complications
Complications are summarised in tables 3 and 4.
Table 3.
Cumulative complications
| Cohort (n=147) |
JIA+ANA (n=58) | JIA−ANA (n=24_ |
ERA (n=9) |
Idiopathic uveitis (n=36) | Sarcoidosis (n=9) |
Other uveitis (n=11) |
|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Patient with a complication | 101 (69) | 43 (72) | 17 (70) | 2 (22) | 24 (67) | 9 | 6 (54) |
| OHT | 57 (39) | 29 (50) | 10 (42) | 2 (22) | 8 (22) | 4 (44) | 4 (36) |
| OHT surgery | 13 (23) | 7 (24) | 4 (40) | – | 2 (25) | – | – |
| Cataract | 32 (22) | 17 (29) | 10 (42) | – | 2 (5) | 1 (11) | 2 (18) |
| Cataract surgery | 13 (38) | 4 (23) | 4 (40) | – | 2 (100) | 1 (100) | 2 (100) |
| Band keratopathy | 19 (13) | 10 (17) | 4 (17) | – | 5 (13) | – | – |
| Macular oedema | 19 (13) | 7 (12) | 3 (12) | – | 4 (11) | 3 (33) | 2 (18) |
| Optic disk oedema | 37 (26) | 11 (19) | 5 (21) | – | 6 (17) | 9 | 6 (54) |
ANA, antinuclear antibody; ERA, enthesitis-related arthritis; JIA, juvenile idiopathic arthritis; OHT, ocular hypertension.
Table 4.
Evolution of visual impairment according to the aetiology and disease outcome
| Cohort (n=147) | JIA+ANA (n=58) | JIA−ANA (n=24) | ERA (n=9) | Idiopathic uveitis (n=36) | Sarcoidosis (n=9) | Others uveitis (n=11) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Irreversible impairement | |||||||
| Legal blindness (LogMAR=1), monocular blindness and LogMAR between 0.9 and 0.4 in fellow eye | 8 (5) | 3 (5) | 2 (8) | 0 | 1 (3) | 1 (11) | 1 (9) |
| Isolated monocular blindness | 9 (6) | 2 (3) | 3 (13) | 1 (11) | 2 (5.5) | 0 | 2 (18) |
| Visual impairment, both eyes (LogMAR between 0.9 and 0.4) | 8 (5) | 3 (5) | 1 (4) | 0 | 2 (5.5) | 1 (11) | 2 (18) |
| Visual impairment, one eye (LogMAR between 0.9 and 0.4) | 6 (4) | 3 (5) | 0 | 0 | 3 (8.3) | 0 | 0 |
| Transient loss | |||||||
| Legal blondness | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Visual impairment, both eyes (LogMAR between 0.9 and 0.4) | 6 (4) | 2 (3) | 0 | 0 | 3 (8.3) | 0 | 1 (9) |
| Visual impairment, one eye (LogMAR between 0.9 and 0.4) | 8 (5) | 1 (1.5) | 1 (14) | 2 (22) | 3 (8.3) | 0 | 1 (9) |
| Total | |||||||
| Overall | 45 (30) | 14 (24) | 7 (38) | 3 (33) | 14 (38) | 2 (22) | 7 (63) |
| Long-term loss | 31 (21) | 11 (19) | 6 (25) | 1 (11) | 8 (22) | 2 (22) | 5 (45) |
| Transient loss | 14 (10) | 3 (5) | 1 (4) | 2 (22) | 6 (16) | 2 (16) | |
ANA, antinuclear antibody; ERA, enthesitis-related arthritis; JIA, juvenile idiopathic arthritis.
The most frequent complication was OHT, which affected 53 patients (36 %) and 111 eyes. In 16 patients, it was already present at the time of diagnosis, in 34 cases it was observed at 12 months, and later in 13 other cases. Thirty-one patients developed cataract, which was present at diagnosis in 8, at 12 months in 9 and at 24 months in 14. Band keratopathy was observed in 19 patients; at diagnosis in 10, at 12 months in 3 and at 24 months in 6. Seventeen patients had a macular oedema, 14 patients were diagnosed during the first ophthalmological consultation and 5 were diagnosed at 12 months. Thirty-seven patients had optic disc oedema, 31 at diagnostic and 6 at 12 months.
VA impairment is shown in table 4. No patient fulfilled the criteria of bilateral legal blindness (ie, best corrected VA <0.1 decimal Snellen in the better eye). Unilateral blindness was documented in 17/147 (11%). Six patients had unilateral VA impairment (VA <0.3 decimal Snellen), and eight patients had bilateral VA impairment. The most frequent cause of blindness was macular oedema, and the most frequent causes of visual impairment were macular oedema and cataract.
Discussion
In this retrospective study, we report a large cohort of patients with non-infectious paediatric-onset chronic or recurrent uveitis. We observed that uveitis was bilateral, anterior, idiopathic or associated to ANA+JIA in most cases. The study shows that a high proportion of patients achieve inactive uveitis, with or without complications, essentially after treatment with MTX or biologics.
This higher rate of JIA in our study compared with other reports5 6 21–23 may be related to a selection bias; indeed, patients had been referred to two paediatric rheumatology centres.
We observed a high level of complication; this rate is comparable for patients with JIA-associated uveitis with that found by Marvillet et al24; Paroli et al1 found a comparable rate of complications as well. Sardar et al25 also found a similar rate also in non-JIA uveitis. Regarding patients’ visual outcome, results from different reports were not always consistent together: Kump et al reported a VA <20/40 in 23% of children with uveitis, de Boer et al reported a VA <20/200 in 19% of cases. Thorne et al reported a VA <20/200 in 23%.2 4 26
In this study, at the end of the follow-up period, only 8% of the eyes had a VA <20/200 and no patients only had a VA <20/200. In our study, visual outcomes in children with AIJ-related uveitis are good in our study, as shown in other recent series.4 26–28
Among the complications, OHT was documented more frequently than in other publications.1 5 27 29 30 Most cases of OHT occurred in patients with JIA-associated or sarcoidosis-associated uveitis. Also, the rate of this complication increases in patients with a longer period between uveitis onset and the referral to the tertiary care centre, especially after several years of active uveitis, as described in other series.30 31
Chronic inflammation and long-lasting local steroid treatment are the two causes widely reported in the OHT literature. We know that one of the particularities in children is the high frequency of cortisone OHTs. In children, it is difficult to distinguish whether symptoms are treatment related or a result of chronic inflammation. Similarly, cataracts were mostly diagnosed in patients with long-lasting active uveitis, who were also undergoing prolonged local steroid treatment.
We noticed a high prevalence of papilloedema, synechiae and CMO at diagnosis. We found that there are two peaks of incidence of complications: the diagnosis of uveitis and 12–18 months of evolution of uveitis under treatment. This result is interesting as this finding supports the importance of early diagnosis and management of effective and steroid-sparing treatments in pNICU, whatever the aetiology.
We found more complications in the ANA+JIA group than in any other groups, which is in line with the difficulty of early diagnosis in such patients.26 32–34
The frequency of use of CSs (orally or intravenously) is quite high in this current series. For uveitis not associated with JIA, this result is not surprising and is consistent with other studies.25 35 For the AJI group, this result is high, but the patients involved are in the majority of cases either severe forms (articular and ocular) or patients whose follow-up began in the early 2000s, where there were little therapeutic alternatives.
The high proportion of patients who received non-biological or biological DMARDs may be linked to a selection bias, as these patients had been referred to tertiary care paediatric rheumatology centres. However, the increase in the use of DMARDs, particularly biological DMARDs, after 2010 indicates a tendency to use such treatments more frequently in this context, as also reported in other countries.5 36 37 The more frequent and earlier introduction of DMARDs in patients treated after 2010 was likely linked to a better communication between ophthalmologists and paediatricians. Indeed, in recent years, thanks to the work of associations, a national effort for rare diseases has been conducted in France. This allowed the development of more common efforts, such as collaborative trials14 and common web conferences. So even in the absence of joint outpatient clinics, the communication between ophthalmologists and paediatricians has improved. It was associated in our series with a lower rate of complications at the latest follow-up, although the follow-up duration was shorter than that in patients treated before 2010. As recent controlled trials demonstrated the efficacy of the anti-TNF antibody adalimumab in controlling pediatric-onset idiopathic or JIA-associated uveitis14 15 and other efforts are on-going with other biologics, more and more patients should receive such treatments in the long run.
Among the biological therapies, we have a majority of adalimumab, with good efficiency. The second biological therapy is etanercept. This treatment, which was well tolerated in the majority of cases, was less used than adalimumab. In the literature, etanercept has been suspected of causing uveitis,38 but it is not clear whether uveitis, during etanercept monotherapy, is a paradoxical effect or an inadequate response to biological therapy.39 In our cohort, we did not see evidence that etanercept precipitated a flare in uveitis, but rather a lack of efficacy that led to a change in biological therapy.
Looking into the occurrence of long-term complications while under biotherapy, we have not seen a difference between biological biotherapies (infliximab, etanercept or adalimumab), but this should be nuanced by the different sizes of the groups.
Only a few patients had infliximab in our series. This is probably due to the fact that 60% of our patients had uveitis related to JIA, and in this type of uveitis, although infliximab is an option, adalimumab is more often used. Indeed, even before it was authorised for JIA-associated uveitis, adalimumab had been approved for the treatment of JIA with polyarticular involvement and an inadequate response to MTX.
In conclusion, we report a large descriptive, retrospective cohort of non-infectious paediatric uveitis. The complication rate in our cohort was high, but tended to be lower in more recently diagnosed patients who likely benefited from earlier introduction of highly effective treatments such as monoclonal anti-TNF alpha antibodies. In recently diagnosed patients, a longer follow-up is needed to confirm the long-term efficacy and safety of these treatments.
Footnotes
Contributors: All authors were involved in the drafting and revising of the manuscript. All authors gave approval of the final version to be published and agreed to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Patient consent for publication: Not required.
Ethics approval: According to French regulation, no ethics committee approval was required for this non-interventional, retrospective study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository.
References
- 1.Paroli MP, Spinucci G, Liverani M, et al. . Uveitis in childhood: an Italian clinical and epidemiological study. Ocul Immunol Inflamm 2009;17:238–42. 10.1080/09273940802702561 [DOI] [PubMed] [Google Scholar]
- 2.Kump LI, Cervantes-Castañeda RA, Androudi SN, et al. . Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology 2005;112:1287–92. 10.1016/j.ophtha.2005.01.044 [DOI] [PubMed] [Google Scholar]
- 3.Edelsten C, Reddy MA, Stanford MR, et al. . Visual loss associated with pediatric uveitis in english primary and referral centers. Am J Ophthalmol 2003;135:676–80. 10.1016/S0002-9394(02)02148-7 [DOI] [PubMed] [Google Scholar]
- 4.de Boer J, Wulffraat N, Rothova A. Visual loss in uveitis of childhood. Br J Ophthalmol 2003;87:879–84. 10.1136/bjo.87.7.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke LAL, Guex-Crosier Y, Hofer M. Epidemiology of uveitis in children over a 10-year period. Clin Exp Rheumatol 2013;31:633–7. [PubMed] [Google Scholar]
- 6.Smith JA, Mackensen F, Sen HN, et al. . Epidemiology and course of disease in childhood uveitis. Ophthalmology 2009;116:1544–51. 10.1016/j.ophtha.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gritz DC, Wong IG. Incidence and prevalence of uveitis in northern california; The Northern california epidemiology of uveitis study. Ophthalmology 2004;111:491–500. 10.1016/j.ophtha.2003.06.014 [DOI] [PubMed] [Google Scholar]
- 8.Acharya NR, Tham VM, Esterberg E, et al. . Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol 2013;131:1405–12. 10.1001/jamaophthalmol.2013.4237 [DOI] [PubMed] [Google Scholar]
- 9.Schwartzman S. Advancements in the management of uveitis. Best Pract Res Clin Rheumatol 2016;30:304–15. 10.1016/j.berh.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 10.Wentworth BA, Freitas-Neto CA, Foster CS. Management of pediatric uveitis. F1000Prime Rep [Internet], 2014. Available: https://www-ncbi-nlm-nih-gov.gate2.inist.fr/pmc/articles/PMC4047950/ [Accessed 7 Mar 2017]. [DOI] [PMC free article] [PubMed]
- 11.Bodaghi B, Wechsler B, Du-Boutin LTH, et al. . Uvéites chroniques sévères : classification, démarche diagnostique et principes thérapeutiques. La Revue de Médecine Interne 2003;24:794–802. 10.1016/S0248-8663(03)00140-1 [DOI] [PubMed] [Google Scholar]
- 12.Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol 2005;32:362–5. [PubMed] [Google Scholar]
- 13.Biester S, Deuter C, Michels H, et al. . Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol 2007;91:319–24. 10.1136/bjo.2006.103721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quartier P, Baptiste A, Despert V, et al. . ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis 2018;77:1003–11. 10.1136/annrheumdis-2017-212089 [DOI] [PubMed] [Google Scholar]
- 15.Ramanan AV, Dick AD, Jones AP, et al. . Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med 2017;376:1637–46. 10.1056/NEJMoa1614160 [DOI] [PubMed] [Google Scholar]
- 16.Jaffe GJ, Dick AD, Brézin AP, et al. . Adalimumab in patients with active noninfectious uveitis. N Engl J Med 2016;375:932–43. 10.1056/NEJMoa1509852 [DOI] [PubMed] [Google Scholar]
- 17.Sheppard J, Joshi A, Betts KA, et al. . Effect of adalimumab on visual functioning in patients with noninfectious intermediate uveitis, posterior uveitis, and panuveitis in the visual-1 and visual-2 trials. JAMA Ophthalmol 2017;135:511–8. 01 10.1001/jamaophthalmol.2017.0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messiaen C, Le Mignot L, Rath A, et al. . CEMARA: a Web dynamic application within a N-tier architecture for rare diseases. Stud Health Technol Inform 2008;136:51–6. [PubMed] [Google Scholar]
- 19.Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. . Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. . Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–16. 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol 2005;89:444–8. 10.1136/bjo.2004.050609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoffel PB, Sauvain MJ, von Vigier RO, et al. . Non-infectious causes of uveitis in 70 Swiss children. Acta Paediatr 2000;89:955–8. 10.1111/j.1651-2227.2000.tb00416.x [DOI] [PubMed] [Google Scholar]
- 23.Clarke SLN, Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J 2016;14 10.1186/s12969-016-0088-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marvillet I, Terrada C, Quartier P, et al. . Ocular threat in juvenile idiopathic arthritis. Joint Bone Spine 2009;76:383–8. 10.1016/j.jbspin.2008.10.015 [DOI] [PubMed] [Google Scholar]
- 25.Sardar E, Dusser P, Rousseau A, et al. . Retrospective study evaluating treatment decisions and outcomes of childhood uveitis not associated with juvenile idiopathic arthritis. J Pediatr 2017;186:131–7. 10.1016/j.jpeds.2017.03.052 [DOI] [PubMed] [Google Scholar]
- 26.Thorne JE, Woreta F, Kedhar SR, et al. . Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol 2007;143:840–6. 10.1016/j.ajo.2007.01.033 [DOI] [PubMed] [Google Scholar]
- 27.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, et al. . Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332–6. 10.1136/bjo.80.4.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grassi A, Corona F, Casellato A, et al. . Prevalence and outcome of juvenile idiopathic arthritis-associated uveitis and relation to articular disease. J Rheumatol 2007;34:1139–45. [PubMed] [Google Scholar]
- 29.Kothari S, Foster CS, Pistilli M, et al. . The risk of intraocular pressure elevation in pediatric noninfectious uveitis. Ophthalmology 2015;122:1987–2001. 10.1016/j.ophtha.2015.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbert HM, Viswanathan A, Jackson H, et al. . Risk factors for elevated intraocular pressure in uveitis. J Glaucoma 2004;13:96–9. 10.1097/00061198-200404000-00003 [DOI] [PubMed] [Google Scholar]
- 31.Blum-Hareuveni T, Seguin-Greenstein S, Kramer M, et al. . Risk factors for the development of cataract in children with uveitis. Am J Ophthalmol 2017;177:139–43. 10.1016/j.ajo.2017.02.023 [DOI] [PubMed] [Google Scholar]
- 32.Berk AT, Koçak N, Ünsal E. Uveitis in juvenile arthritis. Ocul Immunol Inflamm 2001;9:243–51. 10.1076/ocii.9.4.243.3959 [DOI] [PubMed] [Google Scholar]
- 33.Gritz DC, Schwaber EJ, Wong IG. Complications of uveitis: The northern california epidemiology of uveitis study. Ocul Immunol Inflamm 2017;43:1–11. 10.1080/09273948.2016.1247174 [DOI] [PubMed] [Google Scholar]
- 34.Foster CS, Havrlikova K, Baltatzis S, et al. . Secondary glaucoma in patients with juvenile rheumatoid arthritis-associated iridocyclitis. Acta Ophthalmol Scand 2000;78:576–9. 10.1034/j.1600-0420.2000.078005576.x [DOI] [PubMed] [Google Scholar]
- 35.Mercier A-E, Ribeiro E, Korobelnik J-F, et al. . Efficacy of anti-tnf-α therapy for the treatment of non-infectious uveitis: a retrospective study of 21 patients. Ocul Immunol Inflamm 2016:1–8. [DOI] [PubMed] [Google Scholar]
- 36.Cann M, Ramanan AV, Crawford A, et al. . Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol Online J 2018;16 10.1186/s12969-018-0266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara G, Mastrangelo G, Barone P, et al. . Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Pediatr Rheumatol 2018;16 10.1186/s12969-018-0255-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taban M, Dupps WJ, Mandell B, et al. . Etanercept (enbrel)-associated inflammatory eye disease: case report and review of the literature. Ocul Immunol Inflamm 2006;14:145–50. 10.1080/09273940600659393 [DOI] [PubMed] [Google Scholar]
- 39.Wendling D, Paccou J, Berthelot J-M, et al. . New onset of uveitis during anti-tumor necrosis factor treatment for rheumatic diseases. Semin Arthritis Rheum 2011;41:503–10. 10.1016/j.semarthrit.2011.05.005 [DOI] [PubMed] [Google Scholar]