Abstract
The programmed cell death protein 1 (PD-1) signaling axis is among the most important therapeutic targets in modern oncology. Aurigene Discovery Technologies Ltd. (Aurigene) has patented a series of peptidomimetic small molecules derived from the PD-1 protein sequence for use in targeting the interaction between PD-1 and its ligand, PD-L1. We evaluated three of Aurigene’s most potent compounds in SPR binding assays. Our results showed that these compounds—each of which is known to be potently effective in a splenocyte recovery assay—do not directly inhibit the PD-1/PD-L1 interaction nor do they appear to bind to either of the constituent proteins, indicating that another mechanism is at play. As a result of these studies and upon consideration of structural features within the PD-1/PD-L1 complex, we hypothesize that the Aurigene molecules may interact with a currently unknown protein capable of regulating the PD-1 axis.
Keywords: PD-1, PD-L1, protein−protein interaction inhibitors, SPR
Programmed cell death protein 1 (PD-1) and its ligand (PD-L1) are transmembrane immunosuppressive checkpoint proteins.1 PD-1 is expressed on activated T cells, and PD-L1 is expressed on somatic tissue and antigen-presenting cells.2,3 The binding interaction between PD-1 and PD-L1 causes a down-regulation of T cell proliferative gene expression, thus disrupting cytotoxic activity. Certain aggressive cancers, such as pancreatic cancer, breast cancers, and nonsmall lung carcinomas, have evolved to overexpress PD-L1 as a means of immune evasion.3,4 In these cases, PD-L1 expresses constitutively, allowing the tumor to masquerade as immune privileged tissue and evade detection.5−8 Overexpression of PD-L1 is therefore a strong prognostic biomarker in oncology.2−12
Pharmaceutical companies have sought to develop modulators for the PD-1/PD-L1 interaction to recover lymphocyte activity. Current treatments are antibody-based therapies targeting either PD-1 or PD-L1 (Table 1). Despite the remarkable therapeutic success of these antibodies,13,17−25 there are considerable drawbacks such as poor bioavailability, immunogenicity, and the high cost of large-scale production.13,26,27 Small molecule inhibitors have the potential to overcome these obstacles, and multiple research groups are pursuing this objective.28−30 However, there are currently no FDA-approved small molecule inhibitors capable of blocking the PD-1/PD-L1 interaction.31,32
Table 1. List of Current FDA-Approved Immunotherapeutics That Block the PD-1/PD-L1 Interaction13−17.
| name | company | target | FDA approval year |
|---|---|---|---|
| nivolumab | Bristol-Myers Squibb | PD-1 | 2014 |
| pembrolizumab | Merck | PD-1 | 2014 |
| atezolizumab | Genentech/Roche | PD-L1 | 2016 |
| avelumab | Merck | PD-L1 | 2017 |
| durvalumab | AstraZeneca | PD-L1 | 2017 |
| cemiplimab | Regeneron/Sanofi | PD-1 | 2018 |
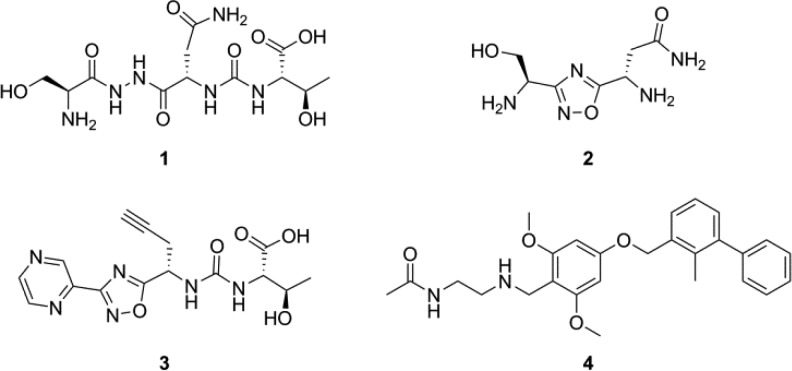
Aurigene has an extensive patent portfolio of peptides and peptidomimetic small molecules that mimic various regions of the PD-1 protein sequence. The most promising peptidomimetic compounds are reported to exhibit nanomolar potency in a phenotypic cell-based splenocyte recovery assay that Aurigene uses to scout for PD-1/PD-L1 inhibitors.33−37 However, no direct protein binding experiments have been reported for this family of small molecules. We selected three of the most promising compounds (Figure 1) from three recent patents35−37 and aimed to characterize them using new surface plasmon resonance (SPR)-based methods. Each test compound was chosen with an eye toward maximizing both potency and drug-like properties, while optimally representing the compounds claimed within each patent.
Figure 1.
Compounds used for the present study. Compounds 1–3 are patented immunomodulators from Aurigene, hypothesized to disrupt the PD-1/PD-L1 interaction. Compound 4 is a known inhibitor developed by Bristol-Myers Squibb, employed here as a positive control.
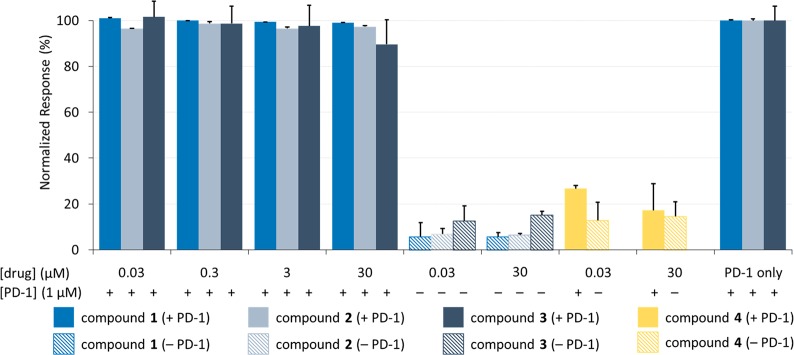
Using SPR, we developed inhibition assays to detect how binding between the extracellular domain of PD-1 and the extracellular domain of PD-L1 is affected in the presence of the Aurigene compounds (refer to Figures S1 and S2 in the Supporting Information for sensorgrams showing PD-1/PD-L1 binding). In our first inhibition assay (Figure 2), biotinylated PD-L1 was adsorbed on a streptavidin-coated gold chip (SA chip). Varied concentrations (ranging from 30 nM to 30 μM) of Aurigene compounds 1–3 were titrated over a flow cell in the presence of recombinant soluble PD-1 (corresponding to residues P34–E150 of the native protein).
Figure 2.
Aurigene compounds (1–3) show no inhibition between 30 nM and 30 μM for PD-1 flowing over PD-L1. Soluble PD-1 was flowed across surface-bound PD-L1 with and without test compounds at varying concentrations. The response is normalized to the control protein interaction (PD-1 only). Responses were measured in triplicate, and error bars represent standard deviation.
The binding responses were normalized to PD-1 binding to PD-L1 (control). Compounds acting as inhibitors of the PD-1/PD-L1 interaction would decrease the binding response relative to the control. To validate the assay, we used a known PD-1/PD-L1 inhibitor from Bristol-Myers Squibb as a positive control (compound 4, data shown in yellow).29 Previous studies have shown that compound 4 induces a dimerization of the PD-L1 proteins, preventing PD-1 from binding.38−40 As expected, compound 4 showed effective in vitro inhibition at both low concentrations (30 nM) and high concentrations (30 μM). For titration data associated with 4, see Figures S3 and S4.
When compounds 1–3 were tested in this assay, we observed no change in binding between the two proteins (Figure 2). Moreover, in the absence of PD-1 protein (data shown in hashed columns) the Aurigene compounds 1–3 also elicited little signal variance between 30 nM and 30 μM, suggesting that there is no directly observable binding to PD-L1. While a very small signal was likewise observed for 4 in the absence of PD-1, this is consistent with the known 2:1 binding mode between PD-L1 and compound 4.38 Such behavior would effectively double the molecular weight of the receptor (since 4 binds to a PD-L1 homodimer, rather than to monomeric PD-L1), while reducing the density of receptor on the surface of the chip (since not all surface-bound proteins would be able to homodimerize). The result would be very weak signal since SPR response is proportional to both the ratios of the molecular weights of the two interacting species and to the immobilization density.
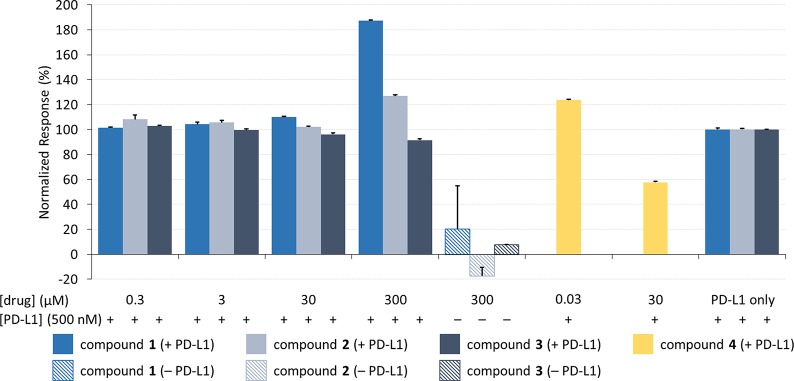
We repeated the inhibition experiment, this time attaching biotinylated PD-1 to the gold surface and flowing recombinant PD-L1 protein (corresponding to residues A18-T239, chosen to represent both extracellular domains of the native protein) across the chip along with various concentrations of 1–4 (Figure 3). This type of reciprocal binding assay serves as an important control for binding artifacts in SPR experiments. Once again, the positive control molecule (4) was observed to inhibit the interaction (data shown in yellow), although in this case higher concentrations of 4 were required because of the high concentration of PD-L1 required in the experiment. These data provide additional support for the earlier study determining PD-L1 as the biological target for compound 4.39
Figure 3.
Aurigene compounds (1–3) show no inhibition between 300 nM and 300 μM for PD-L1 flowing over PD-1. Soluble PD-L1 was flowed across surface-bound PD-1 with and without test compounds at varying concentrations. The response is normalized to the control protein interaction (PD-L1 only). Responses were measured in triplicate, and error bars represent standard deviation.
As in the previous assay, however, the Aurigene compounds 1–3 showed no inhibition of the PD-1/PD-L1 interaction (Figure 3) nor did they appear to elicit any statistically significant binding to adsorbed PD-1. At very high concentrations (300 μM), Aurigene compounds 1 and 2 gave an increase in SPR response (i.e., negative inhibition), but this is likely due either to molecules nonspecifically adsorbing to the attached protein or else to compound precipitation. The same results were observed in our assay for soluble PD-1 flowed over another adsorbed binding partner, PD-L2 (Figure S6). Compound 4 was also shown to not inhibit the PD-1/PD-L2 interaction and showed no sign of binding directly to PD-L2, highlighting the specificity of the BMS compound.
Seeking confirmation of these results in an orthogonal assay type, we also briefly explored the function of compound 1 in a commercial ELISA assay for PD-1/PD-L1 inhibitors (Figure S8). Once again, however, we saw no significant inhibitory activity. Positive controls (including compound 4 and two related inhibitors from Bristol-Myers-Squibb, as well as a known antibody inhibitor) all worked as expected, once again validating the assay.
Aurigene characterized the efficacy of their lead compounds using a phenotypic cell-based assay built around splenocyte recovery as a proxy for immune activation. Compounds 1–3 triggered high recovery in this experiment (68%, 93%, and 92%, respectively, relative to an uninhibited positive control) at 100 nM.35−37 The limited experimental data in Aurigene’s patents (including controls with PD-L1 in the absence of small molecule) suggest that the phenotype was PD-L1 specific. But as we have shown above, the results from Aurigene’s cell-based assays cannot be attributed to direct inhibition of PD-1/PD-L1 binding. Instead, it would appear that an alternative mechanism is responsible for the observed phenotypic effect.
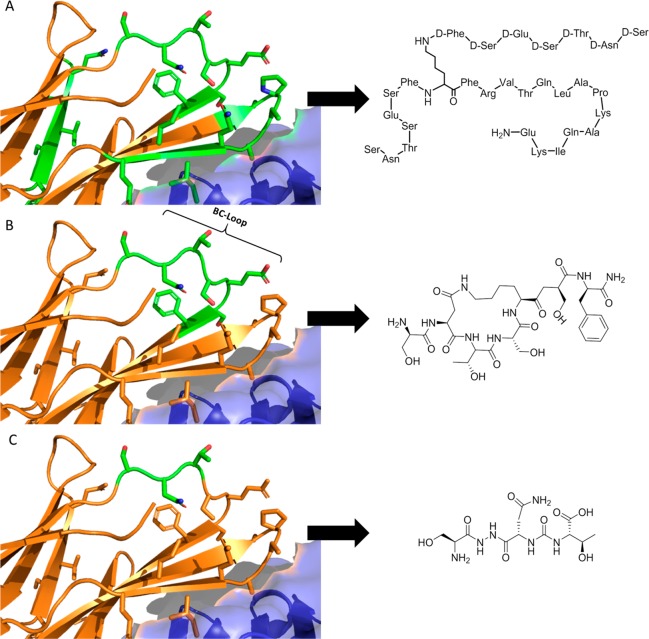
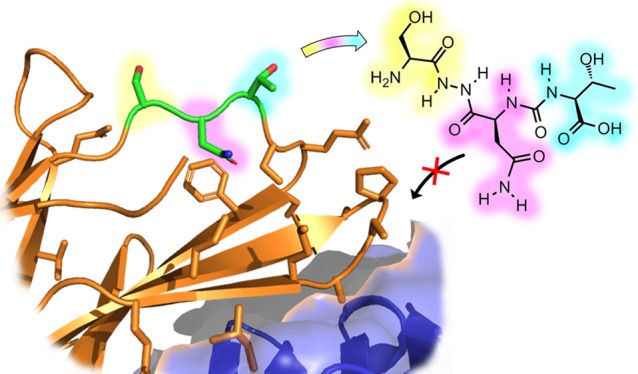
Despite the recent appearance of a number of papers related to small molecule modulation of the PD-1/PDL-1 interaction,14,28,31,41−44 there is little explanation of how the Aurigene compounds exert their function. However, a close reading of Aurigene’s patent portfolio provides some insight into the development of this class of small molecules. Aurigene’s early patent filings claimed large peptides that mimic a significant portion of the PD-1 extracellular domain (Figure 4A).33 This includes much of the PD-L1 binding interface, and so it is likely that these large peptides would be competitive inhibitors of the PD-1/PD-L1 interaction. Attempts to achieve more drug-like properties, however, led to subsequent filings describing the development of macrocyclic peptides that mimic the 7-residue BC-loop (Figure 4B).34 The most recent filings further refine the structure (and, presumably, improve the pharmacokinetic properties) by developing small tripeptides and peptidomimetic analogues that mimic the central serine-asparagine-threonine tripeptide of this loop (Figure 4C).35−37 This is the strategy that ultimately led to small molecules 1–3.
Figure 4.
Binding interface between PD-1 and PD-L1 (from PDB: 4ZQK), together with an illustration of the discovery process leading to Aurigene’s lead compounds. PD-1 and PD-L1 are shown in cartoon representation (orange) and surface representation (blue), respectively. Residues highlighted in sticks are the residues the patent compounds are derived from. (A) Left: Residues (57–63, 95–99, 127–136) of the PD-1 protein. Right: Patented peptide derived as a structural analogue to the region. (B) Left: Residues (57–63) of the PD-1 protein. Right: Patented macrocyclic compound derived as a structural analogue to the region. (C) Left: Residues (57–59) of the PD-1 protein. Right: Small molecule peptidomimetic derived as a structural analogue to the region.
Critically, however, the BC-loop (and particularly the region mimicked by 1–3) points away from the PD-L1 binding interface (Figure 4B), and so it is unclear why these molecules would be expected to be direct binding inhibitors, notwithstanding their apparent potency in cell-based assays and the fact that they are referred to (with little supporting data) in Aurigene’s patents35−37 and subsequent reviews28,42,45 as inhibitors of the PD-1/PD-L1 interaction.
While it is possible that there is some key difference (e.g., glycosylation state) between our in vitro system and the “real-life” PD-1 and PD-L1 proteins expressed on the surface of T cells and antigen presenting cells, a more likely scenario is that the compounds do mimic the PD-1 surface as intended, but that this serves not to directly disrupt binding with the PD-L1 protein as has been assumed, but perhaps to modulate the function of some other PD-1 binding partner. Both PD-1 and PD-L1 are thought to participate in regulatory binding interactions with other proteins,46,47 and we hypothesize that one or more of these might be the true biological target of 1–3.
In 2015, Curis Inc. initiated clinical trials with a small molecule called CA-170, which had been developed at Aurigene.48 The exact structure of CA-170 has yet to be disclosed, but this lead candidate apparently emerged from a focused library at Aurigene that was designed to exploit hotspots within the PD-1/PD-L1 complex.49 In a recent review by two of the inventors of the Aurigene molecules 1–3, it is stated that CA-170 was designed to target one or more conserved pockets found in PD-L1 and VISTA, a nonredundant immunosuppressing protein of the B7-superfamily.28 These authors are careful to not indicate whether CA-170 is structurally related to compounds 1–3, but two subsequent reviews (from two different groups) both speculate that CA-170 is related to Aurigene’s earlier disclosed compounds.50,51 One of these recent reviews asserts that CA-170 is an oxadiazole (like compounds 2 and 3),51 while the other describes CA-170 as a molecule capable of disrupting the PD-1/PD-L1 complex.50 The existence of a clinical candidate that is likely related to compounds 1–3 and that is thought to function through direct blockade of PD-1/PD-L1 binding provides motivation to better understand the function for this series of small molecules. In this study, we performed surface-plasmon resonance assays to test the hypothesis of direct protein binding inhibition and found that none of the compounds tested can disrupt the interaction between soluble PD-1 and adhered PD-L1 or between soluble PD-L1 and adhered PD-1. Preliminary testing also did not reveal binding of 1–3 to surface-bound VISTA (Figure S7). Based on these data and an analysis of structural features of the PD-1/PD-L1 interaction (and particularly the region of the PD-1 BC-loop from which Aurigene’s lead compounds were derived), we hypothesize that this family of small molecules may regulate the function of some other PD-1 binding partner.52
Acknowledgments
D.J.B. thanks the Polymer Nanoparticles for Drug Delivery (POND) CREATE program for a stipend and the University of Victoria for partial operating support that was used to offset the cost of this research. F.H. and J.W. thank the Canada Research Chairs program for salary support.
Glossary
ABBREVIATIONS
- PD-1
programmed cell death protein 1 (CD279);
- PD-L1
programmed death-ligand 1 (CD274 or B7–H1)
- VISTA
V-domain Ig suppressor of T cell activation
- SPR
surface plasmon resonance
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00221.
Supplementary Figures S1–S8, together with experimental details for protein expression, analytical methods, and compound synthesis, as well as NMR spectra for synthesized compounds 1–4 (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by funding from the Canadian Cancer Society Research Institute (grant # 701684), as well as an NSERC CREATE grant (# 497311–2017) and an NSERC Discovery grant to J.W. (# 4283). Additional support came from the Michael Smith Foundation for Health Research, the Canada Research Chairs program, and the University of Victoria.
The authors declare the following competing financial interest(s): D.A.P. is an employee of Inception Sciences Canada. The remaining authors declare no competing financial interest.
Supplementary Material
References
- Santarpia M.; González-Cao M.; Viteri S.; Karachaliou N.; Altavilla G.; Rosell R. Programmed Cell Death Protein-1/Programmed Cell Death Ligand-1 Pathway Inhibition and Predictive Biomarkers: Understanding Transforming Growth Factor-Beta Role. Transl. Lung Cancer Res. 2015, 4 (6), 728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally A. P. R.; Austin J. W.; Boss J. M. Genetic and Epigenetic Regulation of PD-1 Expression. J. Immunol. 2016, 196 (6), 2431–2437. 10.4049/jimmunol.1502643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S. C.; Latchman Y. E.; Buhlmann J. E.; Tomczak M. F.; Horwitz B. H.; Freeman G. J.; Sharpe A. H. Regulation of PD-1, PD-L1, and PD-L2 Expression during Normal and Autoimmune Responses. Eur. J. Immunol. 2003, 33 (10), 2706–2716. 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- Shi L.; Chen S.; Yang L.; Li Y. The Role of PD-1 and PD-L1 in T-Cell Immune Suppression in Patients with Hematological Malignancies. J. Hematol. Oncol. 2013, 6 (1), 74–79. 10.1186/1756-8722-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L.; Huang D.; Liu J.; Qian Y.; Deng J.; Li D.; Hu Z.; Zhang J.; Jiang G.; Zheng S. B7-H1 up-Regulated Expression in Human Pancreatic Carcinoma Tissue Associates with Tumor Progression. J. Cancer Res. Clin. Oncol. 2008, 134 (9), 1021–1027. 10.1007/s00432-008-0364-8. [DOI] [PubMed] [Google Scholar]
- Soliman H.; Khalil F.; Antonia S. PD-L1 Expression Is Increased in a Subset of Basal Type Breast Cancer Cells. PLoS One 2014, 9 (2), e88557 10.1371/journal.pone.0088557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu C. Y.; Huang J. A.; Chen Y.; Chen C.; Zhang X. G. High Expression of PD-L1 in Lung Cancer May Contribute to Poor Prognosis and Tumor Cells Immune Escape through Suppressing Tumor Infiltrating Dendritic Cells Maturation. Med. Oncol. 2011, 28 (3), 682–688. 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- Azuma K.; Ota K.; Kawahara A.; Hattori S.; Iwama E.; Harada T.; Matsumoto K.; Takayama K.; Takamori S.; Kage M.; Hoshino T.; Nakanishi Y.; Okamoto I. Association of PD-L1 Overexpression with Activating EGFR Mutations in Surgically Resected Nonsmall-Cell Lung Cancer. Ann. Oncol. 2014, 25 (10), 1935–1940. 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Lang J. Soluble PD-1 and PD-L1: Predictive and Prognostic Significance in Cancer. Oncotarget 2017, 8 (57), 97671–97682. 10.18632/oncotarget.18311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R.; Abe H.; Kunita A.; Yamashita H.; Seto Y.; Fukayama M. Overexpression and Gene Amplification of PD-L1 in Cancer Cells and PD-L1 + Immune Cells in Epstein-Barr Virus-Associated Gastric Cancer: The Prognostic Implications. Mod. Pathol. 2017, 30 (3), 427–439. 10.1038/modpathol.2016.202. [DOI] [PubMed] [Google Scholar]
- Qin T.; Zeng Y.; Qin G.; Xu F.; Lu J.; Fang W.; Xue C.; Zhan J.; Zhang X.; Zheng Q.; Peng R.; Yuan Z.; Zhang L.; Wang S. High PD-L1 Expression Was Associated with Poor Prognosis in 870 Chinese Patients with Breast Cancer. Oncotarget 2015, 6 (32), 33792–33981. 10.18632/oncotarget.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.-J.; Jeon Y. K.; Cho Y. M.; Lee J.-L.; Chung D. H.; Park J. Y.; Go H. The Association Between PD-L1 Expression and the Clinical Outcomes to Vascular Endothelial Growth Factor-Targeted Therapy in Patients With Metastatic Clear Cell Renal Cell Carcinoma. Oncologist 2015, 20 (11), 1253–1260. 10.1634/theoncologist.2015-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J.; Chehrazi-Raffle A.; Reddi S.; Salgia R. Development of PD-1 and PD-L1 Inhibitors as a Form of Cancer Immunotherapy: A Comprehensive Review of Registration Trials and Future Considerations. J. Immunother. Cancer 2018, 6 (1), 8–25. 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Li F.; Jiang F.; Lv X.; Zhang R.; Lu A.; Zhang G. A Mini-Review for Cancer Immunotherapy: Molecular Understanding of PD-1/ PD-L1 Pathway & Translational Blockade of Immune Checkpoints. Int. J. Mol. Sci. 2016, 17 (7), 1151–1172. 10.3390/ijms17071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaab H. O.; Sau S.; Alzhrani R.; Tatiparti K.; Bhise K.; Kashaw S. K.; Iyer A. K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan M. M.; Hu X. Q.; Liu X. X.; Ruan B. F.; Xu J.; Liao C. From Monoclonal Antibodies to Small Molecules: The Development of Inhibitors Targeting the PD-1/PD-L1 Pathway. Drug Discov. Drug Discovery Today 2016, 21 (6), 1027–1036. 10.1016/j.drudis.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Markham A.; Duggan S. Cemiplimab: First Global Approval. Drugs 2018, 78 (17), 1841–1846. 10.1007/s40265-018-1012-5. [DOI] [PubMed] [Google Scholar]
- Wojas-Krawczyk K.; Kalinka E.; Grenda A.; Krawczyk P.; Milanowski J. Beyond PD-L1Markers for Lung Cancer Immunotherapy. Int. J. Mol. Sci. 2019, 20 (8), 1915–1932. 10.3390/ijms20081915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghaei H.; Paz-Ares L.; Horn L.; Spigel D. R.; Steins M.; Ready N. E.; Chow L. Q.; Vokes E. E.; Felip E.; Holgado E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373 (17), 1627–1639. 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J.; Reckamp K. L.; Baas P.; Crinò L.; Eberhardt W. E. E.; Poddubskaya E.; Antonia S.; Pluzanski A.; Vokes E. E.; Holgado E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373 (2), 123–135. 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R. S.; Baas P.; Kim D. W.; Felip E.; Pérez-Gracia J. L.; Han J. Y.; Molina J.; Kim J. H.; Arvis C. D.; Ahn M. J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387 (10027), 1540–1550. 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- Reck M.; Rodríguez-Abreu D.; Robinson A. G.; Hui R.; Csőszi T.; Fülöp A.; Gottfried M.; Peled N.; Tafreshi A.; Cuffe S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375 (19), 1823–1833. 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- Rittmeyer A.; Barlesi F.; Waterkamp D.; Park K.; Ciardiello F.; von Pawel J.; Gadgeel S. M.; Hida T.; Kowalski D. M.; Dols M. C.; et al. Atezolizumab versus Docetaxel in Patients with Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet 2017, 389 (10066), 255–265. 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolo A. B.; Infante J. R.; Balmanoukian A.; Patel M. R.; Wang D.; Kelly K.; Mega A. E.; Britten C. D.; Ravaud A.; Mita A. C.; Safran H.; Stinchcombe T. E.; Srdanov M.; Gelb A. B.; Schlichting M.; Chin K.; Gulley J. L. Avelumab, an Anti–Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J. Clin. Oncol. 2017, 35 (19), 2117–2124. 10.1200/JCO.2016.71.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia S. J.; Villegas A.; Daniel D.; Vicente D.; Murakami S.; Hui R.; Yokoi T.; Chiappori A.; Lee K. H.; de Wit M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377 (20), 1919–1929. 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- Chames P.; Van Regenmortel M.; Weiss E.; Baty D. Therapeutic Antibodies: Successes, Limitations and Hopes for the Future. Br. J. Pharmacol. 2009, 157 (2), 220–233. 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. K. H. The History of Monoclonal Antibody Development - Progress, Remaining Challenges and Future Innovations. Ann. Med. Surg. 2014, 3 (4), 113–116. 10.1016/j.amsu.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikumar P. G.; Ramachandra M. Small-Molecule Immune Checkpoint Inhibitors Targeting PD-1/PD-L1 and Other Emerging Checkpoint Pathways. BioDrugs 2018, 32 (5), 481–497. 10.1007/s40259-018-0303-4. [DOI] [PubMed] [Google Scholar]
- Chupak L.; Zheng X. Compounds as Useful Immunomodulators. WO 2015034820 A1, 2014.
- Sharpe A. H.; Butte M. J.; Oyama S. Modulators of Immunoinhibitory Receptor Pd-1, and Methods of Use Thereof. WO 2011082400 A2, 2011.
- Li K.; Tian H. Development of Small-Molecule Immune Checkpoint Inhibitors of PD-1/PD-L1 as a New Therapeutic Strategy for Tumour Immunotherapy. J. Drug Target 2019, 27, 244. 10.1080/1061186X.2018.1440400. [DOI] [PubMed] [Google Scholar]
- Konstantinidou M.; Zarganes-Tzitzikas T.; Magiera-Mularz K.; Holak T. A.; Dömling A. Immune Checkpoint PD-1/PD-L1: Is There Life Beyond Antibodies? Angew. Angew. Chem., Int. Ed. 2018, 57 (18), 4840–4848. 10.1002/anie.201710407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikumar P. G. N.; Ramachandra M.; Vadlamani S. K.; Vemula K. R.; Satyam L. K.; Subbarao K.; Shrimali K. R.; Kandepu S. Immunosuppression Modulating Compounds. US 2011031873 A1, 2011.
- Sasikumar P. G. N.; Ramachandra M. Immunomodulating Cyclic Compounds from the Bc Loop of Human Pd1. WO 2013144704 A1, 2013.
- Sasikumar P. G. N.; Ramachandra M.; Naremaddepalli S. S. S. Peptidomimetic Compounds as Immunomodulators. US 20130237580, 2013.
- Sasikumar P. G. N.; Ramachandra M.; Naremaddepalli S. S. S. 1,2,4-Oxadiazole Derivatives As Immunomodulators. WO 2015033299 A1, 2014.
- Sasikumar P. G. N.; Ramachandra M.; Prasad A.; Naremaddepalli S. S. S. 3-Substituted-1,2,4-Oxadiazole and Thiadiazole Compounds As Immunomodulators. WO 2016142886 A2, 2016.
- Zak K. M.; Grudnik P.; Guzik K.; Zieba B. J.; Musielak B.; Domling A.; Dubin G.; Holak T. A. Structural Basis for Small Molecule Targeting of the Programmed Death Ligand 1 (PD-L1). Oncotarget 2016, 7 (21), 30323–30335. 10.18632/oncotarget.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley R.Inhibitors of the PD1/PD-L1 Interaction: Missteps, Mechanisms, and Mysteries, University of Victoria, 2017. [Google Scholar]
- Skalniak L.; Zak K. M.; Guzik K.; Magiera K.; Musielak B.; Pachota M.; Szelazek B.; Kocik J.; Grudnik P.; Tomala M.; Krzanik S.; Pyrc K.; Dömling A.; Dubin G.; Holak T. A. Small-Molecule Inhibitors of PD-1/PD-L1 Immune Checkpoint Alleviate the PD-L1-Induced Exhaustion of T-Cells. Oncotarget 2017, 8 (42), 72167–72181. 10.18632/oncotarget.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann H. Cancer Immunotherapy: Selected Targets and Small-Molecule Modulators. ChemMedChem 2016, 11 (5), 450–466. 10.1002/cmdc.201500566. [DOI] [PubMed] [Google Scholar]
- Zarganes-Tzitzikas T.; Konstantinidou M.; Gao Y.; Krzemien D.; Zak K.; Dubin G.; Holak T. A.; Dömling A. Inhibitors of Programmed Cell Death 1 (PD-1): A Patent Review (2010–2015). Expert Opin. Ther. Pat. 2016, 26 (9), 973–977. 10.1080/13543776.2016.1206527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiera-Mularz K.; Skalniak L.; Zak K. M.; Musielak B.; Rudzinska-Szostak E.; Berlicki Ł.; Kocik J.; Grudnik P.; Sala D.; Zarganes-Tzitzikas T.; Shaabani S.; Dömling A.; Dubin G.; Holak T. A. Bioactive Macrocyclic Inhibitors of the PD-1/PD-L1 Immune Checkpoint. Angew. Chem., Int. Ed. 2017, 56 (44), 13732–13735. 10.1002/anie.201707707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley R. P.; Horvath S.; An J.; Hof F.; Wulff J. E. Salicylates Are Interference Compounds in TR-FRET Assays. Bioorg. Med. Chem. Lett. 2016, 26 (3), 973–977. 10.1016/j.bmcl.2015.12.050. [DOI] [PubMed] [Google Scholar]
- Chen T.; Li Q.; Liu Z.; Chen Y.; Feng F.; Sun H. Peptide-Based and Small Synthetic Molecule Inhibitors on PD-1/PD-L1 Pathway: A New Choice for Immunotherapy?. Eur. J. Med. Chem. 2019, 161, 378–398. 10.1016/j.ejmech.2018.10.044. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H.; Pauken K. E. The Diverse Functions of the PD1 Inhibitory Pathway. Nat. Rev. Immunol. 2018, 18, 153–167. 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- Butte M. J.; Peña-Cruz V.; Kim M.-J.; Freeman G. J.; Sharpe A. H. Interaction of Human PD-L1 and B7–1. Mol. Immunol. 2008, 45 (13), 3567–3572. 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curis Inc. A Study of CA-170 (Oral PD-L1, PD-L2 and VISTA Checkpoint Antagonist) in Patients With Advanced Tumors and Lymphomas. https://clinicaltrials.gov/ct2/show/NCT02812875 (accessed Mar 25, 2019).
- Yang J.; Hu L. Immunomodulators Targeting the PD-1/PD-L1 Protein-Protein Interaction: From Antibodies to Small Molecules. Med. Res. Rev. 2019, 39 (1), 265–301. 10.1002/med.21530. [DOI] [PubMed] [Google Scholar]
- Huck B. R.; Kötzner L.; Urbahns K. Small Molecules Drive Big Improvements in Immuno-Oncology Therapies. Angew. Chem., Int. Ed. 2018, 57, 4412–4428. 10.1002/anie.201707816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Wu X.; Guo C.; Zhang K.; Xu J.; Li Z.; Jiang S. Development of Inhibitors of the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Signaling Pathway. J. Med. Chem. 2019, 28, 1715–1730. 10.1021/acs.jmedchem.8b00990. [DOI] [PubMed] [Google Scholar]
- Following the submission of this manuscript, a study on the in vitro function of a likely CA-170 candidate (structurally related to 1–3) was disclosed. This study likewise finds that the candidate is inactive in a variety of protein-binding experiments and concludes that an alternative mechanism may be responsible for its biological activity. See: Musielak B.; Kocik J.; Skalniak L.; Magiera-Mularz K.; Sala D.; Czub M.; Holak T. A.; Plewka J.. CA-170 - a Potent Small-Molecule PD-L1 Inhibitor or Not? bioRxiv 662668; 10.1101/662668. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.