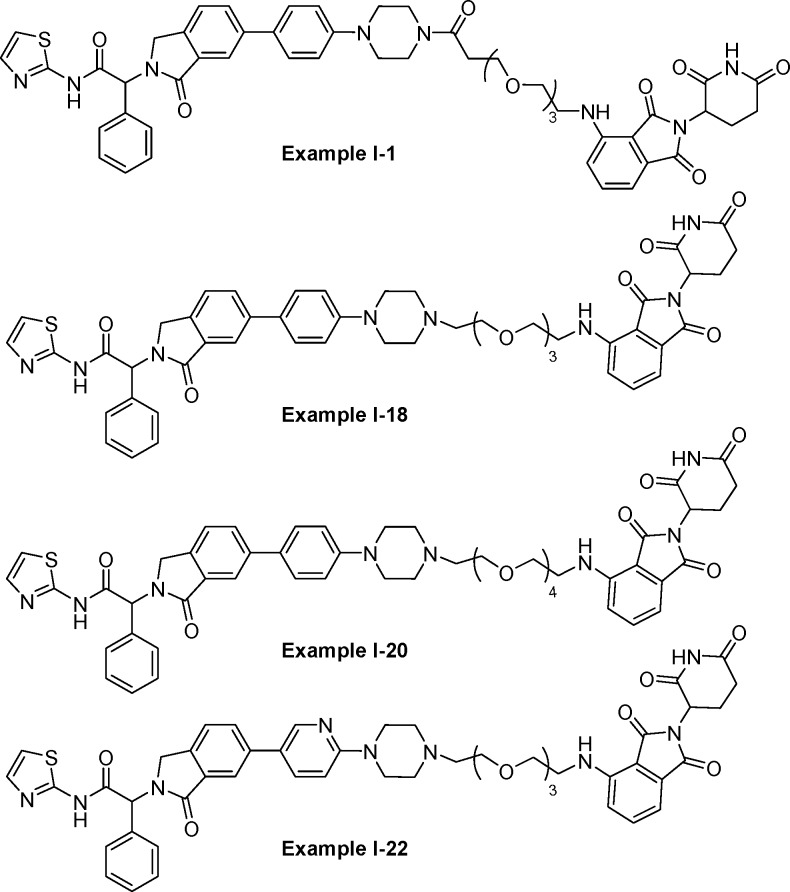
Important Compound Classes
Title
Bifunctional Molecules for Degradation of EGFR and Methods of Use
Patent Application Number
US 20190106417 A1
Publication Date
March 11, 2019
Priority Application
US 16163254
Priority Date
October 17, 2018
Inventors
Gray, N.; Jang, J.; De Clercq, D.; Eck, M.; Janne, P.
Assignee Company
Dana-Farber Cancer Institute, Inc.
Disease Area
Cancer and autoimmune diseases
Biological Target
Epidermal growth factor receptor (EGFR)
Summary
Epidermal growth factor receptor (EGFR) is one of the receptor tyrosine kinases that play a significant role in cellular processes, including adhesion, proliferation, migration, and apoptosis. Binding of the EGF ligand to the membrane protein, which extends into the extracellular space of the EGFR, triggers its activation. In many cancer cells, overexpressed EGFR is associated with multiple types of cancers, and its unregulated activation causes the aberrant cell growth, ligand binding, and downstream signaling, which may result in endocytosis and intracellular terminal trafficking of the EGFR signal. In addition, activated EGFR undergoes internalization to the endosomes by clathrin-dependent and -independent pathways, which occurs until the receptor is degraded in the lysosome or recycled to the membrane. Given the fundamental function of internalization played by EGFR, it has been used as a vital mediator for drug delivery. For example, EGFR antibody conjugate can be ingested by cancer cells and may cause apoptosis. Cetuximab, an antibody drug, targets the extracellular domain of the EGFR, which inhibits receptor activation by binding to the EGF binding site and affects induction of the receptor internalization. Compounds that inhibit EGFR have shown efficacy against cancer cell lines and murine xenograft models, which have led to clinical evaluation of EGFR inhibitors for patients with EGFR-expressing tumors.
Patients with EGFR mutations responded more favorably with gefitinib over chemotherapy for metastatic nonsmall cell lung cancer (NSCLC). Despite the high initial response rates, these patients eventually develop resistance to treatment on EGFR tyrosine kinase inhibitors (TKI). The mechanisms of acquired resistance can be divided into secondary mutations in EGFR (activation of alternative signaling pathways involving the T790M mutation, which account for 50–60% secondary resistance) and the phenotypic or histologic transformation. Furthermore, application of an EGFR inhibitor does not kill all cancer cells, and some cells may survive the exposure to the inhibitor, which could undergo genetic evolution toward resistance.
EGFR drug target landscape consists of drug targets with well-defined active sites suitable for accommodation of a small molecule, which have been the focus of pharmacological intervention. Therefore, EGFR drug development against these active site-containing targets have primarily operated via occupancy-driven pharmacology as the mode of action. Efficacy of these drugs is driven by retaining a high target occupancy in which high drug doses are generally required, often leading to off-target binding, undesired side effects, and resistance to these therapeutics. Consequently, there is a need to develop new drug classes with possibly alternate mode of actions.
On like inhibition, degradation of misfolded or abnormal proteins has received significant attention due to desirable outcome for several therapeutic treatments. The Ubiquitin–Proteasome Pathway (UPP) regulates proteins and degrades misfolded or abnormal proteins. Action of E3 ubiquitin ligases is carried out by the covalent attachment of ubiquitin to specific protein substrates. These ligases have over 500 different proteins and are categorized by the structural element of their E3 functional activity. For instance, Cereblon (CRBN) interacts with damaged DNA binding protein 1 and forms an E3 ubiquitin ligase complex with Cullin 4. This causes the proteins to be recognized by CRBN and are ubiquitinated and degraded by proteasomes. Thalidomide, lenalidomide, and their derivatives have anticancer activities that have been used as immunomodulatory drugs (IMiDs). The action of these compounds is affected by binding to the cereblon protein, which is a substrate receptor of the CRL4A E3 ubiquitin ligase complex. Lenalidomide is the most prominent IMiD and is highly successful in the treatment of multiple myeloma and B cell malignancies. Heterobifunctional PROTAC molecules generally consist of one end that recruits the E3 ligase complex and the other end that engages the target protein. Compounds in this Patent Highlight are bifunctional compounds, which function to recruit targeted proteins to E3 ubiquitin ligase for degradation and are used for the treatment of kinase mediated disorders. The patent application also provides a method for treating or preventing a disease in which EGFR or a mutant thereof plays a role or a disease process that is resistant to an EGFR targeted therapy. In addition, these PROTAC bifunctional molecules are used to treat or prevent conditions selected from autoimmune, inflammatory, proliferative, and hyperproliferative diseases such as cardiovascular, allergies, asthma, and Alzheimer’s diseases.
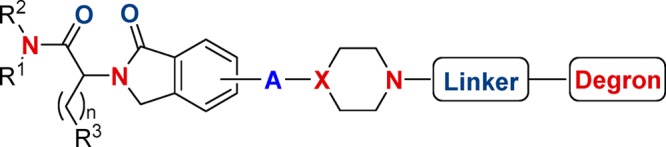
This Patent Highlight describes a bifunctional compound, which could be an enantiomer, diastereomer, stereoisomer, or pharmaceutically acceptable salt.
Definitions
A is phenyl or pyridinyl;
X = CH, C(C1–C3) alkyl, or N;
R1 = H or C(C1–C3) alkyl;
R2 = (C6–C10) aryl or heteroaryl comprising one or two 5- to 6-membered rings;
R4 is independently selected from (C1–C4) alkyl, (C1–C4) haloalkyl, and so forth.
Degron is capable of binding to a ubiquitin ligase;
Targeting ligand is capable of binding to EGFR or a mutant EGFR.
Key Structures
Biological Assay
H1975, H3255, and HaCaT Target Modulation Assays, and EGFR protein degradation was assessed by Western blotting after treatment of T790M/L858R mutant Ba/F3 cell lines. Mouse efficacy studies were carried out and monitored by MRI to quantify lung tumor burden prior to being assigned to various treatment study cohorts.
Biological Data
The compounds in
this Patent Highlight are bifunctional compounds that are potent against
T790M/L858R transformed Ba/F3 cells. The Table shows antiproliferative
activity, where A = EC50 < 500 nM; B = 500 nM < EC50 < 1000 nM; and C = 1000 nM < EC50 <
5000 nM.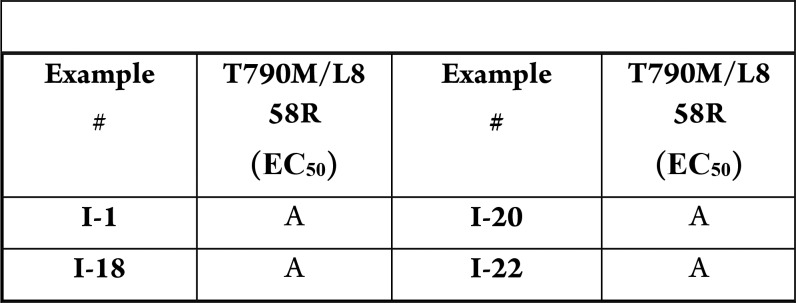
Recent Review Articles
-
1.
Tulchinsky E.; Demidov O.; Kriajevska M.; Barlev N. A.; Imyanitov E.. BBA Rev. Cancer 2019, 1871, 29.
-
2.
Pettersson M.; Crews C. M.. Drug Discov. Today Technol. 2019, 31, 15.
-
3.
Das D.; Hong J.. Eur. J. Med. Chem. 2019, 170, 55.
-
4.
Weng M.-S.; Chang J.-M.; Hung W.-Y.; Yang Y.-C.; Chien M.-H.. J. Exp. Clin. Canc. Res. 2018, 37, 1.
The author declares no competing financial interest.