Abstract
Insulin signaling in the central nervous system influences satiety, counterregulation, and peripheral insulin sensitivity. Neurons expressing the Glut4 glucose transporter influence peripheral insulin sensitivity. Here, we analyzed the effects of insulin receptor (IR) signaling in hypothalamic Glut4 neurons on glucose sensing as well as leptin and amino acid signaling. By measuring electrophysiological responses to low glucose conditions, we found that the majority of Glut4 neurons in the ventromedial hypothalamus (VMH) were glucose excitatory neurons. GLUT4-Cre-driven insulin receptor knockout mice with a combined ablation of IR in Glut4-expressing tissues showed increased counterregulatory response to either 2-deoxyglucose-induced neuroglycopenia or systemic insulin-induced hypoglycemia. The latter response was recapitulated in mice with decreased VMH IR expression, suggesting that the effects on the counterregulatory response are likely mediated through the deletion of IRs on Glut4 neurons in the VMH. Using immunohistochemistry in fluorescently labeled hypothalamic Glut4 neurons, we showed that IR signaling promoted hypothalamic cellular signaling responses to the rise of insulin, leptin, and amino acids associated with feeding. We concluded that hypothalamic Glut4 neurons modulated the glucagon counterregulatory response and that IR signaling in Glut4 neurons was required to integrate hormonal and nutritional cues for the regulation of glucose metabolism.
The reemerging notion that insulin, acting through the central nervous system (CNS), controls peripheral insulin sensitivity raises the possibility of developing CNS-based approaches to treat insulin resistance, obesity, and type 2 diabetes mellitus. Insulin receptor (IR) signaling in the CNS also has roles in the regulation of food intake (1), the counterregulatory response to hypoglycemia (2), gonadotropin release (3), peripheral glucose metabolism (4), leptin sensitivity (5), and neuronal plasticity (6). We previously identified a subset of neurons characterized by Glut4 glucose transporter expression that are important for systemic insulin sensitivity (7). Indeed, mice with a collective ablation of IR in Glut4-expressing tissues [GLUT4-Cre-driven insulin receptor knockout (GIRKO)] develop highly penetrant diabetes that recapitulates cardinal features of the human disease (7).
In this study, we used GIRKO mice to study the role of CNS IR signaling in response to glucose deprivation. Hypoglycemia is commonly observed in association with intensive insulin therapy (8, 9). Restoration of normoglycemia after acute hypoglycemia requires effective sensing of low glucose levels and rapid activation of the counterregulatory hormone responses, particularly glucagon and epinephrine (2), in addition to other key metabolic hormones such as leptin (10). Moreover, recurrent hypoglycemia can decrease the sympathoadrenal response, resulting in greater risk of severe hypoglycemia and hypoglycemia unawareness (8). Thus, understanding CNS-based mechanisms that initiate counterregulatory responses can help reduce or prevent hypoglycemia.
It has been shown that neurons in the rodent mediobasal hypothalamus, which consists of the ventromedial nucleus (VMN) and arcuate nucleus (ARC), are important for glucose sensing and that IR signaling modulates the counterregulatory response to neuroglycopenia (11, 12). Nonetheless, the specific neuronal subtypes involved in this response are unclear (13). We previously showed that Glut4 neurons represent a distinct neuronal population that has a heterogeneous composition of neuronal markers and neurotransmitters (14). Upon toxin-mediated ablation of these neurons in the hypothalamus, mice develop anorexia and reduced insulin secretion that results in impaired glucose tolerance with fasting hyperglycemia (15). These data are consistent with the notion that hypothalamic Glut4 neurons are important mediators of insulin action. In this study, we investigated whether IR signaling on hypothalamic Glut4 neurons participates in regulation of the counterregulatory response to hypoglycemia, using a combination of electrophysiology, hypoglycemic clamps, targeted neuroglycopenia, and analysis of biochemical signaling pathways.
Material and Methods
Mice
Gt(Rosa)26Sor tm9(CAG-tdTomato)Hze, Gt(Rosa)26Sortm3(CAG-EYFP)Hze, ROSA26-EGFP, and wild-type (WT) B6 mice were obtained from The Jackson Laboratories (Bar Harbor, ME). Glut4 neurons were fluorescently labeled by breeding Rosa reporter mice with Glut4-Cre mice. GIRKO mice were generated as previously described (7). For the ventromedial hypothalamus (VMH) IR knockdown studies, C57BL6 mice were procured from Charles River. The procedures were approved by the Indiana University, University of Utah, and Columbia University animal care and utilization committees.
Evaluating the glucose responsiveness of hypothalamic Glut4-expressing neurons
To determine whether hypothalamic Glut4-expressing neurons respond to fluctuating glucose levels, we conducted electrophysiological analyses on coronal brain slices (300 μm) taken through the hypothalamus of three male Glut4-EYFP mice (8 to 10 weeks old). Briefly, the mice were euthanized by decapitation, the brains were rapidly removed, and slices were cut through the hypothalamus using a Leica VT1200 vibratome in an ice-cold, oxygenated (95% O2, 5%CO2) solution (in mM: 10 NaCl, 25 NaHCO3, 2.5 KCl, 10 d-glucose, 1.25 NaH2PO4, 195 sucrose, 2 NaN3, 7 MgCl2, 1 CaCl2, pH 7.3). Three or four slices were prepared from each mouse. Before electrical recordings, the brain slices were maintained at room temperature for at least 1 hour in oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (in mM: 120 NaCl, 5 KCl, 26 NaHCO3, 1.2 NaH2PO4, 2 CaCl2, 1 MgCl2, 5 d-glucose, pH 7.4). Mannitol was used to adjust the osmolality of the low-glucose artificial cerebrospinal fluid (0.1 mM or no glucose). Whole-cell patch recordings were performed using borosilicate glass electrodes with resistance between 4 and 6 M backfilled with an intracellular solution (in mM: 128 K-gluconate, 10 KCl, 10 HEPES, 0.1 EGTA, 2 MgCl2, 3 ATP, and 0.1 GTP, pH 7.3). Glut4 neurons in the VMN or proopiomelanocortin (POMC) neurons in the ARC were identified by fluorescence and patch clamped under infrared differential interference contrast optics. Recordings were acquired with an Axopatch 700B amplifier, digitized, and analyzed with pClamp10 software (Molecular Devices, San Jose, CA).
Evaluating the counterregulatory response to neuroglycopenia
Stereotaxic injections were performed as described previously (16). Briefly, 3- to 4-month-old WT (n = 6) and GIRKO (n = 6) mice were anesthetized, and a unilateral microinjection guide cannula was stereotaxically implanted into the lateral ventricle (−0.3 mm anterior and 1 mm lateral to the bregma and 2.2 mm below the skull surface). The mice were then allowed 1 week for recovery. We verified correct positioning of the cannula by injecting methylene blue (1%) after the animals were euthanized. We induced neuroglycopenia by acutely injecting the nonmetabolizable glucose analog 2-deoxyglucose (2-DG; 1 mg per mouse) intracerebroventricularly into the lateral ventricle. In the first experiment, we monitored blood glucose from a tail nick over the course of 5 hours after injection of 2-DG. In the second experiment, we collected blood samples to measure plasma glucagon levels 30 minutes after intracerebroventricular (icv) 2-DG injection.
Evaluating the counterregulatory hormone response to systemic insulin-induced hypoglycemia in GIRKO mice
Having demonstrated that hypothalamic Glut4 neurons are in fact glucose responsive, we proceeded to establish whether loss of insulin signaling in Glut4 tissues altered the hormone response to hypoglycemia. Approximately 4 to 5 days before the clamp procedure, WT (n = 6) and GIRKO (n = 6) mice were anesthetized with isoflurane, and a single catheter (MRE-25) was implanted into the jugular vein and tunneled subcutaneously as described previously (17). Four to five days later, hypoglycemic clamp studies were performed as described previously (11). Briefly, overnight fasted mice were connected to infusion pumps and allowed to recover for a period of ∼2 hours before baseline blood samples were obtained through a tail nick. Subsequently, a bolus-constant insulin (20 mU/kg/min) and variable 20% dextrose solution were infused intravenously to lower and maintain plasma glucose levels at ∼50 mg/dL for 90 minutes. Blood samples were collected at 30-minute intervals throughout the hypoglycemic clamp portion of the study for measurement of plasma glucagon and catecholamine responses and at 90 minutes for plasma insulin levels. Erythrocytes from a donor mouse were washed, suspended in heparinized-saline, and infused over the course of the study to prevent volume depletion and anemia. At the end of the study, the animals were euthanized with an overdose of sodium pentobarbital.
Identifying whether loss of insulin receptors in the VMH recapitulates the counterregulatory defects observed in GIRKO mice
To better establish whether loss of insulin receptors in the VMH contributed to the amplified glucagon response observed in GIRKO mice, we locally knocked down insulin receptor expression in the VMH using an adeno-associated virus (AAV) that expresses an insulin receptor short hairpin RNA and enhanced green fluorescent protein (GFP) reporter under the control of a U6 promoter (shAAV-262287; Vector Biolabs, Malvern, PA). This viral vector was shown to reduce IR expression in the VMH by ∼45% (18). We microinjected 0.5 μL of the IR short hairpin RNA AAV bilaterally into the VMH (−1.46 mm anteroposteriorly, ± 0.4 mm mediolaterally, and −5.2 mm dorsoventrally at an angle of 0°) of C57BL6 mice (n = 6) under isoflurane anesthesia. An AAV expressing a nonspecific scrambled RNA sequence was used as a negative control (n = 5). Three weeks after injection of the AAV, a vascular catheter was implanted into the jugular vein. The animals then recovered for 4 to 5 days before undergoing a hypoglycemic clamp as described previously.
Hormone analyses
We used ELISA for insulin measurements (Millipore, Burlington, MA) and colorimetric assays for metabolite measurements (16). Plasma insulin and glucagon in blood samples collected during the clamp procedures were measured by RIA (Millipore) (19) or ELISA (Mercodia, Winston-Salem, NC). Catecholamines were measured by ELISA (Eagle Biosciences, Amherst, NH).
Evaluating whether the hypothalamic Glut4 neuronal response to insulin, leptin, and amino acid signaling during feeding is affected in GIRKO mice
To determine whether loss of IR in hypothalamic Glut4-expressing neurons alters their ability to respond to insulin, leptin, and amino acids, we used immunohistochemistry to quantify the activated downstream signaling targets [i.e., phosphorylated Akt (phospho-Akt or pAkt), phosphorylated Stat3 (phospho-Stat3 or pStat3), phosphorylated S6 (phospho-S6 or pS6), respectively] in the ARC. Both WT and GIRKO mice were fasted and refed before they were euthanized. Their brains were harvested and immunohistochemically processed as previously described (16). We quantified the fluorescent signal intensity of pAkt/pStat3/pS6 and GFP from individual cells in the ARC. Image J was used to quantify the fluorescence intensity and mark individual neurons. Glut4 neurons were identified by the green fluorescence, for which the GFP signal intensity was used to identify Glut4+ neurons vs non-Glut4 neurons. Neurons were considered Glut4+ if the GFP integrated density exceeded 0.300 (arbitrary units), whereas Glut4− neurons had a GFP integrated density ≤0.300.
pAkt, Stat3, and S6 were used to gauge the activity of insulin, leptin, and amino acid signaling pathways, respectively. Plotting the results in groups of Glut4+ neurons vs non-Glut4 neurons from WT and GIRKO mice allowed us to quantify the signaling activity in individual cells, which serves as an unbiased way to assay hormone and nutrient sensitivity in Glut4+ vs non-Glut4 neurons. The antibodies used were procured from Cell Signaling Technology (Danvers, MA) and Thermo Fisher Scientific (Waltham, MA) and include pAkt (Ser473) (D9E) XP Rabbit monoclonal antibody [mAb (catalog no. 4060)] [RRID: AB_2315049 (20)], pS6 ribosomal protein (Ser235/236) (catalog no. D57.2.2E) XP Rabbit mAb (catalog no. 4858) [RRID: AB_916156 (21)], pStat3 (Tyr705) (catalog no. D3A7) XP Rabbit mAb (catalog no. A27039) [RRID: AB_2536100 (23)].
Food intake
We measured food intake and feeding response as previously described (24, 25).
Statistical analyses
We analyzed data with the Student t test or one-way or two-way ANOVA using GraphPad Prism software (GraphPad, San Diego, CA). We used the customary threshold of P < 0.05 to declare statistical significance.
Results
Glut4 neurons in the hypothalamus were glucose responsive
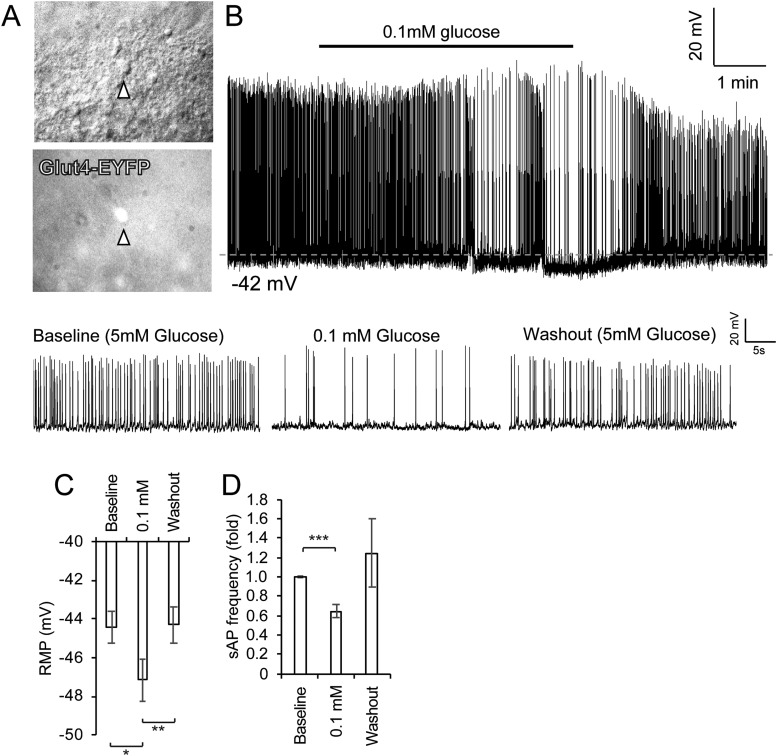
We characterized the glucose-responsive nature of the Glut4 neurons using electrophysiology. We first validated the whole-cell patch-clamp technique using hypothalamic POMC neurons (18), an established type of glucose-excited neurons (26–28). GFP-labeled POMC neurons responded to glucopenia with hyperpolarization and reduced spontaneous firing frequency (18). This is consistent with previous reports that identified POMC neurons as glucose-excitatory neurons (26–28), thus validating our experimental system. Next, we measured the electrophysiological response of Glut4 neurons to changing glucose concentrations by patch clamping EYFP-labeled Glut4 neurons (with intact IR signaling) in the VMN of the hypothalamus (Fig. 1). When baseline glucose (5 mM) was lowered to 0.1 mM, Glut4 neurons responded with hyperpolarization and reduced spontaneous firing frequency (Fig. 1A and 1B), which was restored to baseline measurement during the washout phase (Fig. 1C and 1D). More measurements revealed that hypothalamic Glut4 neurons were a mixed population and that the majority of these neurons were glucose-excited neurons (61.5%), whereas a smaller fraction of these Glut4 neurons were either glucose-inhibited (15.4%) or nonresponsive (23.1%).
Figure 1.
Glut4 neurons are glucose-responsive neurons. (A) Glut4 neurons were identified using a fluorescent microscope. Arrowheads point to representative fluorescent Glut4 neurons. (B) Spontaneous action potentials (sAPs) were recorded in the whole-cell current-clamp mode in Glut4 neurons perfused sequentially with aCSF containing 5 mM glucose (baseline), 0.1 mM glucose, and 5 mM glucose (washout). Representative 30-s sections of a current-clamp recording from the same neuron in aCSF containing different extracellular glucose concentrations are shown below. (C) Mean changes in membrane potential with changes in extracellular glucose concentration are shown. (D) Mean fold-changes in sAP frequency with changes in extracellular glucose concentration are shown. Data show means ± SEM (n = 8). *P < 0.05; **P < 0.01; ***P < 0.001; paired t test. aCSF, artificial cerebrospinal fluid; RMP, resting membrane potential.
Augmented hyperglycemic response to neuroglycopenia in GIRKO mice
We also assessed the glucagon response to neuroglycopenia in GIRKO mice after an icv injection of 2-DG. After icv injection of 2-DG, WT mice developed hyperglycemia, a response that was greatly amplified in GIRKO mice throughout the 5-hour observation period (Fig. 2A). The rise in glucose was accompanied by a nearly 50% increase in glucagon levels in the GIRKO mice compared with the WT mice (Fig. 2B).
Figure 2.
pAkt signaling after neuroglycopenia challenge. (A) Time course study of the hyperglycemic response after 2-DG icv injection in WT and GIRKO mice (n = 7 to 10 per group). (B) Serum glucagon measurement after saline or 2-DG icv injection. Data show means ± SEM (n = 9). (C) Western blot shows increased pAkt in the GIRKO hypothalamus. (D) Quantification of the distribution of pAkt+ cells and Glut4 neurons in WT and GIRKO mice after neuroglycopenia are shown. Glut4 neurons are labeled with "+", and non-Glut4 neurons are labeled with "–". Neurons stained positive for pAkt are labeled with "+", and neurons stained negative for pAkt are labeled with "–". Data show means ± SEM (n = 5). *P < 0.05; **P < 0.01; ****P < 0.0001; two-way ANOVA/Sidak multiple-comparison test.
To examine the signaling pathways mediating the amplified counterregulatory response seen in GIRKO mice, we measured levels of pAkt by western blotting of hypothalamic extracts dissected after icv injection of 2-DG. Unexpectedly, overall pAkt was markedly increased in GIRKO hypothalamic samples (Fig. 2C). In contrast, hippocampi collected from the GIRKO and control mice showed comparable amounts of pAkt (Fig. 2C). This supports the notion that the hypothalamus is a critical site for sensing glucose. Next, we examined pAkt by immunohistochemistry to localize the activation to a specific hypothalamic cell type. In these experiments, we used GIRKO mice in which Glut4 neurons had been labeled with Rosa26-tdTomato (14). Using double-staining with pAkt and Tomato (to label Glut4 neurons), we found that the increase in pAkt occurred largely, if not exclusively, in non-Glut4 neurons of GIRKO mice. Quantitative analysis of the results showed the percentage of pAkt-positive Glut4 neurons were approximately one-third lower in the ARC of GIRKO mice, whereas the percentage of pAkt-positive non-Glut4 neurons was approximately sixfold greater in GIRKO mice (Fig. 2D). The aberrant Akt signaling in the non-Glut4 neurons may explain the exaggerated counterregulatory response in GIRKO mice that experienced neuroglycopenia.
GIRKO mice had an enhanced counterregulatory response to systemic insulin-induced hypoglycemia
Glut4 is expressed in glucose-sensing neurons (29), but it is not known whether IR signaling in Glut4 neurons regulates glucose sensing. To address this question, we investigated the hormonal response to systemic insulin-induced hypoglycemia in GIRKO mice. Our previous work showed that GIRKO mice develop diabetes with age (7). To avoid the potential confounders of hyperglycemia and β-cell failure, we selected chow-fed euglycemic GIRKO and littermate controls for these studies.
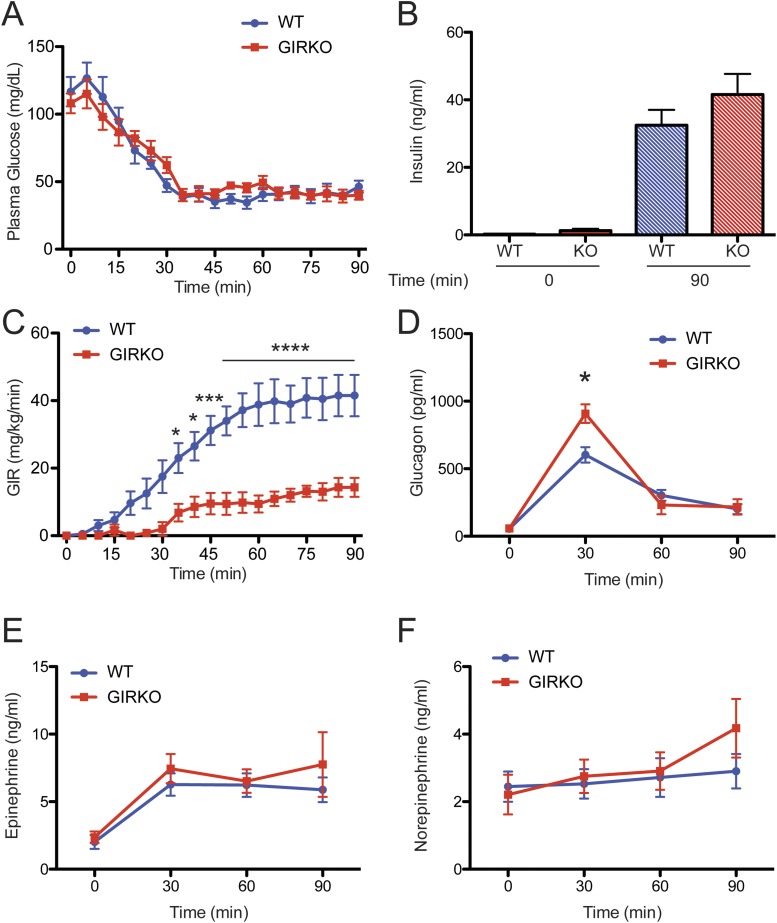
Despite matched plasma glucose and insulin concentrations during the hypoglycemia clamp (Fig. 3A and 3B), the glucose infusion rate (GIR) needed to maintain plasma glucose levels at ∼50 mg/dL in the GIRKO mice was nearly 75% lower than that for WT mice (Fig. 3C). The decrease in the GIR corresponded to an increase in glucagon secretion (Fig. 3D) during the hypoglycemic clamp, but the epinephrine and norepinephrine responses were not affected.
Figure 3.
Increased counterregulatory response in GIRKO mice in hypoglycemic clamp studies. (A) Plasma glucose and (B) insulin concentration during the hypoglycemic clamp procedure were well matched between WT and GIRKO mice. (C) Despite this, glucose infusion rates (GIRs) were significantly lower in the GIRKO mice than in the WT mice. (D) The glucagon response was significantly increased in the GIRKO mice, but (E) plasma epinephrine and (F) norepinephrine responses were not significantly different. Data show means ± SEM (n = 6). *P < 0.05; ***P < 0.001; ****P < 0.0001; two-way ANOVA/Sidak multiple-comparison test.
Improvements in glucagon response to hypoglycemia stemmed from a reduction in VMH IR expression
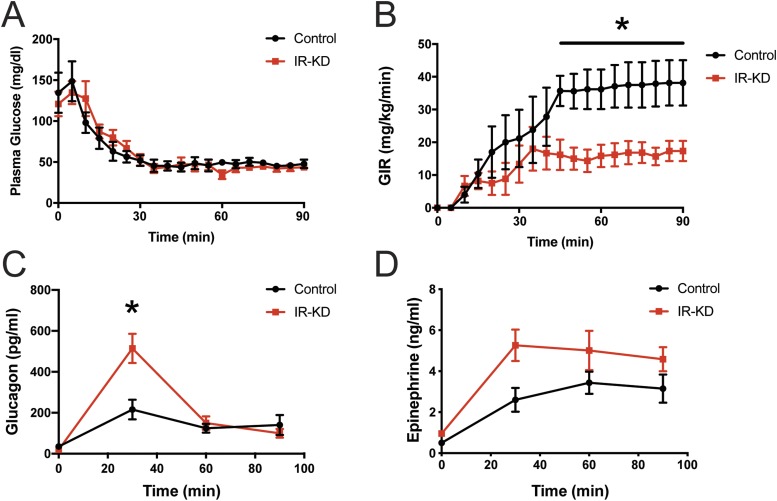
Our results showed that GIRKO mice responded to insulin-induced hypoglycemia with an increased counterregulatory response. However, GIRKO mice have global IR deficiency, and the defect is not confined to the brain. To rule out the possibility that systemic insulin resistance led to improvements in glucagon secretion during hypoglycemia in GIRKO mice, we performed hypoglycemic clamp studies on mice with VMH-specific insulin receptor knockdown (IR-KD). This nucleus was targeted because our previous work showed that, within the hypothalamus, Cre activity of the Glut4-Cre transgene was concentrated in the VMH (15). Therefore, we selectively knocked down IRs in the VMH. Despite similar plasma glucose levels (Fig. 4A), VMH IR-KD mice required significantly less exogenous glucose during the clamp (Fig. 4B). The reduction in GIR observed in the VMH IR-KD mice corresponded to improvements in the glucagon response (Fig. 4C). Plasma epinephrine responses were not significantly different during the clamp (Fig. 4D). These results were consistent with those from the clamp studies in GIRKO mice (Fig. 3). Therefore, we concluded that the exaggerated glucagon response to hypoglycemia observed in GIRKO mice was most likely due to a loss of insulin signaling in the VMH rather than increased peripheral insulin resistance.
Figure 4.
Increased counterregulatory response in IR-KD mice in hypoglycemic clamp studies. (A) Plasma glucose concentrations were matched between control and VMH IR-KD mice during the hypoglycemic clamp. (B) Glucose infusion rates were significantly lower in IR-KD mice. (C) Plasma glucagon and (D) epinephrine were measured during the clamp. Data show means ± SEM (n = 5 or 6 per group). *P < 0.05; two-way ANOVA/Sidak multiple-comparison test.
Regulation of feeding-associated hormonal and nutrient signaling by IR in Glut4 neurons
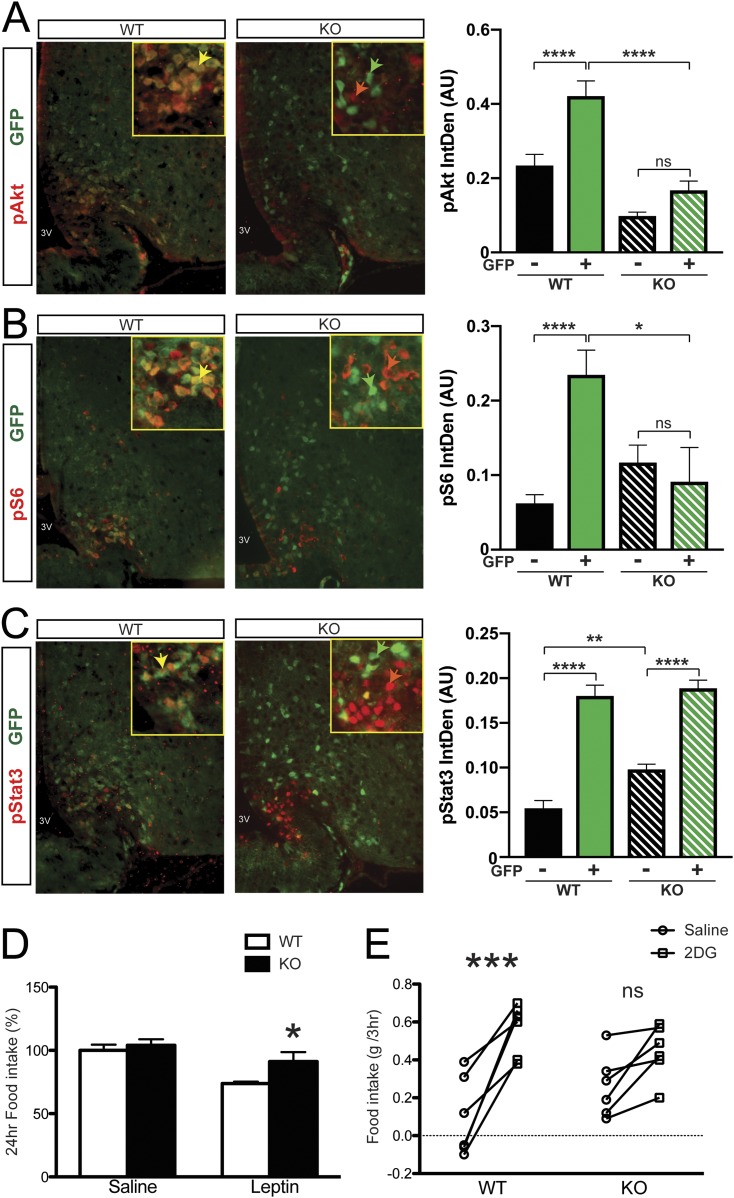
Thus far, we established that Glut4 neurons play an important role in regulating the glucagon response to hypoglycemia. We then asked whether Glut4 neurons respond to other hormonal and nutrient-related signals associated with feeding, such as leptin and amino acids. We took advantage of the ability to label Glut4 neurons in WT and GIRKO mice using a Rosa-GFP reporter allele to investigate these questions. During fasting, leptin and amino acid levels are low, resulting in low basal signaling activity. After refeeding, the increased availability of nutrients triggers hormone release; therefore, how animals respond to the surge reflects the nutritional and hormonal sensitivity. We decided to use the fast-refeeding paradigm as opposed to injecting hormones directly because this is a more physiologically relevant setting for capturing the in vivo response. We subjected mice to fasting and refeeding, then performed immunohistochemistry on hypothalamic sections using readouts of insulin (pAkt), leptin (pStat3), or amino acid signaling (pS6) (red), as well as GFP to identify Glut4-expressing neurons (green). After refeeding, we detected pAkt (red) in Glut4 neurons (green) of WT mice (Fig. 5A; yellow arrowhead in the inset), whereas in GIRKO mice the intensity and colocalization of pAkt in Glut4 neurons was markedly diminished (Fig. 5A; lower panel, separate green and red arrowheads in the inset). These data indicate that most of the pAkt detected after refeeding localized to IR-containing Glut4 neurons in the ARC of WT mice and that ablation of IR markedly blunted this response, thereby helping to validate the model’s utility for investigating the effects of IR signaling on other signaling pathways.
Figure 5.
Hormone and nutrient signaling in Glut4 neurons from GIRKO mice. (A‒C) Immunohistochemistry with (A) pAkt, (B) pS6, and (C) pStat3 and GFP in the ARC of WT and GIRKO mice. Yellow arrowheads point to representative Glut4 neurons in WT mice with positive phosphorylated signaling molecule staining. Green arrowheads point to representative Glut4 neurons in GIRKO mice without positive phosphorylated signaling molecule staining. Red arrowheads point to representative non-Glut4 neurons in GIRKO mice with positive phosphorylated signaling molecule staining. For bar graphs, Glut4 neurons marked by GFP positive staining are labeled with "+"; non-Glut4 neurons lacking GFP staining are labeled with "–". Following refeeding, integrated density of the fluorescent signal in Glut4− (black bars) and Glut+ (green bars) neurons was measured and plotted on the right. Data show means ± SEM (n = 21 to 138 cells measured per group). *P < 0.05; **P < 0.01; ****P < 0.0001; one-way ANOVA/Tukey multiple-comparisons test. (D) Twenty-four‒hour food intake after leptin injection is shown. Data show means ± SEM (n = 7). *P < 0.05; two-way ANOVA/Bonferroni multiple-comparisons. (E) Food intake measurement after saline or 2-DG ip injection is shown. Data show means ± SEM (n = 6). ***P < 0.001; two-way ANOVA/Bonferroni multiple-comparisons. IntDen, integrated density; ns, not significant.
Amino acid sensing in the CNS occurs primarily through the mTOR-S6K-S6 pathway (30); thus, we assessed pS6 immunohistochemistry in WT and GIRKO samples. Similar to the pStat3 results, we detected a strong correlation between pS6 and Glut4 neurons in refed WT mice (Fig. 5B; yellow arrowhead in the inset), whereas in GIRKO mice samples, pS6 immunoreactivity was markedly attenuated, with a trend of increase in a subset of non-Glut4 neurons (Fig. 5B; separate green and red arrowheads in the inset).
Next, we analyzed pStat3 as a means to gauge leptin sensitivity. Although pStat3 and Glut4 (GFP) immunoreactivity showed substantial overlap (Fig. 5C; yellow arrowhead in the inset), we observed a bimodal pStat3 pattern in refed GIRKO mice. pStat3 immunoreactivity was still detected in Glut4 neurons, whereas there was marked activation in a subset of non-Glut4 neurons (Fig. 5C; separate green and red arrowheads in the inset). The increased leptin sensitivity in non-Glut4 neurons of GIRKO mice might reflect (i) a compensatory increase or (ii) removal of an IR-dependent inhibitory signal arising from Glut4 neurons.
Given the bimodal response of pStat3 to leptin (Fig. 5D) in GIRKO mice, we assessed leptin responsiveness in vivo by measuring food intake after leptin injection in euglycemic WT and GIRKO mice. The GIRKO mice exhibited a blunted response to leptin (Fig. 5D), suggesting that ablation of IR signaling in Glut4 neurons impairs leptin sensitivity despite the changes in non-Glut4 neurons.
Taken together, GIRKO mice appeared to have an abnormal response to nutrient availability, which may lead to aberrant feeding behavior. For instance, although neuroglycopenia increased food consumption in WT mice, the food consumption of GIRKO mice in response to both central saline and 2-DG administration was similar (Fig. 5E).
Discussion
In previous studies, we showed that Glut4 neurons are indeed insulin-sensitive neurons and that they may play a role in integrating olfacto-sensory responses with metabolic cues (14). Moreover, in cell ablation experiments, we showed that hypothalamic Glut4 neurons regulate satiety, glucose homeostasis, and CNS sensing of hormones and nutrients (15). The present findings extend our previous work showing that hypothalamic Glut4 neurons are mostly glucose-excited neurons and that inhibition of IR function in the VMN of rodents increases the glucagon response to hypoglycemia (11).
To better understand the characteristic of hypothalamic Glut4-expressing neurons, we performed electrophysiological studies to determine whether they are glucose responsive. The majority of these Glut4 neurons in the VMN decreased their activity in response to a reduction in glucose in the medium, suggesting they are glucose-excited neurons. A previous study showed that insulin stimulates glucose uptake and promotes translocation of Glut4 to the neuron surface (31). This suggests that during the early physiological response to hypoglycemia, when insulin secretion declines, a reduction in insulin action can decrease the translocation of GLUT4 glucose transporters to the membrane in hypothalamic Glut4 neurons, thereby reducing glucose uptake and silencing neuronal activity. If these hypothalamic glucose-excited neurons are indeed inhibitory GABAergic neurons, as was shown previously (32), then a decrease in inhibitory output can enhance the counterregulatory hormone response. In the case of the GIRKO mice, a chronic reduction in IRs may decrease the activity of these glucose-excited GABAergic neurons and ultimately augment the counterregulatory response during hypoglycemia.
The current data provide evidence that insulin may influence the activity of glucose-responsive Glut4 neurons in the hypothalamus to modulate the counterregulatory hormone response to hypoglycemia. Specifically, loss of insulin receptors and signaling on Glut4 neurons in GIRKO mice resulted in an exaggerated glucagon response to insulin-induced hypoglycemia. Because IRs were also knocked out in peripheral tissues of GIRKO mice, peripheral insulin resistance becomes a confounding factor. For this reason, we knocked down IR expression in the VMH of C57BL6 mice and found that this manipulation recapitulated the enhanced glucagon response observed in the GIRKO mice. It is important to note that the chronic loss of IRs in the VMH can lead to impaired glucose tolerance after 12 weeks of knockdown, but not after 6 weeks of knockdown (33). In our VMH IR-KD mice, IR KD was only maintained for a period of 4 weeks; therefore, the development of peripheral insulin resistance and impaired glucose tolerance was likely not a factor that augmented the glucagon response. Moreover, when we centrally administered the glucoprivic agent 2-DG into the brains of GIRKO mice, we observed an exaggerated hyperglycemic response that was coupled with an augmented glucagon response. These observations are consistent with the idea that the loss of IRs in the brain and, more specifically, in hypothalamic Glut4 neurons leads to a decrease in inhibitory output, which enhances glucagon secretion.
In addition, these studies show that in response to neuroglycopenia, there was greater phosphorylation of Akt in the hypothalamus of GIRKO mice than in the hippocampus. Interestingly, although the cell-autonomous decrease of pAkt in Glut4 neurons of GIRKO mice was expected in this scenario, the marked increase of pAkt in non-Glut4 neurons has not been described. This change in pAkt could be engaged independently of the IR and may be due to many factors. For instance, this may be a result of the hormonal/metabolite feedback inputs to these non-Glut4 neurons. Nevertheless, further studies are required to ascertain if this ectopic Akt activation in non-Glut4 neurons is either necessary or sufficient to initiate the counterregulatory response.
An unexpected finding of this work is the paradoxical activation of Stat3 and S6 signaling in non-Glut4 neurons after IR ablation in Glut4 neurons, suggesting that insulin signaling in Glut4 neuron dampens leptin and amino acid signaling in a cell-autonomous fashion. The finding emphasizes the integrative role of Glut4 neurons in energy homeostasis (34), while raising the possibility that the identification of the additional feedback cues emanating from these neurons will provide actionable points to increase leptin or nutrient sensitivity in the treatment of metabolic disorders (35).
The findings of the present work enhance our understanding of the contribution of insulin and Glut4 toward the counterregulatory response to hypoglycemia and nutrient sensing. We conclude that (i) Glut4 neurons are glucosensing neurons in the CNS; (ii) a decrease in IR signaling in Glut4 neurons blunts the glucagon response to hypoglycemia; and (iii) IR signaling in Glut4 neurons has unexpected effects on leptin and amino acid signaling in hypothalamic Glut4 as well as non-Glut4 neurons.
Future work is warranted to investigate the circuitry of Glut4 neurons mediating the response to hypoglycemia, as Glut2 neurons of the nucleus tractus solitarius have reportedly linked hypoglycemia detection to counterregulatory response (36). The finding that hyperglycemic response to 2-DG was not concordant with blunted feeding response in the GIRKO mice is surprising. Similar findings were reported in the hindbrain catecholamine neurons (37). Therefore, whether the hindbrain is involved in the circuitry of Glut4 neurons mediating the hypoglycemia response will also be investigated.
Acknowledgments
We thank Drs. Robert Sherwin and Sachin Paranjape for their critical input during the project development phase.
Financial Support: This study is supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK58282 and DK63608 (to D.A., Columbia University); the Showalter Trust Scholarship, NIH P&F funding from P30DK097512 and UL1TR002529, and NIDDK Grant DK098294 (to H.R., Indiana University); and NIDDK Grant DK099315 (to O.C.; University of Utah). H.R. was supported by a mentor-based postdoctoral fellowship from the American Diabetes Association and by a Berrie Diabetes Research Fellowship.
Author Contributions: D.A., H.R., and O.C. designed and conducted the experiments, analyzed the data, and wrote the article. S.Y., A.V.-d.-A., and A.M.R. conducted the experiments and analyzed the data. A.M.R. and H.R. bred the mice, performed the GIRKO neuroglycopenic experiments, and analyzed the hypothalamic signaling pathways. S.Y. conducted the electrophysiology experiments. A.V.-d.-A. and O.C. validated the brain-specific IR knockdown using a viral approach, performed the hypoglycemic clamp studies, and measured the counterregulatory hormones during the clamp. O.C. and H.R. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviations:
- 2-DG
2-deoxyglucose
- AAV
adeno-associated virus
- ARC
arcuate nucleus
- CNS
central nervous system
- GFP
green fluorescent protein
- GIR
glucose infusion rate
- GIRKO
GLUT4-Cre-driven insulin receptor knockout
- icv
intracerebroventricular
- IR
insulin receptor
- IR-KD
insulin receptor knockdown
- mAb
monoclonal antibody
- pAkt
phosphorylated Akt
- POMC
proopiomelanocortin
- pS6
phosphorylated S6
- pStat3
phosphorylated Stat3
- VMH
ventromedial hypothalamus
- VMN
ventromedial nucleus
- WT
wild-type
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282(5738):503–505. [DOI] [PubMed] [Google Scholar]
- 2. Sherwin RS. Bringing light to the dark side of insulin: a journey across the blood-brain barrier. Diabetes. 2008;57(9):2259–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–2125. [DOI] [PubMed] [Google Scholar]
- 4. Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–449. [DOI] [PubMed] [Google Scholar]
- 5. Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Brüning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11(4):286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodd GT, Michael NJ, Lee-Young RS, Mangiafico SP, Pryor JT, Munder AC, Simonds SE, Brüning JC, Zhang ZY, Cowley MA, Andrikopoulos S, Horvath TL, Spanswick D, Tiganis T. Insulin regulates POMC neuronal plasticity to control glucose metabolism. eLife. 2018;7:e38704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin HV, Ren H, Samuel VT, Lee HY, Lu TY, Shulman GI, Accili D. Diabetes in mice with selective impairment of insulin action in Glut4-expressing tissues. Diabetes. 2011;60(3):700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369(4):362–372. [DOI] [PubMed] [Google Scholar]
- 9. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C; Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 10. Flak JN, Patterson CM, Garfield AS, D’Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JT, Germani M, Jones JC, Rajala M, Satin L, Rhodes CJ, Olson DP, Kennedy RT, Heisler LK, Myers MG Jr. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci. 2014;17(12):1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paranjape SA, Chan O, Zhu W, Horblitt AM, McNay EC, Cresswell JA, Bogan JS, McCrimmon RJ, Sherwin RS. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes. 2010;59(6):1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan O, Sherwin RS. Hypothalamic regulation of glucose-stimulated insulin secretion. Diabetes. 2012;61(3):564–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53(10):2521–2528. [DOI] [PubMed] [Google Scholar]
- 14. Ren H, Yan S, Zhang B, Lu TY, Arancio O, Accili D. Glut4 expression defines an insulin-sensitive hypothalamic neuronal population. Mol Metab. 2014;3(4):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ren H, Lu TY, McGraw TE, Accili D. Anorexia and impaired glucose metabolism in mice with hypothalamic ablation of Glut4 neurons. Diabetes. 2015;64(2):405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren H, Orozco IJ, Su Y, Suyama S, Gutiérrez-Juárez R, Horvath TL, Wardlaw SL, Plum L, Arancio O, Accili D. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake [published correction appears in Cell. 2013;153(5):1166]. Cell. 2012;149(6):1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim JD, Toda C, D’Agostino G, Zeiss CJ, DiLeone RJ, Elsworth JD, Kibbey RG, Chan O, Harvey BK, Richie CT, Savolainen M, Myöhänen T, Jeong JK, Diano S. Hypothalamic prolyl endopeptidase (PREP) regulates pancreatic insulin and glucagon secretion in mice [published correction appears in Proc Natl Acad Sci USA. 2014;111(38):14003]. Proc Natl Acad Sci USA. 2014;111(32):11876–11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ren H, Vieira-de-Abreu A, Yan S, Reilly AM, Chan O, Accili D. Data from: Altered central nutrient sensing in male mice lacking insulin receptors in Glut4-expressing neurons. IUPUI ScholarWorks 2015. Deposited 29 April 2019. http://hdl.handle.net/1805/18992. [DOI] [PMC free article] [PubMed]
- 19. Chan O, Paranjape S, Czyzyk D, Horblitt A, Zhu W, Ding Y, Fan X, Seashore M, Sherwin R. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes. 2011;60(5):1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. RRID:AB_2315049, https://scicrunch.org/resolver/AB_2315049.
- 21. RRID:AB_916156, https://scicrunch.org/resolver/AB_916156.
- 22. RRID:AB_2491009, https://scicrunch.org/resolver/AB_2491009.
- 23. RRID:AB_2536100, https://scicrunch.org/resolver/AB_2536100.
- 24. Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, DePinho RA, Wardlaw SL, Accili D. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15(10):1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4(5):507–512. [DOI] [PubMed] [Google Scholar]
- 26. Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology. 2003;144(4):1331–1340. [DOI] [PubMed] [Google Scholar]
- 27. Hu J, Jiang L, Low MJ, Rui L. Glucose rapidly induces different forms of excitatory synaptic plasticity in hypothalamic POMC neurons. PLoS One. 2014;9(8):e105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449(7159):228–232. [DOI] [PubMed] [Google Scholar]
- 29. Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53(8):1959–1965. [DOI] [PubMed] [Google Scholar]
- 30. Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. [DOI] [PubMed] [Google Scholar]
- 31. Bakirtzi K, Belfort G, Lopez-Coviella I, Kuruppu D, Cao L, Abel ED, Brownell AL, Kandror KV. Cerebellar neurons possess a vesicular compartment structurally and functionally similar to Glut4-storage vesicles from peripheral insulin-sensitive tissues. J Neurosci. 2009;29(16):5193–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu W, Czyzyk D, Paranjape SA, Zhou L, Horblitt A, Szabó G, Seashore MR, Sherwin RS, Chan O. Glucose prevents the fall in ventromedial hypothalamic GABA that is required for full activation of glucose counterregulatory responses during hypoglycemia. Am J Physiol Endocrinol Metab. 2010;298(5):E971–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paranjape SA, Chan O, Zhu W, Horblitt AM, Grillo CA, Wilson S, Reagan L, Sherwin RS. Chronic reduction of insulin receptors in the ventromedial hypothalamus produces glucose intolerance and islet dysfunction in the absence of weight gain. Am J Physiol Endocrinol Metab. 2011;301(5):E978–E983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121(6):2087–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91(2):389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lamy CM, Sanno H, Labouèbe G, Picard A, Magnan C, Chatton JY, Thorens B. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab. 2014;19(3):527–538. [DOI] [PubMed] [Google Scholar]
- 37. Li AJ, Wang Q, Dinh TT, Powers BR, Ritter S. Stimulation of feeding by three different glucose-sensing mechanisms requires hindbrain catecholamine neurons. Am J Physiol Regul Integr Comp Physiol. 2014;306(4):R257–R264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.