Abstract
Although androgen deprivation therapy (ADT) and immunotherapy are potential treatment options in men with metastatic prostate cancer (CaP), androgen has conventionally been proposed to be a suppressor of the immune response. However, we herein report that DHT activates macrophages. When the murine macrophage cell line (RAW 264.7), human monocyte cell line (THP-1), and human peripheral blood monocytes were cultured with androgen-resistant CaP cell lines, DHT increased cytotoxicity of macrophages in a concentration-dependent manner. Further studies revealed that DHT induced M1 polarization and increased the expression levels of TNF-related apoptosis-inducing ligand (TRAIL) in macrophages and that this effect was abrogated when TRAIL was neutralized with a blocking antibody or small interfering RNA. Subsequent experiments demonstrated that induction of TRAIL expression was regulated by direct binding of androgen receptor to the TRAIL promoter region. Finally, an in vivo mouse study demonstrated that castration enhanced the growth of an androgen-resistant murine CaP tumor and that this protumorigenic effect of castration was blocked when macrophages were removed with clodronate liposomes. Collectively, these results demonstrate that DHT activates the cytotoxic activity of macrophages and suggest that immunotherapy may not be optimal when combined with ADT in CaP.
Immunotherapy based on dendritic cells has been incorporated into the armamentarium against advanced prostate cancer (CaP) (1). Castration has classically been used as the cornerstone in treating men with metastatic CaP. Therefore, determining the precise impact of androgen will likely be necessary to optimize the effectiveness of immunotherapies in CaP patients. In this regard, testosterone is generally considered to have immunosuppressive effects. For example, systemic androgen removal has been reported to increase peripheral T lymphocytes (2, 3) and reduce regulatory T cells (4). In addition, Drake et al. (5) demonstrated that androgen ablation results in the expansion and development of prostate-specific T cells after vaccination. Most recently, enhanced dendritic cell function has been correlated with low serum testosterone levels (6).
Among various immune cells, macrophages have numerous functions related to inflammation, immunity, tumor growth, and progression. These divergent effects are due to the heterogeneity of macrophage differentiation and phenotypes (7). Broadly, these “polarization” states are categorized as a proinflammatory (classically activated, M1) phenotype stimulated by lipopolysaccharide (LPS) or IFN-γ (8, 9), or as an anti-inflammatory (M2) phenotype induced by IL-4 and IL-13 (10). M1 macrophages are generally considered potent effector cells that kill microorganisms and tumor cells and produce proinflammatory cytokines. In contrast, M2 macrophages are able to temper inflammatory responses and adaptive Th2 immunity, promote angiogenesis, and scavenge debris (11). In cancer, it has been proposed that the tumor microenvironment tips the macrophage polarization balance in favor of the M2 phenotype.
In the context of macrophages and inflammation, the role of androgens has been controversial. Specifically, it has been demonstrated that testosterone replacement therapy decreases endogenous inflammatory cytokines in men with hypogonadism (12, 13). Likewise, androgen suppresses cytotoxic activity of macrophages and pharmacologic levels of DHTinhibit the generation of superoxides in rat macrophages (14). On the other hand, in a mouse model of wound healing, the proinflammatory cytokine TNF-α at the site of injury was downregulated by castration or flutamide treatment (15). Similarly, in vitro studies found that lipopolysaccharide (LPS)-induced TNF-α production in macrophages was enhanced by testosterone (7).
TNF, a cytokine involved in acute and chronic inflammation and endotoxin-induced shock (16), has a cytotoxic effect on tumor cells and causes hemorrhagic necrosis of tumors in mouse (17). However, TNF’s unacceptable toxicity profile has limited the factor’s systemic use in patients with advanced cancer (18). More recently, TNF-related apoptosis-inducing ligand (TRAIL) has been identified as a member of the TNF superfamily that contains TNF-α and Fas-ligand (19, 20). TNF-α is produced by T cells, natural killer cells, and activated macrophages, whereas TRAIL is expressed by lymphocytes, spleen, prostate, ovary, colon, and placenta (1). It has been suggested that both TNF-α and TRAIL may serve as potential antiprostate cancer agents (2, 3). However, TRAIL is considered more promising than TNF-α because of TRAIL’s lower toxicity (21). Currently, TRAIL-based treatment is being investigated in clinical trials (4, 5).
In this framework, we have investigated the role of DHT on cytotoxic activity of macrophages. We report that the tumoricidal effect of macrophages is stimulated by DHT via TRAIL.
Materials and Methods
Cell culture and reagents
THP-1, RAW264.7, DU145, PC3, LNCaP, 22Rv1, TRAMP-C1, and TRAMPC-2 were purchased from the American Type Culture Collection (ATCC; Manassas, VA). THP-1, DU145, PC-3, 22Rv1, and LNCaP cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS). RAW264.7, TRAMP-C1, and TRAMP-C2 cells were maintained in DMEM containing 10% FBS. Human peripheral blood mononuclear cells were purchased from Stemcell Technologies (Vancouver, British Columbia, Canada) and maintained in RPMI-1640/10% FBS. Human monocyte THP-1 cells were used as macrophages after differentiation with phorbol 12-myristate 13-acetate (PMA). For differentiation, THP-1 was cultured in RPMI/10% FBS with 10 ng/mL PMA for 24 hours. For coculture studies, DMEM or RPMI-1640 containing 1% FBS was used. To isolate murine peritoneal macrophages, 0.9 g of thioglycollate was dissolved in 30 mL of dH2O and autoclaved. C57BL/6 mice were injected with 2 mL of thioglycollate solution intraperitoneally and euthanized 3 days later. Peritoneal lavage was carried out by using 10 mL of PBS. For DHT experiments, 1% charcoal-stripped FBS (cFBS) was used. When indicated, cell count values were normalized with the corresponding vehicle-treated control group.
For cocultures studies, prostate cancer cells were plated on six-well plates (5 × 105 cells per well). After 24 hours, tissue culture inserts (Greiner Bio-One, Monroe, NC) were placed and macrophages were plated (5 × 105 cells per well). After 3 days, the bottom-layer prostate cancer cells were harvested and RT-PCR and Western blot were carried out. For neutralization experiments, all prostate cancer cells were treated with the indicated neutralizing antibody for 1 hour before the addition of macrophages.
Endotoxin assay
For detection of possible contamination of endotoxin in DHT solution, QUANTI-Blue endotoxin assay kit (catalog no. Rep-qb1; Invivogen, San Diego, CA) was used. After treatment with various concentrations of DHT in the RAW264.7 cells under RPMI1640 media with 1% cFBS for 48 hours, released alkaline phosphatase amounts were detected in culture media. Briefly, 20 μL culture supernatant with 180 μL QUANTI-Blue buffer (InvivoGen) incubated in 37°C incubator for 2 hours. Then, OD values were detected by spectrophotometer with 620- to 655-nm wavelength.
Animals
C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at the Rutgers Robert Wood Johnson Medical School. To establish tumor xenografts, 5 million TRAMP-C2 cells were subcutaneously injected into mice. After a week, predesignated mice were castrated surgically. Macrophages were systemically removed by injection of 0.1 mL clodronate liposomes or equal volume of PBS liposomes intraperitoneally at a dose of 25 mg/kg every 3 days. Clodronate liposomes or control PBS-loaded liposomes were purchased from ClodronateLiposomes.org (Amsterdam, Netherlands). Tumor volume based on caliper measurements was calculated by using the modified ellipsoidal equation (22, 23): tumor volume = 1/2(length × width2).
RNA isolation and RT-PCR
Through use of a Direct-zol RNA purification kit (Zymo Research, Irvine, CA), total RNA was purified and RT was carried out to produce cDNA using the Improm-IITM Reverse Transcription system (Promega, Madison, WI). Resulting cDNA was used for quantitative PCR and semi-quantitative PCR. All primers used in this study are listed in an online repository (24). All quantitative RT-PCR values were normalized to those of the respective vehicle-treated control group.
Immunoblot and ELISA
Cells were collected and lysed with cell lysis buffer (10X) (Cell Signaling Technology, Danvers, MA) and 1 mM phenylmethylsulfonyl fluoride. After removal of cellular debris with centrifugations, supernatant was analyzed. Proteins in the supernatant were separated via SDS-PAGE and incubated with androgen receptor (AR) (25) (catalog no. SC-7305, Santa Cruz Biotechnology, Dallas, TX), TRAIL (26) [for human TRAIL: catalog no. SC-8440, Santa Cruz Biotechnology; for mouse TRAIL (27): catalog no. MAB1121, R&D Systems, Minneapolis, MN)], TNF-α (28) (for human and mouse TNF-α: catalog no. 3707, Cell Signaling Technology) and β-actin antibodies (29) (catalog no. A5441, Sigma-Aldrich, St. Louis, MO). Each antibody was diluted 1:1000 and incubated at 4°C overnight. Following incubation with the appropriate secondary antibody, SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Waltham, MA) was used to visualize the target protein. To measure the protein level of TRAIL and TNF-α, conditioned media from DHT-treated macrophages were collected and a human TRAIL ELISA kit (Abcam, Cambridge, MA) and mouse TNF-α Quantikine ELISA kit (R&D Systems) were used. The recommended protocol provided by the manufacturer was used.
Cytotoxicity assay
For macrophage cytotoxicity assay, a commercially available nonradioactive kit was used (catalog no. CLATOX100-3; Cell Technology, Fremont, CA). The principle of the assay is the quantitative measurement of release of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from mammalian cell lines (30). GAPDH, a homotetrameric enzyme in the glycolysis pathway, catalyzes the oxidative phosphorylation of d-glyceraldehyde 3-phosphate to 1,3-diphosphoglycerate. The release of GAPDH from dying cells leads to ATP production, which is then coupled with the luciferase/luciferin bioluminescence as the final readout. In setting up the tissue culture plates, outer rows and columns were not used to reduce any “edge effects.” Following the manufacturer’s recommended protocol, 5000 target cells (TRAMP-C2) were incubated with varying numbers of effectors (RAW264.7) at indicated DHT concentrations to achieve the specified effector-to-target ratio. The reaction was allowed to proceed for 3 days at 37°C. Next, the reaction plate was allowed to equilibrate to RT for 15 minutes and centrifuged in a micro plate centrifuged for 1 minute at 750g. Then, 50 μL of enzyme assay diluent was added to all reaction wells and supernatants were transferred onto a new plate and incubated with 100μL of the 2X enzyme assay reagent containing G3P. Finally, 50 μL of 1X detection reagent was added to each well, and the plate was read by using a luminometer immediately.
The equation used to calculate percentage cytotoxicity is as follows.
where $ refers to values from three wells that were averaged; one asterisk refers to maximum target, obtained by completely lysing 5000 target cells (target cell number used for all data points); and two asterisks refer to media only.
Cytokine antibody array
After treatment with RAW264.7 with DHT 100 nM for 24 hours, cytokines in the conditioned medium were measured by using Mouse Cytokine Array C2000 and Mouse Cytokine Array C7 (RayBiotech, Norcross, GA). Precise details of the cytokine antibody array are shown in an online repository (31, 32). Arrays were used following the manufacturer’s recommendation. Briefly, antibody array membranes were incubated with blocking solution for 30 minutes. Then, 1 mL of conditioned medium was added and incubated for 5 hours at room temperature. After washing three times with PBS, biotinylated antibody cocktail was added and incubated for 2 hours at room temperature. Again, the membrane was washed three times and incubated for 2 hours at room temperature after the addition of horseradish peroxidase streptavidin solution. Finally, signal was detected with a ChemiDoc touch machine (Bio-Rad, Hercules, CA).
Chromatin immunoprecipitation
THP-1 cells were differentiated with PMA and washed twice with sterilized PBS. The cells were cultured in RPMI-1640/1% cFBS with 100 nM DHT for 2 days and cross-linked with 1% formaldehyde and lysed with lysis buffer (20 mM Tris-HCl (pH, 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin). Chromatin was sheared and immunoprecipitated with anti-AR antibody (Santa Cruz Biotechnology) or control normal IgG. The immunoprecipitated DNAs were subjected to quantitative PCR. The primers used in this study were hTRAIL ChIP1 (forward: 5′-GGCTGTGTGTCTTGTGCATT-3′; reverse: 5′-TGAACCTAACACTATTTGAACACACTT-3′) and hTRAIL chromatin immunoprecipitation (ChIP) negative (forward: 5′-CCTGAGCAACTTGCACTTGA-3′; reverse: 5′-TCCTCCTGAAATCGAAAGTATGT-3′), mTRAIL ChIP1 (forward: 5′- GAGCACGAGGACCTTAGCTT-3′; reverse: 5′- AGCTCACACAGGGATCTTCC-3′) and mTRAIL ChIP negative (forward: 5′-AGGGCATGCATCTGGAAATGA-3′; reverse: 5′-CAAGCTAGAGAAGCAAGCCA-3′).
Apoptosis assay
Apoptotic level was measured using Thermo Fisher ApoDETECT Annexin V-FITC kit (catalog no. 33-1200). The protocol recommended by the vendor was used. Briefly, after treatment with various concentrations of DHT for 24 hours, cells were fixed with 80% ethanol and washed with PBS three times. Then, fixed cells were incubated with Annexin V-FITC in PBS solution for 30 minutes at room temperature. After washing three times with PBS, cells were treated with 300 nM DAPI in PBS for 5 minutes at room temperature. Finally, after washing three times with PBS, mounting solution (catalog no. H-1400; Vector Laboratories, Burlingame, CA) was added to stained cells and visualized by using immunofluorescence microscopy.
For paraffin-embedded xenograft mice tumor tissue staining, a modified protocol was used. Briefly, after elimination of paraffin, Annexin V-FITC (1:20 dilution) solution was incubated overnight 4°C degree. Then it was washed three times with PBS. Using antifade mounting medium with DAPI (catalog no. H-1200; Vector Laboratories), the stained slide was mounted.
TRAIL neutralization
Abcam antibodies were used for TRAIL neutralization [for humans (33), catalog no. ab9959; for mice (34), catalog no. ab10516; Abcam]. Two μg of each antibody was used. After seeding of 50,000 TRAMP-C2 or DU145 cells in 24-well plates (DMEM 1% FBS for TRAMP-C2/RAW264.7 or RPMI1640 1% FBS for DU145/THP-1), the same numbers of RAW264.7 or activated THP-1 (with 5 ng/mL PMA for 24 hours) were added by using a Boyden chamber (catalog no. 662641; Greiner Bio-one, Kremsmunster, Austria). Cells were cocultured for 48 hours and TRAMP-C2 and DU145 were counted.
For the human and mouse small interfering RNA (siRNA) study, lipofectamine 3000 reagent and Qiagen siRNAs were used (for human, catalog no. ENSG00000121858; for mouse, catalog no. ENSMUSG00000039304; Qiagen, Germantown, MD). Briefly, 100 nM siRNA and 3 μL lipofectamine 3000 solution were mixed and incubated for 10 minutes. Next this mix was added to RAW264.7 or THP-1 and cultured for 24 hours. Then a coculture study was carried out as above.
Immunofluorescence (IF) for mouse tumor and quantification
All harvested tumors from mice were fixed with formalin, embedded in paraffin, and stained with hematoxylin and eosin. These sections were stained with TRAIL antibody (35) (1:100 dilution, 4°C overnight; Bioss, Woburn, MA) and mouse F4/80 antigen purified antibody (36) (1:100 dilution, 4°C overnight, Affymetrix eBioscience, San Diego, CA). Briefly, each antibody was incubated with 1:100 dilution, 4°C overnight, and washed three times with PBS. All stained slides were scanned by an Olympus VS120 Florescence/Bright-Field whole-slide scanner (Olympus Scientific Solutions Americas Corp., Waltham, MA) and individually quantified by ImageJ V1.50i software (National Institutes of Health, Bethesda, MD). Values are shown as mean fluorescence intensity.
Luciferase assay
RAW 264.7 cells (1 × 105 cells per well) were plated onto six-well plates and transfected with 1 μg of plasmids with androgen response element containing prostate-specific antigen (PSA) promoter-luciferase (PSA-lux) or androgen response element tandem repeated sequence of the probasin promoter-luciferase plasmid (ARR-lux) and 0.2 μg of Renilla luciferase plasmids using lipofectamine 2000 (Thermo Fisher Scientific). Forty-eight hours later, cells were treated with the indicated concentrations of DHT for 48 hours. Luciferase level was measured by using the Dual-Luciferase Reporter Assay System (Promega). All luciferase assay values were calculated to fold changes after normalizing to those of the vehicle-treated control group.
Flow cytometry analysis for macrophage infiltration in TRAMP-C2 xenograft mice tumor
Briefly, xenografts were digested by collagenase type II (catalog no. LS004202; Worthington Biochemical Corp., Lakewood, NJ), and resulting cells were divided into aliquots as a 100-μL suspension with F4/80-FITC conjugated antibody (catalog no. MA5-16628; Thermo Fisher Scientific). After incubating for 30 minutes, cells were washed with PBS, resuspended in 0.2 mL of 0.5% paraformaldehyde/PBS, and subjected to flow cytometry.
Statistical analyses
For all statistical analyses, a Student t test was used. P < 0.05 was considered to indicate a statistically significant finding. All in vitro experiments were performed at least three times.
Supplemental Data
Supplemental tables and figures can be found in an online repository (31, 32, 37).
Results
Macrophages express conventional androgen receptor
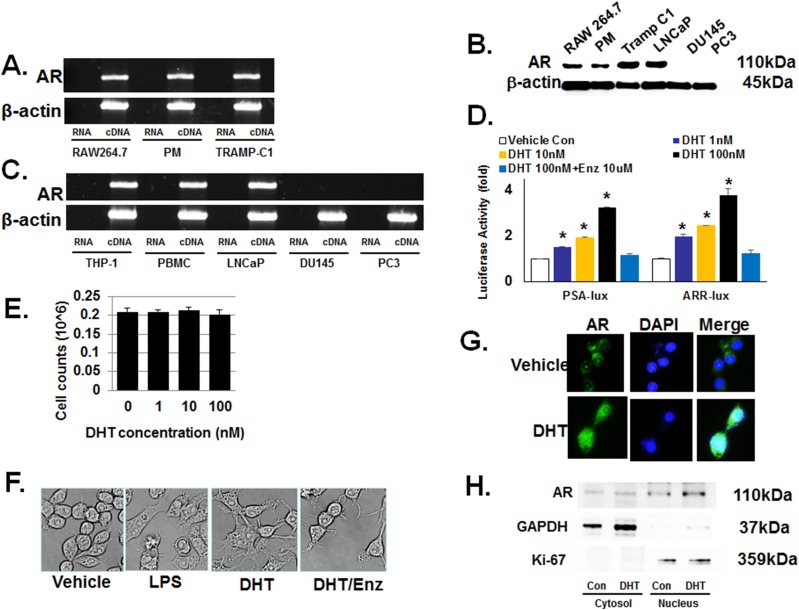
Previously, it was suggested that macrophages express membrane bound and not the conventional AR (6, 12). Therefore, we initially examined the status of the conventional AR expression in the murine macrophage cell line RAW 264.7 and primary murine peritoneal macrophages. The AR-negative human prostate cancer cell lines DU145 and PC3 were used as negative controls. In both RAW 264.7 and peritoneal macrophages, RT-PCR and immunoblot demonstrated the expression of conventional AR (Fig. 1A and 1B, respectively). The human peripheral blood monocytes and monocyte THP-1 also expressed AR mRNA (Fig. 1C). The authenticity of the RT-PCR product was confirmed by sequencing (31, 32).
Figure 1.
Macrophages have a functional conventional AR. Unless specified, cells were treated with the indicated concentrations of dihydrotestosterone (DHT) for 24 h. (A) Through use of RT-PCR, AR mRNA was detected in the murine macrophage cell line RAW264.7 and primary peritoneal macrophages (PM). The androgen-sensitive murine prostate cancer cell line, TRAMP-C1, was used as the positive control cells. Primer sequences are provided in an online repository (25). (B) Immunoblot detected 110-kDa AR protein in RAW 264.7, PM, Tramp C1 (positive control), LNCaP (positive control), DU145 (negative control), and PC3 (negative control). (C) RT-PCR also showed AR mRNA expression in human macrophages. THP-1 human monocyte cell line was differentiated into macrophages by using PMA before isolating mRNA for RT-PCR. LNCaP is a human prostate cancer cell line used as a positive control; DU145 and PC3 are human prostate cancer cell lines used as negative controls. (D) Androgen signaling axis is functional in macrophages. The androgen response reporter vectors, PSA-lux and ARR-lux, were transfected into RAW 264.7 cells and cultured with 0 to 100 nM of DHT and/or enzalutamide (Enz) 10 μM for 24 h. At 1 nM DHT, luciferase activity increased 50% to 100% and a concentration-dependent increase was observed. Enzalutamide at10 μM reversed the induction of luciferase activity. (E) DHT has no significant effect on the proliferative capacity of macrophages. After treatment of RAW264.7 with DHT up to 72 h, no significant change in cell number was detected. The data represent a 72-h study. (F) DHT induced a profound morphological change in macrophages. When RAW264.7 was treated with 100 nM DHT for 24 h, cells developed multiple processes as well as intracellular vacuole-like structures. The morphological change elicited by DHT was nearly identical to that by LPS. Enzalutamide at10 μM inhibited the cellular morphological change by DHT. (G) Nuclear translocation of AR in RAW264.7 after DHT treatment. After treatment with 100 nM DHT for 6 h, AR nuclear translocation was detected by immunofluorescence microscopy. (H) Immunoblot demonstrated increased AR protein in the nuclear but not cytosol fraction. As controls, GAPDH was used for the cytosol fraction and Ki-67 for nuclear fraction. Error bars indicate average ± SEM. *P < 0.05 compared with vehicle-treated control group in all experiments. Control (Con) means only vehicle treatment group. PBMC, human peripheral blood mononuclear cell.
Effects of DHT on RAW 246.7 cells
To determine whether the conventional AR is functional in macrophages, RAW 264.7 was transiently transfected with luciferase reporter constructs containing either the PSA promoter (PSA-lux) or the probasin promoter (ARR-lux) (38). When transfected cells were treated with DHT for 24 hours, luciferase activity increased in a concentration-dependent manner without affecting the cellular proliferation (Fig. 1D and 1E). After treatment for 24 hours with the physiologically relevant DHT concentration of 1 nM, luciferase activity increased by 50% and 100% following transfection with PSA-lux and ARR-lux, respectively. This DHT-stimulated luciferase activity was completely reversed when cells were treated with the antiandrogen enzalutamide at 10 μM. Morphologically, RAW 264.7 cells treated with DHT resembled macrophages activated by LPS (Fig. 1F). Again, this morphological change was largely blocked by coincubating with 10 μM enzalutamide. Furthermore, immunofluorescence microscopy revealed the nuclear translocation of AR in RAW 264.7 cells when treated with 100 nM DHT for 6 hours (Fig. 1G). In addition, immunoblot against the cellular compartments demonstrated that DHT treatment increased AR protein in the nucleus but not cytosol (Fig. 1H). To exclude the possibility of an endotoxin effect of DHT, we measured released alkaline phosphatase by using QUANTI-Blue endotoxin assay kit after treating RAW264.7 with various concentrations of DHT. The results showed no endotoxin effect by DHT treatment (31, 32). These results collectively suggest that there is an active androgen signaling pathway in macrophages through the conventional AR.
Effect of DHT on cytotoxic activity of macrophages
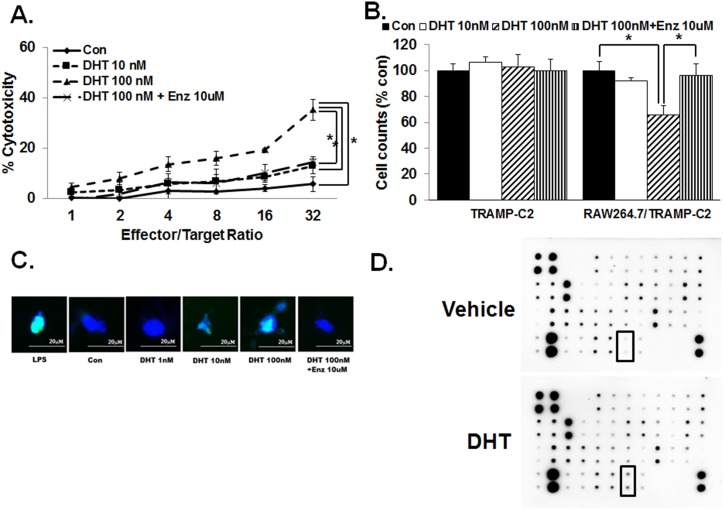
Because cytotoxicity is one commonly assessed function of macrophages, we next investigated the effect of DHT on the cytotoxic activity of RAW 264.7 cells using a coculture model with the androgen-resistant murine CaP cell line, Tramp C2. Using the commercially available assay based on GAPDH release, we found that cell death induced by increasing effector/target ratio is DHT concentration–dependent after 24 hours; this increase in cytotoxicity was reversed by the addition of 10 μM enzalutamide (Fig. 2A). To determine whether a direct cell-cell contact is necessary for this DHT-induced cytotoxic effect of RAW 264.7 on TRAMP-C2 cells, the coculture study was carried out in the presence of tissue culture inserts. The results demonstrated that TRAMP-C2 cell count again decreased significantly when cultured with RAW 264.7 and increasing concentrations of DHT (Fig. 2B). Again, 10 μM enzalutamide treatment reversed the decrease in cell count following exposure to DHT. As previously reported (13), DHT at 10 and 100 nM had no observable effect on the cell number of TRAMP-C2 cells when cultured alone. Next, an identical experiment was carried out by using a THP-1 human monocyte cell line and tissue culture inserts. DU145 and PC3 androgen-independent human CaP cell lines were used as the target. As with that of RAW 264.7, coculture with THP-1 decreased DU145 and PC3 cell numbers with increasing concentrations of DHT; 10 μM enzalutamide treatment completely blocked the decrease in DU145 and PC3 cell count when cocultured with THP-1 at 100 nM DHT for 24 hours (31, 32).
Figure 2.
DHT increases cytotoxic activity of macrophages and TRAIL expression. (A) When TRAMP-C2 CaP cells and RAW 264.7 cells were cocultured for 3 d with increasing concentration of DHT (0 to 100 nM) and GAPDH release was measured as an indicator of cell viability, there was a concentration-dependent and effector/target ratio-dependent increase in cytotoxic effect. Enzalutamide (Enz) treatment, 10 μM, reversed the increase of cytotoxicity by DHT. Cocultures were carried out for 24 h. Each well contained 5000 target cells and varying number of RAW264.7 cells based on the indicated ratio. (B) Direct cell-cell contact is not required for DHT-induced cytotoxic activity of murine macrophages. When TRAMP-C2 was cocultured with RAW264.7 in the presence of a cell culture insert for 24 h, DHT again decreased the count of TRAMP-C2 in a concentration-dependent manner. Enzalutamide treatment, 10μM, completely reversed the increase of cytotoxic activity in macrophages at 100 nM DHT. (C) Upon DHT treatment, macrophages induced apoptosis in TRAMP-C2 cells. After cocultures RAW264.7 and TRAMP-C2 using the tissue culture inserts, Annexin V-FITC assay was carried out on TRAMP-C2 after removal of the macrophages. The results demonstrated an increase in apoptosis level with increased concentrations of DHT. Enzalutamide, 10 μM, totally abrogated the macrophage-mediated apoptosis induced by 100 nM DHT. Blue indicates DAPI staining; green indicates DNA fragments. (D) Cytokine antibody array including TRAIL was analyzed. After treatment of RAW264.7 cells with 100 nM DHT for 24 h, cell extracts were used to assess the expression of various cytokines. In this array, increased TRAIL protein was detected. Error bars indicate average ± SEM. *P < 0.05 in all experiments. Control means only vehicle treatment group. Con, TRAMP-C2 with vehicle without RAW264.7.
DHT-stimulated cytotoxic activity and M1 macrophage polarization
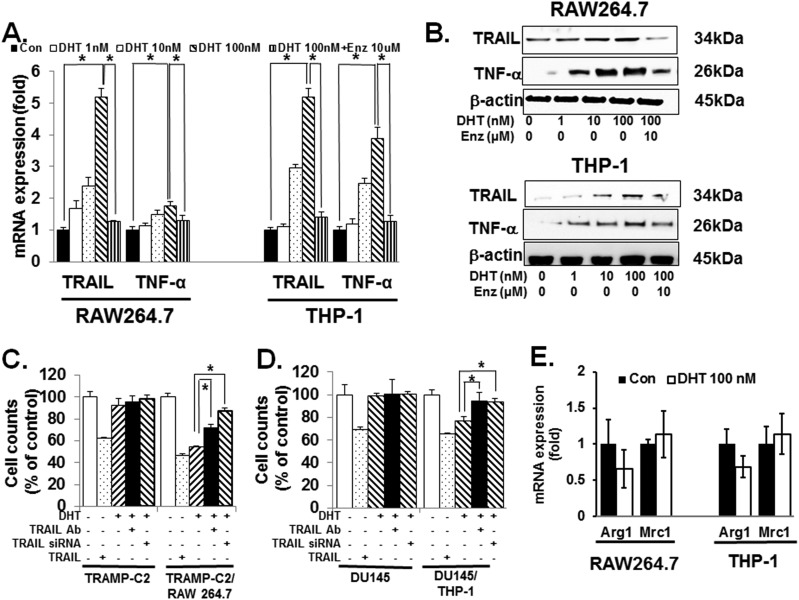
To determine the underlying mechanism for the observed effect of DHT on macrophages, we assessed whether the decrease in prostate cancer cell count was due to apoptosis. As shown in Fig. 2C, TUNEL assay demonstrated an increase in apoptosis of TRAMP-C2 when cocultured with RAW 264.7 at 100 nM DHT for 24 hours. Again, treatment with 10 μM enzalutamide completely blocked the effect on apoptosis. In the TRAMP-C2 xenografted mice experiment, castration decreased apoptosis. These observations again suggest that in AR-unresponsive prostate cancer cells, castration may not be optimal (31, 32). Next, we treated RAW 264.7 with 100 nM DHT for 24 hours and investigated the expression status of cytokines using the RayBio® Mouse Cytokine Array C2000 (RayBiotech) and Mouse Cytokine Array C7 (RayBiotech). The results demonstrated that DHT significantly increased the expression of TRAIL and TNF-α (Fig. 2D) (31, 32). Quantitative PCR and immunoblot revealed that DHT after 24 hours increased TRAIL and TNF-α both at the mRNA and protein levels in a concentration-dependent manner in RAW 264.7 and THP-1 cells (Fig. 3A and 3B). As expected, enzalutamide at 10 μM abrogated this increase in TRAIL and TNF-α induction by DHT in macrophages.
Figure 3.
DHT induces M1 polarization of macrophages. (A) DHT induced the expression of TNF-α and TRAIL mRNAs in both the murine and human macrophages (RAW264.7 and THP-1, respectively) and enzalutamide (Enz; 10 μM) treatment completely neutralized the increase in TNF-α and TRAIL mRNAs. Cells were cultured with 100 nM DHT for 24 h. Then, RNA was isolated and quantitative PCR was carried out. (B) DHT increased TNF-α and TRAIL protein levels in both RAW264.7 and THP-1 cells. After culturing cells with 100 nM DHT for 24 h, immunoblot was carried out. Again, 10 μM enzalutamide partially blocked the increase of TNF-α and TRAIL proteins. (C) RAW264.7/TRAMP-C2 cocultures using tissue culture inserts were pretreated with the TRAIL neutralizing antibody for 1 h. Subsequently, 100 nM DHT was added and cocultures were incubated for 24 h. After removal of RAW264.7 cells in the top compartment, remaining TRAMP-C2 cells in the lower chamber were counted. As a complementary approach, TRAIL short hairpin RNA was used. After transfecting RAW264.7 cells with short hairpin RNA and incubating for 24 h, they were added to the top compartment of the coculture. Twenty-four h later, the top chamber was removed and the remaining TRAMP-C2 was counted. As control, TRAMP-C2 was cultured without RAW264.7. Where denoted negative (−), appropriate volume of vehicle was used. As a positive control, 100 ng/mL of TRAIL was used. The results demonstrated that both the neutralizing antibody and short hairpin RNA partially restored the decrease in cell count seen in the presence of RAW264.7 and 100 nM DHT. (D) Identical study was carried out using the human system: THP-1 and DU145. Again, TRAIL neutralizing antibody and short hairpin RNA partially reversed the decrease in DU145 cell numbers in the presence of THP-1 and 100 nM DHT. To prevent cell-cell contact, tissue culture inserts were used. (E) After culturing RAW264.7 and THP-1 with 100 nM DHT for 24 h, mRNA was isolated and M2 polarization markers Arg1 and Mrc1 were quantitated with qPCR, and there was no significant effect on M2 polarization of macrophages. Error bars indicate average ± SEM. *P < 0.05 in all experiments. Control means only vehicle treatment group.
To investigate whether TNF-α or TRAIL mediates the DHT-induced cytotoxic activity of macrophages, we carried out the aforementioned coculture studies with neutralizing antibodies and siRNAs. The results revealed that both the pretreatment with TRAIL neutralizing antibody and transfection with TRAIL short hairpin RNA reversed the cytotoxic activity of RAW 264.7 on TRAMP-C2 cells (Fig. 3C). The control in which TRAMP-C2 was treated directly with TRAIL (100 ng/mL) showed a ∼40% decrease in cell count after 24 hours. A similar result was obtained with the human system (THP-1/DU145 coculture) (Fig. 3D). The effect of siRNA on blocking TRAIL expression in RAW 264.7 and THP-1 was confirmed by using an immunoblot (31, 32). To study the DHT-stimulated cytotoxic activity of macrophages against AR-positive prostate cancer cells, the human prostate cancer cell line 22Rv1 was cocultured with THP-1 cells. The magnitude of cytotoxic effect was similar to that of TRAMP-C2/RAW264.7 and 22Rv1/THP-1 coculture studies (31, 32). In contrast to blocking TRAIL, blocking TNF-α with a neutralizing antibody in coculture had no effect on CaP cell count (data not shown). Because increased TNF-α and TRAIL expression levels are consistent with M1 differentiation (7, 14, 39), we next assessed the effect of DHT on the M2 markers, Arg1 and Mrc1. In contrast to the M1 factors TNF-α and TRAIL, DHT had no significant effect on Arg1 and Mrc1 mRNA expression levels in both RAW 264.7 and THP-1 cells (Fig. 3E). Collectively, these results indicate that DHT increases cytotoxic activity of macrophages on CaP cells via stimulating the polarization of macrophages toward the M1 phenotype.
Induction of TRAIL expression by DHT
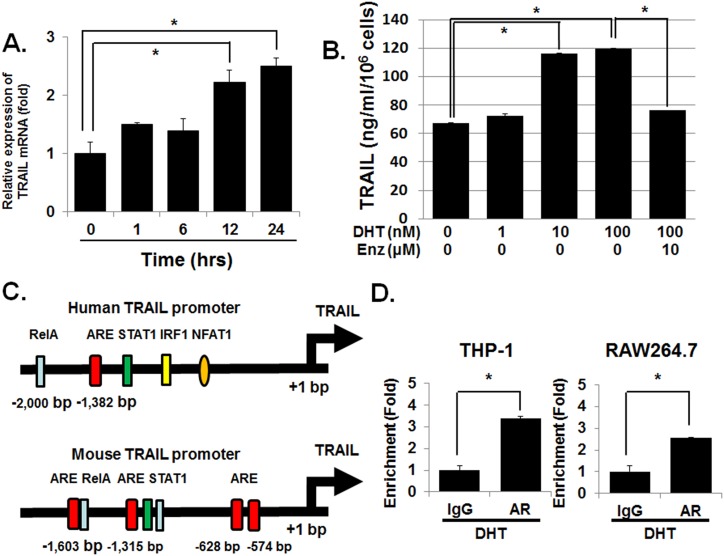
To determine the underlying mechanism for the observed effect of DHT on macrophages’ cytotoxic activity, we investigated the kinetics of DHT-stimulated TRAIL expression. The results demonstrated that 100 nM of DHT induced TRAIL mRNA expression in 6 to 12 hours (Fig. 4A). ELISA confirmed that 100 nM DHT increased TRAIL protein level by more than twofold in the conditioned medium of RAW 264.7 cells within 24 hours (Fig. 4B). Enzalutamide at 10 μM again inhibited the increase in TRAIL protein in the RAW 264.7-conditioned medium. In contrast, the effect of DHT on TNF-α in the conditioned media was more modest (31, 32).
Figure 4.
AR directly activates the transcription of TRAIL mRNA in macrophages. (A) RAW264.7 was treated with 100 nM DHT and cells were harvested after the indicated time and mRNA isolated. TRAIL mRNA was measured by using qPCR. TRAIL mRNA expression values were normalized by subtracting the levels of the vehicle-only group for each time point. The results demonstrated that DHT induced TRAIL mRNA in a time-dependent manner. (B) To measure the effect of DHT on TRAIL expression at the protein level, ELISA was carried out using the conditioned media. RAW264.7 was treated with 1 to 100 nM DHT for 24 h and the conditioned media were collected. The results demonstrated that DHT increased TRAIL protein levels in the conditioned media in a concentration-dependent manner. Enzalutamide (Enz; 10 μM) treatment decreased the DHT-induced TRAIL secretion. (C) There are potential AR binding sites within the human and mouse TRAIL promoter. By using the computer program PROMO, the consensus androgen-responsive element within the TRAIL promoter was assessed. (D) RAW264.7 and THP-1 were treated with 100 nM DHT for 24 h. Then, genomic DNA was isolated ChIP was carried out. The results demonstrated that AR binding to the TRAIL promoter region increased upon treatment with DHT 100 nM. Error bars indicate average ± SEM. *P < 0.05. ARE, androgen response element; Rel-A, transcription factor p65.
Because the time frame within which DHT increases the expression of TRAIL mRNA suggests a direct activation of transcription, we next analyzed the human TRAIL promoter region located within −2000 bp of the transcription start site with the computer program PROMO (40, 41). We found one potential AR element on the human TRAIL promoter at ∼ −1,382 bp (Fig. 4C; AR element marked as a red box). An analysis of the murine counterpart revealed several putative AR elements (−1603 bp, −1315 bp, −628 bp, and −574 bp). ChIP confirmed direct binding of AR to TRAIL promoter when THP-1 and RAW264.7 were treated with 100 nM of DHT for 24 hours (Fig. 4D). These results support the concept that DHT directly activates the transcription of TRAIL, which, in turn, mediates the cytotoxic effect of macrophages on CaP cells.
Castration decreases antitumor activity of macrophages in mice
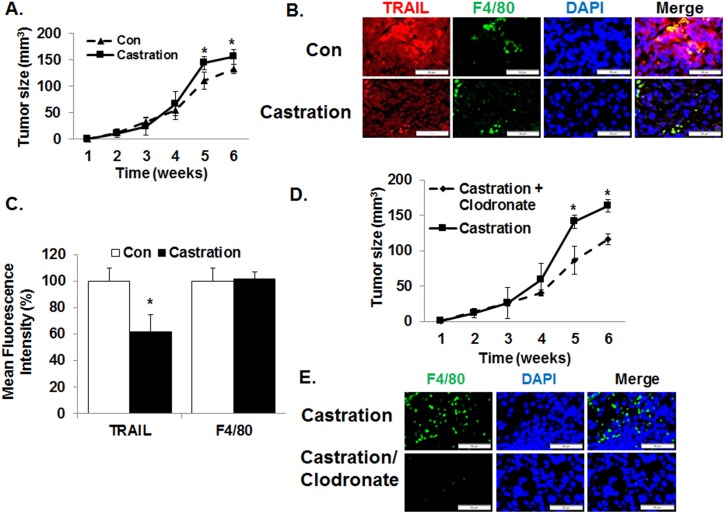
To test the effect of DHT on the cytotoxic effect of macrophages in vivo, we carried out a mouse xenograft study using the TRAMP-C2 androgen-resistant murine CaP cell line in syngeneic mice. Although TRAMP-C2 cells are not responsive to DHT in tissue culture, castration increased the tumor growth rate when compared with the control sham group (Fig. 5A). No significant histopathological differences were found in tumors obtained from the two groups (31, 32). Immunofluorescence study revealed that the level of TRAIL was higher in control tumors than in castrated tumors, but the magnitude of macrophage infiltrations (F4/80) was not significantly different grossly between the two groups (Fig. 5B and 5C). Next, we repeated the castration study after the removal of macrophages by administering clodronate liposomes (42, 43). Results demonstrated that the depletion of macrophages reduced tumor size when compared with the control group (Fig. 5D). Again, there was no significant difference in histology (31, 32). As expected, the level of infiltrated macrophages was decreased in clodronate-treated tumor (Fig. 5E). To rule out the possibility that the decrease in tumor volume following clodronate was due to the absolute reduction of macrophages, flow cytometry was carried out after dissociating the tumor xenografts. The results demonstrated that the fraction of macrophages (F4/80) decreased from 1.3% to 0.3% of the tumors (31, 32). Such small values suggest that the decreased tumor volume is not likely due to an absolute reduction in tumor-infiltrating macrophages.
Figure 5.
Macrophages enhance tumor growth of androgen-resistant prostate cancer cells following castration in vivo. (A) To determine the impact of androgens on macrophages in vivo, C57B/6 mice with established androgen-resistant TRAMP-C2 xenograft were castrated and tumor volume was measured weekly. The results demonstrated that castration increased tumor growth. n = 5 per group. (B) At the end of 6 wk, all tumor xenografts were harvested and analyzed. Immunofluorescence microscopy demonstrated a significant increase in the protein levels of TRAIL within the tumor xenografts following castration. However, no detectable effect was seen on the magnitude of macrophage infiltration (F4/80). (C) To quantify the immunofluorescence, mean fluorescence intensity was measured by using ImageJ V1.50i software. TRAIL fluorescence intensity but not that of F4/80 significantly increased with castration. (D) When macrophage was removed with intraperitoneal injections of clodronate liposome every 3 d following castration, the protumorigenic effect of castration in mice with TRAMP-C2 xenograft was reversed. Control mice were injected with PBS liposome. (E) Administration of clodronate-liposome decreased macrophage infiltration in TRAMP-C2 xenografts. Again, the mean fluorescence intensity levels of TRAIL and F4/80 were quantified by using ImageJ V1.50i software. Values are means ± SEM. n = 5 per group. *P < 0.05.
Finally, because we previously reported that macrophages regulate neuroendocrine differentiation (44), we assessed the expression status of the neuroendocrine markers, parathyroid hormone-related protein and chromogranin A, as well as AR (31, 32). The results demonstrated that castration increased the expression levels of parathyroid hormone-related protein and chromogranin and decreased that of AR.
Discussion
In this study, we demonstrated that DHT increases the cytotoxic activity of macrophages via the upregulation of TRAIL. Simultaneously, DHT induced morphological changes similar to that of LPS treatment and M1 polarization. In vivo, we have observed that castration induces the proliferation of androgen-resistant CaP cells by decreasing the activity of macrophages. Taken together, our findings show that androgens activate macrophages, and the current observations potentially have important implications on the implementation of immunotherapy in CaP.
Immunotherapy is emerging as the next frontier in cancer treatment. Accordingly, the effect of androgens on the immune system is a critical question for men with end-stage CaP because androgen ablation is a first-line treatment of metastatic CaP. Classically, androgens have been considered to be immune suppressors and not activators. For example, compared with women, men show low susceptibility to autoimmune diseases, such as systemic lupus erythematosus and Hashimoto thyroiditis (45, 46). Similarly, it has been reported that castration activates T-cell activity (47, 48). In this framework, immunotherapy in CaP has largely been disappointing. With the exception of sipuleucel-T, no other therapeutics that target the immune system in CaP have shown significant clinical activity (49). Indeed, the most recent advances in immunotherapy that target programmed cell death-1 and programmed cell death ligand-L1 have shown minimal clinical activity in castration-resistant prostate cancer (50–53). Given that castration is usually continued in these patients undergoing immunotherapy, it is possible that low serum androgen levels may not be optimal for immune-based therapeutics in CaP. Consistent with this hypothesis, it has recently been reported that sipuleucel-T is more effective when sequenced before ADT (54). Efficacy of sipuleucel-T can also be better prior to ADT because its immunogen prostate acid phosphatase is an AR-target gene whose expression is likely to decrease following ADT (54).
In support of the concept of androgens as an activator of the immune response, results of the current study suggest that androgens stimulate rather than suppress antitumor activity in macrophages. Specifically, we have demonstrated, using both tissue culture and animal studies, that DHT increases the cytotoxic activity of macrophages. Importantly, with androgen-resistant CaP xenografts, castration increased the tumor growth rate and this protumorigenic effect of castration was largely reversed by the removal of macrophages. Taken together, these observations question the continuation of ADT in patients with CaP receiving immunotherapy.
Macrophages play a critical role in regulating the immune response. Previously, it has been reported that testosterone and DHT regulate the production of IL-1 and IL-6 in macrophages (55, 56). In addition, functional AR was identified in macrophage-like synoviocytes and infiltrating macrophages in skin (57, 58). Accordingly, these observations suggest that sex steroids likely have an important effect on the immune system and macrophages (59). In this context, we have found that DHT stimulates M1 polarization and production of TRAIL. TRAIL is a member of the death receptor ligand superfamily and triggers apoptotic signaling via receptor-mediated death through interaction with death receptors on cancer cells (60). Of interest, different CaP cell lines have differential sensitivity to TRAIL-induced cell death. Androgen-insensitive human CaP cells, DU145 and PC-3, are sensitive to TRAIL-induced cell death, but the androgen sensitive LNCaP is resistant (61). It has been reported that androgens sensitize LNCaP cells to TRAIL-induced cell death by upregulating death receptors of cancer cells in a dose-dependent manner (62). In the current study, we used androgen-insensitive human CaP cell lines DU145 and PC3. Therefore, we have excluded the direct effect of androgens on CaP cells and confirmed the induction of TRAIL-mediated immune response via AR activation in macrophages. This indicates that the cytotoxic effect of androgen in our cocultures is achieved, in part, by the direct interaction between androgens and macrophages rather than androgens and cancer cells.
The implication of the current study on sequencing immunotherapy and ADT contradicts the recommendations made based on the reported effects of androgens on T-cell activity (47, 48). Specifically, it has been demonstrated that castration enhances antitumor T-cell activity. Thus, it is entirely possible that androgens have an opposite effect on T cells and macrophages. Accordingly, additional studies will be necessary to determine the overarching effect of androgens on the immune system. Another note of caution in interpreting our data is that most functional studies involving macrophages were conducted by using the higher-than-physiologic DHT concentration of 100 nM. Such a high DHT concentration was used to ensure that the AR signaling capacity was fully saturated within the time frame of our experiments (24 hours). Nevertheless, the findings of the current report are clinically relevant because the significant effect of DHT on the androgen-responsive promoter activity of PSA and probasin was observed at DHT concentrations as low as 1 nM. In addition, 1 nM DHT treatment of 24 hours increased the protein levels of TNF-α in both RAW 264.7 and THP-1. Finally, results of the in vivo studies demonstrated decreased levels of TRAIL with castration, thereby supporting our hypothesis that androgens stimulate TRAIL production. Indeed, the precise reason for the in vivo mice castration experiments was to demonstrate that our in vitro study is not an artifact of the tissue culture system.
In conclusion, we have demonstrated that DHT stimulates the cytotoxic activity of macrophages. Mechanistic study revealed that DHT stimulates the activation of androgen signaling and upregulates TRAIL expression by directly binding to its promoter. Our future study will focus on investigating the effect of castration on immunotherapy in CaP.
Acknowledgments
Financial Support: This work was supported by the cancer center grant from the National Cancer Institute (Grant P30CA072720) and generous support from the Marion and Norman Tanzman Charitable Foundation and Mr. Malcolm Wernik (to I.Y.K.).
Author Contributions: Data collection and analysis and manuscript writing: G.T.L., J.H.K., S.J.K., M.N.S., M.M.K., S.B., J.H.H., N.N.; data analysis and manuscript writing: W.J.K. and I.Y.K.; overall management of project: I.Y.K.
Glossary
Abbreviations:
- ADT
androgen deprivation therapy
- AR
androgen receptor
- ARR-lux
androgen response element tandem repeated sequence of the probasin promoter-luciferase plasmid
- CaP
prostate cancer
- cFBS
charcoal-stripped fetal bovine serum
- ChIP
chromatin immunoprecipitation
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- LPS
lipopolysaccharide
- PMA
phorbol 12-myristate 13-acetate
- PSA
prostate-specific antigen
- PSA-lux
prostate-specific antigen promoter-luciferase
- siRNA
small interfering RNA
- TRAIL
TNF-related apoptosis-inducing ligand
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. [DOI] [PubMed] [Google Scholar]
- 2. Hesry V, Piquet-Pellorce C, Travert M, Donaghy L, Jégou B, Patard JJ, Guillaudeux T. Sensitivity of prostate cells to TRAIL-induced apoptosis increases with tumor progression: DR5 and caspase 8 are key players. Prostate. 2006;66(9):987–995. [DOI] [PubMed] [Google Scholar]
- 3. Nakajima Y, DelliPizzi AM, Mallouh C, Ferreri NR. TNF-mediated cytotoxicity and resistance in human prostate cancer cell lines. Prostate. 1996;29(5):296–302. [DOI] [PubMed] [Google Scholar]
- 4. Koschny R, Walczak H, Ganten TM. The promise of TRAIL--potential and risks of a novel anticancer therapy. J Mol Med (Berl). 2007;85(9):923–935. [DOI] [PubMed] [Google Scholar]
- 5. Mahalingam D, Szegezdi E, Keane M, de Jong S, Samali A. TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat Rev. 2009;35(3):280–288. [DOI] [PubMed] [Google Scholar]
- 6. Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell. 1999;10(10):3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. [DOI] [PubMed] [Google Scholar]
- 8. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–355. [DOI] [PubMed] [Google Scholar]
- 10. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 11. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. [DOI] [PubMed] [Google Scholar]
- 12. Guo Z, Benten WP, Krücken J, Wunderlich F. Nongenomic testosterone calcium signaling. Genotropic actions in androgen receptor-free macrophages. J Biol Chem. 2002;277(33):29600–29607. [DOI] [PubMed] [Google Scholar]
- 13. Jeet V, Ow K, Doherty E, Curley B, Russell PJ, Khatri A. Broadening of transgenic adenocarcinoma of the mouse prostate (TRAMP) model to represent late stage androgen depletion independent cancer. Prostate. 2008;68(5):548–562. [DOI] [PubMed] [Google Scholar]
- 14. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. [DOI] [PubMed] [Google Scholar]
- 15. Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119(12):3739–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen YL, Le Vraux V, Giroud JP, Chauvelot-Moachon L. Anti-tumor necrosis factor properties of non-peptide drugs in acute-phase responses. Eur J Pharmacol. 1994;271(2-3):319–327. [DOI] [PubMed] [Google Scholar]
- 17. Havell EA, Fiers W, North RJ. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med. 1988;167(3):1067–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown ER, Charles KA, Hoare SA, Rye RL, Jodrell DI, Aird RE, Vora R, Prabhakar U, Nakada M, Corringham RE, DeWitte M, Sturgeon C, Propper D, Balkwill FR, Smyth JF. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer. Ann Oncol. 2008;19(7):1340–1346. [DOI] [PubMed] [Google Scholar]
- 19. Nair PM, Flores H, Gogineni A, Marsters S, Lawrence DA, Kelley RF, Ngu H, Sagolla M, Komuves L, Bourgon R, Settleman J, Ashkenazi A. Enhancing the antitumor efficacy of a cell-surface death ligand by covalent membrane display. Proc Natl Acad Sci USA. 2015;112(18):5679–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smulski CR, Decossas M, Chekkat N, Beyrath J, Willen L, Guichard G, Lorenzetti R, Rizzi M, Eibel H, Schneider P, Fournel S. Hetero-oligomerization between the TNF receptor superfamily members CD40, Fas and TRAILR2 modulate CD40 signalling. Cell Death Dis. 2017;8(2):e2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mérino D, Lalaoui N, Morizot A, Solary E, Micheau O. TRAIL in cancer therapy: present and future challenges. Expert Opin Ther Targets. 2007;11(10):1299–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31(4):229–234. [DOI] [PubMed] [Google Scholar]
- 23. Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. [DOI] [PubMed] [Google Scholar]
- 24. Lee GT, Kim JH, Kwon SJ, Stein MN, Hong JH, Nagaya N, Billakanti S, Kim MM, Kim W-J, Kim IY. Data from: Dihydrotestosterone increases cytotoxic activity of macrophages on prostate cancer cells via TRAIL. figshare 2019. Accessed 23 March 2019. https://figshare.com/s/b3580f71eee4e0e5c517. [DOI] [PMC free article] [PubMed]
- 25.RRID : AB_626671, http://scicrunch.org/resolver/AB_626671.
- 26.RRID : AB_2205932, http://scicrunch.org/resolver/AB_2205932.
- 27.RRID : AB_2205929, http://scicrunch.org/resolver/AB_2205929.
- 28.RRID : AB_2240625, http://scicrunch.org/resolver/AB_2240625.
- 29.RRID : AB_476744, http://scicrunch.org/resolver/AB_476744.
- 30. Corey MJ, Kinders RJ, Poduje CM, Bruce CL, Rowley H, Brown LG, Hass GM, Vessella RL. Mechanistic studies of the effects of anti-factor H antibodies on complement-mediated lysis. J Biol Chem. 2000;275(17):12917–12925. [DOI] [PubMed] [Google Scholar]
- 31. Lee GT, Kim JH, Kwon SJ, Stein MN, Hong JH, Nagaya N, Billakanti S, Kim MM, Kim W-J, Kim IY. Data from: Dihydrotestosterone increases cytotoxic activity of macrophages on prostate cancer cells via TRAIL. figshare 2019. Accessed 23 March 2019. https://figshare.com/s/46b8045ffda070ac217f. [DOI] [PMC free article] [PubMed]
- 32. Lee GT, Kim JH, Kwon SJ, Stein MN, Hong JH, Nagaya N, Billakanti S, Kim MM, Kim W-J, Kim IY. Data from: Dihydrotestosterone increases cytotoxic activity of macrophages on prostate cancer cells via TRAIL. figshare 2019. Accessed 23 March 2019. https://figshare.com/s/7269f0c6066c9c01ebf0. [DOI] [PMC free article] [PubMed]
- 33. RRID :AB_2205816, http://scicrunch.org/resolver/AB_2205816.
- 34. RRID :AB_297257, http://scicrunch.org/resolver/AB_297257.
- 35. RRID :AB_11092156, http://scicrunch.org/resolver/AB_11092156.
- 36. RRID :AB_2735037, http://scicrunch.org/resolver/AB_2735037.
- 37. Lee GT, Kim JH, Kwon SJ, Stein MN, Hong JH, Nagaya N, Billakanti S, Kim MM, Kim W-J, Kim IY. Data from: Dihydrotestosterone increases cytotoxic activity of macrophages on prostate cancer cells via TRAIL. figshare 2019. Accessed 23 March 2019. https://figshare.com/s/157bcd564f16b2ea837e. [DOI] [PMC free article] [PubMed]
- 38. Seethammagari MR, Xie X, Greenberg NM, Spencer DM. EZC-prostate models offer high sensitivity and specificity for noninvasive imaging of prostate cancer progression and androgen receptor action. Cancer Res. 2006;66(12):6199–6209. [DOI] [PubMed] [Google Scholar]
- 39. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. [DOI] [PubMed] [Google Scholar]
- 40. Messeguer X, Escudero R, Farré D, Núñez O, Martínez J, Albà MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18(2):333–334. [DOI] [PubMed] [Google Scholar]
- 41. Farré D, Roset R, Huerta M, Adsuara JE, Roselló L, Albà MM, Messeguer X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31(13):3651–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee GT, Jung YS, Ha YS, Kim JH, Kim WJ, Kim IY. Bone morphogenetic protein-6 induces castration resistance in prostate cancer cells through tumor infiltrating macrophages. Cancer Sci. 2013;104(8):1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee JH, Lee GT, Woo SH, Ha YS, Kwon SJ, Kim WJ, Kim IY. BMP-6 in renal cell carcinoma promotes tumor proliferation through IL-10-dependent M2 polarization of tumor-associated macrophages. Cancer Res. 2013;73(12):3604–3614. [DOI] [PubMed] [Google Scholar]
- 44. Hong EH, Yun HS, Kim J, Um HD, Lee KH, Kang CM, Lee SJ, Chun JS, Hwang SG. Nicotinamide phosphoribosyltransferase is essential for interleukin-1beta-mediated dedifferentiation of articular chondrocytes via SIRT1 and extracellular signal-regulated kinase (ERK) complex signaling. J Biol Chem. 2011;286(32):28619–28631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem. 1989;34(1-6):241–251. [DOI] [PubMed] [Google Scholar]
- 46. Schuurs AH, Verheul HA. Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990;35(2):157–172. [DOI] [PubMed] [Google Scholar]
- 47. Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, Arredouani MS. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci USA. 2014;111(27):9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thara E, Dorff TB, Averia-Suboc M, Luther M, Reed ME, Pinski JK, Quinn DI. Immune response to sipuleucel-T in prostate cancer. Cancers (Basel). 2012;4(2):420–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rekoske BT, McNeel DG. Immunotherapy for prostate cancer: False promises or true hope? Cancer. 2016;122(23):3598–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR; CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Antonarakis ES, Kibel AS, Yu EY, Karsh LI, Elfiky A, Shore ND, Vogelzang NJ, Corman JM, Millard FE, Maher JC, Chang NN, DeVries T, Sheikh NA, Drake CG, Investigators S. Sequencing of sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. Clin Cancer Res. 2017;23(10):2451–2459. [DOI] [PubMed] [Google Scholar]
- 55. Angele MK, Knöferl MW, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol. 1999;277(1):C35–C42. [DOI] [PubMed] [Google Scholar]
- 56. Friedl R, Brunner M, Moeslinger T, Spieckermann PG. Testosterone inhibits expression of inducible nitric oxide synthase in murine macrophages. Life Sci. 2000;68(4):417–429. [DOI] [PubMed] [Google Scholar]
- 57. Khalkhali-Ellis Z, Handa RJ, Price RH Jr, Adams BD, Callaghan JJ, Hendrix MJ. Androgen receptors in human synoviocytes and androgen regulation of interleukin 1beta (IL-1beta) induced IL-6 production: a link between hypoandrogenicity and rheumatoid arthritis? J Rheumatol. 2002;29(9):1843–1846. [PubMed] [Google Scholar]
- 58. Yamamoto Y, Saito H, Setogawa T, Tomioka H. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect Immun. 1991;59(11):4089–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Matsuyama T, Ishimaru T. Estrogen and progesterone receptor expression in macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Hum Reprod. 2005;20(7):2004–2013. [DOI] [PubMed] [Google Scholar]
- 60. Walczak H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb Perspect Biol. 2013;5(5):a008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Munshi A, Pappas G, Honda T, McDonnell TJ, Younes A, Li Y, Meyn RE. TRAIL (APO-2L) induces apoptosis in human prostate cancer cells that is inhibitable by Bcl-2. Oncogene. 2001;20(29):3757–3765. [DOI] [PubMed] [Google Scholar]
- 62. Wang D, Lu J, Tindall DJ. Androgens regulate TRAIL-induced cell death in prostate cancer cells via multiple mechanisms. Cancer Lett. 2013;335(1):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.