Abstract
The in utero maternal metabolic environment is important relative to both short and long term development of the offspring. Although poor fetal growth remains a significant factor relative to long-term outcome, fetal overgrowth is assuming greater importance because of the increase in obesity in the world’s populations. Maternal obesity and gestational diabetes are the most common metabolic complications of pregnancy related to fetal overgrowth and more specifically adiposity.
Women with gestational diabetes have increased insulin resistance and inadequate insulin response compared with weight-matched controls. Gestational diabetes increases the risk of maternal hypertensive disease (preeclampsia) as well as cesarean delivery. At birth the neonate has increased adiposity and is at risk for birth injury. Multiple studies have reported that children of women with gestational diabetes have a greater prevalence childhood obesity and glucose intolerance; even at glucose concentrations less than currently used to define gestational diabetes, compared with normoglycemic women.
Obese women also have increased insulin resistance, insulin response and inflammatory cytokines compared with average weight women both before and during pregnancy. They too are at increased risk for the metabolic syndrome-like disorders during pregnancy that is hypertension, hyperlipidemia, glucose intolerance and coagulation disorders. Analogous to women with gestational diabetes, neonates of obese women are heavier at delivery because of increased fat and not lean body mass. Similarly, these children have an increased risk of childhood adiposity and metabolic dysregulation. Hence, the preconceptional and perinatal period offers a unique opportunity to modify both short and long term risks for both the woman and her offspring.
Keywords: gestational diabetes, obesity, pregnancy
Introduction
Despite these difficult economic times, there is a continued effort on the part of the world’s populations, whether they are in industrialized countries or those less developed, to improve the standard of living of their people. Although poverty remains a substantial issue in many areas, with improving living standards, and greater access to affordable, though not necessarily healthier nutrition, the problem of obesity and resultant complications are more frequently being recognized as global problems.1 The medical consequence of the increase in rates of obesity are the related problems of the metabolic syndrome; that is hypertension, diabetes, hyperlipidemia and atherosclerotic vascular disease, etc (Table 1).2–5 These are the chronic medical conditions which in the coming decades will consume an ever increasing proportion of resources that less fortunate countries can ill afford. To date, however, much of the emphasis of the medical community has been on treatment and less on prevention. The perinatal period is certainly a time when a better understanding of the underlying physiology may provide a better insight into the factors which might prove amenable to prevention; not only for the women but also for her offspring as well. In this review, we will attempt to examine the underlying physiology and clinical factors relating to short and long-term impact of gestational diabetes mellitus (GDM) and obesity on the woman and her offspring.
Table 1A.
ATP III clinical identification of the metabolic syndrome2
| Risk factor | Defining level |
|---|---|
| Abdominal obesity, given as waist circumference | |
| Men | >102 cm (>40in) |
| Women | >88cm (>35in) |
| Triglycerides | >150mg/dl |
| HDL cholesterol | |
| Men | <40 mg/dl |
| Women | <50mg/dl |
| Blood pressure | >130/>85 mmHg |
| Fasting glucose | >110 mg/dl |
HDL, high-density lipoprotein.
Gestational diabetes
Pathophysiology
Gestational diabetes has been defined by the 4th International Workshop-Conference on GDM is: ‘carbohydrate intolerance of varying degrees of severity, with onset or first recognition during pregnancy’.6 However, in 2009 the International Association of Diabetes in Pregnancy Study Groups, an international consensus group of diabetologists and obstetricians, including the American Diabetes Association, recommended that high-risk women found to have diabetes at their initial visit, received a diagnosis of overt and not GDM using the criteria recommended in Table 2.7 In contrast to women with normal glucose tolerance, the underlying pathophysiology of GDM is present before pregnancy. Those destined to develop GDM exhibit decreased insulin sensitivity, before pregnancy, the latter likely to being overweight and obese.8 These women also have an inadequate insulin response to maintain normoglycemia,9 much as one would find in individuals with type 2 diabetes. A very small number of cases of GDM may represent either the onset of type 1 diabetes or inherited genetic defects such as can be found in maturity onset diabetes of the young.10
Table 1B.
| Insulin resistance, identified by one of the following: |
|
| Plus two of any of the following: |
|
HDL, high-density lipoprotein; BMI, body mass index.
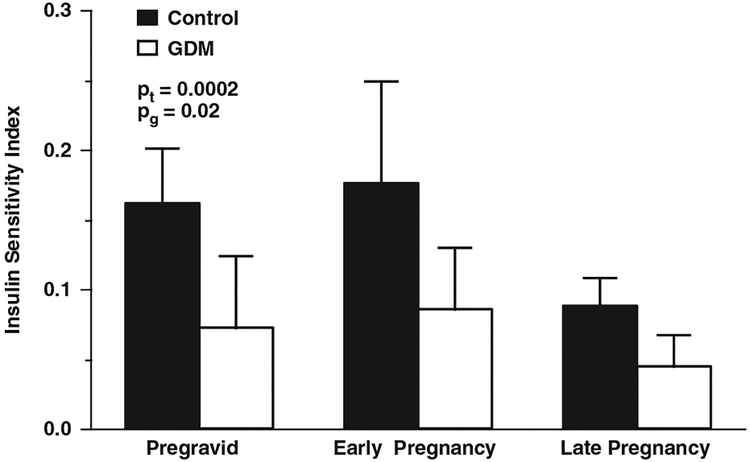
Pregnancy is, and of itself, a condition of decreased insulin sensitivity with a 50%–60% decrease in insulin sensitivity and two to three fold increase in insulin response in women with normal glucose tolerance11 (Figs 1 and 2). Hence, women at risk for GDM most often have decreased pregravid insulin sensitivity, including such problems as being overweight or obese. Because of their underlying subclinical decreased insulin sensitivity and β-cell dysfunction, the aforementioned metabolic stress of pregnancy makes clinically manifest the clinical disorder defined as GDM. Although, we primarily conceive of diabetes as a disorder of glucose metabolism, in women with GDM there are also reported abnormalities in lipid and amino acid metabolism, which may also affect the fetal growth.12 The decrease in insulin sensitivity during pregnancy have been associated with maternal cytokines, particularly circulating tumor necrosis factor-α (TNF-α) concentrations13 as well as the elevated free fatty acid concentrations.14 As insulin sensitivity significantly improves immediately post delivery,15 the placenta has been implicated to play a significant role in the alterations of maternal metabolism during human pregnancy.
Fig. 1.
The longitudinal changes in insulin sensitivity women with gestational diabetes (GDM) and normal glucose tolerance (control). Pt, changes over time; Pg, differences between groups. (Adapted from Catalano, Am J Obstet Gynecol, 1999).
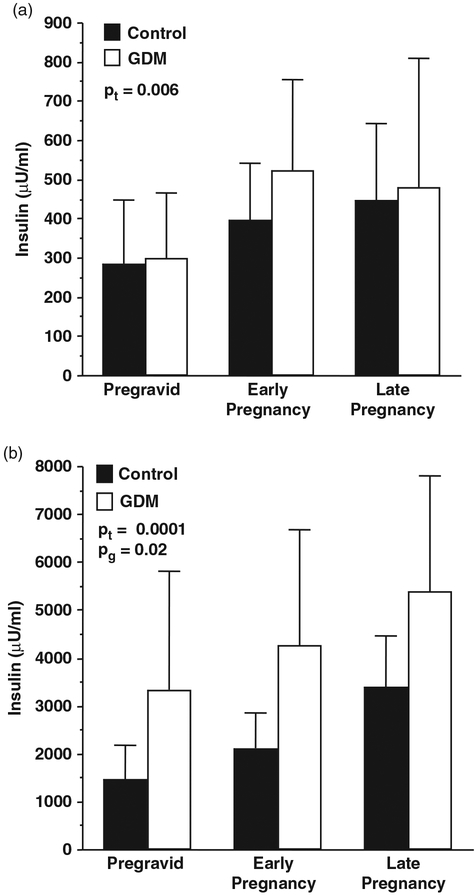
Fig. 2.
The longitudinal changes in insulin response to an intravenous glucose challenge in women with gestational diabetes (GDM) and normal glucose tolerance (control). (a) first phase insulin response, (b) second phase insulin response. Pt, changes over time; Pg, difference between groups. (Adapted from Catalano, Am J Obstet Gynecol, 1999).
Maternal
Women, who develop GDM, are at increased risk for adverse perinatal outcomes. For example, women with GDM have a significantly greater risk of developing hypertensive disorders of pregnancy such as preeclampsia.16 This risk is analogous to the increased risk of chronic hypertensive disorders observed in insulin resistant individuals with type-2 diabetes. Recently concluded hyperglycemia and adverse pregnancy outcome (HAPO) study also reported an increase in the risk of primary cesarean delivery in women with elevated glucose concentrations. Of note, the HAPO study also confirmed that glucose concentrations less than those currently used to define GDM are associated with other adverse perinatal outcomes such as preeclampsia and birth injury.17 Hence, just as there has been an increase in the number of individuals classified as having either impaired fasting glucose (33.8%) or impaired glucose tolerance (15.4%),18 the number of women with GDM presently 5%–10% of the population19 may significantly increase pending results of recently held HAPO consensus conference. Consistent with the underlying pathophysiology of GDM, multiple studies have reported that 50%–60% of women with previously diagnosed GDM will develop type 2 diabetes in 10 years20 as well as other evidence of the metabolic syndrome. Thus pregnancy remains a metabolic stress test for women with underlying metabolic dysfunction.
Fetal
The infant of the women with GDM is often characterized as large or macrosomic. The term large for gestational age (LGA) is commonly defined as weight greater than the 90th percentile for gestational age, gender and racial group. Macrosomia is variously defined as birth weight greater than 4000 g to 4500 g. Infants of women with GDM, particularly if born LGA or macrosomic are at increased risk for short-term complications. During delivery because of their large size and difficulty in transit through the birth canal, shoulder dystocia, occasionally followed by Erb’s Palsy may occur.21 Immediately after birth they are at increased risk for hypoglycemia because of the in utero hyperinsulinemia.22 Other morbidities in the infant of the woman with GDM include hypocalcemia, hyperbilirubinemia and respiratory distress.23
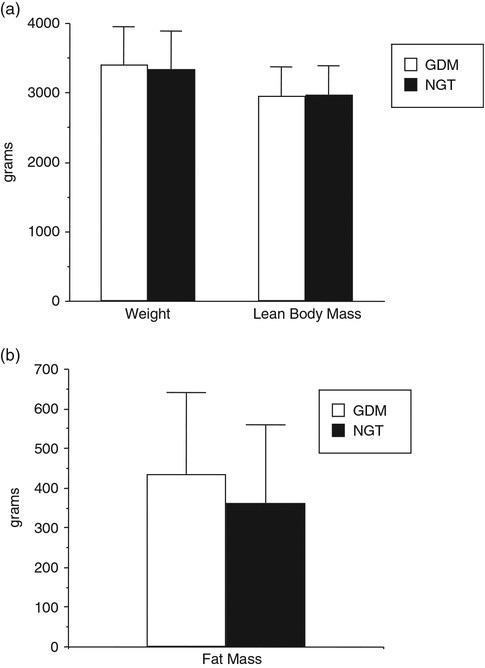
In assessing the body composition of infants of women with GDM, these neonates are heavier at birth because of increases in fat mass but not because of increases in lean body mass24 (Fig. 3). Again based on the results of the HAPO study, increasing maternal glucose concentrations, less than those currently used to define GDM, were also associated not only with an increase in birth weight but specifically and increase in neonatal adiposity.25 Of note, when examining the gene array profile of the placenta from obese GDM and normal glucose tolerant women, there is an increase in placental gene expression of enzymes related to lipid metabolism suggesting the potential of lipids as a nutrient sources of increased neonatal adiposity.26 Those alterations in neonatal growth/adiposity may be one of the prime factors resulting in the long term complications observed in children of women with GDM.
Fig. 3.
Birth weight, lean body mass and fat mass of full term neonates of women with gestational diabetes (GDM) n = 195 and normal glucose tolerant women (NGT), n = 220. Weight P = 0.26, lean body mass P = 0.74 and fat mass P = 0.0002. (Adapted from Catalano, Am J Obstet Gynecol, 2003).
Child
Children of women with GDM are at increased risk for long-term obesity and glucose intolerance. On the basis of early studies by Pettitt et al. children of Pima women with diabetes during pregnancy had significant increases in both diabetes and obesity.27 This risk persisted when the offspring of the women with diabetes where compared with their siblings born before the mother developed glucose intolerance.28
These studies were later confirmed and expanded upon by Dabelea et al. also in a Pima Indian population.29 In fact the strongest risk factor for diabetes in Pima Indian children is maternal diabetes in utero, independent of maternal obesity and birth weight.30–32 In a primarily Caucasian population, Boney et al. reported that not only did the large for gestational age children of the women with GDM had an increased risk of diabetes and obesity but that 50% of those children had evidence of the metabolic syndrome.33 In a recent multiethnic study, Hiller et al. reported that with increasing levels of hyperglycemia, particularly fasting hyperglycemia less than diagnostic of GDM, this was associated with an increased risk of childhood obesity.34
More recently, Clausen et al. reported that the risk of being overweight was doubled in the offspring, age 18–27 years, of women with diet trusted GDM or type 1 diabetes compared with a control group from the same background population. Furthermore, the risk of the metabolic syndrome in these same offspring was increased four-fold in comparison to the same background population.35 In summary, the infant of the women with GDM has a significantly greater risk of being heavier at birth because of increased adiposity. These children are also at increased risk for the childhood obesity, glucose intolerance and associated metabolic dysregulation. However, based on clinical trials by Crowther36 and Landon37 treatment of women with GDM during pregnancy can decrease some of the adverse neonatal outcomes. The potential improvement in long-term childhood outcomes awaits further study.
Maternal obesity
Pathophysiology
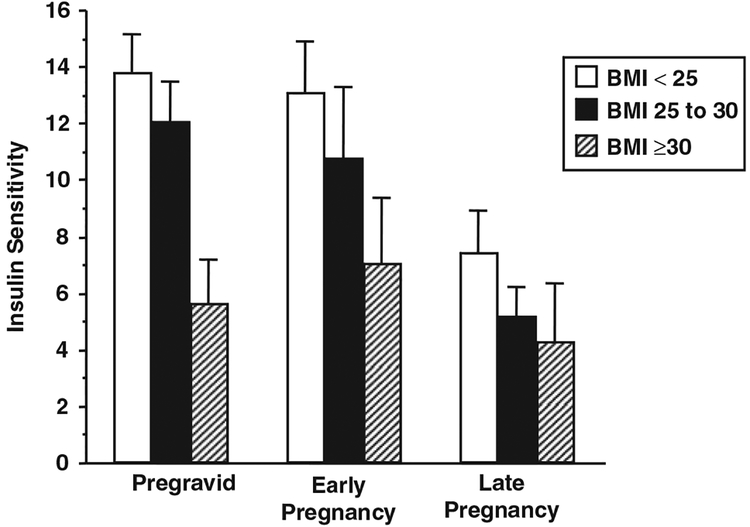
The WHO classification of obesity endorsed by Institute of Medicine (IOM),38 based on maternal pre-pregnancy body mass index, (BMI, weight/height2). In pregnant women there is a strong correlation between maternal BMI and percent body fat in early pregnancy,39 much as there is in non-pregnant individuals. The correlation between maternal BMI and percent body fat is less robust, however, in later pregnancy because of the significant increases in total body water, that is the 40% increase in plasma volume and approximately 1 L of amniotic fluid. However, for clinical purposes maternal BMI has been accepted as a measure of obesity during pregnancy. There is a similar metabolic profile relationship between obese normoglycemic and lean normal glucose tolerant women as there is between women with GDM and those with normal glucose tolerance when matched for the degree of pre-pregnancy obesity. Relative to normal weight women, obese women with normal glucose tolerance, have decreased insulin sensitivity, both peripheral and hepatic as compared with normal weight women (Fig. 4). In addition, normoglycemic obese women have an increase in insulin response 1glucose concentrations in obese as compared with average weight pregnant women. Similar to what has been observed in GDM v. normal glucose tolerant BMI matched women, there is also an increase in lipid concentrations and decreased ability of insulin to suppress lipolysis in obese as compared with normal weight pregnant women in late gestation.
Fig. 4.
The longitudinal changes in insulin sensitivity in average, overweight and obese women, before conception (pregravid) and in early (12–14 weeks) and late (34–36 weeks) gestation. Change over time, P = 0.0001. The obese women were less insulin sensitive than the average weight women (P = 0.001) and overweight women (P = 0.0004). (Adopted from BJOG, 2006). BMI indicates body mass index.
As noted previously there is an improvement in insulin sensitivity immediately post delivery.15 Similarly, when comparing the alterations in insulin sensitivity 1 year post delivery, there was a significant improvement in insulin sensitivity in normal weight women who returned to their pre-pregnancy weight.40 In contrast, in obese women with normoglycemia in pregnancy who fail to return to their pre-pregnancy weight, these women have little or no change in their insulin sensitivity compared with their late pregnancy studies. Additionally, plasma TNF-α concentrations and the expression of TNF-α gene expression in skeletal muscle of these women remain higher 1 year post partum compared with normal weight controls.41 These data provide a plausible link between, obesity, inflammation and insulin resistance both during pregnancy and in non pregnant individuals as risk factors for glucose intolerance.
Maternal
Obese women have many of the perinatal problems present in women with GDM. This should not be surprising as maternal obesity is a significant risk factor for both GDM as well as type 2 diabetes. In early pregnancy obese women have an increased risk of spontaneous miscarriage.42 Similar to what has been observed in women with preexisting glucose intolerance. In the fetuses of obese, non-diabetic women, there is also an increased risk of congenital anomalies; particularly neural tube and cardiac lesions.43 Obese women also have an increased risk of the ‘metabolic syndrome of pregnancy’. They have an increased risk of glucose intolerance (GDM), hypertensive disorders (preeclampsia), hyperlipidemia and circulating inflammatory markers.44 At delivery obese women are at greater risk of requiring cesarean delivery, both primary and failed trial of labor after a previous cesarean delivery. This increased risk of cesarean delivery may be related to fetal size (to be discussed later) or maternal soft tissue dystocia. The force of uterine contractions, however, appears to be similar to that estimated in non-obese women.45 Because of the increase in tissue disruption in obese women, there is an increase in the risk of post-cesarean wound infections in these women as compared with non-obese women. Additional post-partum risks include an increased risk of deep vein thrombophlebitis which may represent another manifestation of the ‘metabolic syndrome of pregnancy’.44
Fetal
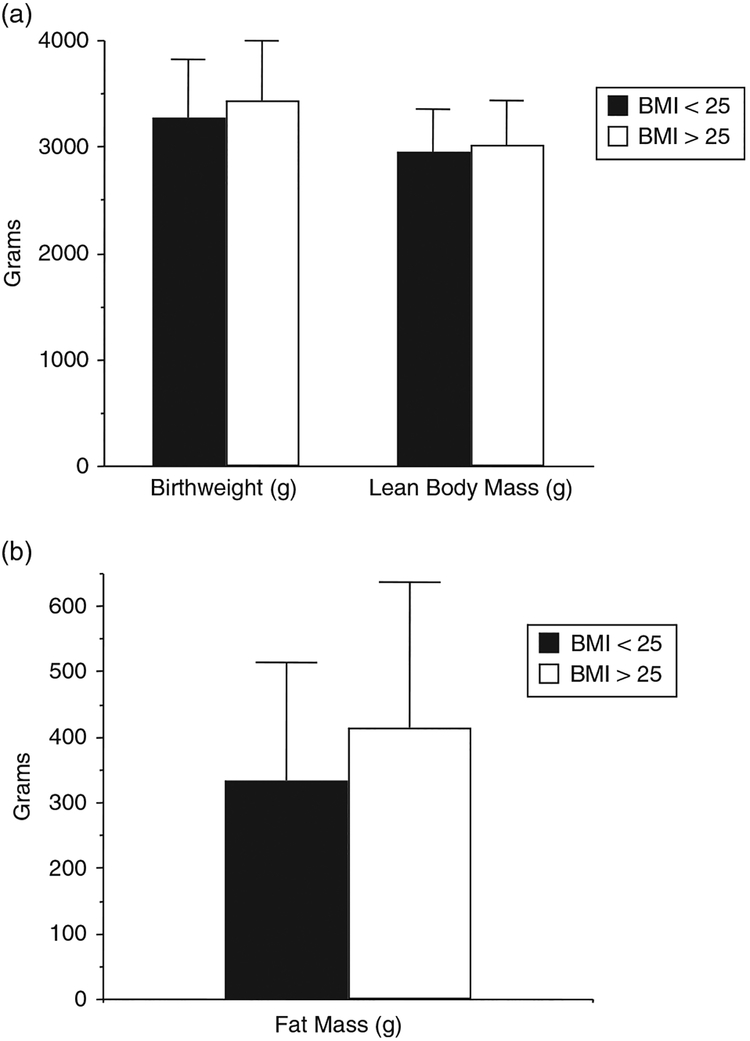
In parallel with the increase in maternal obesity in the past few decades, there has been an increase in birth weight reported in both European and North American populations.46,47 Ananth and Wen have reported that there has been a 5%–10% increase in large for gestational age infants and an 11%–12% decrease in small for gestational age infants from the decade from 1985–1998. At birth, the term infant of obese women weighs on average of 150 g more than that of a normal weight woman. This increase in weight is primarily accounted for by an increase in fat mass and not lean body mass48 as was reported in the infant of the woman with GDM (Fig. 5).24 Of interest, based on population based studies49,50 obese women have less total weight gain in pregnancy as compared with average weight women, although their babies are heavier and fatter at birth. However, an increase in gestational weight gain in obese women is an additional risk factor related to fetal fat accretion.48 In contrast to underweight or normal weight women where low or poor maternal weight gain in pregnancy is associated with small for gestational age (SGA) babies, in obese women poor weight gain in pregnancy is much less of a risk factor for a SGA infant.38 Because of large size or macrosomia the infant of the obese women is also at risk for shoulder dystocia, although usually at higher birth weights than observed in the infant of the women with GDM.51 This may relate to the central distribution of body fat in the infant of the woman with GDM.
Fig. 5.
Birthweight, lean body mass and fat mass of full term neonates of average (BMI < 25) n-144 and overweight(BMI > 25) n = 76 women. Weight P 5 0.051, lean body mass P = 0.22, fat mass P = 0.006. (Adapted from Sewell, Am J Obstet Gynecol, 2006).
The infant of the obese women is not only heavier at birth because of increased adiposity, but has evidence of increased insulin resistance using homeostasis model assessment estimates of insulin resistance compared with neonates of average weight women. Additionally, the cord blood leptin and interlukin-6 concentrations of the fetuses of obese women were significantly greater than the cord blood of the fetuses of the lean women. There was a strong correlation between fetal adiposity and insulin resistance.52 These data suggest that maternal obesity in and of itself poses a significant risk for the next generation with metabolic compromise already apparent at birth.
Child
Both maternal pre-pregnancy obesity and excessive weight gain during pregnancy have been related to increased obesity in children. There is now substantial evidence that the offspring of the obese women is at increased risk for childhood obesity. Whittaker reported that children born to obese women had a 2.5-fold increase increased the risk of obesity at 2–4 years of age based on Center for Disease Control and Prevention criteria compared with those children born to non-obese mothers.53 Similarly, Boney et al. reported that the children of obese women had a two-fold risk of not only obesity but the metabolic syndrome at an age of 11 years, compared with children of non-obese women.33 Lastly, Mingrone et al. in a follow-up study of young adults, noted that the offspring of obese women were more obese and insulin resistant as compared with the offspring of normal weight women at the time of their birth.54 Additionally, Oken et al. reported that increased gestational weight gain was associated with an increase in offspring BMI and skinfold measures at an age of 3 years. Women with adequate or excessive weight gain (IOM 1990 criteria) had a four-fold increase odd of having an overweight child (BMI > 95 percentile) using 2000 CDC reference data.55
On the basis of our own long term follow-up studies of children of women with normal glucose tolerance and GDM during pregnancy, maternal obesity is a significant risk factor for childhood obesity and metabolic dysregulation. There was no significant difference in Center for Disease Control and Prevention weight percentiles or body composition between the children of the women with normal glucose tolerance in pregnancy and GDM at age nine. The strongest perinatal predictor for a child being in the upper tertile of obesity based on dual energy X-ray absorptometry methodology was maternal pregravid BMI odds ratio (OR) 5.45 (95% CI1.62–18.4, P = 0.006). This relationship improved after adjusting for gender (OR 6.36; 95% CI 1.77–22.88, P = 0.004) and/or gender and if the mother had GDM (OR7.75; 95% CI 1.51–37.74, P = 0.01). The strength of these relationships did not change when measures of neonatal adiposity were included in the analysis. Maternal pregravid obesity, independent of maternal glucose status was the strongest predictor of childhood obesity.56
Although the review has been focused exclusively on the human model, there have been numerous animal studies explaining the mechanisms related to the observed physiologic changes. Van Assche et al. investigated the metabolic affects of a maternal cafeteria diet using a rat model. The diet induced obesity resulted in an increase in insulin resistance in the non-pregnant cafeteria fed rat, which was aggravated by pregnancy. The effect of obesity was greater then the effect of pregnancy on insulin resistance in this model. By using a similar murine model, Samuelsson et al. reported that diet-induced obesity in female mice leads to offspring obesity associated with adipocyte hypertrophy and altered mRNA expression of β-adrenoceptor 2 and 3, 11 BHSD-1 and PPAR-δ2. These alterations in adipocyte metabolism may very well play a role in offspring adiposity. The long-term effects including dysregulation of cardiovascular and metabolic function.58 Lastly, Plagemann has reported that depending on critical periods of development, hormones such as insulin and leptin can act as ‘endogenous functional teratogens’. For example hyperinsulinemia may affect malprogramming of neuroendocrine systems, regulating appetite, body weight and energy expenditure.59
Summary
Maternal GDM and obesity share multiple metabolic abnormalities and may well represent a spectrum of maternal metabolic dysregulation. For the mother obesity and GDM increases the risk of the ‘metabolic syndrome of pregnancy’, that is an increased risk of hypertensive, lipid and coagulation disorders, which increases perinatal morbidity and mortality. The offspring of these women are also at increased risk for both neonatal morbidity and long-term sequela related to an abnormal in utero metabolic environment. Ideally life style modification of diet and activity to achieve normal weight before conception is the ideal goal to decrease the risk of these problems.38 However, achieving recommended weight gain during pregnancy and avoiding excessive gestational weight gain as recently noted in the revised IOM guidelines may decrease perinatal morbidities such as maternal weight retention and hence reduced pregravid weight is subsequent pregnancies.38 The perinatal period offers a window of opportunity to improve the short and long-term health of a woman and her offspring. If the goal is to prevent rather than treat chronic metabolic disease, then optimal care of women before and during pregnancy is a necessary component of health and well-being.
Table 1C.
AACE Clinical criteria for diagnosis of the insulin resistance syndrome5
| Risk factor components | Cut points for abnormality |
|---|---|
| Overweight/obesity | BMI>25 kg/m2 |
| Elevated triglycerides | >150mg/dl (1.69mmol/l) |
| Low-HDL cholesterol | |
| Men | <40mg/dl (1.04mmol/l) |
| Women | <50mg/dl (1.29mmol/l) |
| Elevated blood pressure | >130/85mmHg |
| A 2-hour post-glucose | >140mg/dl |
| challenge | |
| Fasting glucose | Between 110 and 126mg/dl |
| Other risk factors | Family history of type 2 diabetes, hypertension, or CVD |
| Polycystic ovary syndrome | |
| Sedentary lifestyle | |
| Advancing age | |
| Ethnic groups having high risk for type 2 diabetes or CVD |
AACE, American Association of Clinical Endocrinologists; BMI, body mass index; HDL, high-density lipoprotein; CVD, cardiovascular disease.
Table 2.
Criteria for the diagnosis of overt diabetes in pregnant women at their initial prenatal visit
| 1. A1C>6.5%. The test should be performed in a laboratory using a method that is NGSP certified and standardized to the DCT assay* |
| Or |
| 2. FPG>126mg/dl (7.0mmol/L). Fasting is defined as no caloric intake for at least 8 h* |
| Or |
| 3. A 2h plasma glucose >200mg/dl (11.1 mmol/1) during an OGTT. The test should be performed as described by the World Health Organization, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water.* |
| Or |
| 4. In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose >200mg/dl (11.1 mmol/1) |
DCT, direct contact test; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test.
In the absence of unequivocal hyperglycemia, criteria 1–3 should be confirmed by repeat testing.
Acknowledgement
This study was supported by NIH-NICHD HD22965, and Clinical Research Unit NCRR CTSA UL1 RR 024989.
Footnotes
Statement of Interest
The author declares no conflict of interest regarding the contents of this manuscript.
References
- 1.World Health Organization. Obesity: preventing and management of a global epidemic. World Health Organization Technical Report Ser. 2000; 894, 1–4. [PubMed] [Google Scholar]
- 2.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final report. Circulation, 2002; 106, 3143–3421. [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus: provisional report of a WHO consultation. Diabetes Med. 1998; 15, 539–553. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. World Health Organization: Geneva, Switzerland: 1999. Retrieved December 12, 2003, fromhttp://whqlibdoc.who.int/hq/1999/WHO_NCD-NCS_99.2.pdf [Google Scholar]
- 5.Einhorn D, Reaven GDM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003; 9, 237–252. [PubMed] [Google Scholar]
- 6.Metzger BE, Coustan DR. Summary and recommendations of the fourth international workshop-conference on gestational diabetes mellitus. Diabetes Care. 1998; 21(Suppl 2), B161–B167. [PubMed] [Google Scholar]
- 7.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33(Suppl 1), 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999; 180, 903–916. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TA. Pancreatic β-cell defects in gestational diabetes: Implications for the pathogenesis and prevention of type 2 diabetes. JCEM. 2001; 86, 989–993. [DOI] [PubMed] [Google Scholar]
- 10.Catalano PM, Buchanan T. Metabolic changes during normal and diabetic pregnancies In Diabetes Mellitus in Women (eds. Reece EA, Coustan D, Gabbe S), 2004; pp. 129–147. Lippincott Williams & Wilkins: Philadelphia. [Google Scholar]
- 11.Catalano PM, Tyzbir ED, Roman NM, Amini SE, Sims EAH. Longitudinal changes in insulin release and insulin resistance in non-obese pregnant women. Am J Obstet Gynecol. 1991; 165, 1667–1672. [DOI] [PubMed] [Google Scholar]
- 12.Freinkel N Banting Lecture, 1980 of pregnancy and progeny. Diabetes. 1980; 29, 1003–1035. [DOI] [PubMed] [Google Scholar]
- 13.Kirwan JP, Hauguel-de Mouzon S, Lepercq J, et al. TNFα is a predictor of insulin resistance in human pregnancy. Diabetes. 2002; 51, 2207–2213. [DOI] [PubMed] [Google Scholar]
- 14.Xiang AH, Peters RK, Trigo E, et al. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes. 1991; 48, 848–854. [DOI] [PubMed] [Google Scholar]
- 15.Ryan EA, O’Sullivan MJ, Skyler JS. Insulin action during pregnancy: studies with the euglycemic clamp technique. Diabetes. 1985; 34, 80–89. [DOI] [PubMed] [Google Scholar]
- 16.Joffe GM, Esterlitz J, Levine RJ, et al. ; for the Calcium for Preeclampsia Prevention (CPEP) Study Group. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Am J Obstet Gynecol. 1998; 179, 1032–1037. [DOI] [PubMed] [Google Scholar]
- 17.Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358, 1991–2002. [DOI] [PubMed] [Google Scholar]
- 18.Center for Disease Control and Prevention, National Diabetes Fact Sheet, 2007. [Google Scholar]
- 19.American Diabetes Discussion: Clinical Practice Recommendations – 2007. Diagnosis and classification of diabetes. Diabetes Care. 1997; 30(Suppl 1), 542–547. [Google Scholar]
- 20.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. Diabetes Care. 2002; 25, 1862–1868. [DOI] [PubMed] [Google Scholar]
- 21.Athukorala C, Crowther CA, Willson K. Women with gestational diabetes mellitus in the ACHOIS Trial: Risk factors for shoulder dystocia. Aust NZ J Obstet Gynaecol. 2007; 47, 37–41. [DOI] [PubMed] [Google Scholar]
- 22.Alam M, Raza SJ, Sherali AR, Akhtor AS. Neonatal complications in infants born to diabetic mothers. J Coll Physicians Surg Pak. 2006; 16, 212–215. [PubMed] [Google Scholar]
- 23.Cordero L, Trener SH, Landon MB, et al. Management of infants of diabetic mothers. Arch Pediatr Adolesc Med. 1998; 152, 249–254. [DOI] [PubMed] [Google Scholar]
- 24.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in-utero development. Am J Obstet Gynecol. 2003; 189, 1698–1704. [DOI] [PubMed] [Google Scholar]
- 25.The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome (HAPO) study. Associations with neonatal anthropometrics. Diabetes. 2009; 58, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radaelli T, Varastehpour A, Catalano PM, Hauguel-de MouzonS. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003; 52, 2951–2958. [DOI] [PubMed] [Google Scholar]
- 27.Pettitt DJ, Knowler WC, Baird HR, Bennett PH. Gestational diabetes: infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care. 1980; 3, 458–460. [DOI] [PubMed] [Google Scholar]
- 28.Pettitt DJ, Aleck KA, Baird HR, et al. Congenital susceptibility to NIDDM: role of the intrauterine environment. Diabetes. 1988; 37, 622–628. [DOI] [PubMed] [Google Scholar]
- 29.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to obesity conveys risks for type 2 diabetes and obesity: a study of discordant sib ships. Diabetes. 2000; 49, 2208–2211. [DOI] [PubMed] [Google Scholar]
- 30.Dabelea D, Pettitt DJ. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab. 2001; 14, 1085–1091. [DOI] [PubMed] [Google Scholar]
- 31.Pettitt DJ, Baird HR, Aleck KA, et al. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983; 308, 242–245. [DOI] [PubMed] [Google Scholar]
- 32.Pettitt DJ, Knowler WDC, Bennett PH, et al. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 1987; 10, 76–80. [DOI] [PubMed] [Google Scholar]
- 33.Boney CM, Verner A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity and gestational diabetes mellitus. Pediatrics. 2005; 115, E290–E296. [DOI] [PubMed] [Google Scholar]
- 34.Hillier TA, Pedula KC, Schmidt MM, et al. Childhood obesity and metabolic imprinting. Diabetes Care. 2007; 30, 2287–2292. [DOI] [PubMed] [Google Scholar]
- 35.Clausen TD, Mathiesen ER, Hansen T, et al. Overweight and the metabolic syndrome in adult offspring of women with diettreated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009; 94, 2464–2470. [DOI] [PubMed] [Google Scholar]
- 36.Crowther CA, Hillier JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005; 352, 2477–2486. [DOI] [PubMed] [Google Scholar]
- 37.Landon MB, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development MFMU Network. A prospective multicenter treatment trial of mild gestational diabetes (GDM). Society for maternal fetal medicine abstract #2. Am J Obstet Gynecol. 2008(Suppl) 52.18313635 [Google Scholar]
- 38.Rasmussin KM, Yaktine AL(eds.). Weight Gain During Pregnancy: Reexamining the Guidelines Institute of Medicine, 2009. National Academy Press: Washington, DC. [PubMed] [Google Scholar]
- 39.Sewell MF, Huston-Presley L, Amini S, Catalano PM. Body mass index, a true indicator of body fat in obese gravidas.J Reprod Med. 2007; 52, 907–911. [PubMed] [Google Scholar]
- 40.Kirwan JP, Varastehpour A, Jing M, et al. Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J Clin Endocrinal Metab. 2004; 89, 4678–4684. [DOI] [PubMed] [Google Scholar]
- 41.Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes. 2008; 57, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lashen H, Fear K, Sturdee DW. Obesity is associated with increased first trimester miscarriage: Matched case-control study. Human Reprod. 2004; 19, 1644–1646. [DOI] [PubMed] [Google Scholar]
- 43.Strothard KJ, Tennant BWG, Bill R, Ramin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009; 301, 636–650. [DOI] [PubMed] [Google Scholar]
- 44.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007; 109, 419–433. [DOI] [PubMed] [Google Scholar]
- 45.Buhimschi CS, Buhimschi IA, Malinow AM, Weiner CP. Intrauterine pressure during the second stage of labor in obese women. Obstet Gynecol. 2004; 103, 225–230. [DOI] [PubMed] [Google Scholar]
- 46.Surkan PJ, Hsieh CC, Johansson AL, Diceman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004; 104, 720–726. [DOI] [PubMed] [Google Scholar]
- 47.Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada. Semin Perinatol. 2002; 26(4), 260–267. [DOI] [PubMed] [Google Scholar]
- 48.Sewell MF, Huston-Presley L, Super DM, Catalano PM. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. AJOG. 2006; 195, 1100–1103. [DOI] [PubMed] [Google Scholar]
- 49.Nohr EA, Vaeth M, Baker JL, et al. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008; 87, 1750–1759. [DOI] [PubMed] [Google Scholar]
- 50.Chu SY, Callaghan WM, Bisch CC, D’Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: fueling future obesity. Am J Obstet Gynecol. 2009; 200, 271–277. [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb AG, Galan HL. Shoulder dystocia: an update. Obstet Gynecol Clin North Am. 2007; 34, 501–531. [DOI] [PubMed] [Google Scholar]
- 52.Catalano P, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009; 32, 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004; 114, e29–e36. [DOI] [PubMed] [Google Scholar]
- 54.Mingrone G, Manco M, Mora MEU, et al. Influence of maternal obesity on insulin sensitivity and secretion of the offspring. Diabetes Care. 2008; 31, 1872–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007; 196, 322e1–322e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009; 90, 1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holemans K, Caluwaerts S, Poston L, Van Assche FA. Dietinduced obesity in the rat: a model for gestational diabetes mellitus. Am J Obstet Gynecol. 2004; 190, 858–865. [DOI] [PubMed] [Google Scholar]
- 58.Samuelsson A-M, Matthews PA, Argenton M, et al. Dietinduced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008; 51, 383–392. [DOI] [PubMed] [Google Scholar]
- 59.Plagemann A Perinatal programming and functional teratogenesis: impact on body weight regulation and obesity. Physiol Behav. 2005; 86, 661–668. [DOI] [PubMed] [Google Scholar]