Abstract
Objective:
The purpose of this study was to examine differences in velopharyngeal structures between adults with repaired cleft palate and normal resonance and adults without cleft palate.
Design:
Thirty-six English-speaking adults, including six adults (two males and four females) with repaired cleft palate (M = 32.5 years of age, SD = 17.4 years) and 30 adults (15 males and 15 females) without cleft palate (M = 23.3 years of age, SD = 4.1 years), participated in the study. Fourteen velopharyngeal measures were obtained on magnetic resonance images and compared between groups (cleft and noncleft).
Results:
After adjusting for body size and sex effects, there was a statistically significant difference between groups for 10 out of the 14 velopharyngeal measures. Compared to those without cleft palate, participants with repaired cleft palate had a significantly shorter hard palate height and length, shorter levator muscle length, shorter intravelar segment, more acute levator angles of origin, shorter and thinner velum, and greater pharyngeal depth.
Conclusion:
Although significant differences were evident in the cleft palate group, individuals displayed normal resonance. These findings suggest a wide variability in velopharyngeal anatomy can occur in the presence of normal resonance, particularly for those with repaired cleft palate. Future research is needed to understand how anatomic variability impacts function, such as during speech.
Keywords: Magnetic resonance imaging, anatomy, velopharyngeal function
Introduction
The levator veli palatini (levator) muscle is responsible for retraction and elevation of the velum against the posterior pharyngeal wall during swallowing and oralized speech sounds (Hoopes et al., 1969; Dickson and Dickson, 1972; Bell-Berti, 1976; Moon et al., 1994). In normal anatomy, the levator muscle courses across the middle one-third of the velum forming a cohesive sling (Kuehn and Moon, 2005). Individuals with unrepaired cleft palate display an anterior bony attachment at the posterior hard palate. The goal of primary palatoplasty is to create separation between the oral and nasal cavities, establish correct placement of the levator sling for normal speech production while also improving middle ear health and reducing upper respiratory infections.
Studies have examined how postsurgical anatomy relates to noncleft anatomy. Ha et al. (2007) used magnetic resonance imaging (MRI) to investigate velopharyngeal anatomy among four male adults with repaired cleft palate. However, the levator muscle was reported for only three of the four participants. The authors concluded that the three males with repaired cleft palate displayed shorter and thinner levator muscle values compared to mean values for noncleft adults reported by Ettema et al. (2002). Ha et al. (2007) observed in one adult participant with repaired cleft palate who presented and hypernasal speech displayed an incomplete levator sling and irregular curvature of the levator muscular sling as it comes into the body of the velum. These observations were unique to this single participant with hypernasal speech and not observed in the other three adults with repaired cleft palate who had normal resonance. The authors suggested such anatomic variability (incomplete levator sling and morphology variations) may be contributing factors to the presence of hypernasal speech.
Tian et al. (2010) similarly examined variations between individuals with repaired cleft palate, cleft palate and hypernasal speech, and noncleft controls. However, this study was among child participants. The authors demonstrated significant differences among those with cleft palate (with and without hypernasality) when compared to the noncleft controls. Minimal differences were observed between the two cleft palate groups, suggesting that anatomic differences alone may not be able to differentiate those with and without hypernasal speech. However, unlike the findings by Ha et al. (2007), Tian et al. (2010) observed all child participants to have a cohesive levator sling with no midline separation or irregularity in the levator morphology. Based on these studies, it is evident that variations in cleft anatomy exist when compared to noncleft controls. Perry et al. (2016) suggested that significant deviations from normative values may be related to aberrant function for normal resonance during speech. However, it is unclear how much anatomic variation is acceptable while still enabling normal oral-nasal balance.
Study design limitations impact the scope of the conclusions possible from the results of Ha et al. (2007). The comparison of the repaired cleft palate adults (Ha et al., 2007) to the control group (Ettema et al., 2002) were not within the same study, and groups displayed differences in participant sex, age, and race. Numerous studies have demonstrated significant sex and race effects for velopharyngeal variables among adults (McKerns and Bzoch, 1970; Bae et al., 2011; Perry et al., 2014; Perry et al., 2016). Given these known sex and race differences in velopharyngeal variables, comparisons between cleft and noncleft adult study groups should control for such effects. The group of normal participants (Ettema et al., 2002) consisted of five Caucasian males and five Caucasian females ranging from 21 to 53 years of age; whereas, the group with cleft palate (Ha et al., 2007) consisted of four males (no females) of different ethnicity (Hispanic and non-Hispanic) ranging from 22 to 43 years of age. Levator muscle length was only measured in three of the four adults with cleft palate (Ha et al., 2007). The lack of a within-study comparison and the uncontrolled influence of sex and race on prior research findings is a limitation that this current study aims to address.
The purpose of this study was to examine differences in velopharyngeal structures between adults with repaired cleft palate and normal resonance and adults without cleft palate. We hypothesized that adults with repaired cleft palate would display significant differences in levator muscle and velopharyngeal structures compared to adults with normal anatomy, even with normal resonance (Ha et al., 2007; Tian et al., 2010). Specifically, we expected that adult participants with repaired cleft palate would exhibit increased variability in levator and velopharyngeal structural shape and form, specifically shorter levator muscle length, shorter velar length, and shorter hard palate length indicating a greater velopharyngeal ratio of velar length to pharyngeal depth than adult participants with normal anatomy. The overarching goal of this study was to provide insight into the amount of acceptable anatomic variability in the presence of normal oral-nasal balance. Such outcomes support our understanding of cleft palate speech and anatomic variability.
Methods
Participants
In accordance with the local Institutional Review Boards, 36 English-speaking adults between 19–66 years of age were recruited to participate in this study. Participants with a history of cleft palate were recruited from the local hospital by contacting adults who have received treatment as part of the hospital’s cleft palate craniofacial team. Participants were provided a flyer by email and requested to contact the study investigator, if interested in participation. Selection of participants for this study was based on the existence of normal oral-nasal balance and representative of one race to control for the known impact of race on velopharyngeal structures (Perry et al., 2016). Participants included six adults (two males and four females) with repaired cleft palate (M = 32.5 years of age, SD = 17.4 years) and 30 adults (15 males and 15 females) without cleft palate (M = 23.3 years of age, SD = 4.1 years). We used a matched case-control study design of 5:1 (control:participants with cleft palate). The use of matching in case-control study design is recommended as a method to ensure comparability between the controls and the cases (i.e., those with cleft palate) to reduce variability and confounders that may influence study outcomes (Garey, 2004; Everitt and Palmer, 2005; Song and Chung, 2010). Of those with repaired cleft palate, two had histories of bilateral complete cleft lip and palate (BCLP), one had unilateral cleft lip and palate (UCLP), and three had cleft palate only (CPO). Participants with cleft palate were included if they had a repaired cleft palate, reported an absence of syndrome diagnosis, and judged to have normal resonance. Participants without a history of cleft palate were recruited as part of a larger study that examined race variations in the velopharyngeal system among adults with normal anatomy (Perry et al., 2016). Selections of participants from the larger study (N = 88) were based on a set of inclusionary criteria and to provide a similarly age- and race-matched control group. Inclusion criteria for the participants without cleft palate included absence of a history of hearing, neurological, swallowing, craniofacial, or musculoskeletal disorders. To control for the effect of race on velopharyngeal measures (Perry et al., 2016), only Caucasian English-speaking adults were included in the study. Body mass index (BMI) was collected on all prospective participants to ensure that he/she was not too large to fit into the magnet scanning bore. Classifications of BMI were consistent with guidelines from the National Heart, Lung, and Blood Institute (NIH, 2015).
Participants with cleft palate reported primary cleft palate repair between 8 and 18 months of age. However, because cleft surgeries were performed in different hospitals, documentation regarding the surgical procedures were not provided. Resonance and articulation was rated by a speech-language pathologist (senior author) with over 15 years of experience in cleft palate assessments using a 5-point rating scale during conversational speech. Ratings were performed on both cleft and noncleft participants to ensure that they did not present with abnormalities in the oral-nasal balance. All participants indicated no hearing loss or middle ear infections or diseases at the time of their participation in the study. Participants without cleft palate were similarly rated by the same speech-language pathologist for assessments of resonance and were determined to displayed normal oral-nasal balance and presented with no observable oral structural abnormalities, as seen through an intra-oral examination. Those with repaired cleft palate had normal resonance (rated as 1 on a 5-point scale), normal articulation, and had no nasal air emission. No participants with cleft palate had received a secondary surgery, such as a pharyngeal flap or sphincterpharyngoplasty, nor presented with an oronasal fistulae at the time of the MRI study. Using an independent samples t-test, we observed there were no significant differences between the two study groups for height (p = 0.082) or head circumference (p = 0.614). However, weight was significantly different between groups (Cleft palate group M = 185 pounds, Median = 184 pounds, SD = 26 pounds; noncleft palate group M = 151 pounds, Median = 152 pounds, SD = 26 pounds; t(34) = −2.865, P = .007).
Magnetic Resonance Imaging
Two MRI scanners were used for this study because the study involved a large scale investigation done at one facility and cleft palate patients were recruited in a different geographic region. MRI protocols were designed to produce similar images between scanners by establishing sequences across the two scanners that yielded a similar in-plane isotropic resolution. All noncleft palate participants and four participants with cleft palate were imaged using a Siemens 3 Tesla Trio (Erlangen, Germany) MRI scanner and a 12-channel Siemens Trio head coil. A T2-weighted 3D turbo spin echo (TSE) anatomical scan called Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE; Henning, 1988; Busse et al., 2006 Siemens AG, 2007) sequence was acquired with .8 mm isotropic spatial resolution (repetition time of 2,500, echo time of 268) in 4:52 minutes. Long spin-echo trains, particularly when using three dimensional slabs or volumes, have been shown to produce adequate signal to noise ratio, clinically useful contrast among tissue, and substantially reducing imaging time (Mugler et al., 2000; Bae et al., 2011; Perry et al., 2014, 2016). Two participants with cleft palate were imaged using a General Electric HDxt 1.5 Tesla system (Milwaukee, Wisconsin) with a head neck spine (HNS) GE 8 channel head coil. A T2-weighted CUBE 3D sequence was acquired in 6;09 minutes with repetition time of 2,500, echo time of 151, and acquired with .83 mm isotropic resolution.
An adult male (not a participant from this study) was imaged at both MRI sites to ensure consistency in the internal reliability between the two MRI systems used in the present study. This was used to ensure variations observed within the study were not attributed to the difference between the MRI magnets. Measures were within 1 mm (and under 1 degree for measures represented as degrees) of each other across the two scanners.
An elastic strap was attached to the head coil and passing over the participant’s head at the level of the glabella. Foam wedges were placed in the space between the individual’s head and the head coil and a mirror was used to allow for a fixed eye gaze onto a stable point. Collectively these steps reduced body movement and resulting motion artifacts within the images. Participants were all imaged in the supine position and instructed to breathe through their nose and remain at rest without speech or swallowing. These instructions reduced motion artifacts and ensured consistency of rest/non-speech positioning of velopharyngeal measures between participants for consistencies in measures.
Image Analyses
Digital Imaging and Communications in Medicine (DICOM) raw data were imported into Amira 5.4.0 Visualization and Volume Modeling Software (Visage Imaging, GmbH, Berlin, Germany). Three-dimensional MRI data were analyzed to produce a serial stack of images along the midsagittal, coronal, axial, and oblique coronal planes for each participant. The midsagittal image plane was selected as the image most clearly depicting the midline of the velar body, maximum velar length, posterior nasal spine (or bony terminal end of hard palate, as in cleft palate participants), fourth ventricle, and genu of the corpus callosum (Ettema et al., 2002). The oblique coronal image plane was determined by rotating the image slice such that the slice coursed through the bulk of the velum and in the plane depicting the levator muscle from origin to insertion (Ettema et al., 2002). The true coronal plane was used to identify the palate width and height measures, obtained at the lingual gingival margin of the first molar.
Velopharyngeal parameters of interest and methods for obtaining measurements were maintained using prior published studies using MRI data (Tian et al., 2010; Bae et al., 2011; Perry et al., 2011) and measures are described in Table 1 and visualized in Figure 1. This includes 14 velopharyngeal measures that represent structures that are likely impacted by the presence of a cleft palate and thus are hypothesized to show variability from the noncleft palate participants. This included measures of the hard palate, levator muscle, and velopharyngeal portal. One cleft palate participant (cleft palate participant 2 of Figure 2) wore an upper fixed lingual retainer, which obscured the view of the anterior nasal spine and thus the hard palate length was not measured on this patient. Cleft palate participant 6 also wore a dental device, as evident by the artifact near the tongue tip. However, the anterior nasal spine was able to be seen in this participant.
Table 1.
Descriptions of variables.
| Parameter | Description |
|---|---|
| Hard Palate Measures | |
| Palate width | Distance (mm) between the free lingual gingival margin of the first molar on each side as seen on the coronal image plane |
| Palate height | Height (mm) of the hard palate measured perpendicular to the palatal width line extending to the roof of the hard palate in the region of the palatal vault as seen on the coronal image plane |
| Palate length | Palate length (mm) measured as the distance between the anterior and posterior borders of the hard palate as seen on the sagittal image |
| Levator Measures | |
| Levator muscle length | Average of right and left levator muscle length measures (mm) from origin at cranial base to terminal end of the levator bundle as seen on the oblique coronal image plane |
| Extravelar levator muscle length | Average of right and left levator muscle fibers (mm) from origin at either side of the cranial base to insertion of levator muscle into either side of the velum as seen on the oblique coronal image plane |
| Intravelar levator muscle length | Length (mm) of the levator muscle within the velum as seen on the oblique coronal image plane |
| Velar insertion distance | Distance (mm) between points of levator insertion into the velum as seen on the oblique coronal image plane |
| Origin to origin | Distance (mm) between the right levator muscle origin at the cranial base and left levator muscle origin on the other side as seen on the oblique coronal image plane |
| Angle of levator muscle origin | Average of right and left angles (degrees) between origin to origin width and course of muscle fibers at cranial base as seen on the oblique coronal image plane |
| Velopharyngeal Portal Measures | |
| Effective velar length | Functional portion of the velum measured as the distance (mm) between the posterior border of the hard palate and point of levator muscle insertion into the velum as seen on the sagittal image plane |
| Velar length | Curvilinear length (mm) of the velum extending from the posterior border of the hard palate through the middle of the velum to the inferior tip of the uvula from the sagittal image plane |
| Velar thickness | Distance (mm) between oral surface of velum and velar knee at the estimated location of the velar knee (region of greatest velar thickness) and velar dimple (oral location) as seen on the midsagittal image plane |
| Pharyngeal depth | Distance (mm) from the posterior border of the hard palate to the posterior pharyngeal wall along the hard palate plane as seen on the sagittal image plane |
| Velopharyngeal ratio | (velar length) / (pharyngeal depth) |
Figure 1.
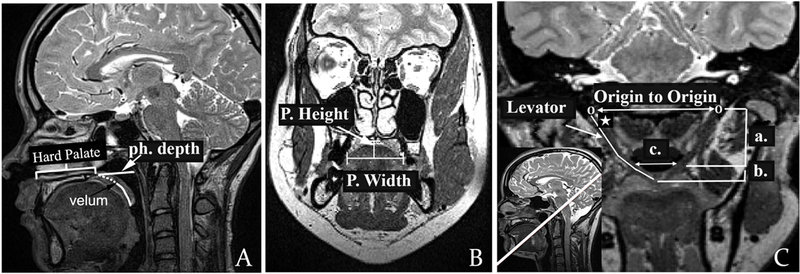
(A): Midsagittal view showing the effective velar length (dotted line), full velar length (dotted line plus full line extending to uvula), pharyngeal depth (line from posterior hard palate to posterior pharyngeal wall, labeled “ph. depth”), and hard palate length as anterior nasal spine to the point at which the velum begins. (B): Coronal view showing the palate width (P. width) and height (P. height); (C): Oblique coronal plane displaying full sling of the levator muscle, origin to origin, angle of origin (noted by white star), extrinsic levator segment (a), intrinsic levator segment (b), and velar insertion distance (c).
Figure 2.
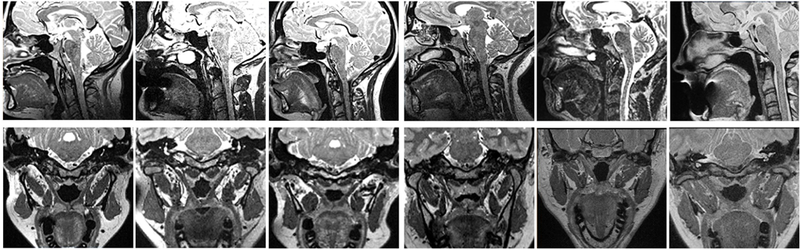
Midsagittal (top row) and oblique coronal (bottom row) images from participants with repaired cleft palate.
Statistical Analysis
Using a factor analysis correlation matrix, height and weight showed a weak correlation (R = .306). However, height and head circumference (R = .549) and weight and head circumference (R = .536) showed a moderate correlation. Therefore, a principal component analysis was used to reduce the three variables into one variable to represent a single component score to define body size. This was also performed to reduce the degrees of freedom and create a component that is based on the shared variance of the three variables representing body size. Sex was also treated as a covariate given the known differences among variables used in the present study based on sex (Perry et al., 2014; Perry et al., 2016).
An analysis of covariance (ANCOVA) was used to determine whether there are any statistically significant group differences on the dependent variable after adjusting for the covariates of sex and body size (IBM SPSS, version 21.0; IBM corp, Armonk, NY). There was homoscedasticity and homogeneity of variances, as assessed by visual inspection of scatterplot and Levene’s test of homogeneity of variance (P > .05 for all variables), respectively. There were no outliers in the data, as assessed by no cases with standardized residuals greater than ±3 standard deviations. Unadjusted means are reported unless otherwise stated. An alpha level of .05 was used to determine significance.
Results
A primary rater with over 15 years of experience (senior author) in measuring MRI data completed the initial measures. The primary rater repeated measures (two months after the initial measures) on 10 randomly selected participants (30%) to estimate intra-rater reliability. Intra-class correlation coefficient (ICC) estimates and their 95% confidence intervals were calculated using SPSS statistical package version 23 (SPSS Inc, Chicago, IL) based on a 2-way mixed-effects model. Measures across the two time points indicated excellent (defined as .90 and higher; Portney and Watkins, 2000) reliability with agreement between .924 and .994. The mean difference between the first and second time points for measures was less than 1.3 mm for all repeated linear measures and within 1 degree for angle of origin measure. A second rater with 4 years of prior experience in measuring MRI data was trained using a dataset obtained from a prior research study. The two raters compared and established excellent reliability using the training set before the second rater measured the data from the present study. The second trained rater completed measurements on 10 randomly selected participants (30%) to examine the inter-rater reliability. Inter-rater reliability using an ICC ranged from .761 and .864 indicating good (defined as .75 to .90; Portney and Watkins, 2000) reliability with the lowest reliability for intravelar levator muscle length (.761). Agreement between raters was within 2.1 mm for linear measures and 2 degrees for the angle of origin measure.
The primary aim of this study was to determine whether adults with repaired cleft palate presenting with normal resonance demonstrate significant differences in velopharyngeal structures when compared to those without cleft palate. Unadjusted means for group means of velopharyngeal variables are reported in Table 2 along with the ANCOVA results for the grouping variable after adjusting for body size and sex effects.
Table 2.
Unadjusted Means and Standard Deviations for Velopharyngeal Variables between Groups. Values listed are in mm, with the exception of the angle (measured as degrees) and velopharyngeal (VP) ratio measures.
| Group means (SD) | F | p-value | Partial Eta Squared |
||
|---|---|---|---|---|---|
| Cleft Group n = 6 |
Noncleft Group n = 30 |
||||
| Palate width | 35.0 (5.7) | 33.4(2.8) | 1.913 | .176 | .056 |
| Palate height | 8.3 (1.5) | 13.2 (2.5) | 20.248 | <.001** | .388 |
| Palate length (n = 5) | 48.7 (5.9) | 59.8 (5.1) | 16.215 | <.001** | .343 |
| Levator muscle length | 41.2 (5.4) | 46.2 (4.7) | 9.244 | .005** | .256 |
| Extravelar levator muscle length | 30.1 (3.2) | 29.6 (3.6) | .115 | .736 | .004 |
| Intravelar levator muscle length | 22.9 (5.7) | 33.1 (4.3) | 16.11 | <.001** | .349 |
| Velar insertion distance | 21.7 (4.1) | 25.0 (2.7) | 23.017 | <.001** | .434 |
| Origin to origin | 53.1 (3.8) | 56.3 (5.7) | 2.904 | .098 | .083 |
| Angle of levator muscle origin | 55.6 (4.6) | 58.0 (3.5) | 8.885 | .006** | .229 |
| Effective velar length | 11.5 (2.2) | 12.9 (1.9) | 2.948 | .096 | .084 |
| Velar length | 24.4 (5.0) | 35.7 (4.5) | 31.005 | <.001** | .492 |
| Velar thickness | 8.1 (1.7) | 10.7 (1.5) | 16.243 | <.001** | .337 |
| Pharyngeal depth | 27.2 (2.1) | 20.8 ( 2.9) | 23.975 | <.001** | .428 |
| VP ratio | .9 (.2) | 1.7 (.3) | 40.372 | <.001** | .558 |
**p < .001
*p < .05
Velopharyngeal Observations
After adjusting for body size and sex effects, there was a statistically significant difference between groups for 10 out of the 14 velopharyngeal measures. There was a statistically significant difference in palate height between study groups, F(1,32) = 20.248, P < .001, partial η2 = .388. Specifically, palate height was greater among those in the control group (M = 13.2 mm, SD = 2.5 mm) compared to participants with repaired cleft palate (M = 8.3 mm, SD = 1.5 mm). Palate length was also significantly different between groups, F(1,32) = 16.215, P < .001, partial η2 = .343, with those in the control group showing a greater palate length (M = 59.8 mm, SD = 5.1 mm) compared to those with repaired cleft palate, minus the one participant who wore a dental device thus obscuring the view of the anterior nasal spine (M = 48.7 mm, SD = 5.9 mm). These findings suggest that those with repaired cleft palate display a significantly shorter hard palate and a more flattened hard palate vault than those without cleft palate. The observation of a shorter hard palate can be visually appreciated by comparing the midsagittal images from participants with repaired cleft palate (Figure 2) to a sample of three participants from the control group (Figure 3).
Figure 3.
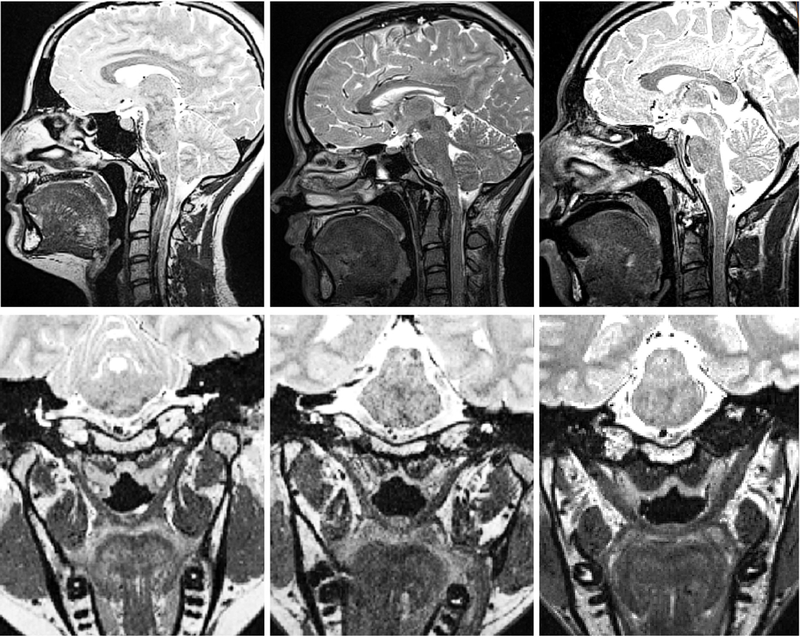
Midsagittal (top row) and oblique coronal (bottom row) images from a sample of three participants from the noncleft control group of 30.
Numerous levator muscle differences were also apparent among the groups. Levator muscle length was significantly shorter for those with cleft palate (M = 41.2 mm, SD = 5.4 mm) compared to participants without cleft palate (M = 46.2 mm, SD = 4.7 mm), F(1,30) = 9.244, P = 0.005, partial η2 = .256. When examining the difference between the segment of the levator muscle that is within the body of the velum (intravelar levator muscle length) and outside the velar body (extravelar levator muscle length), the intravelar segment was the only portion that displayed a significant group effect, F(1,30) = 16.11, P < .001, η2 =.349 with those having cleft palate showing a shorter intravelar levator muscle compared to noncleft controls. Among those with repaired cleft palate, separation and/or sparse fibers are seen through the levator sling at the midline of the velum. All participants with normal anatomy exhibited a cohesive levator muscle sling with no midline separation and a visible musculus uvulae bundle on the nasal surface of the velum.
Participants with repaired cleft palate also displayed a significantly shorter velar insertion distance height (M = 21.7 mm, SD = 4.1 mm) compared to those in the control group (M = 25.0 mm, SD = 2.7 mm), F(1,30) = 23.017, P < .001, partial η2 = .434. The smaller distance between the insertion points of the levator muscle into the velum can likely be explained, in part, by the variations in the angle of the muscle origins. The angle of origin, representing the angle at which the muscle converges from the cranial base into the velar midline was significantly different with those in the cleft palate group showing more acute angles (M = 55.6 degrees, SD = 4.6 degrees) than those without cleft (M = 58.0 degrees, SD = 3.5 degrees). In summary, the shorter levator muscle length apparent in the cleft palate group can be explained largely by the variation within the intravelar segment of the muscle. Additionally, the levator muscle shows a more acute angle of origin resulting in a smaller distance between the insertion points of the levator muscle into the velum.
Lastly, differences were evident in the measures related to the velopharyngeal portal. There was a statistically significant difference between groups for measures of velar length (F(1,32) = 31.002, P < .001, partial η2 = .492), velar thickness (F(1,32) = 16.243, P < .001, partial η2 = .337), and pharyngeal depth (F(1,32) = 23.975, P < .001, partial η2 = .428). Specifically, the cleft palate group displayed a significantly thinner velum (M = 8.1 mm, SD = 1.7 mm) and a greater pharyngeal depth (M = 27.2 mm, SD = 2.1 mm), than the noncleft control group (M = 10.7 mm, SD = 1.5 mm; M = 20.8 mm, SD = 2.9 mm, respectively). Irregularities in the velum are visually apparent in Figures 2 and 3. Of interest, the effective velar length was not impacted by the grouping variable, suggesting no significant differences between groups after accounting for the effect of body size and sex. The velopharyngeal ratio (VP ratio) was significantly smaller for those with cleft palate (M = .9, SD = .2) compared to the noncleft palate group (M = 1.7, SD = .3; P(1,32) = 40.372, P < .001, partial η2 = .558).
Discussion
Significant differences were observed for hard palate, levator muscle, and velopharyngeal portal measures. A decrease in hard palate height and length was observed in this study. A decreased growth of maxillary width and length has been observed consistently in studies of adults and children with a history of cleft palate (Wada and Miyazaki, 1975; Crabb and Foster, 1977; Zajac et al., 2012). Abnormal development of the surgically repaired dental arch and potential for it to collapse has been proposed to explain, in part, the shorter palate height measures observed for participants with repaired cleft palate in the present investigation (Reichert, 1970). However, maxillary growth differences are said to be localized defects which cannot be accounted for by surgical trauma alone (Crabb and Foster, 1977). Although the sample size does not permit comparison by cleft type, those with UCLP and BCLP were observed to display a trend toward a shorter hard palate length compared to those with CPO. This may be due to abnormalities in the positioning of the anterior nasal spine due to the cleft involvement of the primary palate. Future research using a larger sample is needed to examine the influence of cleft type on these variables.
Similar to findings reported by Ha et al. (2007) among three adult males, it was observed in the present study that individuals with repaired cleft palate display a shorter levator muscle length. Whereas Tian et al. (2010) did not observe such differences between cleft and noncleft palate child groups with normal resonance, the authors observed a greater velar insertion width in those with cleft palate. Perry et al. (2011) also observed a broader “U-shaped” muscle, indicating a wider velar insertion distance in infants with cleft palate following cleft palate repair. In contrast to this observation, it was observed in the present study that a smaller velar insertion distance existed. This difference may be related to individual variations (as seen in the noncleft palate controls) and surgical procedures used. A “U-shaped” muscle configuration might be related to variations in surgical procedures related to dissection in the region of the hamulus. Future studies using diffusion tensor MR imaging may be a valuable imaging tool to investigate the fiber distribution relative to the hamular processes. Additionally, longitudinal studies investigating surgical procedures with and without dissection in the hamular region may also provide insight into the effect of the lateral levator tethering.
Perry et al. (2013) used three-dimensional computer reconstructions from MRI to quantify the differences in the circumference and volume of the extravelar muscle segment and the intravelar muscle segment among adults with normal anatomy. Results demonstrated consistency in the extravelar segment of the muscle with the greatest amount of variability within the midline segment of the levator sling. At the middle of the velum, the levator diameter ranged from 4.6 mm to 12.6 mm and circumference ranged from 13.61 mm to 30.27 mm between participants. Using a similar method, Kotlarek et al. (2017) compared volumetric analyses of the levator muscle between five adults with cleft palate and 10 adults without cleft palate. Results demonstrated those with cleft palate displayed a significantly smaller total levator muscle volume and the most significant difference in muscle diameter and circumference was noted at the velar midline. Individuals with cleft palate showed a dehiscence (or a lack of surgical connectivity initially) of the levator sling at the midline.
Findings from the present study are in agreement with these previous studies in which the overall levator muscle length varied significantly and the difference was apparent primarily for the intravelar segment (Perry et al., 2013). Of particular interest, the mean extravelar segment length for the cleft palate group was actually longer than that for the noncleft palate group. However, the intravelar segment was significantly smaller for the cleft palate group, so much that it resulted in the overall muscle length also showing a statistically significant difference compared to the noncleft palate controls. The intravelar muscle segment also demonstrated significant variability in morphology ranging from being present, irregular or sparse, or not continuous. The segment of the levator muscle that is outside of the velar body may be of greater importance for normal velopharyngeal function. In such, surgeries that dissect the extrinsic muscle portion (Nguyen et al., 2015) may result in a more favorable muscle position and function. Further imaging research is needed to understand the impact of surgical procedures on muscle function.
Significant differences were also apparent for the velopharyngeal portal measures in the present study. Studies have consistently demonstrated significantly shorter velar length measures in children and adults with repaired cleft palate compared to normal anatomy (Coccaro et al., 1962; Akgüner et al., 1998; Özgür et al., 2000; Satoh et al., 2002). Findings from the present study further support this observation. Of interest, one factor that appeared similar between groups was the effective velar length, which in the cleft palate group is representative of the amount of posterior positioning of the levator sling at the time of primary palatoplasty and presumably the location to which the muscle has migrated following surgery. An appropriately retropositioned levator sling is likely of critical importance in creating normal velopharyngeal function. The distance from the posterior bony palate to the velar eminence is functionally the most important portion of the velar body. As described by Kuehn and Kahane (1990), the bulk of the muscular segment of the velum occupies the central one-third of the velum. This segment is noted as the velar eminence which serves as the point of contact against the posterior pharyngeal wall during velopharyngeal closure. Posteroinferiorly to the velar eminence, the velum shows greater variability in tissue composition between individuals and is noted by a marked decrease in muscle fibers thus demonstrating its limited role in speech (Kuehn and Kahane, 1990).
The significantly greater pharyngeal depth and shorter velum explains the significant difference in the VP ratio between groups. Previous investigations of velopharyngeal ratio in participants with normal anatomy provide a range from 1.2 to 1.43 (Subtelny, 1957; Tian et al., 2010). Hoopes et al. (1969) reported individuals with normal anatomy exhibited an average VP ratio of 1.35, whereas those with insufficient velopharyngeal closure exhibited an average VP ratio of 1.05. The participants without cleft palate in the present study presented a range from 1.17 to 2.86, whereas those with repaired cleft palate (presenting with normal resonance) ranged from .7 to 1.21. Although VP ratios for those in the cleft palate group fell well below the mean range provided by Subtelny (1957), all adult participants in the study had normal resonance. Tian et al. (2010) observed children without cleft palate had a VP ratio of 1.5, and those with cleft palate, regardless of the presence or absence of hypernasal speech, had a VP ratio of 1.1. These findings suggest that the VP ratio alone cannot explain acceptable velopharyngeal function for speech.
As demonstrated, adults with repaired cleft palate display numerous significant differences in the velopharyngeal structures even in the presence of normal resonance. This raises the question of whether anatomic variations can appropriately define those with cleft palate who will have normal speech and those that will not. Given the aforementioned variability found in adults without cleft palate in the intravelar segment (Perry et al. 2013), it is questionable as to whether or not the variation within the adults with cleft palate in the present study is of any clinical significance. The observation of a non-continuous levator sling being associated with normal resonance in the present study supports the notion that there are likely more significant factors beyond a certain characteristic anatomy that are required for producing acceptable velopharyngeal function. Because this study did not include adults with a history of cleft palate and hypernasality, conclusions related to the cause of velopharyngeal dysfunction cannot be drawn.
Although groups were matched, weight was found to be significantly different between groups with adults with a history of cleft palate weighing significantly more (greater mean and median, no outliers) than those without cleft palate. There was association between head circumference and weight, suggesting a possible influence of weight on measures. However, measures with significant differences all revealed lesser values that adults without cleft palate. This is an opposite effect of what would be expected if weight independently had an influence on variables. If any effect, it would be expected that matching groups by weight in future studies would yield a greater difference in the values between study groups. Consideration of age- and sex-matched cohorts is of great importance in future investigations.
Muscle configuration, morphology, and velopharyngeal function are likely due to surgical procedures or the effects of these with maturation (Law and Fulton, 1959; McGowan et al., 1992; Ishikawa et al., 1998; Khanna et al., 2012). The most notable limitations of the present study is the lack of surgical details for participants with cleft palate. In such, conclusions about anatomic configurations as a result of surgery cannot be established. A significant increase in the sample size would also be necessary for such comparison. It is possible that variations in scar pattern are a result of surgical techniques and may yield differences in the velopharyngeal anatomic parameters. Future research is needed to examine the impacts of scar patterns on velopharyngeal anatomy and function. In this study, a 5-point resonance rating scale was the only means for determining normal oral-nasal balance. Because ratings were done live at the time of the study, only one rater performed these assessments of speech. As a result, reliability of the speech rating was not established. The lack of quantitative assessment tools, such as pressure-flow or nasometry, is an additional limitation of this study.
Conclusion
Findings suggest that, compared to participants with normal anatomy, individuals with repaired cleft palate display significant differences in the hard palate, levator muscle, and velopharyngeal portal measures. Although significant differences were evident in the cleft palate group, individuals displayed normal resonance. Future research is needed to understand how anatomic variability impacts function, such as during speech.
Acknowledgments
This study was made possible by grant number 1R03DC009676–01A1 from the National Institute of Deafness and Other Communicative Disorders. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health.
REFERENCES
- Akgüner M, Karaca C, Barutçu A, Özaksoy D, Yurt A, Vayvada H. Evaluation of velopharyngeal pathophysiology and velopharyngeal insufficiency with magnetic resonance imaging. Eur J Plast Surg.1998;21:118–128. [Google Scholar]
- Bae Y, Kuehn DP, Sutton BP, Conway CA, Perry JL. Three-dimensional magnetic resonance imaging of velopharyngeal structures. J Speech Hear Res. 2011;54:1538–1545. [DOI] [PubMed] [Google Scholar]
- Bell-Berti F An electromyographic study of velopharyngeal function in speech. J Speech Hear Res. 1976;19:225–240. [DOI] [PubMed] [Google Scholar]
- Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: Design of variability refocusing flip angle schedules and generation of clinical T2 contrasts. Magn Reson Med. 2006;55:1030–1037. [DOI] [PubMed] [Google Scholar]
- Coccaro PJ, Subtelny JD, Pruzansky S. Growth of soft palate in cleft palate children: serial cephalometric study. Plast Reconstr Surg. 1962;30:43–55. [DOI] [PubMed] [Google Scholar]
- Crabb JJ, Foster TD. Growth defects in unrepaired unilateral cleft lip and palate. Oral Surg Oral Med Oral Path. 1977;44:329–335. [DOI] [PubMed] [Google Scholar]
- Dickson DR, Dickson WM. Velopharyngeal anatomy. J Speech Hear Res. 1972;15(2):372–381. [DOI] [PubMed] [Google Scholar]
- Ettema SL, Kuehn DP, Perlman AL, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate Cranio J. 2002;39:130–144. [DOI] [PubMed] [Google Scholar]
- Garey KW. The role of matching in epidemiologic studies. Am J Pharmaceut Ed. 2004;68:1–7 [Google Scholar]
- Ha S, Kuehn DP, Cohen M, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle in speakers with repaired cleft palate. Cleft Palate Cranio J. 2007;44:494–501. [DOI] [PubMed] [Google Scholar]
- Henning J Multiecho imaging sequences with low refocusing flip angle. J Magn Reson. 1988;78:397–407. [Google Scholar]
- Hoopes JE, Dellon AL, Fabrikant JI, Soliman AH. The locus of levator veli palatini function as a measure of velopharyngeal incompetence. Plast Reconstr Surg. 1969;44:155–160. [PubMed] [Google Scholar]
- Ishikawa H, Nakamura S, Misaki K, Kudoh M, Fukuda H, Yoshida S. Scar tissue distribution on palates and its relation to maxillary dental arch form. Cleft Palate Cranio J. 1998;35(4);313–319. [DOI] [PubMed] [Google Scholar]
- Khanna R, Tikku T, Wadhwa J. Nasomaxillary complex in size, position and orientation in surgically treated and untreated individuals with cleft lip and palate: a cephalometric overview. Indian J Plast Surg. 2012;45(1);68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarek KJ, Perry JP, Fang X. Morphology of the Levator Veli Palatini Muscle in Adults with Repaired Cleft Palate. J Craniofac Surg. 2017;28:833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn DP, Kahane JC. Histologic study of the normal human adult soft palate. Cleft Palate J. 1990;27:26–34. [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Moon JB. Histologic study of intravelar structures in normal human adult specimens. Cleft Palate Cranio J. 2005;42:481–489. [DOI] [PubMed] [Google Scholar]
- Law FE, Fulton JT. Unoperated oral clefts at maturation. Am J Public Health. 1959;49(11);1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JC, Hatabu H, Yousem DM, Randall P, Kressel HY. Evaluation of soft palate function with MRI: Application to the cleft palate patient. J Compt Assist Tomogr. 1992;16(6):877–882. [DOI] [PubMed] [Google Scholar]
- McKerns D, Bzoch KR. Variations in velopharyngeal valving: the factor of sex. Cleft Palate Cranio J. 1970;7;652–662. [PubMed] [Google Scholar]
- Moon JB, Smith AE, Folkins JW, Lemke JH, Gartlan M. Coordination of velopharyngeal muscle activity during positioning of the soft palate. Cleft Palate Craniofac J. 1994;31:45–55. [DOI] [PubMed] [Google Scholar]
- Mugler JP, Kiefer B, Brookeman JR. Three-dimensional T2-weighted imaging of the brain using very long spin-echo trans. Proc Int Soc Magn Reson Med. 2000;8:687. [Google Scholar]
- National Institutes of Health. Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. 2015; retrieved from https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html
- Nguyen DC, Kamlesh BP, Skolnick GB, Skladman R, Grames LM, Stahl MB, Marsh JL, Woo AS. Progressive tightening of the levator veli palatini muscle improves velopharyngeal dysfunction in early outcomes of primary palatoplasty. Plast Recontr Surg. 2015;136:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özgür F, Tunçbilek G, Cila A. Evaluation of velopharyngeal insufficiency with magnetic resonance imaging and nasoendoscopy. Ann Plast Surg. 2000;44:8–13. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP. Morphology of the levator veli palatini muscle using magnetic resonance imaging. Cleft Palate Cranio J. 2013;50:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Gamage JK. Sexual dimorphism of the levator veli palatini muscle: An imaging study. Cleft Palate Cranio J. 2014;51:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Gamage JK, Fang X. Anthropometric analysis of the velopharynx and related craniometric dimensions in three adult populations using MRI. Cleft Palate Cranio J. 2016;53:e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Goldwasser MS, Jerez AD. Craniometric and velopharyngeal assessment of infants with and without cleft palate. J Craniofac Surg. 2011;22:499–503. [DOI] [PubMed] [Google Scholar]
- Perry JL, Sutton BP, Kuehn DP, Gamage JK. Using MRI for assessing velopharyngeal structures and function. Cleft Palate Cranio J. 2014;51:476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portney LG Watkins MP. Foundations of clinical research: Application to practice. 2000; Prentice Hall: New Jersey. [Google Scholar]
- Reichert H Osteoplasty in complete clefts of the secondary palate. Br J Plast Surg. 1970;23:45–49. [DOI] [PubMed] [Google Scholar]
- Satoh K, Wada T, Tachimura T, Shiba R. The effect of growth of nasopharyngeal structures in velopharyngeal closure in patients with repaired cleft palate and controls without clefts: a cephalometric study. Br J Oral Maxillofac Surg. 2002;40:105–109. [DOI] [PubMed] [Google Scholar]
- Siemens AG. Syngo SPACE. Retrieved from https://usa.healthcare.siemens.com/magnetic-resonance-imaging/options-and-upgrades/clinical-applications/syngo-space [Google Scholar]
- Subtelny JD. A cephalometric study of the growth of the soft palate. Plast Reconstr Surg.1957;19:49–62. [DOI] [PubMed] [Google Scholar]
- Tian W, Yin H, Li Y, Zhao S, Zheng Q, Shi B. Magnetic resonance imaging of velopharyngeal structures in Chinese children after primary palatal repair. J Cranio Surg. 2010;21:568–577. [DOI] [PubMed] [Google Scholar]
- Wada T, Miyazaki T. Growth and changes in maxillary arch form in complete unilateral cleft lip and cleft palate children. Cleft Palate J. 1975;12:115–130. [PubMed] [Google Scholar]
- Wada T, Satoh T, Tachimura T, Tatsuta U. Comparison of nasopharyngeal growth between patients with clefts and noncleft controls. Cleft Palate Cranio J. 1997;34:405–409. [DOI] [PubMed] [Google Scholar]
- Zajac DJ, Cevidanes L, Shah S, Haley KL. Maxillary arch dimensions and spectral characteristics of children with cleft lip and palate who produce middorsum palatal stops. J Speech Hear Res. 2012;55:1876–1886. [DOI] [PubMed] [Google Scholar]


