Abstract
Background:
Parabens are synthetic preservatives present in many consumer products. Their antimicrobial and endocrine- disrupting properties have raised concerns that they might play a role in respiratory and allergic diseases; however, studies exploring these associations are scarce.
Objective:
We examined the cross-sectional association between parabens and asthma morbidity among 450 children with asthma and with asthma prevalence among 4023 children in the US general population participating in the National Health and Nutrition Examination Survey (2005–2014).
Methods:
We conducted multivariable logistic regression to examine associations between urinary paraben biomarker concentrations (butyl paraben, ethyl paraben, methyl paraben [MP], and propyl paraben [PP]) and asthma attacks and emergency department visits among children with asthma and with a current asthma diagnosis among all children. We also examined heterogeneity of associations by sex.
Results:
We observed an increased prevalence odds of reporting emergency department visits for every 10-fold increase in MP and PP concentrations among boys with asthma (adjusted prevalence odds ratio, 2.61 [95% CI, 1.40–4.85] and 2.18 [95% CI, 1.22–3.89, respectively; Pinteraction-MP = .002 and Pinteraction-PP = .003); associations remained after adjusting for other phenolic compounds previously linked to respiratory outcomes. No other dimorphic effects of exposure by sex were observed. Among children in the general population, no overall associations with current asthma were observed, although there was a positive trend with PP and a current asthma diagnosis. Conclusion: We identified differential effects of exposure to select parabens by sex on asthma morbidity. Further studies are needed to replicate these findings and elucidate mechanisms by which parabens could affect respiratory health and elicit dimorphic effects by sex.
Keywords: Parabens, children, asthma, respiratory, antimicrobials, endocrine disruptors
Parabens are synthetic preservatives widely used in personal care products, medications, and foods.1–4 In the United States exposure to parabens is widespread, with select parabens detected in more than 90% of the general population.5 The main route of exposure to parabens is considered to be dermal absorption from personal care product use, although other routes and sources of exposure are possible.3,6,7 Their widespread detection in the general population has raised concerns about their potential health risks given they are antimicrobial agents and endocrine- disrupting compounds (EDCs) exhibiting weak estrogenic and antiandrogenic activity.8–11 Of emerging concern is their potential effects on pediatric respiratory health given children’s developing immune and respiratory systems, and their unique vulnerabilities to environmental contaminants.
Results from limited in vivo and in vitro studies support the hypothesis that parabens could play a role in modulating immune and allergic responses.12–18 EDCs can influence immune cell activation and survival and modulate cytokine production, TH1/TH2 balance, and IgE production.12 In addition, it is plausible that the antimicrobial properties of parabens could promote an allergic phenotype by altering the microbiome of the gut, respiratory tract, or both.19–24
To date, few epidemiologic studies have examined the role of paraben exposure on the risk of pediatric respiratory and allergic disease, and findings have been inconsistent. In 2 cross-sectional studies conducted on children from the US general population participating in the 2005–2006 National Health and Nutrition Examination Survey (NHANES),25,26 exposure to select parabens was positively associated with aeroallergen sensitization, an important risk factor for development, morbidity, and severity of asthma and allergic diseases.25,27–30 Dimorphic effects of paraben exposure by sex on allergic sensitization have also been reported,31 although no consistent associations with asthma and wheeze have been identified.26,31 To our knowledge, no other studies have examined these associations or whether exposure to parabens is associated with worse asthma-related outcomes among asthmatic patients.
In this study, we sought to address current knowledge gaps and examine whether exposure to 4 parabens (butyl paraben [BP], ethyl paraben [EB], methyl paraben [MP], and propyl paraben [PP]) commonly used in consumer products is associated with increased morbidity (ie, increased prevalence odds of asthma attacks and emergency department [ED] visits for asthma) among children with asthma from a larger subset of the US general population participating in NHANES (2005–2014). We also examined the association between exposure to parabens and the prevalence of a current asthma diagnosis among all children. Lastly, we assessed whether the effect of paraben exposure on our outcomes varied by sex given reported sex differences with paraben exposure and risk of allergic sensitization.31
METHODS
Data source for the study population
Our study population consisted of children between the ages of 6 and 19 years participating in NHANES, a population-based, cross-sectional survey assessing the general health and nutritional status of the US noninstitutionalized population. NHANES uses a complex, stratified, multistage probability sample design to be representative of the general population. All study activities were approved by the National Center for Health Statistics (NCHS) institutional review board, and proper consenting procedures were followed prior to any data collection.32 Information on participants was collected through a household interview and a standardized physical examination.33 Paraben exposure measurements were conducted on a random one-third subsample of participants 6 years of age and older between 2005 and 2014.
Exposure assessment of parabens
Urinary biomarker concentrations for BP, EP, MP, and PP in spot urine samples provided by study participants were used to assess paraben exposure. Total concentrations (free plus conjugated species) for each of the 4 parabens were measured by using a validated laboratory method described previously.34 Limits of detection (LODs) were 1.0 μg/L (MP and EP) and 0.2 μg/L (PP and BP). Urinary creatinine concentrations were also measured and used in our analyses to correct for renal function.35
Respiratory outcome assessment
As part of a medical examination, participants or their caregivers completed a questionnaire that asked about several medical conditions. As part of this questionnaire, participants were asked the following: “Has a doctor or other health professional ever told you that you have asthma?” Participants who answered affirmatively to this question were then asked, “Do you still have asthma?,” which was hereafter referred to as “current asthma.” If participants reported having current asthma, they were then asked the following: “During the past 12 months, have you had an episode of asthma or an asthma attack?” (hereafter referred to as asthma attack[s]) and “During the past 12 months, have you had to visit an emergency room or urgent care center because of asthma?” (hereafter referred to as an ED visit for asthma). For our analyses, we focused on the following outcomes: (1) current asthma (yes/no) among all children and, among the subset of children with current asthma, whether the child experienced (2) asthma attacks (yes/no) or (3) had an ED visit or visits for asthma (yes/no). For current asthma, the comparison group was children who never received an asthma diagnosis or who reported formerly having asthma.
Of 4338 children aged 6 to 19 years with paraben biomarker data for cycle years 2005–2014, 4023 had complete data on current asthma diagnosis and main covariates, and among these 4023 children, 450 who reported having a current asthma diagnosis had complete data on asthma attacks, ED visits, and main covariates to assess morbidity (see Fig E1 in this article’s Online Repository at www.jacionloine.org).
Statistical analysis
All analyses were conducted in Stata 14.0 software (StataCorp, College Station, Tex). We applied NCHS-created sampling weights, strata, and primary sampling units in our statistical analyses according to NCHS guidelines, unless otherwise noted, to yield unbiased point estimates and to account for the complex, stratified, multistage probability sample design. We calculated descriptive statistics to summarize demographic characteristics and urinary biomarker concentrations (eg, numbers, detection frequencies [DFs], geometric means, weighted percentiles, and maximum concentrations). To assess whether there were any significant differences in demographic characteristics between children who reported having the target outcomes and those who did not, we conducted χ2 tests. We assessed differences in biomarker concentrations (for frequently detected parabens) and DFs (for less frequently detected parabens) based on outcome status and sex by conducting t tests and χ2 tests, respectively.
To examine associations between urinary paraben biomarker concentrations and each outcome of interest, we used logistic regression models to estimate crude prevalence odds ratios and adjusted prevalence odds ratios (aPORs) and corresponding 95% CIs. We constructed separate models for each paraben and each binary outcome of interest. Crude models included log10-transformed creatinine concentrations as a covariate to account for urinary dilution, whereas adjusted models included log10-transformed creatinine concentrations in addition to other covariates, as described below. Because of their low DFs (DF < 50%), both BP and EP were modeled as dichotomous independent variables (ie, < LOD vs ≥LOD). For MP and PP, we used restricted cubic splines with 3 df to assess the linearity of the dose-response relationship with each respiratory outcome by using log10-transformed urinary concentrations. None of the digressions from linearity tests were significant, and therefore we expressed MP and PP concentrations using log10-transformed concentrations in our models.
To increase the statistical power and precision of our effect estimates, we replaced MP and PP concentrations less than the LOD with LOD/√2.36,37 To assess effect modification by sex, we included a single multiplicative interaction term (sex*biomarker concentration) in separate adjusted models.
Our criteria for statistical significance were set at α levels of .05 and .10 for main effects and effect measure modification, respectively. Given that the outcome measures we assessed are not completely independent of one another and the exploratory nature of our study,38 we did not perform adjustment for multiple comparisons.
Covariates
In our adjusted models we controlled a priori for several covariates that were identified as potential confounders by using directed acyclic graphs (not shown) or that were expected to be strong predictors of our outcome measures. These covariates included, sex, age (years), race/ethnicity (non-Hispanic white, non-Hispanic Black, Mexican American, and “other,” which included those who self-identified as multiracial, Asian Pacific, or of other Hispanic descent), poverty income ratio (modeled as a continuous variable), and survey cycle year (2005–2006, 2007–2008, 2009–2010, 2011–2012, and 2013–2014). We excluded health insurance as a covariate in our models given that it was not a significant predictor of any of our outcomes in our study population.
Sensitivity analyses
We also conducted sensitivity analyses to examine the robustness of our results in the presence of other model adjustments. First, because tobacco smoke exposure from active or passive smoking has been strongly linked to respiratory outcomes, including asthma-related symptoms,39,40 we ran models with and without serum cotinine as a covariate for the subset of participants with available data. We considered addition of cotinine as a covariate as part of our sensitivity analyses rather than inclusion in our main models because data on this covariate were missing for up to 30% of our study population. Although there is increasing evidence that obesity might play a role in asthma diagnosis, control, and exacerbation severity,41 we did not include body mass index (BMI) in any of our primary models because it is a potential intermediate of an association between parabens and asthma.24,42,43 However, as part of our sensitivity analyses, we included age- and sex-standardized BMI z scores as a continuous covariate in our primary models. Because other phenolic compounds (eg, triclosan, bisphenol A, and 2,5-dichlorophenol) have been previously linked to respiratory outcomes, including asthma development and morbidity,44–49 we also ran models including log10-transformed urinary biomarker concentrations for each of these phenols as covariates to assess the independent effects of parabens on each of the target respiratory outcomes assessed. Lastly, to further examine and confirm dose-response relationships, we also categorized concentrations of frequently detected parabens into tertiles of exposure and reran the main logistic regression models when significant associations were observed with our continuous exposure measures.
RESULTS
Study population characteristics
Of the 4465 children with data on current asthma diagnosis, 4023 had data available on parabens and covariates included in our analyses. Weighted demographic characteristics for the 4023 children with complete data were similar to those of the larger population of children with available data on current asthma diagnosis (n = 4465; Table I). The mean age of children included in our analyses was 13.0 (SD, 4.0) years, and approximately 52% were male. More than half of the children were non-Hispanic white, and approximately 24% reported a household income of less than the poverty level.
TABLE I.
Weighted demographic characteristics for children 6 to 19 years of age from the US general population with data on current asthma (NHANES 2005–2014)
| Children with data on current asthma, regardless of paraben or covariate data available (n = 4465) | Children in the present study with data on parabens, current asthma, and covariates (n = 4023) | |||
|---|---|---|---|---|
| No. | Percent | No. | Percent | |
| Sex | ||||
| Boys | 2263 | 50.9 | 2068 | 51.6 |
| Girls | 2202 | 49.1 | 1955 | 48.4 |
| Age (y)* | ||||
| 6–11 | 2043 | 42.1 | 1832 | 42.7 |
| 12–19 | 2422 | 57.9 | 2191 | 57.3 |
| Race | ||||
| Non-Hispanic white | 1264 | 57.1 | 1159 | 58.0 |
| Non-Hispanic black | 1195 | 14.6 | 1101 | 14.7 |
| Mexican American | 1158 | 14.2 | 1032 | 13.9 |
| Other | 848 | 14.1 | 731 | 13.3 |
| Poverty income ratio | ||||
| <1.0 | 1371 | 23.8 | 1434 | 23.9 |
| ≥1.0 | 2786 | 76.2 | 2689 | 76.2 |
| Missing | 308 | — | ||
Mean age in years among the 4465 children with data on current asthma diagnosis was 12.6 (SD, 4.0) versus 12.5 (SD, 4.0) for the 4023 children in our present study with complete data on current asthma diagnosis, parabens, and covariates used in our analyses.
Prevalence of respiratory outcomes
The prevalence for each target outcome assessed is displayed in Table II. There were no significant sex or age group differences between children who reported having a current asthma diagnosis versus those who did not, and non-Hispanic black children were more likely to report having current asthma. Among the 450 children with a current asthma diagnosis, 233 (53.4%) reported having experienced an asthma attack, and 81 (16.2%) reported having an ED visit or visits in the prior 12 months. Among children with asthma, male subjects were more likely than female subjects to report having experienced asthma attacks in the prior 12 months, although these findings were not statistically significant. The prevalence of self-reported ED visits did not significantly differ by sex. Also, older children (12–19 years of age) with asthma were more likely to report an asthma attack in the prior 12 months compared with younger children; no age group differences were observed for ED visits.
TABLE II.
Prevalence of respiratory outcomes by demographic characteristics among children aged 6 to 19 years (NHANES 2005–2014)*
| All children, regardless of outcome status | Children with a current asthma diagnosis† | Children who reported ED visit(s) in the prior 12 mo† | Children who reported asthma attacks(s) in the prior 12 mo† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percent | No. | Percent | P value‡ | No. | Percent | P value‡ | No. | Percent | P value‡ | |
| All children | 4023 | — | 450 | 10.9 | — | 81 | 16.2 | — | 233 | 53.4 | — |
| Sex | |||||||||||
| Boys | 2068 | 51.6 | 248 | 51.6 | .99 | 46 | 47.6 | .58 | 135 | 55.4 | .21 |
| Girls | 1955 | 48.4 | 202 | 48.4 | 35 | 52.4 | 98 | 44.6 | |||
| Age (y) | |||||||||||
| 6–11 | 1832 | 42.7 | 206 | 41.2 | .57 | 52 | 52.1 | .17 | 119 | 47.0 | .03 |
| 12–19 | 2191 | 57.3 | 244 | 58.8 | 29 | 47.9 | 114 | 53.0 | |||
| Race | |||||||||||
| Non-Hispanic white | 1159 | 58.0 | 123 | 55.4 | <.001 | 16 | 47.6 | .27 | 71 | 57.6 | .22 |
| Non-Hispanic black | 1101 | 14.7 | 181 | 23.5 | 36 | 25.2 | 89 | 21.2 | |||
| Mexican American | 1032 | 13.9 | 75 | 9.8 | 10 | 9.6 | 31 | 8.3 | |||
| Other§ | 731 | 13.3 | 71 | 11.3 | 19 | 17.6 | 42 | 12.9 | |||
| Poverty income ratio | |||||||||||
| <1.0 | 1434 | 23.9 | 170 | 28.4 | .07 | 41 | 33.9 | .31 | 90 | 29.6 | .58 |
| ≥1.0 | 2689 | 76.2 | 280 | 71.6 | 40 | 66.1 | 143 | 70.4 | |||
Data presented are for children with complete data on current asthma diagnosis, parabens, and covariates (ie, age, sex, race/ethnicity, poverty income ratio, and creatinine concentrations). Values displayed are weighted to take into account the complex NHANES survey design.
Current asthma is defined as an affirmative response to the following 2 questions: “Has a doctor or other health professional ever told you that you have asthma?” and “Do you still have asthma?” ED visits are defined as affirmative responses to the following question: “During the past 12 months, have you had to visit an emergency room or urgent care center because of asthma?” Asthma attacks are defined as affirmative responses to the following question: “During the past 12 months, have you had an episode of asthma or an asthma attack?”
P values reported are from χ2 tests used to determine whether demographic characteristics differed based on outcome status.
The “other” category includes children who self-identified as multiracial or of Asian Pacific or other Hispanic descent.
Paraben biomarker concentrations
Summary statistics for paraben biomarker concentrations in our study population are displayed in Table III. Among the 4023 children with complete data on covariates, paraben biomarker measurements, and current asthma diagnosis, we observed that BP and EP were not widely detected (DF < 40%), whereas MP and PP were detected in more than 95% of children. Geometric mean concentrations for both MP and PP were significantly higher (P = .02 and P = .008, respectively) among children reporting a current asthma diagnosis compared with children with no current asthma diagnosis. Geometric mean concentrations for these frequently detected parabens were generally statistically significantly higher for female compared with male subjects, regardless of current asthma diagnosis status.
TABLE III.
Summary statistics for urinary paraben biomarker concentrations in children aged 6 to 19 years from the US general population (NHANES 2005–2014; in nanograms per milliliter)*
| No. | Percent detected | GM | Median (p25-p75) | p95 | Max | P value | |
|---|---|---|---|---|---|---|---|
| BP | |||||||
| All children | 4,023 | 36.1 | — | <LOD (<LOD-0.3) | 9.2 | 1,240 | |
| Male subjects | 2,068 | 24 | — | <LOD (<LOD-0.1) | 1.7 | 1,240 | <.001† |
| Female subjects | 1,955 | 49.1 | 0.4 | <LOD (<LOD-0.7) | 17.6 | 353 | |
| Children with asthma | 450 | 33.5 | — | <LOD (<LOD-0.3) | 19.6 | 1,240 | .35‡ |
| Male subjects with asthma | 248 | 24.4 | — | <LOD (<LOD-<LOD) | 1.9 | 1,240 | .004§ |
| Female subjects with asthma | 202 | 43.2 | 0.4 | <LOD (<LOD-0.5) | 24.5 | 90 | |
| Children with no asthma | 3,573 | 36.5 | — | <LOD (<LOD-0.3) | 8.3 | 493 | |
| Male subjects with no asthma | 1,820 | 24 | — | <LOD (<LOD-0.1) | 1.7 | 493 | <.001∥ |
| Female subjects with no asthma | 1,753 | 49.8 | 1.9 | <LOD (<LOD-0.8) | 14.5 | 353 | |
| EP | |||||||
| All children | 4,023 | 34.6 | — | <LOD (<LOD-1.9) | 34.5 | 1,981 | |
| Male subjects | 2,068 | 26.3 | — | <LOD (<LOD-1.0) | 10.7 | 1,981 | <.001† |
| Female subjects | 1,955 | 43.4 | 1.1 | <LOD (<LOD-3.2) | 60.8 | 1,760 | |
| Children with asthma | 450 | 38.3 | — | <LOD (<LOD-2.2) | 60.3 | 1,670 | .16‡ |
| Male subjects with asthma | 248 | 30 | — | <LOD (<LOD-1.3) | 34.5 | 1,110 | .005§ |
| Female subjects with asthma | 202 | 47.3 | 2.0 | <LOD (<LOD-3.4) | 63.3 | 1,670 | |
| Children with no asthma | 3,573 | 34.1 | — | <LOD (<LOD-1.8) | 31.3 | 1,981 | |
| Male subjects with no asthma | 1,820 | 25.9 | — | <LOD (<LOD-1.0) | 10.4 | 1,981 | <.001∥ |
| Female subjects with no asthma | 1,753 | 42.9 | 1.9 | <LOD (<LOD-3.2) | 60.8 | 1,760 | |
| MP | |||||||
| All children | 4,023 | 98.5 | 38.0 | 31.4 (10.3–136.0) | 868 | 149,000 | |
| Male subjects | 2,068 | 98.2 | 25.2 | 18.9 (7.8–66.5) | 654 | 149,000 | <.001¶ |
| Female subjects | 1,955 | 98.7 | 58.9 | 56.2 (17.0–209.0) | 1,000 | 132,000 | |
| Children with asthma | 450 | 99.5 | 49.4 | 41.2 (12.1–174.0) | 1,050 | 149,000 | .02# |
| Male subjects with asthma | 248 | 100 | 39.2 | 30.5 (9.2–107.0) | 1,170 | 149,000 | .07# |
| Female subjects with asthma | 202 | 99 | 63.2 | 64.0 (16.7–261.0) | 1,020 | 132,000 | |
| Children with no asthma | 3,573 | 93.3 | 36.8 | 30.1 (10.2–129.0) | 847 | 135,000 | |
| Male subjects with no asthma | 1,820 | 98.1 | 23.9 | 18.3 (7.7–63.8) | 621 | 135,000 | <.001¶ |
| Female subjects with no asthma | 1,753 | 100 | 58.4 | 55.3 (17.0–201.0) | 995 | 125,000 | |
| PP | |||||||
| All children | 4,023 | 95.2 | 4.7 | 3.7 (0.9–20.5) | 208 | 4,150 | |
| Male subjects | 2,068 | 93.3 | 2.6 | 1.9 (0.7–7.9) | 120 | 3,320 | <.001¶ |
| Female subjects | 1,955 | 97.2 | 8.7 | 8.3 (1.8–43.4) | 279 | 4,150 | |
| Children with asthma | 450 | 97.2 | 6.7 | 4.9 (1.2–34.2) | 283 | 2,650 | .008# |
| Male subjects with asthma | 248 | 95.9 | 3.9 | 2.5 (1.0–12.0) | 210 | 2,020 | <.001¶ |
| Female subjects with asthma | 202 | 98.5 | 11.7 | 11.6 (1.9–68.3) | 381 | 2,650 | |
| Children with no asthma | 3,573 | 94.9 | 4.5 | 3.5 (0.9–18.6) | 200 | 4,150 | |
| Male subjects with no asthma | 1,820 | 93 | 2.5 | 1.8 (0.6–7.6) | 100 | 3,320 | <.001¶ |
| Female subjects with no asthma | 1,753 | 97 | 8.4 | 8.0 (1.8–40.1) | 276 | 4,150 | |
GM, Geometric mean; <LOD, summary statistic value of less than the detection limit for the respective paraben; Max, maximum concentration observed.
LODs were 0.2 ng/mL (BP and PP) and 1.0 ng/mL (EP and MP). In accordance with US Centers for Disease Control and Prevention guidelines, a geometric mean is only reported when the DF is greater than 40% for the specified subgroup, and all statistics reported have been weighted to account for the complex NHANES survey design.
P value from χ2 test for differences in DF based on sex among all 4023 children.
P value from χ2 test for differences in DF based on current asthma status among all 4023 children.
P value from χ2 test for differences in DF based on sex among the 450 children with current asthma.
P value from χ2 test for differences in DF based on sex among the 3573 children without current asthma.
P values reported are from t tests examining whether paraben biomarker concentrations differed by sex among the specified subgroups (ie, differences in concentrations between boys and girls among those with asthma and differences in concentrations between boys and girls among those without asthma).
P values reported from t tests examining whether paraben biomarker concentrations among children without a current asthma diagnosis differed from those observed among children with a current asthma diagnosis.
Associations between paraben exposure and morbidity among children with asthma
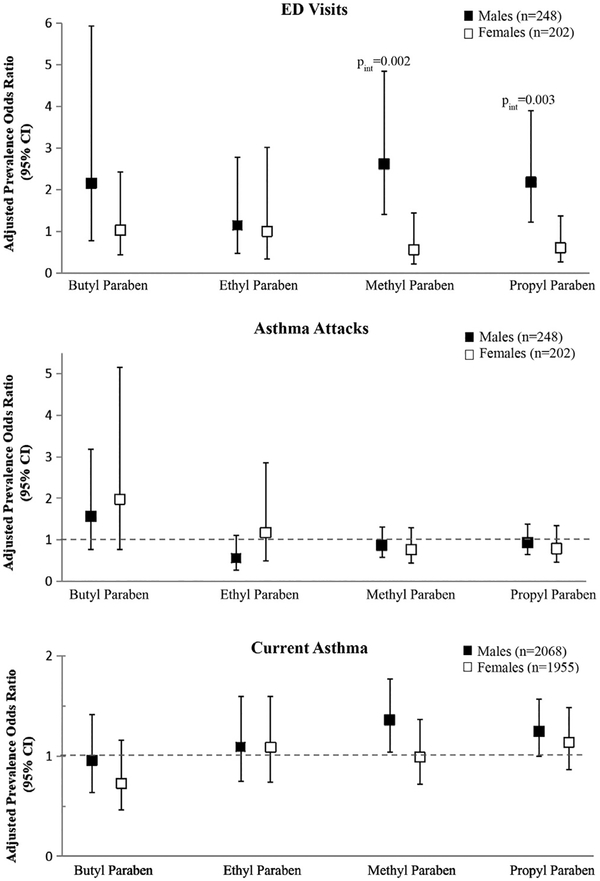
Among children with asthma, we did not observe overall associations between any of the parabens and reporting of asthma attacks or ED visits in the prior 12 months in either unadjusted or adjusted analyses (Table IV). However, we did observe effect modification by sex for ED visits for asthma in the prior 12 months for both MP and PP (Pint≤.003), with statistically significant positive associations observed among boys with asthma (Fig 1 and Table V). For every 10-fold increase in MP and PP concentrations, we observed a respective 2.61 (95% CI, 1.40–4.85; P =.003) and 2.18 (95% CI, 1.22–3.89; P = .01) increased prevalence odds of reporting an ED visit in the prior 12 months among boys with current asthma. We also observed a positive and statistically significant dose-response trend with ED visits among boys when using tertiles of exposure for both MP and PP (MP: aPORTertile2 of 3.02[95% CI, 0.99–9.16] and aPORTertile3 of 4.02 [95% CI, 1.35–11.94], Ptrend = .01; PP: aPORTertile2 of 2.34 [95% CI, 0.68–7.93] and aPORTertile3 of 6.56 [95% CI, 1.49–28.76], Ptrend = .01). No other dimorphic effects by sex were observed.
TABLE IV.
Associations of exposure to parabens with respiratory morbidity measures and current asthma prevalence among all children aged 6 to 19 years from the US general population, NHANES 2005–2014*
| cPOR | 95% CI | P value | aPOR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Morbidity | ||||||
| ED visits for asthma† (n = 450) | ||||||
| BP (<LOD vs ≥LOD) | 1.43 | 0.78–2.63 | .24 | 1.44 | 0.73–2.84 | .28 |
| EP (<LOD vs ≥LOD) | 1.07 | 0.56–2.05 | .84 | 1.04 | 0.52–2.22 | .85 |
| MP (log10, ng/mL) | 1.22 | 0.70–2.14 | .48 | 1.22 | 0.63–2.38 | .55 |
| PP (log10, ng/mL) | 1.10 | 0.72–1.66 | .66 | 1.15 | 0.64–2.07 | .64 |
| Asthma attacks† (n = 450) | ||||||
| BP (<LOD vs ≥LOD) | 1.52 | 0.86–2.68 | .15 | 1.78 | 0.94–3.39 | .08 |
| EP (<LOD vs ≥LOD) | 0.69 | 0.41–1.16 | .16 | 0.81 | 0.46–1.42 | .46 |
| MP (log10, ng/mL) | 0.76 | 0.55–1.05 | .09 | 0.81 | 0.55–1.18 | .26 |
| PP (log10, ng/mL) | 0.77 | 0.56–1.06 | .11 | 0.86 | 0.59–1.25 | .41 |
| Prevalence | ||||||
| Current asthma† (n = 4023) | ||||||
| BP (<LOD vs ≥LOD) | 0.82 | 0.63–1.08 | .15 | 0.83 | 0.62–1.10 | .19 |
| EP (<LOD vs ≥LOD) | 1.14 | 0.89–1.47 | .29 | 1.09 | 0.84–1.41 | .50 |
| MP (log10, ng/mL) | 1.21 | 1.01–1.45 | .04 | 1.17 | 0.94–1.46 | .15 |
| PP (log10, ng/mL) | 1.21 | 1.04–1.41 | .02‡ | 1.20 | 1.00–1.43 | .05 |
cPOR, Crude prevalence odds ratio.
Crude models were adjusted for log10 creatinine concentrations, and adjusted models were adjusted for age in years, sex (overall crude and adjusted models only), poverty income ratio (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, and “other,” which includes multiracial, Asian, and other Hispanic), survey cycle year (2005–2006, 2007–2008, 2009–2010, 2011–2012, and 2013–2014), and log10 creatinine concentrations.
ED visits are defined as affirmative responses to the following question: “During the past 12 months, have you had to visit an emergency room or urgent care center because of asthma?” Asthma attacks are defined as affirmative responses to the following question: “During the past 12 months, have you had an episode of asthma or an asthma attack?” Current asthma is defined as an affirmative response to the following 2 questions: “Has a doctor or other health professional ever told you that you have asthma?” and “Do you still have asthma?”
P < .05.
FIG 1.
Adjusted associations between children’s urinary paraben biomarker concentrations and respiratory outcomes by child’s sex. We modeled BP and EP exposure as a dichotomous variable (less than LOD vs LOD or greater), whereas we used log10-transformed MP and PP concentrations in models. Pint, P value on interaction term (sex*biomarker concentration). Only significant interaction P values of less than .10 are reported.
TABLE V.
Associations of exposure to parabens with respiratory morbidity measures among children with asthma and with current asthma prevalence stratified by sex*
| aPOR | 95% CI | P value | aPOR | 95% CI | P value | Pint value | |
|---|---|---|---|---|---|---|---|
| Morbidity | Boys (n = 248) | Girls (n = 202) | |||||
| ED visits for asthma† | |||||||
| BP (<LOD vs ≥LOD) | 2.16 | 0.79–5.93 | .13 | 1.03 | 0.44–2.42 | .95 | .28 |
| EP (<LOD vs ≥LOD) | 1.14 | 0.47–2.78 | .76 | 1.01 | 0.34–3.01 | .98 | .86 |
| MP (log10, ng/mL) | 2.61 | 1.40–4.85 | .003‡ | 0.57 | 0.23–1.44 | .23 | .002‡ |
| PP (log10, ng/mL) | 2.18 | 1.22–3.89 | .01‡ | 0.61 | 0.28–1.37 | .23 | .003‡ |
| Asthma attacks† | |||||||
| BP (<LOD vs ≥LOD) | 1.56 | 0.76–3.18 | .22 | 1.98 | 0.76–5.16 | .16 | .68 |
| EP (<LOD vs ≥LOD) | 0.55 | 0.27–1.11 | .09 | 1.18 | 0.49–2.86 | .71 | .19 |
| MP (log10, ng/mL) | 0.86 | 0.57–1.31 | .48 | 0.76 | 0.44–1.30 | .31 | .67 |
| PP (log10, ng/mL) | 0.94 | 0.64–1.38 | .74 | 0.79 | 0.46–1.34 | .37 | .52 |
| Prevalence | |||||||
| Current asthma† | Boys (n = 2068) | Girls (n = 1955) | |||||
| BP (<LOD vs ≥LOD) | 0.96 | 0.64–1.42 | .83 | 0.73 | 0.46–1.16 | .18 | .42 |
| EP (<LOD vs ≥LOD) | 1.09 | 0.75–1.60 | .64 | 1.09 | 0.74–1.60 | .66 | .98 |
| MP (log10, ng/mL) | 1.36 | 1.04–1.77 | .02‡ | 0.99 | 0.72–1.37 | .96 | .12 |
| PP (log10, ng/mL) | 1.25 | 1.00–1.57 | .05 | 1.14 | 0.87–1.49 | .33 | .58 |
cPOR, Crude prevalence odds ratio; Pint, P value for interaction term (sex*biomarker concentration term).
Adjusted models included age in years, poverty income ratio (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, and “other,” wich includes multiracial, Asian, and other Hispanic), survey cycle year (2005–2006, 2007–2008, 2009–2010, 2011–2012, and 2013–2014), and log10 creatinine concentrations as covariates.
ED visits are defined as affirmative responses to the following question: “During the past 12 months, have you had to visit an emergency room or urgent care center because of asthma?” Asthma attacks are defined as affirmative responses to the following question: “During the past 12 months, have you had an episode of asthma or an asthma attack?” Current asthma is defined as an affirmative response to the following 2 questions: “Has a doctor or other health professional ever told you that you have asthma?” and “Do you still have asthma?”
P < .05.
In sensitivity analyses inclusion of BMI z scores and concentrations of other phenolic compounds in our models did not materially affect our results, although inclusion of cotinine led to a significantly increased prevalence odds of asthma attacks among children with detectable concentrations of BP (aPOR, 2.64 [95% CI, 1.32–5.31], P = .01; see Table E1 in this article’s Online Repository at www.jacionloine.org). Lastly, similar to our main models, we observed an increased prevalence odds of ED visits among boys with asthma when controlling for cotinine, BMI z scores, or other phenolic compounds but no other dimorphic effects of paraben exposure by sex among children with asthma (see Tables E1–E3 in this article’s Online Repository at www.jacionloine.org).
Associations between paraben exposures and prevalence of current asthma diagnosis among all children in the general population
After adjustment for confounders, among all children, we did not observe significant associations between exposure to any parabens and self-report of current asthma diagnosis, although the relationship between PP and current asthma diagnosis approached statistical significance (aPOR, 1.20 [95% CI, 1.00–1.43]; P =.05). Interactions between paraben exposure and sex on current asthma diagnosis were not statistically significant (Fig 1 and Table V), and inclusion of other covariates in sensitivity analyses did not materially affect our results (see Tables E1–E3).
DISCUSSION
In this study, we examined cross-sectional associations between exposure to parabens and asthma-related outcomes in a sample of children from the US general population. We did not observe associations between any paraben and asthma prevalence or asthma morbidity in the population as a whole. However, we identified dimorphic effects of paraben exposure by sex among children with asthma. We found that exposure to both MP and PP was associated with increased prevalence odds of reporting ED visits for asthma in the prior 12 months among boys with asthma, despite boys having lower urinary paraben biomarker concentrations.
Although the association between paraben exposure and asthma morbidity has not been previously examined, sex differences in the association between paraben exposures and allergic sensitization have been reported, with male subjects generally at a greater risk than female subjects, including in a smaller subset of our study population.25,31 Sexual dimorphism has also been reported for pediatric asthma and for ED visits, with boys experiencing a greater asthma prevalence and ED visits for asthma exacerbations.50,51 In addition, sex differences have been reported for associations between other EDCs, including some compounds with antimicrobial properties and respiratory outcomes, with more prevalent effects observed among boys.52–54 Given that male subjects are at a greater risk of allergic disease in general,55 one plausible explanation for our findings with ED visits is that exposure to parabens could result in enhancement of the allergic response and increased susceptibility to adverse respiratory effects. The endocrine- disrupting actions of parabens could be more potent in boys given that their hormonal milieu is distinct from girls and existing evidence indicating that sex hormones and environmental agents with endocrine disrupting properties influence function and/or development of the lungs and the immune system.56–60 Additionally, the antimicrobial properties of parabens might have a greater influence on the risk of asthma exacerbations among boys because of their inherent phenotypic asthma features, including greater burden of atopy. It has also been suggested that potential sex differences in the risk of allergic and respiratory outcomes could be due to an interplay between the endocrine disrupting and antimicrobial properties of parabens, resulting from differences in microbiome composition, hormone function, and consequences of microbial interactions.31 However, more studies are needed to confirm our findings and elucidate the potential mechanisms by which parabens could result in dimorphic effects by sex.
Our results of no overall associations between exposure to parabens and current asthma diagnosis were similar to those reported in a previous study conducted by Spanier et al26 in which authors examined the association between exposure to parabens and ever having received an asthma diagnosis based on atopic status among 837 children aged 6 to 18 years participating in the 2005–2006 NHANES cycle. The authors reported no significant associations between exposure to parabens and increased risk of ever having received an asthma diagnosis, regardless of atopic status. Similarly, Lee-Sarwar et al31 also reported null associations between prenatal and early postnatal paraben biomarker concentrations in samples collected approximately between 2011 and 2013 with asthma and wheeze among 460 three-year-old children. Similar to our study, Spanier et al26 and Lee-Sarwar et al31 reported low detection of BP and EP in children’s urine. Geometric mean concentrations for MP and PP in our study were similar to those reported by Spanier et al26 in children participating in the 2005–2006 NHANES cycle (present study: MP, 38 ng/mL; PP, 4.7 ng/mL vs Spanier et al: MP, 42 ng/mL; PP, 5.3 ng/mL). However, median concentrations for MP and PP were lower in our study population compared to those reported among 3-year-olds by Lee-Sarwar et al31 (present study: MP, 31.4 ng/mL; PP, 3.7 ng/mL vs Lee-Sarwar et al: MP, 62.8 ng/mL; PP, 6.8 ng/mL). Differences in concentrations between our study and those reported by Lee-Sarwar et al might be related to study population characteristics, including age and racial/ethnic composition. Nonetheless, our results on asthma prevalence are in agreement with these prior studies.
The lack of consistency in associations between MP and PP and asthma outcomes could suggest spurious findings but could also be due to the fact that each of the 3 major outcomes we evaluated measures distinct manifestations of asthma. For example, current asthma indicates whether someone has the disease currently and does not capture the degree of symptoms or morbidity. Because exposures that can lead to an increased risk in asthma development can differ from those that lead to symptoms, exacerbations, or both among those with established asthma, as reported in prior studies,61–63 it is possible that MP and PP contribute to symptoms among those with disease, but do not contribute to risk of developing asthma. Additionally, an “asthma attack” is defined in NHANES as an affirmative response to the question “During the past 12 months, have you had an episode of asthma or an asthma attack?,” and affirmative responses to this question are much more common than affirmative responses to the question about ED visits. Thus, self-report of “asthma attacks” as queried by NHANES, could be measuring something different and milder than ED visits.
A limitation of this study is its cross-sectional design, limiting our ability to ascribe a direct cause-effect relationship between our exposures and outcomes. In addition, parabens are thought to be largely excreted within 24 hours.64 Biomarker concentrations could thus reflect recent rather than long-term exposures. Although some studies in adults65,66 suggest that a single spot sample might be sufficient to characterize exposure to select parabens over a period of a few months, reliance on a single urine sample for exposure assessment might have led to nondifferential exposure misclassification, potentially attenuating our results if paraben concentrations vary widely among children. While we adjusted for several important confounders, our analyses were also limited by the variables available in this national survey. For example, allergic sensitization is a significant risk factor for asthma development, morbidity, and severity that has been linked to paraben exposure; however, data on sensitization were not available for the cycle years assessed in our analyses. Lastly, it is possible that our findings with ED visits are not representative of the US population of children or are spurious findings based on the modest sample size for an analysis of complex survey design data.
While we examined associations between exposures to parabens and respiratory outcomes among children aged 6 to 19 years, we were not able to assess exposures among younger children or during the prenatal period because paraben biomarker data were unavailable so further studies are warranted to identify critical windows of susceptibility. Innate and adaptive immune responses are immature at birth and undergo constant development during the early postnatal period through the adolescent phase, making these stages vulnerable to environmental exposures, including EDCs.67,68 Although a recent study did not observe associations between prenatal or early postnatal exposure to parabens and pediatric asthma or wheeze during the preschool years,31 the study did not evaluate asthma among school-aged children. Thus, future studies should examine the effects of prenatal and early postnatal paraben exposures in children at different life stages.
Despite our study limitations, our study has several strengths. To our knowledge, this is the first study to examine the association between exposure to parabens and respiratory outcomes among children with asthma. We also conducted our analyses on a large sample of US children. In addition, the availability of cotinine and other phenolic compounds provided the opportunity to adjust for these exposures that have been previously associated with respiratory symptoms, and in general, associations observed remained or became stronger.
In summary, we observed differential effects of exposure to select parabens by sex on asthma morbidity, but did not observe associations between exposure to these parabens and the prevalence of current asthma. Given the cross-sectional study design, future studies are needed to replicate our findings and identify potential windows of susceptibility. Lastly, studies are needed to elucidate the mechanisms by which parabens could impact development, morbidity, or severity of respiratory outcomes, and elicit dimorphic effects by sex.
Supplementary Material
Clinical implications:
Urinary concentrations of the antimicrobial agents MP and PP were associated with ED visits among asthmatic boys. These findings warrant further study given the widespread use of parabens in consumer products.
Acknowledgments
L.Q.-A. was supported by a National Heart, Lung, and Blood Institute (NHLBI) Career Development Award (K01HL138124); N.N.H. was supported by the National Institute of Environmental Health Sciences (NIEHS; P50 ES018176, R01ES022607, and R01ES023500), the National Institute on Minority Health and Health Disparities (NIMHD; (P50MD010431), and the US Environmental Protection Agency (EPA; agreements 83615201, 83451001, and 83615001); M.C.M. was supported by the NIEHS (P50 ES018176 and R21 ES025840), NIMHD (P50MD010431), and EPA (agreement 83615201); and E.C.M. was supported by the National Institute of Allergy and Infectious Diseases (NIAID; K24AI114769) and NIEHS (R01ES023447 and R01ES026170). The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position or views of the National Institutes of Health or the EPA.
Abbreviations used
- aPOR
Adjusted prevalence odds ratio
- BMI
Body mass index
- BP
Butyl paraben
- DF
Detection frequency
- ED
Emergency department
- EDC
Endocrine disrupting compound
- EP
Ethyl paraben
- LOD
Limit of detection
- MP
Methyl paraben
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- PP
Propyl paraben
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). Parabens factsheet 2013. Available at: https://www.cdc.gov/biomonitoring/Parabens_BiomonitoringSummary.html. Accessed January 5, 2018.
- 2.Andersen FA. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol 2008;27(suppl 4):1–82. [DOI] [PubMed] [Google Scholar]
- 3.Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 2005;43:985–1015. [DOI] [PubMed] [Google Scholar]
- 4.Soni MG, Taylor SL, Greenberg NA, Burdock GA. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol 2002;40:1335–73. [DOI] [PubMed] [Google Scholar]
- 5.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect 2010;118:679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Hussein S, Muret P, Berard M, Makki S, Humbert P. Assessment of principal parabens used in cosmetics after their passage through human epidermis-dermis layers (ex-vivo study). Exp Dermatol 2007;16:830–6. [DOI] [PubMed] [Google Scholar]
- 7.Fisher M, MacPherson S, Braun JM, Hauser R, Walker M, Feeley M, et al. Paraben concentrations in maternal urine and breast milk and its association with personal care product use. Environ Sci Technol 2017;51:4009–17. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmacol 2007;221:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oishi S Effects of propyl paraben on the male reproductive system. Food Chem Toxicol 2002;40:1807–13. [DOI] [PubMed] [Google Scholar]
- 10.Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol 1998;153:12–9. [DOI] [PubMed] [Google Scholar]
- 11.Vo TT, Yoo YM, Choi KC, Jeung EB. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod Toxicol 2010;29: 306–16. [DOI] [PubMed] [Google Scholar]
- 12.Chalubinski M, Kowalski ML. Endocrine disrupters—potential modulators of the immune system and allergic response. Allergy 2006;61:1326–35. [DOI] [PubMed] [Google Scholar]
- 13.Balzano G, Fuschillo S, Melillo G, Bonini S. Asthma and sex hormones. Allergy 2001;56:13–20. [DOI] [PubMed] [Google Scholar]
- 14.Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol 2013;13:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canguven O, Albayrak S. Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypotheses 2011;76:585–8. [DOI] [PubMed] [Google Scholar]
- 16.Choi IS. Gender-specific asthma treatment. Allergy Asthma Immunol Res 2011; 3:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadre SK, Zein JG. Serum testosterone levels in children with asthma [abstract]. Am J Respir Crit Care Med 2017;195:A2201. [Google Scholar]
- 18.Laffont S, Blanquart E, Savignac M, Cenac C, Laverny G, Metzger D, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med 2017;214:1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014;384:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CH, Yang XQ, Liu CH, He Y, Wang LJ. Allergic airway response associated with the intestinal microflora disruption induced by antibiotic therapy. Zhonghua Er Ke Za Zhi 2007;45:450–4. [PubMed] [Google Scholar]
- 21.Singanayagam A, Ritchie AI, Johnston SL. Role of microbiome in the pathophysiology and disease course of asthma. Curr Opin Pulm Med 2017;23:41–7. [DOI] [PubMed] [Google Scholar]
- 22.Stiemsma LT, Turvey SE. Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin Immunol 2017;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor SL, Leong LEX, Choo JM, Wesselingh S, Yang IA, Upham JW, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol 2018;141: 94–103.e15. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Raikhel V, Gopalakrishnan K, Fernandez-Hernandez H, Lambertini L, Manservisi F, et al. Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome 2016;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savage JH, Matsui EC, Wood RA, Keet CA. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol 2012;130:453–60.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spanier AJ, Fausnight T, Camacho TF, Braun JM. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc 2014;35:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHugh BM, MacGinnitie AJ. Indoor allergen sensitization and the risk of asthma and eczema in children in Pittsburgh. Allergy Asthma Proc 2011;32: 372–6. [DOI] [PubMed] [Google Scholar]
- 28.Leung R, Ho P, Lam CW, Lai CK. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol 1997;99:594–9. [DOI] [PubMed] [Google Scholar]
- 29.Lombardi C, Savi E, Ridolo E, Passalacqua G, Canonica GW. Is allergic sensitization relevant in severe asthma? Which allergens may be culprit? World Allergy Organ J 2017;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Visness CM, Calatroni A, Gergen PJ, Mitchell HE, Sampson HA. Effect of environmental allergen sensitization on asthma morbidity in inner-city asthmatic children. Clin Exp Allergy 2009;39:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee-Sarwar K, Hauser R, Calafat AM, Ye X, O’Connor GT, Sandel M, et al. Prenatal and early-life triclosan and paraben exposure and allergic outcomes. J Allergy Clin Immunol 2018;142:269–78.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1 2013;(56):1–37. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC). About the National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed January 5, 2018.
- 34.Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction- high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2006;844:53–9. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey data, protocols, and analytic guidelines. Available at: http://www.cdc.gov/nchs/nhanes.htm. Accessed January 5, 2018.
- 36.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. J Appl Occup Environ Hyg 1990;5:46–51. [Google Scholar]
- 37.Cole SR, Chu H, Nie L, Schisterman EF. Estimating the odds ratio when exposure has a limit of detection. Int J Epidemiol 2009;38:1674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] [Google Scholar]
- 39.Sturm JJ, Yeatts K, Loomis D. Effects of tobacco smoke exposure on asthma prevalence and medical care use in North Carolina middle school children. Am J Public Health 2004;94:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.USEPA. Respiratory Health Effects of Passive Smoking: Lung Cancer and Other disorders. Washington (DC): US Environmental Protection Agency; 1992. EPA publication no. 600/6–90/006F. [Google Scholar]
- 41.Mohanan S, Tapp H, McWilliams A, Dulin M. Obesity and asthma: pathophysiology and implications for diagnosis and management in primary care. Exp Biol Med (Maywood) 2014;239:1531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho Y, Shore SA. Obesity, asthma, and the microbiome. Physiology (Bethesda) 2016;31:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quirόs-Alcalá L, Buckley JP, Boyle M. Parabens and measures of adiposity among adults and children from the U.S. general population: NHANES 2007–2014. Int J Hyg Environ Health 2018;221:652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol 2013;131:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jerschow E, McGinn AP, de Vos G, Vernon N, Jariwala S, Hudes G, et al. Dichlorophenol-containing pesticides and allergies: results from the US National Health and Nutrition Examination Survey 2005–2006. Ann Allergy Asthma Immunol 2012;109:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jerschow E, Parikh P, McGinn AP, de Vos G, Jariwala S, Hudes G, et al. Relationship between urine dichlorophenol levels and asthma morbidity. Ann Allergy Asthma Immunol 2014;112:511–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie MY, Ni H, Zhao DS, Wen LY, Li KS, Yang HH, et al. Exposure to bisphenol A and the development of asthma: a systematic review of cohort studies. Reprod Toxicol 2016;65:224–9. [DOI] [PubMed] [Google Scholar]
- 48.Hauptman M, Phipatanakul W, Jhun I, Petty MA, Gold DR, Savage JR. Urinary triclosan levels and asthma exacerbations in inner-city school children [abstract]. J Allergy Clin Immunol 2016;137:AB192. [Google Scholar]
- 49.Savage JH, Johns CB, Hauser R, Litonjua AA. Urinary triclosan levels and recent asthma exacerbations. Ann Allergy Asthma Immunol 2014;112:179–81.e2. [DOI] [PubMed] [Google Scholar]
- 50.Sears MR, Burrows B, Flannery EM, Herbison GP, Holdaway MD. Atopy in childhood. I. Gender and allergen related risks for development of hay fever and asthma. Clin Exp Allergy 1993;23:941–8. [DOI] [PubMed] [Google Scholar]
- 51.Nath JB, Hsia RY. Children’s emergency department use for asthma, 2001–2010. Acad Pediatr 2015;15:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ku HY, Su PH, Wen HJ, Sun HL, Wang CJ, Chen HY, et al. Prenatal and postnatal exposure to phthalate esters and asthma: a 9-year follow-up study of a Taiwanese birth cohort. PLoS One 2015;10:e0123309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Habib MR, Karim MR. Antimicrobial and cytotoxic activity of Di-(2-ethylhexyl) phthalate and anhydrosophoradiol-3-acetate isolated from Calotropis gigantea (Linn.) flower. Mycobiology 2009;37:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buckley JP, Quirόs-Alcalá L, Teitelbaum SL, Calafat AM, Wolff MS, Engel SM. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7 years. Environ Int 2018;115:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alfardan AS, Nadeem A, Ahmad SF, Al-Harbi NO, Al-Harbi MM, AlSharari SD. Plasticizer, di(2-ethylhexyl)phthalate (DEHP) enhances cockroach allergen extract-driven airway inflammation by enhancing pulmonary Th2 as well as Th17 immune responses in mice. Environ Res 2018;164:327–39. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed SA. The immune system as a potential target for environmental estrogens (endocrine disruptors): a new emerging field. Toxicology 2000;150:191–206. [DOI] [PubMed] [Google Scholar]
- 57.Card JW, Zeldin DC. Hormonal influences on lung function and response to environmental agents: lessons from animal models of respiratory disease. Proc Am Thorac Soc 2009;6:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeWitt C, Patisaul HB. Endocrine disruptors and the developing immune system. Curr Opinion Toxicol 2017. [Google Scholar]
- 59.Kuo CH, Yang SN, Kuo PL, Hung CH. Immunomodulatory effects of environmental endocrine disrupting chemicals. Kaohsiung J Med Sci 2012;28(suppl): S37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller MD, Marty MA. Impact of environmental chemicals on lung development. Environ Health Perspect 2010;118:1155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol 2018;141:1468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol 2014;134: 593–601.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol 2013;132:830–5, e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moos RK, Angerer J, Dierkes G, Bruning T, Koch HM. Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Arch Toxicol 2016;90:2699–709. [DOI] [PubMed] [Google Scholar]
- 65.Dewalque L, Pirard C, Vandepaer S, Charlier C. Temporal variability of urinary concentrations of phthalate metabolites, parabens and benzophenone-3 in a Belgian adult population. Environ Res 2015;142:414–23. [DOI] [PubMed] [Google Scholar]
- 66.Pollack AZ, Perkins NJ, Sjaarda L, Mumford SL, Kannan K, Philippat C, et al. Variability and exposure classification of urinary phenol and paraben metabolite concentrations in reproductive-aged women. Environ Res 2016; 151:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holladay SD, Smialowicz RJ. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect 2000;108(suppl 3):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lisciandro JG, van den Biggelaar AH. Neonatal immune function and inflammatory illnesses in later life: lessons to be learnt from the developing world? Clin Exp Allergy 2010;40:1719–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.