Abstract
The global epidemic of obesity has led to an increasing number of obese women of reproductive age. Obesity is associated with reduced fertility, and pregnancies complicated by maternal obesity are associated with adverse outcomes, including increased risk of gestational diabetes, pre-eclampsia, preterm birth, instrumental and caesarean births, infections, and post-partum haemorrhage. The medical and obstetric management of obese women is focused on identifying, addressing, and preventing some of these associated complications, and is a daunting challenge given the high percentage of patients with obesity and few therapeutic options proven to improve outcomes in this population. The UK’s National Institute for Health and Care Excellence guidelines and the American College of Obstetricians and Gynecologists recommend that all pregnant women follow a healthy diet, and consider at least half an hour of moderate physical activity per day during pregnancy. However, although obese women are often directed to seek the advice of a nutritionist and to limit gestational weight gain, guidelines for the management of pregnancy and delivery in this high-risk group are lacking. The post-partum period represents an important opportunity to optimise maternal health before the next pregnancy. As many of the physiological changes of pregnancy associated with maternal obesity are present from early pregnancy onward, reducing maternal obesity before conception is probably the best strategy to decrease the health burden associated with maternal obesity.
Introduction
Maternal obesity, a nutritional and metabolic life-course condition, has become an important public health problem, with consequences to the health and wellbeing of both mother and child (table 1).1–4
Table 1:
Hazards of maternal obesity
| Risk to mother | Risk to offspring | |
|---|---|---|
| Before conception and ante partum | Reduced fertility; pregnancy loss | .. |
| Intra partum | Obstructive sleep apnoea; gestational diabetes; pre-eclampsia; gastro-oesophageal reflux disease; gestational hypertension; preterm delivery | Congenital anomalies; small for gestational age; large for gestational age and macrosomia; preterm delivery |
| Peri partum | Operative delivery; caesarean delivery; failed vaginal birth after caesarean delivery; endometritis; wound disruption | Fetal distress; adiposity; neonatal birth injuries |
| Post partum | Post-partum depression; venous thromboembolism; dyslipidaemia; hypertension; type 2 diabetes; osteoarthritis; obstructive sleep apnoea; malignancies (eg, breast, endometrial, liver, colon, haematological) | Less likely to be breastfed; increased adiposity |
| Long-term impact on offspring4 | .. | Obesity; diabetes; hypertension; dyslipidaemia; autism spectrum disorder; cognitive abnormality |
Traditionally considered a problem of high-income countries, maternal obesity has received greater attention in low-income and middle-income countries (LMICs) in recent years. A systematic review and meta-analysis of population-based cohort studies done in LMICs documents a double burden: maternal underweight is associated with higher risk of preterm, low birthweight, and small for gestational age (SGA) birth, and by contrast, maternal obesity increases the risk of gestational diabetes, pregnancy-induced hypertension, pre-eclampsia, caesarean delivery, and post-partum haemorrhage.5,6 In 2008, there were almost three overweight or obese women of childbearing age for each underweight woman in LMICs,7 which raises the question of whether maternal overweight is now more important than maternal underweight in influencing the long-term chronic disease burden in these countries.8 Table 2 classifies maternal overweight and obesity according to maternal BMI.
Table 2:
Classification of maternal BMI and cutoffs for definition of maternal obesity
| BMI in kg/m2 | |
|---|---|
| Underweight | <18.5 |
| Normal | 18.5–24.9 |
| Overweight | 25–29.9 |
| Obese class I | 30–34.9 |
| Obese class II | 35–39.9 |
| Obese class III | ≥40 |
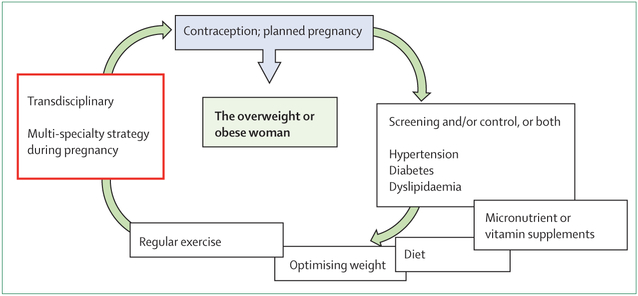
The optimal clinical management of the obese pregnant woman involves care beyond that afforded to the non-obese pregnant woman. Ideally, care should be provided before, during, and after the pregnancy (figure). Although we believe that detailing routine obstetric management is beyond the scope of this Review, we aim to address issues that are of particular concern for the obstetric management of obese women. These issues include preconception weight management to improve metabolic health and fertility and decrease early pregnancy loss, as well as screening for medical problems such as diabetes. In early pregnancy, setting guidelines for gestational weight gain through healthy eating and physical activity, and screening for fetal structural defects is warranted. In mid-pregnancy, screening for obstetric conditions, such as gestational diabetes and pre-eclampsia, needs to be initiated in all pregnancies. As gestation advances, preparation for delivery should include assessment for fetal overgrowth, timing of delivery, anaesthesia consultation, and assurance that the delivery suite is appropriately staffed and equipped. Intra-partum decisions regarding the course of labour, caesarean delivery, and prophylactic antibiotics should be addressed. Post-partum attention in women who are obese should be directed to the increased risk of venous thromboembolism, difficulty with lactation, contraception, and depression. In addition to the need for obstetric and medical expertise, very real practical challenges exist in managing pregnancies in this ever-increasing population, including involving occupational health-care professionals in care delivery, and various logistical challenges regarding the availability of appropriate facilities and equipment that could be needed in providing care to this patient group.
Figure: Optimising care for the overweight or obese woman: before conception, during, and after pregnancy.
Optimal care for women who are obese or overweight should involve a transdisciplinary and multi-specialty care approach, and should not be limited to managing the complications that arise during pregnancy. Recognising this concept is key to improving long-term outcomes for both mother and offspring. This multidisciplinary approach should include both medical (eg, hypertension, diabetes, dyslipidaemia) and social determinants of health (eg, diet, exercise, pregnancy planning).
Nutrition, lifestyle modification, and management of gestational weight gain
Weight gain is an essential aspect of pregnancy, but excessive gestational weight gain is associated with adverse pregnancy outcomes. Based on population-based studies, weight gain in pregnant adults is inversely related to maternal pregravid BMI.9 An essential physiological weight gain during pregnancy is necessary for a healthy pregnancy, and consists of approximately 8 kg of water (increased plasma volume and amniotic fluid), 1 kg of protein (maternal, placental, and fetal lean mass) plus variable amounts (1–6 kg) of adipose tissue.10 The increases in fat mass in normal weight pregnant women are required to meet the increased energy needs of late pregnancy and lactation. Estimates of adiposity in pregnancy should be based on pre-pregnancy measures of height and weight. However, with advancing gestation, BMI becomes a poorer estimate of maternal adiposity11 because of increased water and fetoplacental components of gestational weight gain. On the basis of data published in 2016, excess gestational weight gain beyond Institute of Medicine (IOM) guidelines in obese women will result in increased adipose rather than lean body mass weight accrual.12 Furthermore, because of the differences in body composition between various ethnic groups, WHO has discussed, but not endorsed, using lower criteria (increased risk for type 2 diabetes and cardiovascular disease at BMI between 22 kg/m2 and ≤25 kg/m2, and high risk at BMI between >25 kg/m2 and 31 kg/m2) for the classification of obesity in Asian women.13 These differences in classification are reflected by the higher prevalence of gestational diabetes at a relatively low BMI for Asian populations than people of European descent, and should be considered in clinical practice.14 Additionally, the association between maternal hyperglycaemia and adverse outcomes appears to differ between ethnic groups, with one study proposing different glycaemic thresholds for diagnosis of gestational diabetes in south Asian people.15
In 2009, the IOM revised the gestational weight guidelines on the basis of the increased incidence of obesity in women of reproductive age (table 3).10 Because obese women enter pregnancy with excess adipose tissue, the recommendation was to primarily meet the obligatory accrual of water and protein. Approximately 50–60% of obese pregnant women gain in excess of the IOM recommendations.16 Although some authors have suggested that less weight gain than the current IOM recommendations for obese women could improve some perinatal outcomes,17 inadequate gestational weight gain in obese women might have detrimental effects, including an increased risk of SGA infants,18 lower birthweight and fat mass, and potentially of greater consequence, a decrease in lean body mass, length, and head circumference.19 In general, inadequate weight gain and gestational weight loss should be avoided for obese women.
Table 3:
US Institute of Medicine recommendations for gestational weight gain depending on the before pregnancy BMI
| BMI (kg/m2;WHO criteria) | Range of total weight gain (kg) | Rates of weight gain* in second and third trimesters (mean [range] in kg per week) | |
|---|---|---|---|
| Underweight | <18.5 | 12.7–18.1 | 0.45 (0.45–0.59) |
| Normal weight | 18.5–24.9 | 11.3–15.9 | 0.45 (0.36–0.45) |
| Overweight | 25.0–29.9 | 6.8–11.3 | 0.27 (0.23–0.32) |
| Obese (includes all classes) | ≥30.0 | 5.0–9.1 | 0.23 (0.18–0.27) |
These calculations assume a weight gain of 0.5–2.0 kg in the first trimester.
Although both maternal obesity and excess gestational weight gain in a mother of normal pre-gestational BMI are associated with adverse pregnancy outcomes, obstetric management of obese mothers tends to focus on those who enter pregnancy being obese, whereas those who enter pregnancy at a healthy BMI but gain excess weight during gestation might not be as readily identified. Greater awareness of the hazards of excess gestational weight gain and regular review of gestational weight with advancing gestation could help to address this issue. In the UK, weight is often not routinely measured during pregnancy.20 The use of electronic health records with plotting of gestational weight gain relative to IOM guidelines at each antenatal visit is being considered at many centres; although, a randomised controlled trial (RCT)21 suggested that just repeatedly measuring weight without adequate guidance or support for appropriate gestational weight gain might not suffice.
Various interventions, including diet, lifestyle, and drugs such as metformin, have been assessed with the aim to prevent or reduce obesity or excessive weight gain in pregnancy, and improve maternal and fetal outcomes. The National Institute for Health and Care Excellence (NICE) in the UK, as well as the American College of Obstetricians and Gynecologists (ACOG), recommend a healthy diet and at least half an hour of moderate physical activity per day during pregnancy. WHO defines a healthy diet as one in which the proportion of energy intake from total fat is less than 30%, preferably from unsaturated fats rather than saturated fats, with a fruit and vegetable intake of at least 400 g per day, and at least 25 g of dietary fibre each day.22 In addition to these general recommendations, guidelines issued by the International Federation of Gynecology and Obstetrics (FIGO) have highlighted the need to recognise maternal micronutrient deficiencies (eg, iron, iodine, folate, vitamin B12, calcium, and vitamin D) during all pregnancies, and to address these deficiencies through interventions including dietary diversity, consumption of fortified foods, and supplementation, as appropriate.23 Appropriate nutritional advice for obese pregnant mothers should also take into account cultural issues and diversity, and the fact that no single diet fits all is important to note. Because of the effect of obesity on the distribution and metabolism of folate, obese women can benefit from higher doses of folate supplements (5 mg per day, from at least 1 month before conception and continuing during the first trimester).23
A systematic review on lifestyle interventions to manage weight during pregnancy found a significant reduction in gestational weight gain (mean difference −1.42 kg; 95% CI −0.95 to −1.89 kg; I2=80%) compared with the control group, with some suggestion of improved pregnancy outcomes for pre-eclampsia, shoulder dystocia, gestational hypertension, and preterm birth.24 However, two large-scale RCTs, LIMIT25 and UPBEAT,26 reported modest or no improvements in pregnancy outcomes, such as frequency of gestational diabetes or large for gestational age (LGA) infants, despite improvements in maternal diet and physical activity, highlighting the fact that initiating lifestyle changes during the second and third trimester of pregnancy could be too late to alter the course of pregnancy outcomes.1 The results of the Finnish Gestational Diabetes Prevention Study (RADIEL),27 of 269 obese women with a history of gestational diabetes, found that improvements in physical activity and dietary quality reduced gestational diabetes incidence, but again had no effect on maternal (eg, pre-eclampsia, gestational hypertension) or neonatal (eg, birthweight) outcomes. Interventions that are initiated before conception or during the first trimester might be more effective.27–30 Further discussions of the effect of lifestyle interventions during pregnancy can be found in a comment accompanying this Series.31
Diagnosis and management of gestational diabetes and diabetes in pregnancy
Diagnosis of gestational diabetes is increased in women with class I obesity (BMI 30–34.9 kg/m2; odds ratio [OR] 2.6, 95% CI 2.1–3.4) and class II obesity (BMI ≥35 kg/m2; OR 4.0, 3.1–5.2) compared with women with a BMI less than 30 kg/m2.32 Hyperglycaemia in women with gestational diabetes is a result of an inadequate insulin response relative to decreased insulin sensitivity. In general, obese women have decreased insulin sensitivity before and during pregnancy compared with normal weight women, which partly explains the increased incidence of gestational diabetes in this population.
An international consensus regarding the process and criteria for diagnosis of gestational diabetes remains elusive, although the updated WHO criteria33 are the most widely accepted worldwide. Table 4 shows the major approaches for screening and diagnosis. One point of agreement is that all obese women should be tested. Screening for gestational diabetes is generally recommended at 24–28 weeks’ gestation, and screening for both gestational diabetes and overt diabetes in early gestation should be considered among obese women, women with impaired glucose tolerance, or those with a history of gestational diabetes. The need to detect pre-existing diabetes in pregnancy is highlighted in guidelines from the UK, Europe, USA, and WHO (table 4).34–37 Whether screening or management of gestational diabetes in early pregnancy is of clinical value or is cost-effective is unclear;38 however, studies using the Gestational Diabetes Formulas for Cost-Effectiveness (GeDiForCE) model suggest that standard screening (between the second and third trimester) and treatment is highly cost-effective in both an Indian and Israeli setting, especially if long-term effects are taken into account.39 Studies to examine the benefit of early gestational diabetes diagnosis and treatment are underway (eg, , , and ).
Table 4:
Recommended testing for gestational diabetes in obese pregnant women
| Early pregnancy testing | Standard gestational diabetes test (second to third trimester) | Criteria for diagnosing gestational diabetes | |
|---|---|---|---|
| WHO* (global) | Yes. Diabetes and gestational diabetes | 75 g OGTT | Any of the following: fasting glucose concentration ≥5.1 mmol/L; 1 h OGTT concentration ≥10.0 mmol/L; 2 h OGTT ≥8.5 mmol/L |
| ACOG* (USA) | Yes in presence of risk factors, including maternal BMI ≥30 kg/m2, history of gestational diabetes | Two step: non-fasting 50 g glucose challenge test, followed by 100 g OGTT if glucose challenge test ≥7.8 mmol/L | Fasting glucose concentration ≥5.3 mmol/L; for OGTT: 1 h glucose ≥10.0 mmol/L; 2 h glucose ≥8.6 mmol/L; 3 h glucose ≥7.8 mmol/L (two values ≥ threshold for diagnosis) |
| ADA (USA) | Not specified | WHO or ACOG approach* | WHO or ACOG criteria* |
| Endocrine Society (USA) | Yes. Aimed at detection of overt diabetes | 75 g OGTT | Any of the following: fasting glucose concentration ≥5.1 mmol/L; 1 h OGTT ≥10.0 mmol/L; 2 h OGTT ≥8.5 mmol/L |
| NICE (UK) | Only if previous gestational diabetes | 75 g OGTT | Either of these values: fasting glucose concentration ≥5.6 mmol/L; 2 h OGTT ≥7.8 mmol/L |
| EBCOG (Europe) | Yes. Aimed at detection of overt diabetes | Either WHO or ACOG approach* | Fasting glucose concentration* ≥5.1 mmol/L; 1 h OGTT ≥10.0 mmol/L; 2 h OGTT ≥8.5 mmol/L |
OGTT=oral glucose tolerance test. ACOG=American College of Obstetrics and Gynecology. ADA=American Diabetes Association. NICE=National Institute for Health and Care Excellence. EBCOG=European Board and College of Obstetrics and Gynaecology.
Using the approach or criteria defined by WHO and ACOG.
Hyperglycaemia in pregnancy requires immediate action for its control and for the detection of possible chronic complications.36,40 The primary maternal management outcome in all women with gestational diabetes is to maintain normoglycaemia. However, little evidence exists to support key accepted practices in the treatment of gestational diabetes, and even less to support specific treatment regimens in obese women with the disease. Clinical practice varies substantially within and between countries partly because of the scarcity of the evidence base.36,40 For example, glibenclamide remains the recommended oral drug in the USA,36 whereas metformin appears to be much more commonly prescribed in the UK.34 Normalisation of maternal glucose in pregnancies complicated by gestational diabetes has been shown to decrease the incidence of pre-eclampsia and prevent neonatal outcomes such as stillbirth and fetal over-growth.41,42 No evidence exists with regard to the recommended weight gain specific to pregnancies complicated by diabetes. However, although insulin resistance is a common feature, maternal obesity and gestational diabetes have both independent and additive effects on maternal and neonatal outcomes,43 and thus the specific contribution of each is difficult to disentangle.
Two large RCTs41,42 and a subsequent systematic review44 demonstrated that in women with gestational diabetes, interventions based primarily on dietary modification (with addition of insulin therapy if glycaemic goals were not achieved) reduced the prevalence of LGA infants, preeclampsia, and shoulder dystocia. A secondary analysis of one study45 reported that reduction in excess fetal growth was related to maternal BMI, with greater reductions reported in women in the overweight and obese class I and II BMI categories and no reduction in women of normal weight or with class III obesity. However, long-term follow-up of these studies has not shown any benefit terms of the reduction of childhood obesity.46,47 A multicentre cohort study from Japan48 reported lower rates of LGA infants in obese women than in normal weight or overweight women with gestational diabetes treated by dietary intervention with or without insulin. However, hypertensive disorders, caesarean section, and induction of labour were more common in the obese group than the normal weight group.
Optimising maternal nutrition is the cornerstone for good maternal and offspring outcomes, although this approach becomes even more important in the presence of maternal obesity or gestational diabetes, for which medical nutritional therapy is the first-line treatment.23,40 Information regarding the ideal diet for treatment of gestational diabetes has been reviewed in detail.49 Comparisons of low versus high glycaemic index (GI) diets50–52 or a low GI versus a conventional high fibre, moderate GI diet53 showed no differences in LGA infants, macrosomia, or other key outcomes, although Moses and colleagues52 reported that a low GI diet might reduce the need for insulin therapy. Other dietary options, including energy restriction,54,55 low carbohydrate intake,56 and high monounsaturated fat intake,57 have also not shown specific benefits. A general recommendation for medical nutritional therapy in gestational diabetes is a “carbohydrate-controlled meal plan that promotes adequate nutrition with appropriate weight gain, normoglycaemia, and the absence of ketosis”,58 although data to provide evidence-based recommendations for most of the nutrition interventions are insuffcient.40
Despite not being specifically outlined as a therapy intervention in the two RCTs previously mentioned,41,42 exercise is frequently recommended as a mainstay of treatment in gestational diabetes for pregnant women, with a general recommendation of 30 min of exercise per day.36,40,59,60 But again, evidence is sparse, with the few published clinical trials focusing on glycaemic control rather than on pregnancy outcomes.61–63
Insulin was the only drug used in these two RCTs,41,42 and is clearly the most established drug in women with gestational diabetes who do not meet glycaemic goals after lifestyle modification. The potential use of the oral antidiabetic drugs glibenclamide and metformin in gestational diabetes has been systematically reviewed.64 Glibenclamide was reported to increase risks of LGA infants, macrosomia, and neonatal hypoglycaemia compared with insulin.65–67 In a retrospective analysis of a population-based cohort of 110 879 women with gestational diabetes from a nationwide insurance database from the USA, newborn babies of mothers treated with glibenclamide had an increased risk for neonatal intensive care unit admissions, as well as respiratory distress, hypoglycaemia, birth injury, and LGA infants compared with those treated with insulin.68 Metformin was associated with lower maternal weight gain, more preterm births, less pregnancy-induced hypertension, and less severe neonatal hypoglycaemia compared with insulin.69–73 The retrospective analysis concluded that glibenclamide was clearly inferior to both insulin and metformin in the treatment of gestational diabetes.68 Detailed studies of antidiabetic drugs to inform future clinical practice would be extremely valuable.
Surveillance of congenital malformation
Offspring of obese mothers have an increased risk of congenital anomalies including neural tube defects and cardiovascular anomalies compared with offspring born to mothers of a normal weight.74 The underlying metabolic factors that account for this increased risk are not well understood. Although maternal folate status and preconception glucose intolerance have been primary theories, no definitive mechanism has been identified.75 A multidisciplinary approach before conception is therefore necessary, since many women do not initiate antenatal care until after fetal organogenesis is completed.31
No general guidelines are available on the use of ultrasound during pregnancy for most parts of the world, and local practice often varies depending on available resources and reimbursement policies. In our opinion, all obese patients should be considered to have a detailed fetal anatomy ultrasound scan to screen for anomalies in the mid-second trimester. Routine ultrasound detects 46.2% of structural anomalies with a normal karyotype, but detection rates decreased significantly with an increasing maternal BMI (p=0.007).76 The odds of detection of any anomaly were lower in obese women than those with a normal BMI (adjusted OR 0.77, 95% CI 0.60–0.99; p=0.046).76 Factors associated with the decreased ability to diagnose congenital anomalies include distance from the skin surface to the fetus, resolution or penetration of sonographic equipment, prolonged time to complete the examination, and experience of the sonographer.77 Potential ways to optimise image quality include a vaginal approach in the first trimester, use of the maternal umbilicus as an acoustic window, tissue harmonic imaging, compound imaging, and speckle reduction filters.77,78 Accuracy of fetal weight estimates using ultrasound increases with advancing gestation and is greater in women with a BMI less than 25 kg/m2.79 Referral of obese women to tertiary centres for expert scans might be helpful, if available.
Different options of screening for aneuploidy are now available,80 and first trimester screening in all pregnancies includes nuchal translucency measurement, serum-free β-human chorionic gonadotrophin, or total human chorionic gonadotrophin coupled with pregnancy-associated plasma protein A levels, although cell-free DNA (cfDNA) screening is increasingly being used.80 Obesity affects the measures of serum analytes used to screen for aneuploidies because of the increased plasma volume in obese women. Although weight adjustment for analytes related to neural tube defects and trisomy 18 improves detection, this is not the case for Down’s syndrome.81 In a general population with prenatal testing for trisomies 21 and 18, the false-positive rate with cfNDA was significantly lower than with standard screening. The positive predictive value for cfDNA was also better for trisomy 21 and trisomy 18.80
Coexisting medical problems
Obese women should be screened for pre-existing hypertension and proteinuria at their initial antenatal visit, which is recommended for all women. An appropriate size of arm cuff should be used and the cuff size used should be documented in the medical records.20 Women with suspected obstructive sleep apnoea (snoring, excessive daytime sleepiness, witnessed apnoea, or unexplained hypoxia) should be referred for assess ment.82 Obstructive sleep apnoea can be a problem at delivery because of the potential complications associated with anaesthesia with caesarean delivery. Non-alcoholic fatty liver disease is the most common liver disorder in developed countries, usually presenting as elevated liver function tests.83 This diagnosis is important because unless recognised early in gestation, abnormal liver function tests later in gestation might be confused with complications of pre-eclampsia—ie, HELLP syndrome (haemolysis, elevated liver enzyme scores, and low platelet counts).
The risk of pre-eclampsia is twice as high in pregnant women who are obese compared with those of healthy weight.84 Although no proven methods to prevent preeclampsia are known, avoiding excessive gestational weight gain and tight control of diabetes in obese women could be beneficial.24 WHO has recommended that women at a high risk of pre-eclampsia (including those with previous pre-eclampsia, diabetes, or obesity) from areas with low dietary calcium intake should take calcium supplements during pregnancy, and low-dose aspirin at 75 mg per day should be considered for prophylaxis.85 Obese women are at increased risk of venous thromboembolism,86 and thus thromboprophylaxis assessment should be done in obese pregnant women at clinical encounters throughout the pregnancy and after. Although detailed description of the management of different obstetric complications is beyond the scope of this Review, it is important to note that obstetric complications are more common in obese women, and need to be screened for with greater vigilance, and managed according to established guidelines.
Preterm delivery
Indicated preterm deliveries in overweight and obese women are more common (relative risk [RR] 1.30, 95% CI 1.23–1.37) than in women of normal weight87 because of ante-partum complications. Many studies do not differentiate between indicated and spontaneous preterm delivery, but this distinction is important because of the 2.7 times increased risk of spontaneous preterm birth between 22 weeks and 27 weeks of gestation88 in women with a BMI of 40 kg/m2 or more. The use of progesterone supplementation has been shown to decrease the risk of preterm delivery in women with risk factors (such as previous preterm delivery or short cervical length). A secondary analysis of the Maternal Fetal Medicine Units’ trial of 17-hydroxy-progesterone caproate showed a non-significant increased risk of preterm birth (RR 1.55, 95% CI 0.93–2.89) in women with a BMI over 30 kg/m2.89
Labour and clinical issues of delivery
In pregnant women who are obese, the timing, method of delivery, and the peri-partum management plan should be discussed in light of any other obesity-related obstetric complications, such as gestational diabetes and macrosomia.90 Because of the multiple medical and obstetric problems associated with obesity and pregnancy, scheduled induction of labour is common in obese women. In addition to potential maternal conditions such as pre-eclampsia and gestational diabetes, fetal conditions such as LGA are an additional potential indication for induction of labour. Induction of labour for suspected LGA infants does not increase the risk of caesarean delivery, but reduces the risk of shoulder dystocia and associated morbidity compared with expectant management.91 Unfortunately, the ability of ultrasound to accurately estimate fetal weight at the upper limits of fetal growth is poor, and the reported accuracy range is between 47% and 64%.92 Mothers should be informed about the increased risks involved with induction of labour, including caesarean section, decreased ability to monitor the fetal heart-rate pattern (which reflects fetal metabolic status), wound infection, and primary post-partum haemorrhage.
Infants of obese women are at an increased risk for fetal macrosomia, specifically increased body fat compared with infants of normal weight women.93,94 Although the assessment of macrosomia by clinical examination or ultrasound-based estimates of fetal weight lacks precision, prophylactic caesarean section can be considered for suspected fetal macrosomia with estimated fetal weight greater than 5 kg in women without diabetes or greater than 4.5 kg in women with diabetes to avoid obstructed labour.95
Obese women have an increased risk of stillbirth, shoulder dystocia, peri-partum or post-partum haemorrhage, and of late complications including pelvic floor prolapse and fistulae. The length of labour in nulliparous women is also correlated to maternal BMI;96 in a study adjusting for appropriate covariates, the median duration of labour from 4 cm to 10 cm was significantly longer in obese women compared with women of normal weight (7.5 h vs 6.2 h).97 However, the second stage of labour was not different between normal, overweight, and obese women. The underlying cause of increased length of labour in obese women is unknown.
30% of obese women are at an increased risk of a prolonged pregnancy (>290 days) compared with 17% of normal weight women, and obese women have an increased rate of labour induction.98 Induction failure rates are associated with increasing obesity from 13% in normal weight women to 29% in class III obese women.99 In one meta-analysis, the unadjusted OR of caesarean delivery was 2.05 (95% CI 1.86–2.27) for women with class I obesity, and 2.89 (2.28–3.79) for women with class II or III obesity compared with normal weight women.100 However, studies also support elective induction at term instead of expectant management in those presenting with anticipated LGA neonates.91,101 Nonetheless, the association between induced labour and higher rates of emergency caesarean delivery should be discussed with the mother before elective induction of labour at term.
In view of a high risk of either elective or emergency caesarean delivery, obese women, especially those with obstructive sleep apnoea, should have an anaesthetic assessment in late gestation. Epidural or spinal anaesthesia is recommended, although this procedure might be technically difficult in an obese woman.
Delivery during subsequent pregnancies is also complicated by maternal obesity. Success rates of vaginal births after previous caesarean delivery are inversely related to BMI: less than 19.8 kg/m2 (83.1%), 19.8–26 kg/m2 (79.9%), 26.1–29 kg/m2 (69.3%), and more than 29 kg/m2 (68.2%; p<0.001). Similarly, gestational weight gain exceeding 18 kg is associated with a decreased success rate of vaginal birth after caesarean or trial of labour after caesarean (66.8% vs 79.1%, p<0.001).102 BMI class III women undergoing labour after caesarean had greater composite morbidity (prolonged hospital stay, endometritis, rupture or dehiscence, and neonatal injury including fractures, brachial plexus injuries, and lacerations) compared with class III women having elective caesarean delivery, but absolute morbidities were low in both groups.103 Class III obese women have a significantly increased risk of post-partum atonic haemorrhage (>1000 mL) after a vaginal delivery (5.2%) compared with normal weight women (4.4%), and it is more pronounced after instrumental delivery (13.6%) compared with normal weight women (8.8%).104
Women with a BMI of 45 kg/m2 or more have a two to four times increased risk of postoperative wound infection.105 The choice of skin incision for primary caesarean section in class II and III obese women remains controversial. Although one study reported that a vertical skin incision was associated with a higher rate of wound complications than a transverse incision,106 a multicentre registry study suggested that vertical skin incision was associated with a lower wound complication rate in class III obese patients after adjustment for confounding factors.107 To prevent postoperative wound complications, closure of the subcutaneous layer is recommended when the fat layer is more than 2 cm.108 Placement of a subcutaneous drain is not recommended.109
The standard for prophylactic antibiotics after delivery is 1 g of cefazolin to patients with a BMI less than 30 kg/m2, but 2 g for those with a BMI of 30 kg/m2 or more. The regimen was supported by a study showing adequate antibiotic concentrations (above the minimum inhibitory concentration) in adipose tissue.110 Clinical practice guidelines from Canada, for example, recommend that clinicians consider doubling the antibiotic dose in the setting of a maternal BMI more than 35 kg/m2.111
Finally, given the aforementioned risks, consideration should be made for proper equipment, facilities, and staff for the care and safety of obese pregnant women in labour. These include, but are not limited to, large chairs, blood pressure measuring devices, and birthing beds capable of supporting 200–300 kg or more. Similarly, because of the increased need for caesarean delivery among obese pregnant women, operating tables should accommodate weights up to 400 kg, yet allow surgeons ready access to the operating field. Other considerations for the care of obese woman include doors that are wide enough to rapidly move patients, power lifts to move patients from the bed to the operating table, and appropriately sized operating instruments to access proper tissue planes.
Management of peri-partum complications
Obese women remain at increased risk of complications after delivery. Because of the increased risk of venous thromboembolism, pneumatic compression stockings before and after caesarean delivery should be considered in obese post-partum women, in addition to early ambulation. Weight-adjusted postnatal thromboprophylaxis is now recommended by the Royal College of Obstetrics and Gynaecology for all women with class III obesity, and all other obese women with one or more additional risk factors—eg, advanced maternal age, history of caesarean section, preterm delivery, and post-partum haemorrhage. The duration of thromboprophylaxis with low-molecular-weight heparin should be extended if there are more than three risk factors, or if the risk factors are persistent.86
Successful breastfeeding in obese women has multiple barriers, including the physical issues of large breast size and increased risk of caesarean delivery. The need for neonates of obese women to be assessed in the intensive care nursuries represents an additional barrier to breast-feeding compared with normal weight women. Obese post-partum women can also have a decrease in their first phase of milk production. Despite these obstacles, breastfeeding should be encouraged in obese women, not only because of the potential benefits to neonates, but also potential maternal benefits relating to post-partum weight reduction.112 This process can be facilitated through appropriate specialist advice and support during the ante-natal and postnatal period to help overcome difficulties with breastfeeding, including early termination.20
During pregnancy, prevalence of depression was 33% among obese women and 23% among normal weight women. Post-partum depression was present in 13% of obese women compared with 10% in women of normal weight.113 ACOG recommends that all pregnant women should be screened at least once for depression in the post-partum period. When clinically significant depression is diagnosed the care provider should either initiate treatment or refer the patient as needed.114
The increased pregnancy complications associated with obesity are a harbinger of future metabolic dysfunction in these women. Weight gain in excess of the IOM guidelines, which occurs in 63% of class I obese women,10,115 is a key risk factor for post-partum weight retention.116 In turn, weight gain increases the risk of future metabolic dysfunction and increases pregravid obesity with future pregnancies. Of note, although gestational weight gain and pregravid obesity are interrelated, only increased pregravid obesity is associated with early termination of breastfeeding.117
Post-partum management
The prevention of long-term complications associated with maternal obesity can start in the post-partum period (panel). This period represents an important window of opportunity to encourage overweight and obese women to lose weight at a time when they are usually motivated. The UK NICE guidelines recommend that the 6–8 week postnatal check should be used as an opportunity to discuss the woman’s weight and treatment strategies.118 In a systematic review and meta-analysis of strategies to encourage weight loss in post-partum women, subgroup analysis demonstrated that the most effective interventions in reducing weight were exercise programmes with objectively defined goals, such as the use of a heart rate monitor or pedometer, and exercise programmes coupled with intensive dietary intervention.119
Weight control interventions can be implemented post partum even during lactation. The IOM Subcommittee on Nutrition During Lactation stated that losing as much as 2 kg per month did not affect milk volume, but daily energy intake should not be restricted to less than 1800 kcal.120 Further studies demonstrated that such weight loss can be safely achieved in lactating women who are overweight by restricting the usual energy intake by 500 kcal per day and by doing aerobic exercise 4 days per week.121 The safety and feasibility of these recommendations is also supported by systematic reviews.119,122
A lack of appropriately designed, high-quality studies on weight management in women before pregnancy or between pregnancies was noted in a meta-analysis of interventions to reduce obesity, although data suggest that dietary and physical activity counselling before conception or between pregnancies reduces of gestational weight gain.123 The ACOG recommends that nutrition and exercise counselling should continue post partum and before attempting another pregnancy.90,124
As offspring of obese mothers or mothers with excessive gestational weight gain are at high risk of excessive weight gain in childhood,125 post-partum counselling of mothers should also highlight the need to monitor the child’s health and to encourage a healthy lifestyle for the family. Maternal post-partum weight gain is also an independent predictor of overweight in preschool children.126
Prevention through treatment of obesity before conception
One of the best ways to optimise outcomes in pregnancies in obese mothers is to reduce obesity before conceiving for the first time, or before conceiving again. This approach is due in part to the fact that obese women have increased lipogenesis and inflammation, along with altered maternal or placental function starting in the first trimester, meaning that lifestyle interventions initiated later in pregnancy are rather too late to be effective.1 Lifestyle modification remains the cornerstone of obesity management in all people, and NICE guidelines suggest that women with BMI of 25 kg/m2 or more lose weight before becoming pregnant, at a rate not exceeding 0.5–1 kg per week.118 Additionally, obese women who require fertility interventions are encouraged to reduce bodyweight by at least 5%, a weight reduction associated with substantial improvement in cardiometabolic risk factors, insulin sensitivity, ovulation, and improved success of assisted reproductive technology.90,127
Bariatric or metabolic surgery is currently the most effective approach for sustained weight reduction, although careful selection of appropriate surgical candidates is important to ensure optimal outcome. Successful weight loss after bariatric surgery also has benefits during pregnancy. A systematic review of 75 studies that examined pregnancy outcome following bariatric surgery suggested that the rate of many adverse maternal and fetal outcomes was lower in pregnant women who had undergone bariatric surgery compared with those who had not.128 Recommendations from ACOG suggest that women should avoid conceiving within 18 months of bariatric surgery to avoid pregnancy at a time of rapid weight loss.129 Although the optimal gap for conception after bariatric surgery is still not clear,124 pregnancy is probably best deferred until bodyweight has stabilised after surgery. Obese women might be at risk of nutritional deficiencies including iron, vitamin B12, folate, vitamin D, and calcium, and this risk is of particular concern in women after bariatric surgery, who should be assessed for the need to take supplements. Furthermore, women who have had gastric banding might need adjustment of the band during pregnancy.124
Areas for further research and perspective on prevention
Although obesity is currently defined by an individual’s BMI, the important role of body fat distribution is increasingly recognised, with subcutaneous adiposity considered a different phenotype compared with visceral adiposity.130 The term metabolically healthy obesity has been coined to describe individuals whose obesity appears to differ from traditional obesity.131 The relationship between metabolically healthy obesity and pregnancy outcome and management is not well defined and should be evaluated in future studies.
In terms of establishing optimal weight gain during pregnancy, RCTs are needed to determine whether compliance with IOM recommendations for weight gain improves maternal and fetal outcomes. The global International Weight Management in Pregnancy (i-WIP) collaborative network is undertaking an individual patient data meta-analysis to assess whether various dietary and physical activity interventions have differential effects based on parity, BMI, and pre-existing medical con ditions.132 Future trials should also incorporate modern technologies to help empower behavioural change; a meta-analysis of lifestyle interventions to limit post-partum weight retention noted that few trials used modern technology, such as text messages and apps.133 Large RCTs are also required to assess the effects of nutritional supplements such as probiotics in the prevention of gestational diabetes, and to compare different pharmacological treatments for gestational diabetes.
This Review has highlighted the challenges to clinical management of the obese pregnant mother. Given the global epidemic of obesity, the effect of maternal obesity on reproductive health and public health is alarming. As discussed here and in another Review in this Series, maternal obesity is associated with adverse long-term consequences in the offspring through multiple mechanisms, and early action is needed to break this putative intergenerational passage of risk to subsequent generations. The pregnancy and inter-pregnancy periods are increasingly recognised to represent an important window of opportunity for health-care education and prevention of non-communicable diseases, with several international organisations including WHO, FIGO, and the International Diabetes Federation (IDF), all recognising the importance of these periods to reduce risk of non-communicable diseases in subsequent generations.23,40,134,135 However, treatment of obesity or prevention of obesity in the preconceptional and post-partum periods (in preparation for future pregnancies) might be an even better strategy.
Given the global scale of the problem and the need for effective treatment on a community-wide level, lifestyle modification will remain the mainstay of treatment and prevention of obesity, with surgery reserved for a highly selective subgroup in settings with appropriate expertise and resources. Public education regarding the hazards of maternal obesity is important, but needs to be coupled with action to reduce the obesogenic environment as well as research on how best to implement behavioural modification on a large scale. The focus needs to shift from simply delivering health education to mobilising communities to empower young women towards better health before conception. Improved integration of maternal and child health, along with primary care services and education, is key to tackling many of the current problems. For example, although care in the post-partum period is often focused on the neonate, this period also provides an important opportunity to provide education for the mother on healthy lifestyle choices and contraception, and to ensure that post-partum glycaemic evaluation is performed for women diagnosed with gestational diabetes.136 Given the high-default rate for postnatal oral glucose tolerance test, glycated haemoglobin has been proposed as an alternative for postnatal glycaemic evaluation.34,137 Sub stantial changes in health-care organisation and care delivery models will be required to better integrate the different disciplines such as obstetric, paediatric, medical, and primary care that contribute to postnatal health of both mother and child. More details on public health aspects of obesity prevention before conception and examples of community programmes can be found in other reviews in this Series.3,31 The ability to address these issues, and to implement a transdisciplinary approach involving a variety of health-care providers, including nutritionists, exercise physiologists, and behavioural scientists, will be key to efforts to combat maternal obesity and its long-term effect on non-communicable diseases and other associated complications.
Panel: Summary of management of overweight and obese women before and through pregnancy.
Counselling before conception
Discuss reduced fertility and risks of pregnancy associated with obesity
Advise on lifestyle interventions and goals for weight loss
Refer for nutritional counseling
Folic acid supplementation
Assess for surgical interventions in selected cases
Antenatal care
Record weight, height, and BMI and advise on appropriate targets of gestational weight gain
Counsel on nutrition and physical activity to promote healthy behaviours
Assess social determinants of health
Monitor blood pressure
Screen for gestational diabetes
Screen for obstructive sleep apnoea
Perinatal care
Require obstetric and anaesthesiology expertise
Anaesthesia consultation
Prescribe prophylactic antibiotics for caesarean delivery
Postnatal care
Deep vein thrombosis prophylaxis with caesarean delivery
Encourage breastfeeding
Vigilance for post-partum complications
Post-partum oral glucose tolerance test in patients with gestational diabetes
Education regarding inter-pregnancy weight loss
Education regarding long-term risk in mother and offspring and empowering behavioural modification
Advise on contraception
Search strategy and selection criteria.
We searched MEDLINE (2000–16) for English language papers using the search terms “maternal obesity” and “management” or “treatment” or “epidemiology” or “bariatric surgery” in combination with the terms “offspring” or “outcome”. The date of the last search was Aug 10, 2016. We mainly selected publications in the past 5 years, but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. We cited review articles and book chapters to provide readers with more details and more references than possible in this Review. We modified the reference list further according to suggestions from reviewers.
Acknowledgments
RCWM acknowledges support from the Research Grants Council General Research Fund (CU471713, CU14110415), the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly Collaborative Research Programme, and the Research Grants Council Theme-based Research Scheme (T12–402/13-N).
PMC acknowledges support from the Eunice Kennedy Shriver National Institute of Child Health and Development (HD 11089).
Footnotes
Declaration of interests
HDM reports personal fees from Novo Nordisk, AstraZeneca Australia, and Eli Lilly Australia, outside the submitted work. All other authors declare no competing interests.
References
- 1.Catalano P, de Mouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond) 2015; 39: 642–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma RCW, Gluckman PD, Hanson MA. Maternal obesity and developmental priming of risk In: Mahmood T, Arulkumaran S, eds. Obesity: a ticking time bomb for reproductive health, 1st edn. London: Elsevier, 2013: 193–212. [Google Scholar]
- 3.Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences.Lancet Diabetes Endocrinol 2016; published online Oct 12. 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol 2016; published online Oct 12. 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman MM, Abe SK, Kanda M, et al. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Obes Rev 2015; 16: 758–70. [DOI] [PubMed] [Google Scholar]
- 6.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16: 621–38. [DOI] [PubMed] [Google Scholar]
- 7.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382: 427–51. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Obesity: preventing and managing the global epidemic-report of a WHO consultation WHO technical report series. Geneva: World Health Organization, 2000: 1–268. [PubMed] [Google Scholar]
- 9.Chu SY, Callaghan WM, Bish CL, D’Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: fueling future obesity. Am J Obstet Gynecol 2009;200: 271. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen KM, Yaktine AL; Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press, 2009. [PubMed] [Google Scholar]
- 11.Sewell MF, Huston-Presley L, Amini SB, Catalano PM. Body mass index: a true indicator of body fat in obese gravidas. J Reprod Med 2007; 52: 907–11. [PubMed] [Google Scholar]
- 12.Berggren EK, Groh-Wargo S, Presley L, Hauguel-de Mouzon S, Catalano PM. Maternal fat, but not lean, mass is increased among overweight/obese women with excess gestational weight gain. Am J Obstet Gynecol 2016; 214: 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–63. [DOI] [PubMed] [Google Scholar]
- 14.Hedderson M, Ehrlich S, Sridhar S, et al. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012; 35: 1492–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrar D, Fairley L, Santorelli G, et al. Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: analysis of data from the Born in Bradford cohort. Lancet Diabetes Endocrinol 2015; 3: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deputy NP, Sharma AJ, Kim SY. Gestational weight gain—United States, 2012 and 2013. MMWR Morb Mortal Wkly Rep 2015; 64: 1215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artal R, Lockwood CJ, Brown HL. Weight gain recommendations in pregnancy and the obesity epidemic. Obstet Gynecol 2010;115: 152–55. [DOI] [PubMed] [Google Scholar]
- 18.Kapadia MZ, Park CK, Beyene J, et al. Weight loss instead of weight gain within the guidelines in obese women during pregnancy:a systematic review and meta-analyses of maternal and infant outcomes. PLoS One 2015; 10: e0132650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catalano PM, Mele L, Landon MB, et al. Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol 2014; 211: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modder J, Fitzsimons KJ. CMACE/RCOG joint guideline-management of women with obesity in pregnancy. London: Centre for Maternal and Child Enquiries and the Royal College of Obstetricians and Gynaecologists, 2010. [Google Scholar]
- 21.Brownfoot FC, Davey MA, Kornman L. Routine weighing to reduce excessive antenatal weight gain: a randomised controlled trial. BJOG 2016; 123: 254–61. [DOI] [PubMed] [Google Scholar]
- 22.WHO and Food and Agriculture Organization of the United States. Diet, nutrition and the prevention of chronic diseases WHO technical report series. Geneva: World Health Organization, 2003. [PubMed] [Google Scholar]
- 23.Hanson MA, Bardsley A, De-Regil LM, et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int J Gynaecol Obstet 2015; 131 (suppl 4): S213. [DOI] [PubMed] [Google Scholar]
- 24.Thangaratinam S, Rogozinska E, Jolly K, et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess 2012; 16:1–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodd JM, Turnbull D, McPhee AJ, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014; 348: 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015; 3: 767–77. [DOI] [PubMed] [Google Scholar]
- 27.Koivusalo SB, Rono K, Klemetti MM, et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish Gestational Diabetes Prevention Study (RADIEL): a randomized controlled trial. Diabetes Care 2016; 39: 24–30. [DOI] [PubMed] [Google Scholar]
- 28.Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ 2012; 345: e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simmons D, Jelsma JG, Galjaard S, et al. Results from a European multicenter randomized trial of physical activity and/or healthy eating to reduce the risk of gestational diabetes mellitus:the DALI lifestyle pilot. Diabetes Care 2015; 38: 1650–56. [DOI] [PubMed] [Google Scholar]
- 30.Song C, Li J, Leng J, Ma RC, Yang X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obes Rev 2016; published online July 15. DOI: 10.1111/obr.12442. [DOI] [PubMed] [Google Scholar]
- 31.Hanson M, Barker M, Dodd JM, et al. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol 2016; published online Oct 12. 10.1016/S2213-8587(16)30108-5. [DOI] [PubMed] [Google Scholar]
- 32.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol 2004; 190: 1091–97. [DOI] [PubMed] [Google Scholar]
- 33.Colagiuri S, Falavigna M, Agarwal MM, et al. Strategies for implementing the WHO diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Diabetes Res Clin Pract 2014; 103: 364–72. [DOI] [PubMed] [Google Scholar]
- 34.NICE. Diabetes in pregnancy: management of diabetes and its compliations from preconception to the postnatal period NICE guideline 3. London: National Institute for Health and Care Excellence, National Collaborating Centre for Women’s and Children’s health, 2015. [PubMed] [Google Scholar]
- 35.Benhalima K, Mathieu C, Damm P, et al. A proposal for the use of uniform diagnostic criteria for gestational diabetes in Europe:an opinion paper by the European Board & College of Obstetrics and Gynaecology (EBCOG). Diabetologia 2015; 58: 1422–29. [DOI] [PubMed] [Google Scholar]
- 36.Committee on Practice Bulletins—Obstetrics. Practice bulletin number 137: gestational diabetes. Obstet Gynecol 2013; 122: 406–16. [DOI] [PubMed] [Google Scholar]
- 37.WHO. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva: World Health Organization, 2013. [PubMed] [Google Scholar]
- 38.Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marseille E, Lohse N, Jiwani A, et al. The cost-effectiveness of gestational diabetes screening including prevention of type 2 diabetes: application of a new model in India and Israel. J Matern Fetal Neonatal Med 2013; 26: 802–10. [DOI] [PubMed] [Google Scholar]
- 40.Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 2015; 131 (suppl 3): S173. [DOI] [PubMed] [Google Scholar]
- 41.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005; 352: 2477–86. [DOI] [PubMed] [Google Scholar]
- 43.Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012; 35: 780–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falavigna M, Schmidt MI, Trujillo J, et al. Effectiveness of gestational diabetes treatment: a systematic review with quality of evidence assessment. Diabetes Res Clin Pract 2012; 98: 396–405. [DOI] [PubMed] [Google Scholar]
- 45.Casey BM, Mele L, Landon MB, et al. Does maternal body mass index influence treatment effect in women with mild gestational diabetes? Am J Perinatol 2015; 32: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillman MW, Oakey H, Baghurst PA, et al. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010; 33: 964–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landon MB, Rice MM, Varner MW, et al. Mild gestational diabetes mellitus and long-term child health. Diabetes Care 2015; 38: 445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugiyama T, Nagao K, Metoki H, et al. Pregnancy outcomes of gestational diabetes mellitus according to pre-gestational BMI in a retrospective multi-institutional study in Japan. Endocr J 2014;61: 373–80. [DOI] [PubMed] [Google Scholar]
- 49.Han S, Crowther CA, Middleton P, Heatley E. Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst Rev 2013; 3: CD009275. [DOI] [PubMed] [Google Scholar]
- 50.Balas-Nakash M, Rodriguez-Cano A, Munoz-Manrique C, Vasquez-Pena P, Perichart-Perera O. Adherence to a medical nutrition therapy program in pregnant women with diabetes, measured by three methods, and its association with glycemic control. Rev Invest Clin 2010; 62: 235–43 (in Spanish). [PubMed] [Google Scholar]
- 51.Grant SM, Wolever TM, O’Connor DL, Nisenbaum R, Josse RG. Effect of a low glycaemic index diet on blood glucose in women with gestational hyperglycaemia. Diabetes Res Clin Pract 2011; 91: 15–22. [DOI] [PubMed] [Google Scholar]
- 52.Moses RG, Barker M, Winter M, Petocz P, Brand-Miller JC. Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 2009; 32: 996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Louie JC, Markovic TP, Perera N, et al. A randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care 2011; 34: 2341–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rae A, Bond D, Evans S, et al. A randomised controlled trial of dietary energy restriction in the management of obese women with gestational diabetes. Aust N Z J Obstet Gynaecol 2000; 40: 416–22. [DOI] [PubMed] [Google Scholar]
- 55.Magee MS, Knopp RH, Benedetti TJ. Metabolic effects of 1200-kcal diet in obese pregnant women with gestational diabetes. Diabetes 1990; 39: 234–40. [DOI] [PubMed] [Google Scholar]
- 56.Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness, tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol 2007; 58: 314–19. [PubMed] [Google Scholar]
- 57.Lauszus FF, Rasmussen OW, Henriksen JE, et al. Effect of a high monounsaturated fatty acid diet on blood pressure and glucose metabolism in women with gestational diabetes mellitus. Eur J Clin Nutr 2001; 55: 436–43. [DOI] [PubMed] [Google Scholar]
- 58.American Dietetic Association. Medical nutrition therapy evidence-based guides for practice: nutrition practice guidelinesfor gestational diabetes. Chicago, IL: American Dietetic Association, 2001. [Google Scholar]
- 59.Padayachee C, Coombes JS. Exercise guidelines for gestational diabetes mellitus. World J Diabetes 2015; 6: 1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2013; 98: 4227–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jovanovic-Peterson L, Peterson CM. Is exercise safe or useful for gestational diabetic women? Diabetes 1991; 40 (suppl 2): 179–81. [DOI] [PubMed] [Google Scholar]
- 62.de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am J Obstet Gynecol 2010; 203: 556e1–6. [DOI] [PubMed] [Google Scholar]
- 63.Stafne SN, Salvesen KA, Romundstad PR, et al. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstet Gynecol 2012; 119: 29–36. [DOI] [PubMed] [Google Scholar]
- 64.Balsells M, Garcia-Patterson A, Sola I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350: h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343: 1134–38. [DOI] [PubMed] [Google Scholar]
- 66.Lain KY, Garabedian MJ, Daftary A, Jeyabalan A. Neonatal adiposity following maternal treatment of gestational diabetes with glyburide compared with insulin. Am J Obstet Gynecol 2009; 200: 501e1–6. [DOI] [PubMed] [Google Scholar]
- 67.Anjalakshi C, Balaji V, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract 2007; 76: 474–75. [DOI] [PubMed] [Google Scholar]
- 68.Camelo Castillo W, Boggess K, Sturmer T, et al. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169: 452–58. [DOI] [PubMed] [Google Scholar]
- 69.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008; 358: 2003–15. [DOI] [PubMed] [Google Scholar]
- 70.Ijas H, Vaarasmaki M, Morin-Papunen L, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG 2011; 118: 880–85. [DOI] [PubMed] [Google Scholar]
- 71.Niromanesh S, Alavi A, Sharbaf FR, et al. Metformin compared with insulin in the management of gestational diabetes mellitus:a randomized clinical trial. Diabetes Res Clin Pract 2012; 98: 422–29. [DOI] [PubMed] [Google Scholar]
- 72.Tertti K, Ekblad U, Koskinen P, Vahlberg T, Ronnemaa T. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab 2013; 15: 246–51. [DOI] [PubMed] [Google Scholar]
- 73.Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol 2013; 209: 34. [DOI] [PubMed] [Google Scholar]
- 74.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009; 301: 636–50. [DOI] [PubMed] [Google Scholar]
- 75.Correa A, Marcinkevage J. Prepregnancy obesity and the risk of birth defects: an update. Nutr Rev 2013; 71 (suppl 1): S68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Best KE, Tennant PW, Bell R, Rankin J. Impact of maternal body mass index on the antenatal detection of congenital anomalies. BJOG 2012; 119: 1503–11. [DOI] [PubMed] [Google Scholar]
- 77.Weichert J, Hartge DR. Obstetrical sonography in obese women: a review. J Clin Ultrasound 2011; 39: 209–16. [DOI] [PubMed] [Google Scholar]
- 78.Walsh JM, McAuliffe FM. Foetal ultrasound in obese pregnancy In: Mahmood T, Arulkumaran S, eds. Obesity: a ticking time bomb for reproductive health, 1st edn. London: Elsevier, 2013: 213–22. [Google Scholar]
- 79.Paganelli S, Soncini E, Comitini G, Palomba S, La Sala GB. Sonographic fetal weight estimation in normal and overweight/ obese healthy term pregnant women by gestation-adjusted projection (GAP) method. Arch Gynecol Obstet 2016; 293: 775–81. [DOI] [PubMed] [Google Scholar]
- 80.Anon. Practice bulletin no. 163: screening for fetal aneuploidy. Obstet Gynecol 2016; 127: e123–37. [DOI] [PubMed] [Google Scholar]
- 81.Racusin D, Stevens B, Campbell G, Aagaard KM. Obesity and the risk and detection of fetal malformations. Semin Perinatol 2012; 36: 213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Louis J, Auckley D, Bolden N. Management of obstructive sleep apnea in pregnant women. Obstet Gynecol 2012; 119: 864–68. [DOI] [PubMed] [Google Scholar]
- 83.Page LM, Girling JC. A novel cause for abnormal liver function tests in pregnancy and the puerperium: non-alcoholic fatty liver disease. BJOG 2011; 118: 1532–35. [DOI] [PubMed] [Google Scholar]
- 84.Anderson NH, McCowan LM, Fyfe EM, et al. The impact of maternal body mass index on the phenotype of pre-eclampsia: a prospective cohort study. BJOG 2012; 119: 589–95. [DOI] [PubMed] [Google Scholar]
- 85.WHO. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva: World Health Organization, 2011: 38. [PubMed] [Google Scholar]
- 86.Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. London: Royal College of Obstetricians and Gynaecologists, 2015: 1–40. [Google Scholar]
- 87.McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 2010; 341: c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA 2013; 309: 2362–70. [DOI] [PubMed] [Google Scholar]
- 89.Heyborne KD, Allshouse AA, Carey JC. Does 17-alpha hydroxyprogesterone caproate prevent recurrent preterm birth in obese women? Am J Obstet Gynecol 2015; 213: 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anon. Practice bulletin no 156: obesity in pregnancy. Obstet Gynecol 2015; 126: e112–26. [DOI] [PubMed] [Google Scholar]
- 91.Boulvain M, Senat MV, Perrotin F, et al. Induction of labour versus expectant management for large-for-date fetuses: a randomised controlled trial. Lancet 2015; 385: 2600–05. [DOI] [PubMed] [Google Scholar]
- 92.Sandmire HF. Whither ultrasonic prediction of fetal macrosomia? Obstet Gynecol 1993; 82: 860–62. [PubMed] [Google Scholar]
- 93.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 2006; 195: 1100–03. [DOI] [PubMed] [Google Scholar]
- 94.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol 2008; 198: 416. [DOI] [PubMed] [Google Scholar]
- 95.American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol 2014; 123: 693–711. [DOI] [PubMed] [Google Scholar]
- 96.Nuthalapaty FS, Rouse DJ, Owen J. The association of maternal weight with cesarean risk, labor duration, and cervical dilation rate during labor induction. Obstet Gynecol 2004; 103: 452–56. [DOI] [PubMed] [Google Scholar]
- 97.Vahratian A, Zhang J, Troendle JF, Savitz DA, Siega-Riz AM. Maternal prepregnancy overweight and obesity and the pattern of labor progression in term nulliparous women. Obstet Gynecol 2004; 104: 943–51. [DOI] [PubMed] [Google Scholar]
- 98.Arrowsmith S, Wray S, Quenby S. Maternal obesity and labour complications following induction of labour in prolonged pregnancy. BJOG 2011; 118: 578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolfe KB, Rossi RA, Warshak CR. The effect of maternal obesity on the rate of failed induction of labor. Am J Obstet Gynecol 2011;205: 128. [DOI] [PubMed] [Google Scholar]
- 100.Chu SY, Kim SY, Schmid CH, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev 2007; 8: 385–94. [DOI] [PubMed] [Google Scholar]
- 101.Darney BG, Snowden JM, Cheng YW, et al. Elective induction of labor at term compared with expectant management: maternal and neonatal outcomes. Obstet Gynecol 2013; 122: 761–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juhasz G, Gyamfi C, Gyamfi P, Tocce K, Stone JL. Effect of body mass index and excessive weight gain on success of vaginal birth after cesarean delivery. Obstet Gynecol 2005; 106: 741–46. [DOI] [PubMed] [Google Scholar]
- 103.Hibbard JU, Gilbert S, Landon MB, et al. Trial of labor or repeat cesarean delivery in women with morbid obesity and previous cesarean delivery. Obstet Gynecol 2006; 108: 125–33. [DOI] [PubMed] [Google Scholar]
- 104.Blomberg M Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol 2011; 118: 561–68. [DOI] [PubMed] [Google Scholar]
- 105.Stamilio DM, Scifres CM. Extreme obesity and postcesarean maternal complications. Obstet Gynecol 2014; 124: 227–32. [DOI] [PubMed] [Google Scholar]
- 106.Wall PD, Deucy EE, Glantz JC, Pressman EK. Vertical skin incisions and wound complications in the obese parturient. Obstet Gynecol 2003; 102: 952–56. [DOI] [PubMed] [Google Scholar]
- 107.Marrs CC, Moussa HN, Sibai BM, Blackwell SC. The relationship between primary cesarean delivery skin incision type and wound complications in women with morbid obesity. Am J Obstet Gynecol 2014; 210: 319. [DOI] [PubMed] [Google Scholar]
- 108.Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol 2004; 103: 974–80. [DOI] [PubMed] [Google Scholar]
- 109.Machado LS. Cesarean section in morbidly obese parturients: practical implications and complications. N Am J Med Sci 2012; 4: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Young OM, Shaik IH, Twedt R, et al. Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol 2015; 213: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Schalkwyk J, Van Eyk N. Antibiotic prophylaxis in obstetric procedures. J Obstet Gynaecol Can 2010; 32: 878–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gunderson EP, Hurston SR, Ning X, et al. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann Intern Med 2015;163: 889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Obesity and mental disorders during pregnancy and postpartum: a systematic review and meta-analysis. Obstet Gynecol 2014; 123: 857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee opinion no. 630. Screening for perinatal depression. Obstet Gynecol 2015; 125: 1268–71. [DOI] [PubMed] [Google Scholar]
- 115.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol 2015; 125: 773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr 2011; 94: 1225–31. [DOI] [PubMed] [Google Scholar]
- 117.Baker JL, Michaelsen KF, Sorensen TI, Rasmussen KM. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. Am J Clin Nutr 2007; 86: 404–11. [DOI] [PubMed] [Google Scholar]
- 118.NICE. Weight management before, during and after pregnancy Public health guidelines. NICE guidelines 27. Manchester, UK: National Institute for Health and Care Excellence, 2010. [Google Scholar]
- 119.Nascimento SL, Pudwell J, Surita FG, Adamo KB, Smith GN. The effect of physical exercise strategies on weight loss in postpartum women: a systematic review and meta-analysis. Int J Obes (Lond) 2014; 38: 626–35. [DOI] [PubMed] [Google Scholar]
- 120.Institute of Medicine. Nutrition during lactation. Washington, DC: Institute of Medicine, 1991. [Google Scholar]
- 121.Lovelady C Balancing exercise and food intake with lactation to promote post-partum weight loss. Proc Nutr Soc 2011; 70: 181–84. [DOI] [PubMed] [Google Scholar]
- 122.Amorim AR, Linne YM, Lourenco PM. Diet or exercise, or both, for weight reduction in women after childbirth.Cochrane Database Syst Rev 2007; 3: CD005627. [DOI] [PubMed] [Google Scholar]
- 123.Agha M, Agha RA, Sandall J. Interventions to reduce and prevent obesity in pre-conceptual and pregnant women: a systematic review and meta-analysis. PLoS One 2014; 9: e95132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.American College of Obstetrics and Gynecologists. ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol 2013; 121: 213–17. [DOI] [PubMed] [Google Scholar]
- 125.Mamun AA, O’Callaghan M, Callaway L, et al. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 2009; 119: 1720–27. [DOI] [PubMed] [Google Scholar]
- 126.Robinson CA, Cohen AK, Rehkopf DH, et al. Pregnancy and post-delivery maternal weight changes and overweight in preschool children. Prev Med 2014; 60: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016; 23: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA 2008; 300: 2286–96. [DOI] [PubMed] [Google Scholar]
- 129.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 105: bariatric surgery and pregnancy. Obstet Gynecol 2009; 113: 1405–13. [DOI] [PubMed] [Google Scholar]
- 130.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–87. [DOI] [PubMed] [Google Scholar]
- 131.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med 2013; 159: 758–69. [DOI] [PubMed] [Google Scholar]
- 132.Ruifrok AE, Rogozinska E, van Poppel MN, et al. Study protocol: differential effects of diet and physical activity based interventions in pregnancy on maternal and fetal outcomes—individual patient data (IPD) meta-analysis and health economic evaluation. Syst Rev 2014; 3: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van der Pligt P, Willcox J, Hesketh KD, et al. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev 2013; 14: 792–805. [DOI] [PubMed] [Google Scholar]
- 134.WHO. Interim report of the commission on ending childhood obesity. Geneva: World Health Organization, 2015: 1–30. [Google Scholar]
- 135.International Diabetes Federation. IDF diabetes atlas, 7th edn. Brussels: International Diabetes Federation, 2015. [Google Scholar]
- 136.Keely E An opportunity not to be missed–how do we improve postpartum screening rates for women with gestational diabetes? Diabetes Metab Res Rev 2012; 28: 312–16. [DOI] [PubMed] [Google Scholar]
- 137.Venkataraman H, Sattar N, Saravanan P. Postnatal testing following gestational diabetes: time to replace the oral glucose tolerance test? Lancet Diabetes Endocrinol 2015; 3: 754–56. [DOI] [PubMed] [Google Scholar]