Summary
Background
The optimal duration of extended therapy with aromatase inhibitors in patients with postmenopausal breast cancer is unknown. In the NSABP B-42 study, we aimed to determine whether extended letrozole treatment improves disease-free survival after 5 years of aromatase inhibitor-based therapy in women with postmenopausal breast cancer.
Methods
This randomized, double-blind, placebo-controlled, phase 3 trial was done in 158 centers in the USA, Canada, and Ireland. Postmenopausal women with stage I–IIIA hormone receptor-positive breast cancer, who were disease-free after about 5 years of treatment with an aromatase inhibitor or tamoxifen followed by an aromatase inhibitor, were randomly assigned (1:1) to receive 5 years of letrozole (2·5 mg orally per day) or placebo. Randomization was stratified by pathological node status, previous tamoxifen use, and lowest bone mineral density T score in the lumbosacral spine, total hip, or femoral neck. The primary endpoint was disease-free survival, defined as time from randomization to breast cancer recurrence, second primary malignancy, or death, and was analysed by intention to treat. Primary endpoint was DFS, defined as time from randomization to BC recurrence, second primary malignancy, or death, and analyzed by intention to treat. To adjust for interim analyses, two-sided statistical significance level for DFS was set at 0.0418.
ClinicalTrials.gov, ; active; no longer enrolling patients.
Findings
Between Sept 28, 2006, and Jan 6, 2010, 3966 patients were randomly assigned to receive letrozole (n=1983) or placebo (n=1983). Follow-up information was available for 3903 patients for the analyses of disease-free survival. Median follow-up was 6·9 years (IQR 6·1–7·5). Letrozole treatment did not significantly improve disease-free survival (339 disease-free survival events were reported in the placebo group and 292 disease-free survival events were reported in the letrozole group; hazard ratio 0·85, 95% CI 0·73–0·999; p=0·048). 7-year disease-free survival estimate was 81·3% (95% CI 79·3–83·1) in the placebo group and 84·7% (82·9–86·4) in the letrozole group. The most common grade 3 adverse events were arthralgia (47 [2%] of 1933 patients in the placebo group vs 50 [3%] of 1941 patients in the letrozole group) and back pain (44 [2%] vs 38 [2%]). The most common grade 4 adverse event in the placebo group was thromboembolic event (eight [<1%]) and the most common grade 4 adverse events in the letrozole group were urinary tract infection, hypokalemia, and left ventricular systolic dysfunction (four [<1%] each).
Interpretation
After five years of aromatase inhibitor-based therapy, five years of letrozole therapy did not significantly prolong disease-free survival compared with placebo. Careful assessment of potential risks and benefits is required before recommending extended letrozole therapy to patients with early-stage BC.
Keywords: Letrozole, Extended Adjuvant Therapy, Breast Cancer, Hormone-receptor Positive
Introduction
Patients with hormone-receptor positive early-stage breast cancer are at risk for recurrence long after the first five years from diagnosis. In the Early Breast Cancer Trialist’s Collaborative Group overview analysis, about half of the recurrences and more than two-thirds of the deaths from breast cancer occurred more than 5 years after diagnosis.1,2
Extended adjuvant endocrine therapy after five years of tamoxifen with either tamoxifen or an aromatase inhibitor (AI) has been shown to improve disease-free survival (DFS) in early-stage breast cancer.3–6 In one large trial, breast cancer specific mortality and overall survival (OS) were also improved.5 However, studies evaluating the benefit of extending adjuvant AI therapy beyond five years have only recently been reported, with mixed results7–11
The NSABP B-42 clinical trial aimed to determine whether five years of letrozole vs. placebo would improve DFS in patients who had remained free of breast cancer after completing five years of endocrine therapy with either an AI or initial tamoxifen for up to three years followed by an AI for the remainder of five years.
Methods
Study design and participants
This was a randomised, double-blind, placebo-controlled, phase 3 trial done in 158 centers in the USA, Canada, and Ireland. Eligible patients were postmenopausal women with histologically confirmed, estrogen or progesterone receptor-positive invasive ductal carcinoma (by local assessment), stage I-IIIA at diagnosis, and disease-free after approximately five years of endocrine therapy consisting of either an AI or tamoxifen for ≤3 years followed by an AI for the remainder of the initial five years. For study purposes, postmenopausal was defined as age 56 or older with no spontaneous menses for at least 12 months prior to study entry, or age 55 or younger with no spontaneous menses for at least 12 months prior to study entry and with a documented estradiol level in the postmenopausal range according to local institutional/laboratory standards, or a prior documented bilateral oophorectomy. The B-42 trial was approved by local human investigations committees or institutional review boards in accordance with assurances filed with and approved by the Department of Health and Human Services. Written informed consent was required for participation.
In order to have a predominantly letrozole-treated population for enrollment in B-42, an optional registration program allowed patients who had had a minimum of two years of prior hormonal therapy (either tamoxifen for ≤3 years or an AI) to be offered letrozole at no cost until completion of five years of initial adjuvant endocrine therapy.
Patients could have undergone either breast-conserving therapy or mastectomy with axillary lymph node staging. Estimated life expectancy was not an eligibility criterion, however it was suggested that investigators consider women with a life expectancy less than 10 years, excluding her diagnosis of breast cancer, as potentially unsuitable candidates for the trial. Patients were required to have ECOG performance status of 0-1 and must have consented to participate by signing and dating an appropriate IRB-approved consent form that conformed to federal and institutional guidelines.
Randomization was to occur within 6 months following completion of adjuvant hormonal therapy. The duration of the patient’s adjuvant hormonal therapy following breast cancer diagnosis was required to have been 57-63 months from the first dose regardless of the number of missed doses. At randomization, patients were required to have no clinical evidence of recurrent breast cancer. Bilateral mammogram (when applicable) and bone mineral density (BMD) testing were required within one year. A total cholesterol ≤grade 1 (NCI Common Terminology Criteria for Adverse Events [CTCAE] v3.0) was required within one year or within two years depending on the patient’s history of hypercholesterolemia, use of cholesterol-lowering interventions, or risk factors for cardiovascular events.
Patients were ineligible if they had history of non-traumatic osteoporotic fracture, bilateral breast cancer including DCIS, other malignancies (except carcinoma in situ of the colon or cervix, melanoma in situ, or squamous or basal cell carcinoma of the skin) unless disease-free for ≥5 years prior to randomization and deemed at low risk for recurrence by their physician. Use of sex hormonal therapy or therapy with any hormonal agent for management of osteoporosis made a patient ineligible unless such therapy was discontinued prior to study entry. Administration of any investigational agent within 30 days before entry also made a patient ineligible.
The study was approved by local human investigations committees or institutional review boards in accordance with assurances filed with and approved by the Department of Health and Human Services. Written, informed consent was required from each participant.
Randomisation and masking
Eligible patients were randomized 1:1 to either letrozole or matching placebo (Appendix Figure 1 [Schema] p 2). Assignment to the treatment groups was balanced by pathologic nodal status at diagnosis (negative, positive), use of tamoxifen as component of initial adjuvant therapy (no, yes), and lowest BMD T score in the lumbosacral spine, total hip, or femoral neck (≤−2.0, >−2.0 SD) using a biased-coin minimization algorithm.12 Randomization was done centrally by the statistical center (Pittsburgh, PA, USA). Treatment assignment was double-blinded: both patients and investigators were masked to treatment group. Letrozole and matching placebo was supplied in bottles and each bottle was labelled letrozole 2·5 mg or placebo.
Procedures
Letrozole 2.5 mg/placebo tablet was taken orally once daily. Therapy was to begin within 30 days following randomization and to end 5 years from the date of the first dose regardless of any missed doses. If patients developed grade 3-4 cholesterol high adverse event (AE), the study drug had to be suspended until total cholesterol returned to grade 1 or lower. The study drug had to be discontinued for any of the following: ≥grade 1 stroke, transient ischemic attack; ≥grade 2 acute coronary syndrome, cerebrovascular ischemia; ≥grade 3 myocardial infarction, peripheral ischemia, or visceral arterial ischemia or if osteoporotic fracture occurred with a T-score less than −2.5 while on bisphosphonates or other medication for osteoporosis. Patients were required to have a physical exam annually while on therapy. Patient status was to be updated every 6 months during therapy and every 12 months thereafter. Bilateral mammogram was required every 12 months and BMD testing was required every two years while on treatment. The frequency of lipid panel testing was on a patient-by-patient basis. Adverse events (according to the CTCAE version 3.0 and then according to version 4.0 (insert) from the beginning of January, 2011) were assessed every 6 months during study therapy and 6 months after the last administered dose of the investigational study drug.
Outcomes
The primary endpoint was disease-free survival, defined as time from randomisation to breast cancer recurrence, second primary malignancy, or death. Secondary endpoints were overall survival (time from randomisation to death from any cause), breast-cancer-free interval (time from randomisation to local, regional, or distant recurrence of breast cancer, or contralateral breast cancer as a first event), distant recurrence (time from randomisation to distant recurrence of breast cancer), incidence of osteoporotic fractures (defined as Colles’, hip, or spinal fractures), and incidence of arterial thrombotic events as defined by CTCAE version 4.0 (grade ≥1 stroke or transient ischemic attack; grade ≥2 acute coronary syndrome or cerebrovascular ischemia; grade ≥3 myocardial infarction, peripheral ischemia, or visceral arterial ischemia; and grade ≥4 selected thromboembolic events [cerebrovascular event, arterial insufficiency].
The pathology reports submitted by the sites were reviewed centrally to confirm breast cancer recurrence, contralateral breast cancer, or second non-breast primary malignancy. All time-to-event endpoints were measured from the date of randomization to the date of diagnosis of the specified event. Patients otherwise event-free were censored at their last follow-up. In addition, for the BCFI endpoint, other second primary cancers and death without evidence of recurrence were treated as censored events. Clinical assessment was required for determining patients’ status for all endpoints except OS.
Statistical analysis
The study was designed to have at least 80% power to detect a 20% reduction in the annual DFS hazard rate with letrozole compared to placebo using a 0.05 two-sided significance level. A total of 3,840 patients were to be enrolled. Definitive analysis was planned after the report of the 631th DFS event on both treatment groups combined. Four formal interim analyses were pre-specified in the statistical analysis plan and scheduled after 126, 252, 379, and 505 events were observed. Symmetric stopping boundaries based on the O’Brien-Fleming method13 were originally employed. The futility boundaries were added for the third and fourth interim analyses14 per Data Monitoring Committee recommendations. The original O’Brien-Fleming method was utilized for the one-sided lower boundary for superiority. To adjust for the previous four interim analyses and account for alpha-spending, the adjusted two-sided significance level of 0.0418 was used for the primary analysis.
Differences in primary and secondary endpoints between treatment groups were assessed by stratified log-rank tests, controlling for stratification variables.15 Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were calculated based on stratified Cox proportional hazards model for all time-to-event endpoints.16 Assumption of proportionality of hazards was tested for each time-to-event outcome.17 When the proportional hazards assumption was not satisfied, a “change point” for the relative risk technique was used to identify the optimal point to dichotomize time.15 In secondary analyses, the proportional hazards model was used to estimate and control for the effect of additional prognostic factors. Two-sided P-values of <0.05 were considered significant for analyses of secondary endpoints. Presence of treatment-by-covariate interactions were tested with two-sided P-value of <0.01 used to claim their statistical significance.
For illustration purposes the distribution of DFS and OS endpoints were estimated using the Kaplan-Meier method18 and the cumulative incidence function was used to estimate the proportions of BCFI, DR, OF, and AT events over time to account for competing risks.19 Second primary cancers (other than breast) and death without evidence of recurrence were considered as competing events in estimating cumulative incidence of BCFI events. Death as first event was considered as a competing event in estimating cumulative incidence of DR, OF, and AT events.
Definitive analysis, which was triggered by observing 631 disease-free survival events, was based on the intention-to-treat principle, with all randomly assigned patients analyzed, regardless of eligibility or protocol compliance. Patients with no follow-up and those not at risk for the primary endpoint (metastases at time of randomization or first non-death event within 30 days from randomization) were excluded. All statistical analyses were performed using SAS software (v9.4, Cary, NC). Analyses reported here include all data received as of August 25, 2016. This study is registered with ClinicalTrials.gov number .
Role of the funding source
The sponsors had no role in the study design, collection, analysis, or interpretation of the data, or in the writing of the report. EPM, HB, and JHJ had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Cohort characteristics (Fig 1: Trial profile NSABP B-42)
Figure 1:
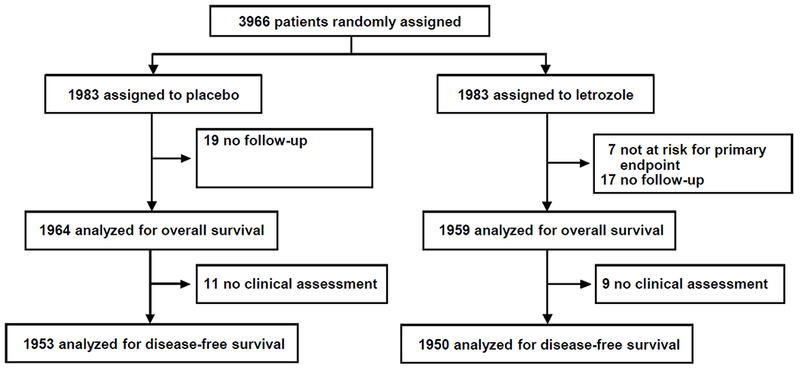
Between September 28, 2006 and January 6, 2010, 3,966 patients were randomly assigned by 158 participating institutions (Appendix [Table 1] p 3). Median time from original diagnosis to randomization was 5.6 years (IQR=5.4-5.8). Distribution of baseline patient, tumor, and prior treatment characteristics were well balanced between the two treatment groups (Table 1). The median duration of AI treatment in all patients in the first five years after diagnosis was 60 months (IQR=40-60). It was 36 months (IQR=31-47) for women also treated with prior tamoxifen and 60 months (IQR=60-61) for women who did not receive prior tamoxifen. Seven patients randomized to the letrozole group were excluded from analyses (not at risk for the primary endpoint), and 36 patients (placebo:19, letrozole:17) were excluded due to no available follow-up. Among 3,923 patients with follow-up, 20 (0.5%) (placebo:11 [0.6%], letrozole:9 [0.5%]) did not have a clinical assessment for the duration of the follow-up and therefore were excluded from the analyses of all disease-related endpoints except for OS. Median follow-up time for 3,923 patients included in efficacy analyses was 6.9 years (IQR=6.1-7.5).
Table 1:
Patient and tumor characteristics: NSABP B-42
| Placebo (n=1983) | Letrozole (n=1983) | |||
|---|---|---|---|---|
| Characteristic | n | % | n | % |
| Age at randomization, yrs | ||||
| <60 | 675 | 34.0 | 685 | 34.5 |
| ≥60 | 1308 | 66.0 | 1298 | 65.5 |
| Race | ||||
| White | 1840 | 92.8 | 1848 | 93.2 |
| Black | 81 | 4.1 | 70 | 3.5 |
| Asian | 39 | 2.0 | 39 | 2.0 |
| Other/Unknown | 23 | 1.2 | 26 | 1.3 |
| Pathologic Nodal Status | ||||
| Negative | 1134 | 57.2 | 1145 | 57.7 |
| Positive | 849 | 42.8 | 838 | 42.3 |
| Lowest BMD T-score | ||||
| ≤−2.0 | 493 | 24.9 | 489 | 24.7 |
| >−2.0 | 1490 | 75.1 | 1494 | 75.3 |
| Duration of Tamoxifen Prior to Randomization, mos | ||||
| 0 | 1212 | 61.1 | 1207 | 60.9 |
| 1 to 12 | 164 | 8.3 | 150 | 7.6 |
| 13 to 24 | 254 | 12.8 | 259 | 13.1 |
| 25 to 36 | 353 | 17.8 | 367 | 18.5 |
| Surgery Type | ||||
| Lumpectomy | 1208 | 60.9 | 1201 | 60.6 |
| Mastectomy | 775 | 39.1 | 782 | 39.4 |
| HER 2 Status | ||||
| Positive | 278 | 14.0 | 287 | 14.5 |
| Negative | 1547 | 78.0 | 1546 | 78.0 |
| Not done/Unknown | 158 | 8.0 | 150 | 7.6 |
| Duration of AI prior to randomization, mos* | ||||
| ≤36 | 412 | 20.8 | 399 | 20.1 |
| 37 to 48 | 192 | 9.7 | 207 | 10.4 |
| 49 to 60 | 992 | 50.0 | 970 | 48.9 |
| >60 | 387 | 19.5 | 407 | 20.5 |
Duration of AI for one patient in placebo group was unknown, presented in the “≤36” category.
Protocol treatment adherence
Median duration of treatment was 59.8 months (IQR=32.6-60.0) in the placebo group and 59.8 months (IQR=32.3-60.0) in the letrozole group. Among 3,923 patients included in the efficacy analysis, 47 (1.2%) (placebo:30 [1.5%], letrozole:17 [0.9%]) did not initiate treatment. Overall, 1228 (63%) of 1964 patients in the placebo group and 1181 (60%) of 1959 patients in the letrozole group completed 5 years of therapy. Main reasons for treatment discontinuation were withdrawal or refusal (250 [13%] of 1964 patients in the placebo group vs 271 [14%] of 1959 patients in the letrozole group), adverse events (140 [7%] vs 189 [10%]), disease progression (102 [5%] vs 81 [4%]), other complicating disease or death (53 [3%] vs 52 [3%]), and declining bone density or osteoporotic fracture (16 [1%] vs 27 [1%]).
Primary endpoint: Disease-free survival (DFS)
There were 631 (16.2%) DFS events recorded among 3,903 patients included in the analyses of DFS (placebo:339 [17.4%], letrozole:292 [15.0%]). Letrozole did not result in a statistically significant increase in DFS compared to placebo (HR=0.85; 95%CI 0.73-0.999, P=0.048), The 7-year disease-free survival point estimates were 81·3% (95% CI 79·3–83·1) for placebo and 84·7% (95% CI 82·9–86·4) for letrozole (figure 2A).
Fig 2:
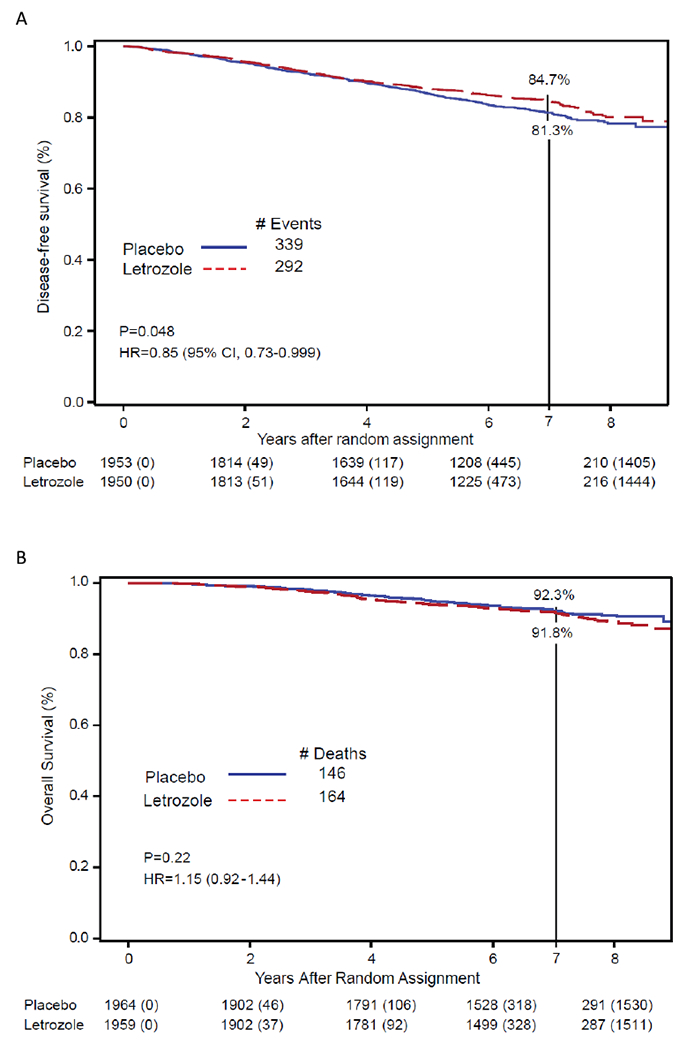
Effect of letrozole vs. placebo on (A) disease-free survival and (B) overall survival. NSABP B-42
The primary differences in the frequency of DFS events between placebo and letrozole were observed in DR (87 vs. 61 events, respectively) and in contralateral breast cancer (59 vs. 30 events, respectively) (Table 2).
Table 2:
Type of first events by treatment group: NSABP B-42
| Placebo (n=1953) | Letrozole (n=1950) | |||
|---|---|---|---|---|
| First event | n | % | n | % |
| Distant recurrence | 87 | 4.5 | 61 | 3.1 |
| Local recurrence | 33 | 1.7 | 36 | 1.8 |
| Contralateral breast cancer | 59 | 3.0 | 30 | 1.5 |
| Second non-breast primaries | 112 | 5.7 | 104 | 5.3 |
| Death | 48 | 2.5 | 61 | 3.1 |
| Total first event | 339 | 17.4 | 292 | 15.0 |
| Alive, event free | 1614 | 82.6 | 1658 | 85.0 |
Secondary endpoints
Overall Survival (OS)
A total of 310 deaths occurred: placebo:146, and letrozole:164. There was no statistically significant difference in OS with letrozole compared to placebo (HR=1.15, 95%CI 0.92-1.44, P=0.22) with 7-year OS point estimates of placebo:92.3% (95%CI 90.9-93.5) and letrozole:91.8% (95%CI 90.4-93.0). (Figure 2B [OS]). Ninety three patients died from breast cancer: placebo:47, letrozole:46. (Appendix [Table 2] p 7).
Breast Cancer-Free Interval (BCFI)
A total of 306 BCFI events were observed (placebo:179, letrozole: 127). Letrozole demonstrated a statistically significant reduction in BCFI events compared to placebo (HR=0.71, 95%CI 0.56-0.89, P=0.0027). The cumulative incidence of BCFI events through 7 years was: placebo:10% (95%CI 8.6-11.5), letrozole:6.7% (95%CI 5.6-8.0). (Figure 3A [BCFI]).
Fig 3:
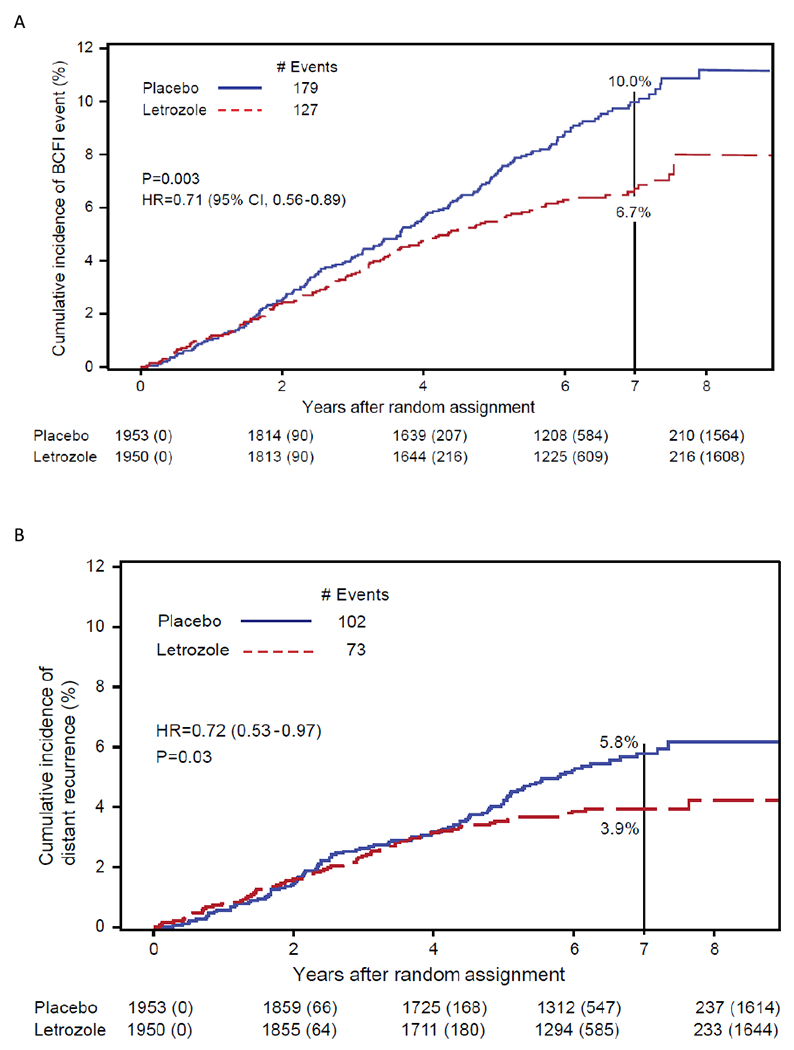
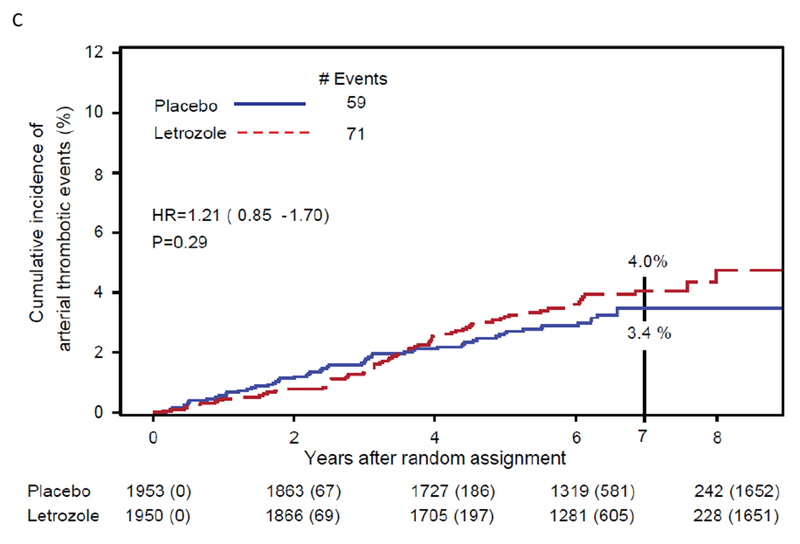
Cumulative Incidence of (A) breast cancer-free interval (BCFI), (B) distant recurrence, and (C) arterial thrombotic events. NSABP B-42
Distant Recurrence (DR)
A total of 175 DRs were observed (placebo:102; letrozole:73) (Appendix [Table 3] p 8). Letrozole resulted in a statistically significant 28% reduction in the rate of DR (HR=0.72, 95%CI 0.53-0.97, P=0.0304) compared to placebo. However, a non-proportionality of hazards in the treatment groups was detected (P=0.012) with a change point for the relative risk of 4.1 years. No difference in the risk of DR events was evident before 4.1 years (HR=1.00, 95%CI 0.70-1.42, P=0.98) but a statistically significant reduction favoring letrozole was observed afterwards (HR=0.32, 95%CI 0.17-0.59, P=0.0003). The cumulative incidence of DRs through 4 years was placebo:3.2% (95%CI 2.5-4.0), letrozole:3.1% (95%CI 2.4-4.0) and through 7 years placebo:5.8% (95%CI 4.7-7.0), letrozole:3.9% (95%CI 3.1-4.9). (Figure 3B [DR]).
Osteoporotic fractures (OF)
There were 169 osteoporotic fractures: placebo:78, letrozole:91, with no statistically significant difference in the development of OF between groups (HR=1.19, 95%CI 0.88-1.60, P=0.27). The cumulative incidence of OF through 7 years was placebo:4.8% (95%CI 3.8-6.0), letrozole:5.4% (95%CI 4.3-6.6).
Arterial thrombotic (AT) events
There were 130 AT events reported (placebo:59, letrozole:71). Although treatment with letrozole did not result in an overall statistically significant increase in AT events compared to placebo (HR=1.21, 95%CI 0.85-1.70, P=0.29), a proportionality of the hazards assumption was not satisfied (P=0.007). A change point of 2.5 years for the relative risk of AT events was identified. There was no significant difference in the risk of arterial thrombotic events with letrozole compared with placebo before 2.5 years (HR=0.55, 95%CI 0.30-1.01, P=0.054), with a statistically significant increase after 2.5 years (HR=1.85, 95%CI 1.18-2.88, P=0.0069). The cumulative incidence of AT events through 2.5 years was placebo:1.6% (95%CI 1.1-2.2) and letrozole:0.9% (95%CI 0.5-1.4) and through 7 years was placebo:3.4% (95%CI 2.6-4.4) and letrozole:4.0% (95%CI 3.1-5.0). (Figure 3C [AT]).
Multivariable and subgroup analyses
The effect of treatment on DFS persisted in multivariable analysis (HR=0.86, 95%CI 0.73-1.00; P=0.0501) after adjustment for other prognostic factors: age (P<0.0001), pathologic nodal status (P=0.0005), prior tamoxifen use (P=0.0035), and type of surgery (P=0.0098) (Table 3)
Table 3:
Multivariable analysis for disease-free survival (DFS): NSABP B-42
| Characteristic | No. of patients (N=3,903) | No. (%) of DFS events | Hazards ratio (95%CI) | P | |
|---|---|---|---|---|---|
| Treatment | Placebo | 1953 | 339 (17.4) | --- | 0.0501 |
| Letrozole | 1950 | 292 (15.0) | 0.86 (0.73,1.00) | ||
| Age | < 60 | 1344 | 163 (12.1) | --- | <0.0001 |
| ≥60 | 2559 | 468 (18.3) | 1.55 (1.29,1.86) | ||
| Pathologic Nodal Status | Negative | 2240 | 322 (14.4) | --- | 0.0005 |
| Positive | 1663 | 309 (18.6) | 1.33 (1.13,1.56) | ||
| Received Tamoxifen | No | 2377 | 421 (17.7) | --- | 0.0035 |
| Yes | 1526 | 210 (13.8) | 0.78 (0.66,0.92) | ||
| Surgery Type | Lumpectomy | 2374 | 348 (14.7) | --- | 0.0098 |
| Mastectomy | 1529 | 283 (18.5) | 1.24 (1.05,1.45) |
There were no significant differences in the letrozole effect on DFS by age, pathologic nodal status, prior tamoxifen use, surgery, or lowest BMD score (Figure 4 [DFS]). The letrozole effect was very similar by pathologic nodal status and patient age. Although no statistically significant treatment by factor interactions were identified, the letrozole effect appeared more pronounced in patients who had received prior tamoxifen vs. not in those who had mastectomy vs. lumpectomy and in those with lowest BMD score of ≤−2.0 vs. >−2.0. Additionally, the absolute 7-year differences in DFS, BCFI event, and DR were minimal: DFS:2.1%−6.4%; BCFI event:2.8%−4.5%, and DR:0.6%−4.6% (Table 4).
Fig 4:
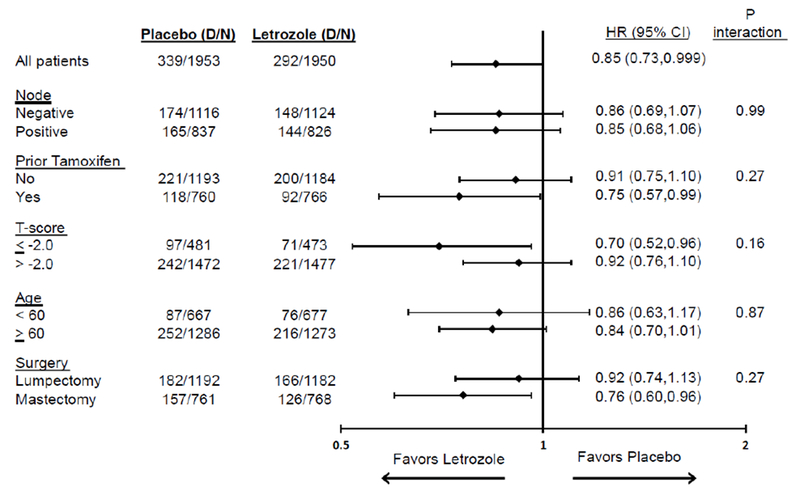
Letrozole effect on disease-free survival (DFS) in subgroups: NSABP B-42
Table 4:
7-yr disease-free survival (DFS), cumulative incidence of breast cancer-free interval (BCFI), and distant recurrence according to select patient characteristics and treatment: NSABP B-42
| Endpoint | 7-Yr DFS (%) | 7-Yr Cum. Incidence BCFI Event (%) | 7-Yr Cum. Incidence Distant Recurrence (%) | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | Placebo | Letrozole | Placebo | Letrozole | Placebo | Letrozole | |
| Pathologic Nodal Status | Negative | 83.4 (80.9, 85.7) | 86.5 (84.2,88.5) | 8.2 (6.5, 10.0) | 5.3 (4.0, 6.8) | 3.6 (2.5, 4.9) | 2.2 (1.4, 3.2) |
| Positive | 78.4 (75.2, 81.3) | 82.4 (79.4, 85.0) | 12.4 (10.1, 15.0) | 8.7 (6.8, 10.8) | 8.7 (6.8, 10.9) | 6.3 (4.7, 8.2) | |
| Prior Tamoxifen | No | 79.8 (77.1, 82.1) | 82.0 (79.4, 84.3) | 9.9 (8.2, 11.8) | 6.8 (5.4, 8.5) | 5.9 (4.5, 7.4) | 3.6 (2.6, 4.9) |
| Yes | 83.7 (80.6, 86.3) | 88.8 (86.2, 91.0) | 10.0 (7.8, 12.5) | 6.5 (4.8, 8.5) | 5.6 (4.0, 7.6) | 4.4 (3.1, 6.1) | |
| Lowest BMD T score | ≤−2.0 | 77.9 (73.4, 81.7) | 84.3 (80.2, 87.6) | 9.3 (6.9, 12.3) | 5.7 (3.6, 8.5) | 7.3 (5.1, 9.9) | 2.7 (1.5, 4.6) |
| >−2.0 | 82.4 (80.1, 84.4) | 84.9 (82.8, 86.7) | 10.2 (8.5, 11.9) | 7.1 (5.8, 8.5) | 5.3 (4.1, 6.6) | 4.3 (3.3, 5.5) | |
| Age, years | < 60 | 86.0 (82.9, 88.6) | 88.1 (85.2, 90.5) | 9.5 (7.3, 12.1) | 6.7 (4.9, 8.9) | 5.9 (4.2, 8.0) | 4.3 (2.9, 6.2) |
| ≥60 | 78.8 (76.3, 81.2) | 83.0 (80.6, 85.1) | 10.2 (8.5, 12.1) | 6.7 (5.4, 8.3) | 5.7 (4.4, 7.2) | 3.7 (2.8, 4.9) | |
| Surgery Type | Lumpectomy | 83.4 (80.9, 85.6) | 85.7 (83.3, 87.7) | 8.7 (7.0, 10.5) | 6.2 (4.8, 7.9) | 3.1 (2.2, 4.3) | 2.5 (1.7, 3.5) |
| Mastectomy | 78.0 (74.6, 81.0) | 83.3 (80.3, 85.9) | 12.0 (9.6, 14.6) | 7.5 (5.7, 9.5) | 10.0 (7.8, 12.4) | 6.1 (4.5, 8.1) | |
In a post-hoc analysis, 1393 (35·7%) of patients reported the use of bisphosphonates (BSP) at baseline (701 in the placebo group and 692 in the letrozole group): 577 (60·5%) of 954 patients with a lowest bone mineral density score of −2 or lower (294 in the placebo group, 283 in the letrozole group) and 816 (27·7%) of 2949 patients with a lowest bone mineral density score of higher than −2 (407 in the placebo group and 409 in the letrozole group) used bisphosphonates at baseline. Among 1393 participants taking bisphonates at baseline, 1381 (99%) planned to continue bisphosphonate use during and after randomisation.
Toxicity
Toxicity information had been received for 3,874 (98.8%) out of 3,923 patients with available follow-up (placebo: 1,933 [98.4%], letrozole: 1,941 [99.1%]) and was similar between both groups (Appendix [Table 4] p 9). The distribution of patients by the highest grade of the most frequent toxicities experienced is summarized in Table 5. The most common grade 3 AEs were arthralgia (placebo=47 [2.4%], letrozole=50 [2.6%] patients) and back pain (placebo=44 [2.3%], letrozole=38 [2.0%] patients). The most common grade 4 adverse event in the placebo group was thromboembolic event (eight [<1%]) and the most common grade 4 events in the letrozole group were urinary tract infection, hypokalemia, and left ventricular systolic dysfunction (four [<1%] each). There were 104 (2.7%) patients who experienced a grade 4 AE as their highest event (placebo:53 [2.7%], letrozole:51 [2.6%]) and 51 (1.3%) who experienced a grade 5 AE as their highest event (placebo:26 [1.3%], letrozole:25 [1.3%]). There were 21 serious adverse events (SAE) reported among 18 patients (placebo:8 [0.4%], letrozole:10 [0.5%]) with treatment-related attribution as possible or probable as reported by the sites. Among these 21 SAEs, there were four grade 3 events (placebo:1, letrozole:3), 16 grade 4 events (placebo:7, letrozole:9), and one grade 5 left ventricular systolic disfunction reported in the letrozole treatment group. The most frequent drug-related SAE reported among patients randomized to placebo was thromboembolic event (three grade 4). The most frequent drug-related SAEs reported among patients randomized to letrozole were other nervous system disorders (two grade 4).
Table 5:
| Term | Placebo (n=1933) | Letrozole (n=1941) | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | |
| Overall | 219 (11.3 %) | 418 (21.6 %) | 53 (2.7 %) | 26 (1.3 %) | 263 (13.5 %) | 486 (25 %) | 51 (2.6 %) | 25 (1.3 %) |
| Arthralgia | 253 (13.1 %) | 47 (2.4 %) | 0 (0 %) | 0 (0 %) | 296 (15.2 %) | 50 (2.6 %) | 0 (0 %) | 0 (0 %) |
| Myalgia | 99 (5.1 %) | 19 (1 %) | 0 (0 %) | 0 (0 %) | 133 (6.9 %) | 16 (0.8 %) | 0 (0 %) | 0 (0 %) |
| Back pain | 0 (0 %) | 44 (2.3 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 38 (2 %) | 0 (0 %) | 0 (0 %) |
| Fracture | 0 (0 %) | 29 (1.5 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 36 (1.9 %) | 2 (0.1 %) | 0 (0 %) |
| Hypertension | 0 (0 %) | 27 (1.4 %) | 2 (0.1 %) | 0 (0 %) | 0 (0 %) | 27 (1.4 %) | 1 (0.1 %) | 0 (0 %) |
| Dyspnea | 0 (0 %) | 22 (1.1 %) | 1 (0.1 %) | 0 (0 %) | 0 (0 %) | 29 (1.5 %) | 2 (0.1 %) | 0 (0 %) |
| Thromboembolic event | 15 (0.8 %) | 9 (0.5 %) | 8 (0.4 %) | 0 (0 %) | 11 (0.6 %) | 9 (0.5 %) | 1 (0.1 %) | 0 (0 %) |
| Hot flashes | 0 (0 %) | 16 (0.8 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 28 (1.4 %) | 0 (0 %) | 0 (0 %) |
| Cataract | 0 (0 %) | 20 (1 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 21 (1.1 %) | 0 (0 %) | 0 (0 %) |
| Urinary tract infection | 0 (0 %) | 17 (0.9 %) | 2 (0.1 %) | 0 (0 %) | 0 (0 %) | 18 (0.9 %) | 4 (0.2 %) | 0 (0 %) |
| Atrial fibrillation | 0 (0 %) | 16 (0.8 %) | 1 (0.1 %) | 0 (0 %) | 0 (0 %) | 20 (1 %) | 0 (0 %) | 0 (0 %) |
| Fatigue | 0 (0 %) | 17 (0.9 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 20 (1 %) | 0 (0 %) | 0 (0 %) |
| Syncope | 0 (0 %) | 16 (0.8 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 21 (1.1 %) | 0 (0 %) | 0 (0 %) |
| Anemia | 0 (0 %) | 11 (0.6 %) | 5 (0.3 %) | 0 (0 %) | 0 (0 %) | 17 (0.9 %) | 2 (0.1 %) | 0 (0 %) |
| Lung infection | 0 (0 %) | 13 (0.7 %) | 2 (0.1 %) | 4 (0.2 %) | 0 (0 %) | 14 (0.7 %) | 0 (0 %) | 0 (0 %) |
| Myocardial infarction | 0 (0 %) | 11 (0.6 %) | 2 (0.1 %) | 2 (0.1 %) | 0 (0 %) | 11 (0.6 %) | 1 (0.1 %) | 0 (0 %) |
| Dizziness | 0 (0 %) | 15 (0.8 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 11 (0.6 %) | 0 (0 %) | 0 (0 %) |
| Diarrhea | 0 (0 %) | 16 (0.8 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 9 (0.5 %) | 0 (0 %) | 0 (0 %) |
| Heart failure | 0 (0 %) | 6 (0.3 %) | 1 (0.1 %) | 1 (0.1 %) | 0 (0 %) | 14 (0.7 %) | 2 (0.1 %) | 1 (0.1 %) |
| Pain | 0 (0 %) | 11 (0.6 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 14 (0.7 %) | 0 (0 %) | 0 (0 %) |
| Depression | 0 (0 %) | 4 (0.2 %) | 6 (0.3 %) | 0 (0 %) | 0 (0 %) | 12 (0.6 %) | 2 (0.1 %) | 0 (0 %) |
| Hyperglycemia | 0 (0 %) | 8 (0.4 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 14 (0.7 %) | 2 (0.1 %) | 0 (0 %) |
| Skin infection | 0 (0 %) | 12 (0.6 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 11 (0.6 %) | 1 (0.1 %) | 0 (0 %) |
| Headache | 0 (0 %) | 11 (0.6 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 12 (0.6 %) | 0 (0 %) | 0 (0 %) |
| Abdominal pain | 0 (0 %) | 9 (0.5 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 13 (0.7 %) | 0 (0 %) | 0 (0 %) |
| Left ventricular systolic dysfunction | 0 (0 %) | 5 (0.3 °b) | 2 (0.1 %) | 0 (0 %) | 0 (0 %) | 10 (0.5 %) | 4 (0.2 %) | 1 (0.1 %) |
| Infections and infestations - Other, specify | 0 (0 %) | 15 (0.8 %) | 0 (0 %) | 1 (0.1 %) | 0 (0 %) | 5 (0.3 %) | 0 (0 %) | 0 (0 %) |
| Surgical and medical procedures - Other, specify | 0 (0 %) | 11 (0.6 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 10 (0.5 %) | 0 (0 %) | 0 (0 %) |
| Hyponatremia | 0 (0 %) | 11 (0.6 %) | 2 (0.1 %) | 0 (0 %) | 0 (0 %) | 6 (0.3 %) | 1 (0.1 %) | 0 (0 %) |
Adverse events occurring in ≥10% of patients and all grade 3-5 events occurring in ≥1% of patients are reported in the table. Per protocol, adverse events were to be reported every 6 months during study therapy, 6 months after the last administered dose, and beyond 6 months after the last administered dose if possibly, probably, or definitely attributed to the investigational agent.
Only the following grade 2 adverse events (and grade 1 as noted in parentheses) had to be reported: acute coronary syndrome, cholesterol high, hypertriglyceridemia, arthralgia, myalgia, ischemia cerebrovascular, stroke (grade 1 and 2), transient ischemia attack (grade 1 and 2), peripheral ischemia, thromboembolic events, and visceral arterial ischemia.
Discussion
The NSABP B-42 trial is the largest trial evaluating extended adjuvant AI therapy in patients who were disease-free after five years of endocrine therapy primarily with an AI. Our findings did not demonstrate a statistically significant prolongation of DFS with extended letrozole therapy according to the protocol statistical plan. At first glance, our results appear discordant to those recently reported from the MA.17R trial,7 which enrolled 1,918 postmenopausal women with primary breast cancer who were free of recurrent disease after receiving 4.5-six years of adjuvant AI therapy, preceded in most patients by five years of tamoxifen. Patients were randomized within two years after completion of AI therapy to five years of placebo or letrozole. With a median follow-up of 6.9 years and 165 DFS events reported, the study demonstrated a statistically significant reduction in DFS events in favor of letrozole (HR=0.66, P=0.01; five-year DFS: placebo:91%, letrozole:95%). However, the MA.17R-definition of DFS included only breast cancer recurrence and contralateral breast cancer as events, which corresponds to the STEEP BCFI-definition employed in B-42 (HR=0.71, P=0.003). In a DFS analysis that also included deaths from any cause as first events, but not other non-breast second primary cancers, the MA.17R trial did not demonstrate a statistically significant improvement with extended letrozole (HR=0.80, P=0.06; five-year DFS: placebo:88%, letrozole:90%). Both trials showed no significant differences in OS with extended letrozole. Thus, although in both B-42 and MA.17R extended letrozole significantly reduced recurrence and distant recurrence, only a modest, statistically non-significant reduction in DFS as defined by the STEEP criteria was shown.20 Although reductions in breast cancer recurrence reflect the biologic effect of extended endocrine therapy, traditionally-defined DFS captures the overall clinical effect in the study populations of postmenopausal patients, some with preexisting co-morbidities and at risk of non-breast cancer-related events.
The B-42 results are also corroborated by two other randomized trials of extended adjuvant endocrine therapy recently reported (The DATA trial9 and the IDEAL trial8). In the DATA trial9, postmenopausal women with hormone receptor-positive early-stage breast cancer and no signs of disease recurrence after 2-3 years of adjuvant tamoxifen were randomly assigned to either 3 or 6 years of anastrozole. The primary study endpoint was DFS starting beyond 3 years after random assignment (adapted DFS). Of 1,860 eligible patients, 1,660 were disease free 3 years after random assignment. The 5-year adapted DFS was 83.1% (95% CI 80.0-86.3) in the 6-year group and 79.4% (76.1-82.8) in the 3-year group (HR=0.79 [95% CI 0.62-1.02]; P=0.066). Based on their findings, the authors concluded that they cannot recommend the use of extended adjuvant aromatase inhibition after 5 years of sequential endocrine therapy in all postmenopausal women with hormone receptor-positive breast cancer. In the IDEAL trial,8 postmenopausal patients with hormone receptor-positive breast cancer were randomly allocated to either 2.5 or five years of letrozole after the initial five years of any endocrine therapy. A total of 1,824 patients were assigned to either 2.5 years (n=909) or five years (n=915) of letrozole. With a median follow-up of 6.6 years there were no statistically significant differences in DFS between both groups (HR=0.92, 95% CI=0.74-1.16). There were also no statistically significant differences in OS or distant metastases-free survival between both groups but there was a statistically significant reduction in the occurrence of second primary breast cancer with five years of treatment (HR=0.39, 95% CI=0.19-0.81). The authors concluded that there was no superiority of five years over 2.5 years of extended adjuvant letrozole after an initial five years of adjuvant endocrine therapy. Because both of the above trials evaluated shorter differences in the duration of extended endocrine therapy compared to the B-42 trial (2.5-3 years vs. 5 years), their findings of no statistically significant DFS improvement with the longer regimen are not surprising in the context of the B-42 results.
More recently, two other trials evaluating extended adjuvant AI therapy were reported (ABCSG-16 and SOLE trials).10,11 In the ABSCG-16 trial,11 3,484 postmenopausal women with stage I-III, hormone-receptor positive breast cancer who had completed 5 years of endocrine therapy with either tamoxifen, an AI, or tamoxifen followed by an AI, were randomized to receive 2 vs. 5 years of anastrozole. With median follow-up of 106 months, there were no significant differences in DFS between the two groups (HR 1.007, 95% CI 0.87-1.16; p=0.925). Based on these findings, the authors concluded that after 5 years of adjuvant endocrine therapy, two additional years of anastrozole were sufficient for extended adjuvant therapy because a further extension to 5 additional years did not yield additional outcome benefit but added toxicity. In the SOLE multicenter, open-label, randomized, phase III trial,10 4,884 postmenopausal women with hormone receptor-positive, lymph node-positive, and operable breast cancer who had completed 4-6 years of adjuvant endocrine therapy were randomly assigned to either continuous letrozole for 5 years or intermittent letrozole for 9 months followed by a 3-month break in years 1-4 and then continuous letrozole for all 12 months of year 5. After a median follow-up of 60 months, there were no significant differences in DFS between the two groups (HR 1.08, 95% CI 0.93-1.26; p=0.31). The rates of AEs were also similar between the two groups. Thus, the results of the SOLE trial support the safety of intermittent administration of extended AI therapy. These two trials help to further refine the optimal duration of extended AI inhibitor therapy after five years of endocrine therapy.
Our finding that the majority of the reduction in DR events occurred after four years is of interest and a potential limitation of this report, because the reduction in DR from extended letrozole therapy may continue to increase with additional follow-up. At the same time, the late increase in AT events with letrozole vs. placebo requires additional follow-up to determine if further increase in the rate of AT events will continue to occur. A recent systematic review of randomized controlled trials that compared AIs and tamoxifen as primary adjuvant endocrine therapy in postmenopausal women showed that longer duration of AI use was associated with increased odds of developing cardiovascular disease (OR=1.26, P< .001) and bone fractures (OR=1.47, P< .001) but a decreased odds of venous thrombosis (OR=0.55, P< .001) and endometrial carcinoma (OR=0.34, P< .001).21 Furthermore, five years of AIs was associated with a non-statistically significant increased odds of death without recurrence compared with 5 years of tamoxifen alone or tamoxifen for 2-3 years followed by an AI for 2-3 years (OR=1.11, P=.09). These observations can also --at least partially-- explain the lack of OS benefit in all of the extended AI trials despite the observed reductions in breast cancer recurrence in some (B-42 and MA.17R).
Given the modest effect of extended letrozole on DFS, it is important to identify patient subgroups at higher risk for recurrence or who receive greater proportional benefit from extended endocrine therapy. Our multivariable analysis demonstrated that age, pathologic nodal status, prior tamoxifen use, and surgical procedure were independent predictors of DFS. Furthermore, the effect of extended letrozole appeared more pronounced in patients who had a mastectomy, those who received prior tamoxifen, and those with lowest BMD score of ≤−2.0, however none of these subgroup differences were statistically significant. Furthermore, there was no evidence to suggest that the effect of extended letrozole in patients was associated with BSP use at baseline. Thus, baseline clinico-pathologic factors and patient/treatment characteristics in B-42 were not particularly useful predictors of which subgroups of patients should be recommended extended endocrine therapy.
During the past few years, several attempts have been made to further refine risk of late recurrence after 5 years of endocrine therapy. These include the development of clinico-pathologic algorithms,22 assessment of circulating tumor cell counts,23 and evaluation of several commercially available genomic classifiers, some of which may also predict benefit from extended endocrine therapy.24–29 Incorporating such approaches into the clinical decision-making algorithm for recommending extended endocrine therapy may improve patient selection and optimize risk vs. benefit ratio. Correlative science studies utilizing B-42 tumor tissue are currently being planned.
Conclusions
Our findings suggest that careful assessment of potential risks and benefits is required before recommending extended letrozole therapy to patients with early-stage breast cancer. This assessment should include patient and tumor characteristics, existing comorbidities, information about bone mineral density, and tolerance of aromatase inhibitor treatment in the initial 5 years of treatment for breast cancer.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before the study
It has been well-established from clinical trials and overview analyses that patients with hormone-receptor positive, early-stage breast cancer are at considerable risk for recurrence well beyond the first five years from diagnosis. At the time when the B-42 trial was being designed (2005), literature search through Pubmed was performed to identify randomized clinical trials that could inform on the state of the art of adjuvant endocrine therapy in English. At that time, adjuvant tamoxifen for five years was the standard of care for premenopausal breast cancer patients. Clinical trials such as the aTTOM and ATLAS were evaluating extended adjuvant tamoxifen therapy for ten years compared to five years but had not reported results at that time. Eventually, these two trials demonstrated that 10 years of tamoxifen improved disease-free survival (DFS) (and breast cancer-specific mortality in the ATLAS trial) compared to five years. For postmenopausal patients, several clinical trials had shown significant improvement in DFS with the use of aromatase inhibitors (AIs) compared to five years of tamoxifen. There were three different approaches for the incorporation of AIs in the adjuvant setting and all three were compared to the standard of five years of tamoxifen: five years of an upfront AI (as evaluated in the AT AC and BIG-1-98 trials), two-to-three years of an AI after two-to-three years of tamoxifen (as evaluated in the ABCSG-8/ARNO 95 and the ITA trials), or five years of an AI after five years of tamoxifen (as evaluated in the MA. 17 and B-33 trials). All three approaches yielded statistically significant improvements in DFS compared to 5 years of adjuvant tamoxifen. Although the MA.17 and B-33 trials evaluated extended AI therapy after five years of tamoxifen, the benefit of extending adjuvant AI therapy beyond five years in patients who have received five years of an AI or two-to-three years of tamoxifen followed by two- to-three years of an AI was unknown at the time our trial started. Therefore, the NSABP B-42 (B-42) trial aimed to determine whether extending therapy past 5 years would improve disease-free survival in these patients.
Added value of this study
The B-42 trial showed that letrozole therapy did not significantly prolong disease-free survival after 5 years of hormonal therapy. However, extended letrozole therapy resulted in statistically significant reduction in breast cancer recurrence and distant recurrence. At first glance, the B-42 results are discordant to those recently reported from the NCIC MA.17R trial, which showed a statistically significant improvement in DFS with extended letrozole therapy in patients who had already received five years of letrozole (preceded in most by five years of tamoxifen). However, DFS in MA.17R included only breast cancer recurrence and contralateral breast cancer as events, which is the definition of breast cancer-free interval by the STEEP criteria. When the DFS endpoint in MA.17R is defined more closely to the STEEP criteria by including deaths as first event, there was a smaller and not statistically significant improvement in DFS with extended letrozole. The results of the B-42 trial are further corroborated by two other randomised trials (DATA and IDEAL), which compared longer and shorter durations of extended aromatase inhibitor therapy.
Implications of all the available evidence
When all the available evidence is taken in its totality, it appears that the benefit from extended AI therapy is modest. Thus, careful assessment of potential risks and benefits is required before recommending extended letrozole therapy to patients with early-stage breast cancer who are disease-free after five years of hormonal therapy, primarily with an AI. Further research is needed to identify biologic markers that predict risk of late recurrence and/or magnitude of benefit from extended AI therapy in order to optimize selection of candidates for extended AI therapy.
Acknowledgements
This work was supported by the National Cancer Institute at the National Institutes of Health, U.S. Department of Health and Human Services, Public Health Service grants U10-CA180868 (NCTN), U10-CA180822 (NRG SDMC), UG1CA189867 (NCORP), and U24-CA196067 (BSB). The Korea Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI13C2162)(SP); and Novartis.
The authors wish to thank Barbara C. Good, PhD, Wendy L. Rea, and Christine I. Rudock for editorial and graphics assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials registration:
Potential conflict(s) of interest
The following authors declare the following potential conflict(s) of interest:
EPM - - served as a consultant and speaker for Genomic Health, Inc, and consultant for Biotheranostics, outside the submitted work..
CEG, Jr. - - reports grants from National Cancer Institute, during the conduct of the study; personal fees from Celgene, personal fees from Myriad, other from AstraZeneca, outside the submitted work.
JMW - - reports personal fees from Genomic Health Inc, Biotheranostics, Roche, and Pfizer, outside the submitted work,
SMS - - reports grants, personal fees, and non-financial support from Genentech/Roche; personal fees from Novartis and AstraZeneca; personal fees and non-financial support from Eli Lilly & Co., Pieris Pharmaceuticals, and Inivata Ltd.; grants from Puma Biotechnology, Pfizer, and Merrimack Pharmaceuticals; and non-financial support from Caris Life Sciences and AstraZeneca.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group: Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351:1451–67, 1998 [PubMed] [Google Scholar]
- 2.Pan H, Gray R, Braybrooke J, et al. : 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 377:1836–1846, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss PE, Ingle JN, Martino S, et al. : A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–802, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Mamounas EP, Jeong JH, Wickerham DL, et al. : Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 26:1965–71, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Davies C, Pan H, Godwin J, et al. : Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805–16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray RG, Rea DW, Handley K, et al. : ATTom: randomized trial of 10 versus 5 years of adjuvant tamoxifen among 6,934 women with estrogen receptor-positive (ER+) or ER untested breast cancer—preliminary results. J Clin Oncol 26 (Suppl 10):Abstract 513, 2008 [Google Scholar]
- 7.Goss PE, Ingle JN, Pritchard Kl, et al. : Extending Aromatase-lnhibitor Adjuvant Therapy to 10 Years. N Engl J Med 375:209–19, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. : Optimal Duration of Extended Adjuvant Endocrine Therapy for Early Breast Cancer; Results of the IDEAL Trial (BOOG 2006-05). J Natl Cancer Inst 110, 2018. doi: 10.1093/jnci/djx134 [DOI] [PubMed] [Google Scholar]
- 9.Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, et al. : Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol 18:1502–11, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Colleoni M, Luo W, Karlsson P, et al. : Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19:127–138, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Gnant M, Steger G, Greil R, et al. : A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy – results from 3,484 postmenopausal women in the ABCSG-16 trial. San Antonio Brest Cancer Symposium, Abstract GS3-01:Abstract GS3-01, 2017 [Google Scholar]
- 12.White SJ, Freedman LS: Allocation of patients to treatment groups in a controlled clinical study. BrJ Cancer 37:849–57, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien PC, Fleming TR: A multiple testing procedure for clinical trials. Biometrics 35:549–56, 1979 [PubMed] [Google Scholar]
- 14.Anderson JR, High R: Alternatives to the standard Fleming, Harrington, and O’Brien futility boundary. Clinical trials 8:270–6, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Klein JP, Moeschberger ML: Survival Analysis: Techniques for Censored and Truncated Data. . Statistics for Biology and Health Eds: Dietz K, Gail M, Krickeberg K, Samet J, Tsiatis A Springer; New York: , 2003 [Google Scholar]
- 16.Cox DR: Regression models and life-tables (with discussion) . J Royal Stat Society B 34:187–202, 1972 [Google Scholar]
- 17.Lin DY, Wei LJ, Ying Z: Checking the Cox Model with Cumulative Sums of Martingale-Based Residuals. Biometrika 80:557–572, 1993 [Google Scholar]
- 18.Kaplan EL, Meier P: Nonparametric-Estimation from Incomplete Observations. J am Stat Asscoc 53:457–481, 1958 [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL: The statistical analysis of failure time data. Hoboken, N.J.: J. Wiley, , 2002 [Google Scholar]
- 20.Hudis CA, Barlow WE, Costantino JP, et al. : Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25:2127–32, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Amir E, Seruga B, Niraula S, et al. : Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 103:1299–309, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Dowsett M, Sestak I, Regan MM, et al. : Integration of Clinical Variables for the Prediction of Late Distant Recurrence in Patients With Estrogen Receptor-Positive Breast Cancer Treated With 5 Years of Endocrine Therapy: CTS5. J Clin Oncol 36: 1941–48, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparano JA, O’Neill A, Alpaugh K, et al. : Circulating tumor cells (CTCs) five years after diagnosis are prognostic for late recurrence in operable stage M-IM breast cancer. San Antonio Brest Cancer Symposium, Abstract GS6-03, 2017 [Google Scholar]
- 24.Dubsky P, Brase JC, Jakesz R, et al. : The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 109:2959–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolmark N, Mamounas EP, Baehner FL, et al. : Prognostic Impact of the Combination of Recurrence Score and Quantitative Estrogen Receptor Expression (ESR1) on Predicting Late Distant Recurrence Risk in Estrogen Receptor-Positive Breast Cancer After 5 Years of Tamoxifen: Results From NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-28 and B-14. J Clin Oncol 34:2350–8, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sgroi DC, Sestak I, Cuzick J, et al. : Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. The Lancet. Oncology 14:1067–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filipits M, Nielsen TO, Rudas M, et al. : The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clinical Cancer Res 20:1298–305, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Blok EJ, Bastiaannet E, van den Hout WB, et al. : Systematic review of the clinical and economic value of gene expression profiles for invasive early breast cancer available in Europe. Cancer Treat Rev 62:74–90, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Sgroi DC, Carney E, Zarrella E, et al. : Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 105:1036–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


