Abstract
The neuroprotective effects of neuregulin-1 (NRG-1) on stroke lesions were assessed longitudinally in rats with middle cerebral artery occlusion (MCAo) using MRI. Sprague-Dawley rats (n=16, 250±20g) underwent permanent MCAo surgery with cerebral blood flow (CBF) monitored by laser doppler flowmetry at ipsilateral side of bregma for 20 minutes post occlusion. A single 50 μl bolus dose of NRG-1 or vehicle was administered into the left internal carotid artery immediately prior to MCAo. The expansion of the ischemic lesion into the cortex was attenuated by NRG-1 over a 48-hour(hr) time span as measured by diffusion weighted imaging (DWI). The final infarct volumes of NRG-1 treated rats were significantly smaller than those of the vehicle treated group at 48 hr (264.8 ± 192.1 vs. 533.4 ± 175.5 mm3, p<0.05). The NRG-1 treated rats were further subdivided into 2 subgroups according to their CBF reduction during stoke surgery: mild ischemia (<70% CBF reduction) or severe ischemia (>70% CBF reduction). In particular, ischemic infarction was not usually observed in the cortex of NRG-1 treated rats with mild ischemia at 3 and 48 hr post occlusion. Histological results validated the imaging findings and demonstrated that NRG-1 treated rats had fewer injured neurons in peri-infarct areas 48 hr post ischemia. In summary, the longitudinal neuroprotective effect of NRG-1 in the pMCAo stroke model was demonstrated by prevention of ischemic lesion expansion, reduced infarct volume and protection of neurons from ischemic damage.
Keywords: NRG-1, MCAo, Rat, MRI, stroke, ischemia
1. Introduction
Stroke is the leading cause of adult disability and fourth leading cause of death in the United States (Towfighi and Saver, 2011). More than 700,000 people have a stroke each year in the United States. Among them, about 80% of strokes are caused by focal cerebral ischemia due to arterial occlusion and the remaining are caused by hemorrhages (Feigin et al., 2003). Currently, the only FDA-approved post-stroke intervention is intravenous thrombolysis using recombinant tissue plasminogen activator (rt-PA) within 4.5 hours (hr) after the symptom onset (Adams et al., 1993; Hacke et al., 2004). Treatment with rt-PA can provide reperfusion of the ischemic territory and results in the salvage of the ischemic penumbra, reduced final infarct size, and improved clinical outcomes. However, such thrombolysis strategy has many limitations including a short treatment time window and increased hemorrhage complication (Hacke et al., 1995; Wolpert et al., 1993). As a result, only few (typically 2% to 5%) patients are able to receive rt-PA reperfusion therapies in clinic practice (Adams et al., 1993; Hacke et al., 2004). Therefore, strategies that can extend the time window for thrombolysis are still being pursued extensively.
Neuregulin-1 (NRG-1) is a family of growth factors with multiple potent effects that includes acetylcholine receptor inducing activity (ARIA), glial growth factor-2, heregulins and neu differentiation factors (Falls et al., 1993). Its neuroprotective effects for ischemic brain injury have been reported (Ford et al., 2006; Li et al., 2007b; Xu et al., 2004; Xu et al., 2006). In a rat model with transient middle cerebral artery occlusion (tMCAo), NRG-1 reduced cortical infarct volume by 90% if administered immediately after the onset of reperfusion and exhibited a therapeutic window of >13 hr (Xu et al., 2004; Xu et al., 2006). Such treatment efficacy of NRG-1 on stroke was evaluated previously using histological analysis within 24 hr but not characterized longitudinally.
Diffusion-weighted imaging (DWI) is the most sensitive MRI modality to detect early stroke injury and has been widely utilized to assess the infarct evolution immediately after stroke onset (Schaefer et al., 2015; Zhang et al., 2014). Meanwhile, the apparent diffusion coefficient (ADC, derived from DWI) demonstrates to be a robust imaging marker to evaluate the stroke lesion evolution and is time-dependent during acute stroke (Schlaug et al., 1997). T2-weighted imaging is sensitive to brain tissue water content and can reveal vascular brain edama during acute stroke and identify the final infarct territory. In particular, quantitative T2 measurement can be used to estimate the water uptake in ischemic tissue (Venkatesan et al., 2000). The various MRI parameters provide complementary information to assess the structural alteration in stroke-damaged brains with or without therapeutic treatment.
The neuroprotective effects of NRG-1 on stroke injury have been demonstrated in our previous reports. However, there is no information reported about the spatial-temporal evolution of ischemic lesion treated by NRG-1 and its relationship with histological findings. The overall goals of this study are therefore to assess the neuroprotective effects of NRG-1 using MRI for better translation of findings in the animal into clinical trials.
2. Methods
Animal model preparation
All procedures followed the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University in accordance with the NIH Guide for Care and Use of Laboratory Animals. All surgical procedures were performed with sterile/aseptic techniques in accordance with institutional guidelines. Adult Sprague–Dawley rats weighing 230–270 g were used in this study. Rats were anesthetized using isoflurane (3% for induction and 2% for maintenance) mixed with O2. Permanent MCAo was induced by the intraluminal suture MCAo method as previously described (Li et al., 2007b; Xu et al., 2006). Briefly, the left common carotid artery (CCA) was exposed through a midline incision and was carefully dissected free from surrounding nerves and fascia. The internal carotid artery (ICA) was isolated and carefully separated from the adjacent vagus nerve, and the pterygopalatine artery was ligated close to its origin with a 6–0 silk suture. Then, a 40 mm 4–0 surgical monofilament nylon suture coated with rubber silicone (diameter 0.39mm length 2.3–2.5mm) was inserted from the external carotid artery (ECA) into the internal carotid artery (ICA) and then into the circle of Willis to occlude the origin of the left middle cerebral artery.
Laser Doppler flowmetry (LDF) (wavelength, 780 nm; probe 407, Perimed, Stockholm, Sweden) was used to continuously monitor cerebral blood flow (CBF) changes prior to, during, and 20 minutes following vessel occlusion to confirm appropriate occlusion. A micro drill was used to make a 0.9 mm diameter partial burr hole in the cranial skull at 2.0 mm posterior and 7.0 mm lateral to bregma. The burr hole did not penetrate the full thickness of the skull and the brain was not exposed in this procedure. The LDF probe was placed in the created defect in the skull and fixed with sterile tissue glue. Following surgery the probe was removed and the muscle and skin was closed. The CBF reduction was calculated as: (pre-occlusion CBF)-(post-occlusion CBF)/pre-occlusion CBF × 100%.
NRG-1 administration
To determine the effects of NRG-1 on ischemic stroke, rats were randomized and injected intra-arterially with a single bolus 50 μl dose of vehicle (1%BSA in PBS) or NRG-1β (20ug/kg, EGF-like domain, R&D Systems, Minneapolis, Minnesota) through a Hamilton syringe as previously described (Li et al., 2007b; Xu et al., 2004). NRG-1 (n=10) or vehicle (n=6) treated rats were administered by bolus injection into the ICA through ECA immediately before pMCAo. All NRG-1 and vehicle treatment studies were performed in a blinded manner. To evalute the potential effects induced by NRG-1, several physiological parameter including heart rate, respiratory rate, SpO2, temperature and CBF were continuously monitored at the baseline, during surgery and 20 minutes after injection of NRG-1 with the Mouse-Ox system (Starr life science, USA). During the surgery and MRI scans, the body temperature was monitored with a rectal probe (Starr life science, USA) and maintained at ~37 °C with a Homeothermic Blanket Control Unit (Harvard Apparatus).
MRI data acquisition
In vivo MRI was performed using a 7T animal MRI scanner (Bruker BioSpin, Billerica, MA) and a surface coil (internal diameter =2.5cm). The rats were immobilized with a custom-made holder during MRI scanning. All rats were imaged immediately after surgery from 0.5 hr to 3 hr and at 48 hr post surgery (Figure 1). Coronal MRI sections were performed from 2 mm anterior to the corpus callosum to the end of the cerebrum. T2-weighted imaging (T2WI) were acquired with the following parameters: field of view (FOV) = 3.0 × 3.0 cm2, matrix size = 256×256, repetition time (TR)=1000 ms and echo time (TE)=50 ms, slice thickness=1.0 mm. DTI was acquired with a four-shot EPI sequence. The imaging parameters were: TR=3000 ms, TE=32 ms, Δ=20 ms, δ=4 ms, field of view=3.0 × 3.0 cm2, slice thickness=1.0 mm, matrix size=128×128, image in-plane resolution=250×250 μm2, NEX=4, 30 gradient directions, b value= 1000 s/mm2, respectively. To determine the stroke lesion area, the ROI was identified by refering to DWI and T2-weighted images. With each rat brain, the area of the stroke lesion and total brain area in every slice was manually measured by ImageJ 1.34 (National Institutes of Health, Bethesda, MD). Then, the total stroke volume was calculated as the sum of the lesion area across all slices, multiplied by the total slice thickness.
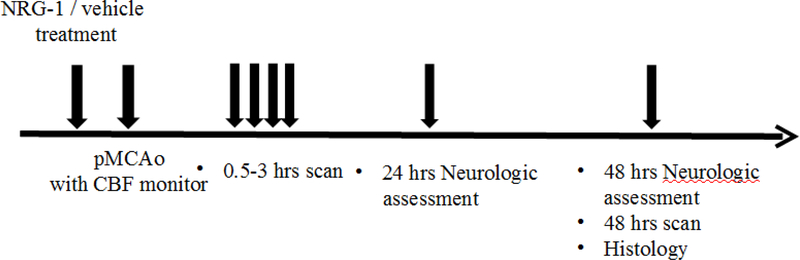
Figure 1.
Experimental protocol.
Experimental protocols of MRI evaluations of NRG-1 neuroprotective treatment response in rats with permanent MCA occlusion (pMCAo).
Histopathology evaluation
Rats were sacrificed for histological evaluation immediately after their last MRI scans (48 hr post stroke). After MCAo, 1.0 mm-thick brain sections were stained with 2% triphenyltetrazolium chloride (TTC) solution and then transferred into a 4% formaldehyde solution for fixation. For immunohistology, brain specimens were processed using standard histological protocols (Li et al., 2007b; Xu et al., 2004; Xu et al., 2005). Briefly, rat brains were perfusion-fixed through the left cardiac ventricle with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. Brains were quickly removed and cryoprotected in 30% sucrose. The brains were then frozen in OCT compound (Sakura Finetek, Torrance, CA) and stored at −80°C until sectioning. Coronal sections of 20 μm thickness were cryosectioned and mounted on slides which were then stored at −80°C until further processed. Brain sections were washed in PBS and incubated with Cy3 conjugated anti-NeuN (1:500, Millipore) overnight at 4°C. Slides were then washed with PBS and mounted with Vectashield mounting medium containing DAPI. For double staining with Fluoro-Jade B (FJB) (Chemicon International, Temecula, CA, USA) double labeling, immunostaining was performed first and then sections were incubated with 0.0015% potassium permanganate for 1 min, washed with distilled water for 2 minutes and treated with 0.0001% FJB in 0.1% acetic acid for 10 min. Sections were then washed and coverslipped in glycerol/0.3% acetic acid mounting medium. All sections were examined with fluorescence microscopy in three random MCA served areas in the inner border of the infarct in the ischemic fronto-parietal cortex of each rat.
Neurological assessment
Standard neurological tests, utilizing a 6-point neurological scale (0= no deficit, 1= failure to extend left forepaw fully, 2= circling to the left, 3= falling to the left, 4= no spontaneous walking with a depressed level of consciousness, and 5= dead), were performed at 24 hr and 48 hr post occlusion (van Swieten et al., 1988).
Statistical Analysis
All results were expressed as mean ± standard deviation (SD). The student t-test was applied to evaluate differences between the stroke volumes of the vehicle and treatment groups. We further analyzed the infarct volumes of the NRG-1 treated rats, by dividing animals into two subgroups based upon the CBF reduction after pMCAo (mean CBF reduction = 76%): the mild ischemia group which consisted of the rats with less than 70% CBF reduction and the severe ischemia group which had the rats with more than 70% CBF reduction during surgery. A one-way analysis of variance (ANOVA) test, followed by the Tukey test, was applied to analyze the statistical differences in infarct volume, DWI and T2 values between vehicle and NRG-1 treatment groups. The Pearson correlation test was used to evaluate correlations between MRI results and CBF reduction. All statistical analysis procedures were performed using the SPSS for Windows statistical package (Version 18, SPSS, Chicago, IL). A p-value of <0.05 was considered statistically significant.
3. Results
Physiological parameters after MCAo and NRG-1 administration
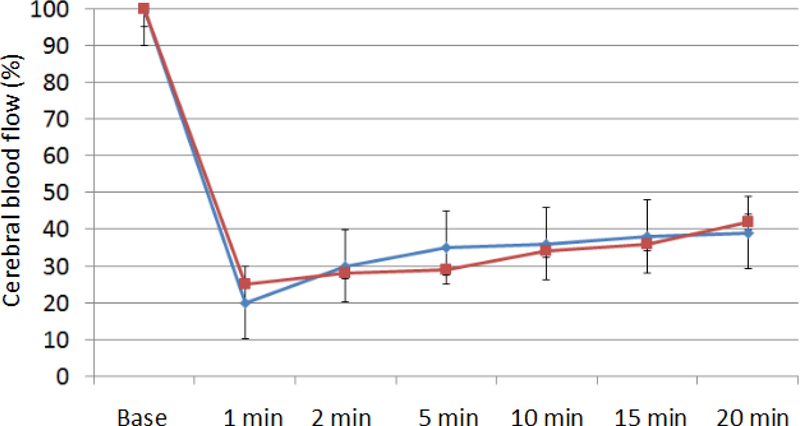
Physiological parameters were continuely monitored at the baseline, during surgery and 20 minutes after injection of NRG-1. NRG-1 did not significantly affect any of the following physiological parameters in our studies, including of heart rate, respiratory rate, SpO2, temperature (Table 1), consistent with our findings using the transient MCAo model (Xu et al., 2006). Also, NRG-1 did not alter CBF significantly, suggesting that vascular blood flow was not affected significantly due to the administration of NRG-1 (Figure 2).
Table 1 –
Physiological parameter including heart rate, respiratory rate, SpO2 and body temperature were continued monitored at the baseline, during surgery and 20 minutes after injection of NRG-1 or vehicle. NRG-1 did not significantly affect any of the following physiological parameters (determined by ANOVA).
| Baseline | During surgery | 20 mins after NRG-1 injection | |
|---|---|---|---|
| HR (per min) | 287±28 | 279±24 | 263±35 |
| Respiratory rate (per min) | 86±13 | 83±7 | 83±12 |
| SpO2 (%) | 98.2±0.7 | 95.6±2.4 | 94.9±2.6 |
| Temperature (ºC) | 36.4±0.4 | 36.6±0.6 | 36.8±0.6 |
Figure 2-.
CBF measurements from LDF
The line chart indices the CBF measurements of LDF from the baseline to 20 min post MCAo. NRG-1 was injected immediately before the MCAo. No significant differences of CBF were found between NRG-1 and vehicle rats. Red line=NRG-1 treated rats, blue line=vehicle rats.
Stroke lesion volume analysis after NRG-1 treatment
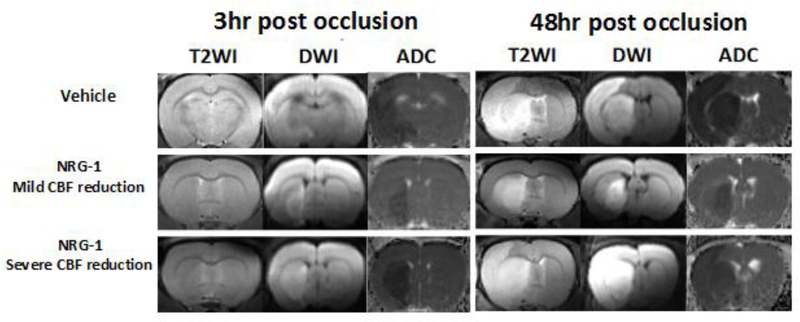
Infarct volumes following MCAo were measured with longtiduainal DWI images (1–3 hr post occlusion) and T2WI at the 48 hr time point. Compared to the vehicle treated group, the infarct volumes of NRG-1 treated group were significantly smaller at 0.5 hr (85.0 ± 20.1 vs. 44.4 ± 21.2 mm3), 1 hr (118.6 ± 70 vs. 56.5 ± 27.1 mm3), 2 hr (147.2 ± 75.6 vs. 75.6 ± 41.2 mm3), 3 hr (211.1 ± 126.9 vs. 83.0 ± 45.6 mm3) and 48 hr (533.4 ± 175.4 vs. 264.8 ± 192.1 mm3) post occlusion (all p<0.05). In the vehicle-treated rats, a brain region with ADC hypointensity and DWI hyperintensity was observed at both striatum (100% of animals) and cortex (83% of animals, 5/6) as early as 0.5 hr post occlusion. On the contrary, NRG-1 treated rats showed ischemic lesions mostly within the striatum (70%, 7/10) at the 3 hr and the later 48 hr time point (Figure 3). It is known that early injury in the striatum is the core ischemic area in the MCAo model (Ginsberg, 2003), therefore the ischemic lesion after NRG-1 treatment suggests its penumbral protection in the cortex.
Figure 3-.
MRI of stroke lesion in NRG-1 and vehicle treated rats
The stroke lesions in the vehicle and treatment group at 3 hr and 48 hr post occlusion. In a rat in the vehicle group (65% CBF reduction during surgery), the stroke volumes are 327.3 mm3 and 660.3 mm3, respectively. In a rat in the treatment group, the ischemic region was only found in the striatum whereas no obvious ischemic region was seen in the cortex at 3 hr and 48 hr post occlusion. There was a 65% CBF reduction during the pMCAo surgery. In a rat with 95% CBF reduction, the ischemic region was only found in the striatum on 3 hr post occlusion. However, the ischemic regions expand to most of ipsilateral brain including cortex.
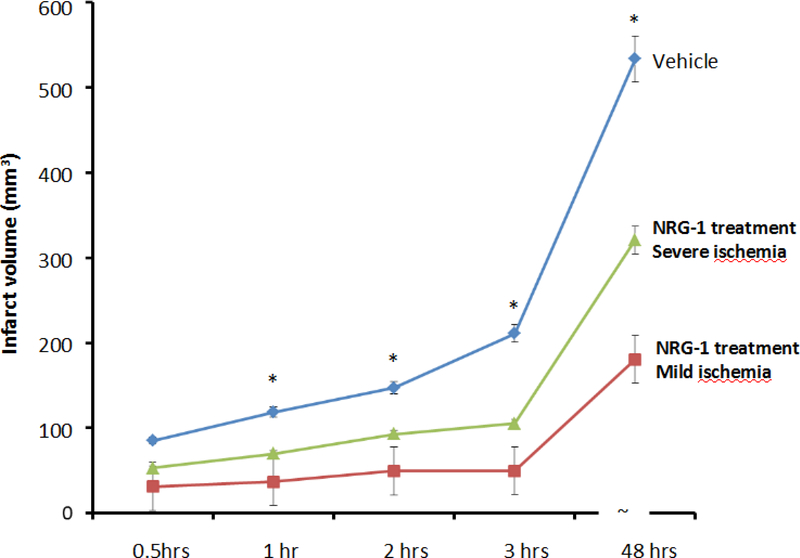
In order to evaluate the treatment efficacy of NRG-1 in rats with different severety of ischemia, we further analyzed the infarct volumes of the NRG-1 treated rats, which were divided into two subgroups based upon the CBF reduction after MCAo and the results are shown in Figure 4.1. NRG-1 treated rats have significantly decreased infarct volume compared to vehicle group (both p<0.05) following both mild and severe CBF reduction. The infarct volumes of NRG-1 treated group were significantly smaller than those in vehicle treated group at all time points post occlusion (all p<0.05). In contrast, no significant difference was observed in the infarct volumes of vehicle group rats divided by the same CBF reduction threshold (p >0.30 at all time points). When comparing the infarct volume in mild and severe ischemia group according to CBF reduction during surgery, the NRG-1 was more effective at neuroprotection in the mild ischemia group. Longitudinally, the mean stroke volume at 48 hr was significantly larger than that at any early points (p<0.001) in both vehicle and treatment group. At 48 hr post occlusion, infarct volume was reduced by 66% in the mild CBF reduction group compared to 40% in the severe group (180.8 ± 70.8 vs. 321.0 ± 232.3 mm3, p<0.05). Overall, significant but weak correlations between the mean stroke volume and CBF reduction during the surgery were observed (p=0.003, r=0.326).
Figure 4.1 –
The final infarct volume was identified by serial DWI images and T2WI on different time points.
The infarct volumes of vehicle group (blue line) were significantly larger than those in NRG-1 treated group at all time points post occlusion (all p<0.05). When comparison of the stroke volume in mild (red line, square marker) and severe ischemia group (green line, triangle marker) according to CBF reduction during surgery, the neuroprotective effects of NRG-1 seems more obvious in the mild ischemia group. At 2 and 3 hr post occlusion, there were 47% and 53% volume reduction in mild ischemic group compared to those in severe goup (p<0.05). On 48 hr post occlusion, 47% volume reduction is noticed in the mild group compared to severe group (180.8 ± 70.8 vs. 321.0 ± 232.3 mm3, p<0.05). The star indicated the significant difference between vehicle and severe ischemia group.
In addition, we evaluated the temporal evolution of stroke infarct volumes with the formula reported previously(Zhang et al., 2015):
| Equation 1 |
In which t is the time post occlusion, V is the lesion volume at time t (t > 0, in hour), C is the growth rate of lesion volume per logarithmic time scale, V0 is the baseline value at the time t = 1, ln is the natural logarithms.
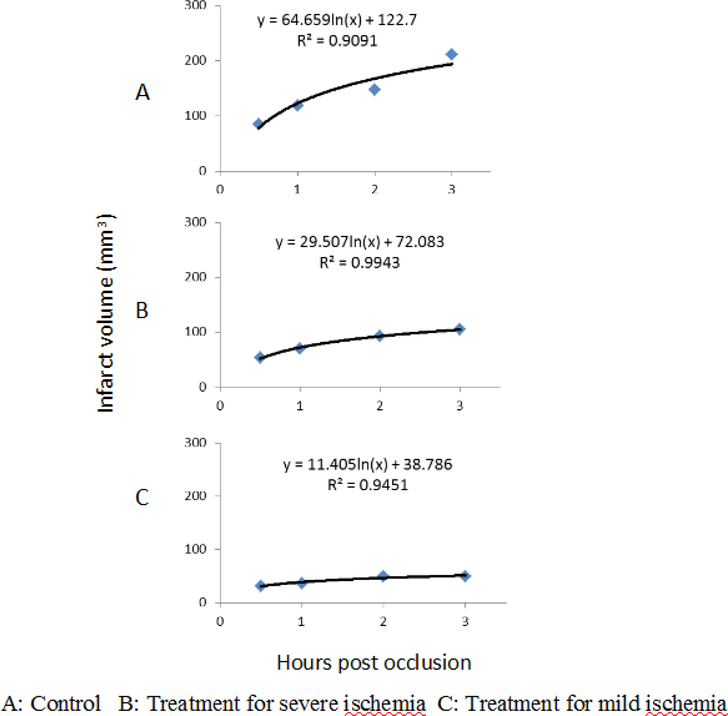
The fitting results are shown in Fig 4.2. It is shown that the evolution of the infarct volume follows the longarithmic pattern in rats without treatment (R2=0.9091), as similar as those reported previously in macaque brains with pMCAo. Such pattern is also seen in rats with NRG-1 treatment with slightly reduced correlation (R2=0.9943 and 0.9451 with severe and mild stroke, respectively). In particular, the infarct growth rates decrease remarkably in rats of treatment groups comparied to that in control group.
Fig 4.2.
The longitudinal evolution of the infarct volume changes are fitted with a longarithmic pattern in rats with/without NRG-1 treatment. Different growth rates are seen in rats without treatment and with treatment for severe and mild ischemia.
Histological evaluation of brains following ischemia and NRG-1 treatment
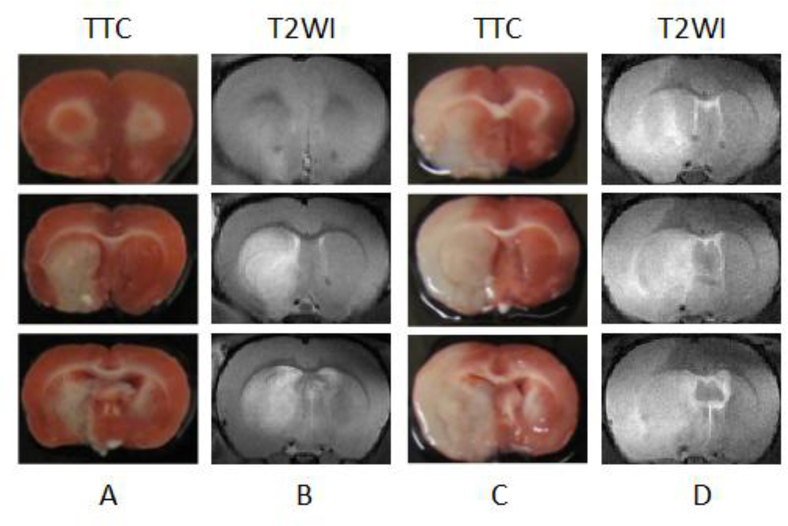
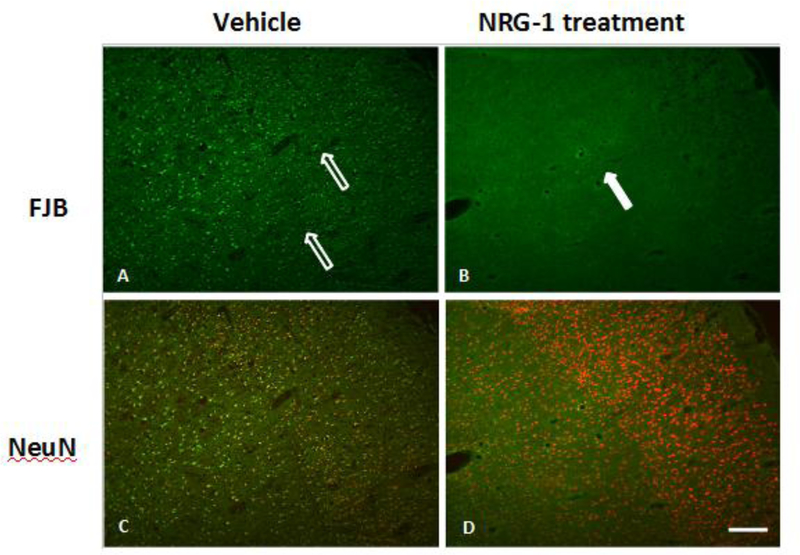
We performed histopathological analysis to examine neuronal injury in rat brains with and without NRG-1 treatment. The infarction territory detected by MRI at 48 hr post stroke correlated well with that seen in TTC staining (Figure 5). The immunohistological results of NRG-1 treated and vehicle rats at 48 hr post occlusion are shown in Figure 6. FJB labeling of brain tissues collected 48 hr after vehicle treament revealed numerous FJB-positive cells in the ischemic cortex and subcortical areas (Figure 6A). in contrast, NRG-1 pretreatment effectively abolished cortical FJB labeling in a similar regional pattern as illustrated in representative photomicrographs (Figure 6B). The injured areas showed high numbers of FJB labeling, which co-localizes with the low or no NeuN expressing cells (Figure 6C). Neighboring neurons that were not injured showed relatively higher levels of NeuN immunoreactivity. NRG-1 treatment rescued NeuN immunoreactivity (Figure 6D).
Figure 5 –
Staining by 2,3,5-triphenyltetrazolium chloride (TTC) matched regions of infarction on MRI at 48 h after MCAo. (A and C) Brain section stained TTC. (B and D) Corresponding T2-weighted MRI. A and B are NRG-1 treated rat. C and D for vehicle rat.
Figure 6 –
FJB and NeuN in cortex and striatum after NRG-1 treatment.
In vehicle group (A), FJB positive cells widely distributed among the cortex where MCA supplied (open arrows). However FJB positive cells could not be observed at cortex in NRG-1 treated group (closed arrow) (B). In vehicle group(C), NeuN positive neuron show obvious morphological changes of cell shrinkage and chromatin condenses. However, relative normal neuron are found in the cortex in the NRG-1 treated rats(D). The immunohistology results of treatment and vehicle pMCAo rats at 48 hr post surgery. Scale bar =100μm.
Neurologic assessment of rats following MCAo and NRG-1 treatment
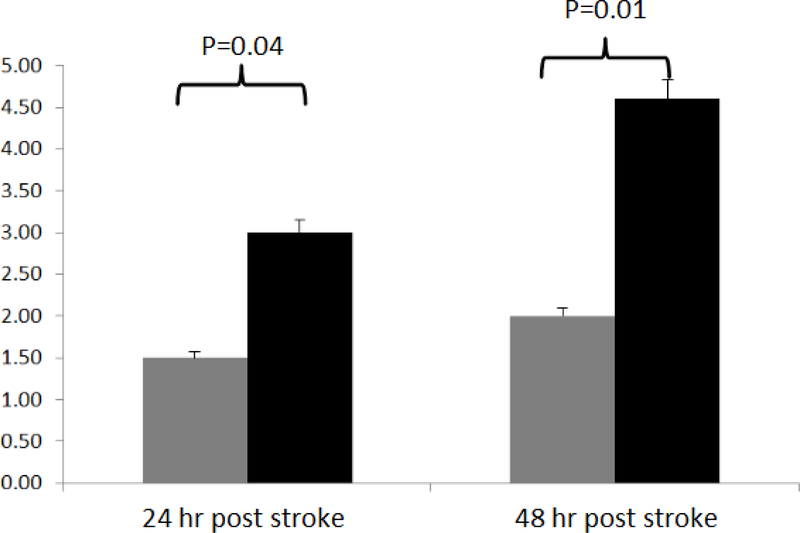
The mean neurological scores of NRG-1 versus vehicle-treated rats were, 1.5 vs. 2.0 at 24 hr (p=0.04), and 3.0 vs. 4.7 at 48 hr post occlusion (p=0.01), respectively (Figure 7). The results indicated that NRG-1 treated rats had less progression of general decline in neurological status compared to vehicle group.
Figure 7 –
Neurological assessment.
Standard neurological tests utilizing a 6-point neurological scale (0= no deficit, 1= failure to extend left forepaw fully, 2= circling to the left, 3= falling to the left, 4= no spontaneous walking with a depressed level of consciousness, and 5= dead) were performed at 24 hr and 48 hr post occlusion. The mean neurological scores of NRG-1 versus vehicle-treated rats were, 1.5 vs. 2.0 at 24 hr (p=0.04), and 3.0 vs. 4.7 at 48 hr post occlusion (p=0.01), respectively. Gray bar=NRG-1 treated rats, black bar=vehicle treated rats.
4. Discussion
We assessed the neuroprotective effects of NRG-1 in a rat model of pMCAo using MRI to determine the spatio-temporal relationships between ischemic insults and NRG-1 treatment effects. We demonstrated that NRG-1 provided neuroprotection during the acute ischemic insult as indicated by reducing the infarct volume and reduced injury of neurons following ischemic insults, consistent with our previous results (Li et al., 2007b; Xu et al., 2004; Xu et al., 2006). In particular, it is found that the efficacy of NRG-1’s neuroprection is related with the severity of stroke insult. As seen in the present report, NRG-1 exhibits significantly stronger neuroprotection in rats with mild ischemia than those with severe ischemia 48 hours post occlusion.
Diffusion MRI has been widely used in animal and clinical studies to evaluate ischemic brain injury because of its unique ability for very early detection of ischemic lesion and the correlation with final infarct volume (Lutsep et al., 1997), a strong predictor of clinical outcome. Early signal increase of DWI images reflects cell swelling in ischemic brain tissue due to energy failure of Na+-K+ ATP pump. In addition, ADC allows for quantitatively characterizing ischemic lesion. After initial decrease of ADC values in acute phase, ADC gradually recovers to baseline value due to water influx from vessels to brain tissue, namely vasogenic edema (Olah et al., 2000). NRG-1 treated group showed relatively slow ADC recovery until 48 hr, especially in the mild ischemic lesion group, indicating the mitigated impact of the vessel occlusion on neuronal injury compared to vehicle group. Histological evaluation also indicated that there were much fewer injured neurons in the NRG-1 treated group compared to vehicle group.
It has been reported that the infarct volume evolution in macaque brains with pMCAo follows a logarithmic pattern during acute stroke for up to 48 hours post occlusion (Zhang et al., 2015). In the present study, the formula was also applied to fit the infarct evolution in rats with pMCAo to examine the temporal-spatial changes of infarct with/without treatment. As seen in Fig 4.2, the temporal changes of infarct volume in control and treatment groups exhibits a good fitting of logarithmic pattern, as similar as those seen in macaque brains with pMCAo (without treatment). After NRG-1 treatment, the growth rates of infarct volume dramatically decrease (64.6, 29.5, and 11.4 in control group, treatment groups with severe and mild ischemia, respectively. unit: mm3 per hour (log scale)), indicating the treatment intervention alters the course of infarct evolution remarkably. Also, the growth rate is much smaller in rats with mild ischemia in which higher neuroprotection efficacy of NRG-1 was observed in the same rats, suggesting that the growth rate may be a complementary and sensitive indicator to assess the efficacy of pharmaceutical treatment in subjects with pMCAo.
The exact mechanism(s) of the NRG-1 neuroprotective effects remain inconclusive. Without supply of oxygen and glucose, the cell death occurs due to the energy failure, which leads to shut down of ATP dependent Na+-K+ATPase. Consequently, massive Na+ and Ca2+ cytoplasmic accumulation leads to overactivation of the NMDA subtype of glutamate receptors. Finally, cytoplasmic accumulation or Ca2+ untimately leading to necrotic cell death (Lipton, 1999). NRG-1 may block NMDA receptors which prevents glutamate-mediated excitotoxic as well as necrotic neuronal death (Gwag et al., 1995). Another possible mechanism is that NRG-1 may play a key role in inhibition of apoptotic and pro-inflammatory responses (Falls et al., 1993; Xu et al., 2004; Xu et al., 2005; Xu et al., 2006). NRG-1 prevented ischemia-induced mononuclear infiltration, astrocyte activation and cytokine production, which are known to be associated with delayed neuronal death following ischemia(Falls et al., 1993; Xu et al., 2004; Xu et al., 2005; Xu et al., 2006).
Another important question is that whether physiological parameters, including CBF are altered after NRG-1 administration. Xu et al (Xu et al., 2006) analyzed physiological parameters in vehicle and NRG-1-treated animals before and after transient MCAo and showed that NRG-1 did not significantly affect any of the physiological parameters including pH, pCO2, PO2, Hct (%), Na+, K+, Ca2+, heart rate, mean arterial pressure and blood pressure. Simlarly, our current data suggested that NRG-1 did not affect physiological parameters or CBF during the MCAo procedure. Further perfusion measurements with MRI may be employed to validate the CBF results in the future study.
The neuroprotective effects of NRG-1 was partially contingent on the initial severity of ischemic insults. Our results show that NRG-1 neuroprotective effects are pronounced in mild ischemic insults (i.e., less than 70% CBF reduction) compared to severe ischemic insults. The CBF threshold was determined empirically based upon the present study. Initial CBF reduction status after stroke insult may be a key factor to determine the NRG-1 neuroprotective efficacy. Additionally, the neuroprotective effects of NRG-1 at current dose were associated with the severity of injury and the CBF threshold and could be a dose-dependent effect. The dosage effect of NRG-1 will be further studied to determine the optimal dosage for maximal neuroprotection during mild and severe ischemia.
Our findings suggest that NRG-1 given before pMCAO can reduce the infarction. The results from other models in our laboratory also indicate that a single administration of NRG-1 is sufficient to block the downstream mechanisms of acute brain injury, even in the presence of the insult. We reported previously that NRG-1 blocked downstream neurotoxic and pro-inflammatory responses in animal models of nerve agent exposure and experimental cerebral malaria without affecting the levels of AChE inhibition or parasite numbers, respectively (Li et al., 2007a; Solomon et al., 2014). Therefore, it is not unexpected that NRG-1 prevents neuronal death despite the persistence of CBF reduction. We propose that NRG-1 blocks the pro-inflammatory responses downstream of ischemia that lead to secondary neuronal death.
NRG-1 was deliveded intra-arterially (i.a.) before pMCAo in the present study. With regards to i.a. administration, there is clear precedence for its utility in a number of neurological disorders, including stroke (Connors, 2002; Higashida et al., 2003). In fact, rt-PA, the only drug FDA approved for stroke therapy, was shown in clinical trials to be safe and efficacious 6 hours after stroke compared to the 3–4 hour therapeutic window for intravenous (i.v.) administration (Ellis et al., 2011). However, we expect that i.v. administration of NRG-1 will similarly provide neuroprotection as previously demonstrated in other models of acute brain injury (Iaci et al., 2010; Lok et al., 2007). In addition, ongoing pilot studies are examining whether NRG-1 can also be delivered i.v. and intra-nasally to treat ischemic neuronal injury.
In summary, the longitudinal, spatio-temporal neuroprotective effects of NRG-1 were demonstrated in a rat model of stroke using MRI and histological evaluations. More studies are needed to fully understand the mechanisms of NRG-1 neuroprotective effects and to optimize the treatment strategy in stroke patients. In vivo multiparametric MRI could serve as a valuable monitor tool in this endeavor.
Highlights.
NRG-1 shows significant neuroprotective effects in pMCAo model.
NRG-1 treated rats have smaller infraction volume and better neurological recovery.
NRG-1 treatment effect was influenced by CBF reduction during stroke.
Acknowledgements
The authors thank Ruth Connelly, Paul Chen, and Doty Kempf (DVM) for animal handling. This project was supported in part by NCRR and currently by the Office of Research Infrastructure Programs of NIH (OD P51OD011132, P51RR000165).
Footnotes
Conflict of interest statement:
Patent related to the work being reported is held by the author without direct corporate involvement at the time.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams H, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh E.r., 1993. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 24, 35–41. [DOI] [PubMed] [Google Scholar]
- Connors JJ 3rd, 2002. Interventional stroke therapy: the potential benefit of direct intra-arterial infusion. Rev Cardiovasc Med. 3 Suppl 2, S92–9. [PubMed] [Google Scholar]
- Ellis JA, Youngerman BE, Higashida RT, Altschul D, Meyers PM, 2011. Endovascular treatment strategies for acute ischemic stroke. Int J Stroke. 6, 511–22. [DOI] [PubMed] [Google Scholar]
- Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD, 1993. Aria, a Protein That Stimulates Acetylcholine-Receptor Synthesis, Is a Member of the Neu Ligand Family. Cell. 72, 801–815. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CMM, Bennett DA, Anderson CS, 2003. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurology. 2, 43–53. [DOI] [PubMed] [Google Scholar]
- Ford G, Xu ZF, Gates A, Jiang J, Ford BD, 2006. Expression Analysis Systematic Explorer (EASE) analysis reveals differential gene expression in permanent and transient focal stroke rat models. Brain Research. 1071, 226–236. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, 2003. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection: the 2002 Thomas Willis Lecture. Stroke. 34, 214–23. [DOI] [PubMed] [Google Scholar]
- Gwag BJ, Lobner D, Koh JY, Wie MB, Choi DW, 1995. Blockade of Glutamate Receptors Unmasks Neuronal Apoptosis after Oxygen-Glucose Deprivation in-Vitro. Neuroscience. 68, 615–619. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, Vonkummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH, Hennerici M, 1995. Intravenous Thrombolysis with Recombinant Tissue-Plasminogen Activator for Acute Hemispheric Stroke - the European Cooperative Acute Stroke Study (Ecass). Jama-Journal of the American Medical Association. 274, 1017–1025. [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, Von Kummer R, Broderick J, Brott T, Frankel M, Grotta J, Haley E Jr, 2004. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 363, 768–774. [DOI] [PubMed] [Google Scholar]
- Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D, 2003. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 34, e109–37. [DOI] [PubMed] [Google Scholar]
- Iaci JF, Ganguly A, Finklestein SP, Parry TJ, Ren J, Saha S, Sietsma DK, Srinivas M, Vecchione AM, Caggiano AO, 2010. Glial growth factor 2 promotes functional recovery with treatment initiated up to 7 days after permanent focal ischemic stroke. Neuropharmacology. 59, 640–9. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu Z, Ford GD, Croslan DR, Cairobe T, Li Z, Ford BD, 2007a. Neuroprotection by neuregulin-1 in a rat model of permanent focal cerebral ischemia. Brain Res. 1184, 277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YG, Xu ZF, Ford GD, Croslan DR, Cairobe T, Li ZZ, Ford BD, 2007b. Neuroprotection by neuregulin-1 in a rat model of permanent focal cerebral ischemia. Brain Research. 1184, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P, 1999. Ischemic cell death in brain neurons. Physiol Rev. 79, 1431–568. [DOI] [PubMed] [Google Scholar]
- Lok J, Wang H, Murata Y, Zhu HH, Qin T, Whalen MJ, Lo EH, 2007. Effect of neuregulin-1 on histopathological and functional outcome after controlled cortical impact in mice. J Neurotrauma. 24, 1817–22. [DOI] [PubMed] [Google Scholar]
- Lutsep HL, Albers GW, DeCrespigny A, Kamat GN, Marks MP, Moseley ME, 1997. Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol. 41, 574–80. [DOI] [PubMed] [Google Scholar]
- Olah L, Wecker S, Hoehn M, 2000. Secondary deterioration of apparent diffusion coefficient after 1-hour transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 20, 1474–82. [DOI] [PubMed] [Google Scholar]
- Schaefer PW, Souza L, Kamalian S, Hirsch JA, Yoo AJ, Kamalian S, Gonzalez RG, Lev MH, 2015. Limited reliability of computed tomographic perfusion acute infarct volume measurements compared with diffusion-weighted imaging in anterior circulation stroke. Stroke. 46, 419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S, 1997. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology. 49, 113–9. [DOI] [PubMed] [Google Scholar]
- Solomon W, Wilson NO, Anderson L, Pitts S, Patrickson J, Liu M, Ford BD, Stiles JK, 2014. Neuregulin-1 attenuates mortality associated with experimental cerebral malaria. J Neuroinflammation. 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi A, Saver JL, 2011. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 42, 2351–5. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J, 1988. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 19, 604–7. [DOI] [PubMed] [Google Scholar]
- Venkatesan R, Lin W, Gurleyik K, He YY, Paczynski RP, Powers WJ, Hsu CY, 2000. Absolute measurements of water content using magnetic resonance imaging: preliminary findings in an in vivo focal ischemic rat model. Magn Reson Med. 43, 146–50. [DOI] [PubMed] [Google Scholar]
- Wolpert SM, Bruckmann H, Greenlee R, 1993. Neuroradiologic Evaluation of Patients with Acute Stroke Treated with Recombinant Tissue-Plasminogen Activator (Vol 14, Pg 3, 1993). American Journal of Neuroradiology. 14, 562–562. [PMC free article] [PubMed] [Google Scholar]
- Xu ZF, Jiang J, Ford G, Ford BD, 2004. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochemical and Biophysical Research Communications. 322, 440–446. [DOI] [PubMed] [Google Scholar]
- Xu ZF, Ford GD, Croslan DR, Jiang J, Gates A, Allen R, Ford BD, 2005. Neuroprotection by neuregulin-1 following focal stroke is associated with the attenuation of ischemia-induced pro-inflammatory and stress gene expression. Neurobiology of Disease. 19, 461–470. [DOI] [PubMed] [Google Scholar]
- Xu ZF, Croslan DR, Harris AE, Ford GD, Ford BD, 2006. Extended therapeutic window and functional recovery after intraarterial administration of neuregulin-1 after focal ischemic stroke. Journal of Cerebral Blood Flow and Metabolism. 26, 527–535. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tong F, Li CX, Yan Y, Nair G, Nagaoka T, Tanaka Y, Zola S, Howell L, 2014. A fast multiparameter MRI approach for acute stroke assessment on a 3T clinical scanner: preliminary results in a non-human primate model with transient ischemic occlusion. Quant Imaging Med Surg. 4, 112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tong F, Li CX, Yan Y, Kempf D, Nair G, Wang S, Muly EC, Zola S, Howell L, 2015. Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. PLoS One. 10, e0117290. [DOI] [PMC free article] [PubMed] [Google Scholar]