Abstract
Background
While accumulating evidence suggests that balance and gait impairments are commonly seen in patients with essential tremor (ET), questions remain regarding their prevalence, their relationship with normal aging, whether they are similar to the impairments seen in spinocerebellar ataxias, their functional consequences, and whether some ET patients carry greater susceptibility.
Methods
We conducted a literature search (until December 2018) on this topic.
Results
We identified 23 articles on gait or balance impairments in ET. The prevalence of balance impairment (missteps on tandem walk test) was seven times higher in ET patients than controls. Gait impairments in ET included reduced speed, increased asymmetry, and impaired dynamic balance. While balance and gait problems worsened with age, ET patients were more impaired than controls, independent of age. The pattern of impairments seen in ET was qualitatively similar to that seen in spinocerebellar ataxias. Balance and gait impairments resulted in greater number of near falls in ET patients. Factors associated with balance and gait impairments in ET included age, presence of tremor in midline structures, and cognitive dysfunction.
Discussion
Accumulating evidence suggests that balance and gait impairments are common in ET patients and occur to a greater extent in controls. Thus, they represent a disease-associated feature. These impairments, which are qualitatively similar to those seen in spinocerebellar ataxias, are not merely subclinical but result in difficulty performing functional tasks and increase falls risk. A subset of patients is more susceptible to balance and gait impairments. The full spectrum of impairments remains to be characterized.
Keywords: Essential tremor, mobility, gait, balance, posture, ataxia
Introduction
Essential tremor (ET) is among the most common movement disorders, and its primary feature is action (i.e., kinetic and postural) tremor.1 This tremor occurs mainly in the arms, although it may also be seen in the neck and other parts of the body.2 Historically, ET was considered a monosymptomatic disorder. However, results from a number of studies in the past decade have challenged this view. ET is now recognized as a disease, or a family of diseases, with both motor and non-motor features.3,4
While tremor is the defining feature of ET, impairments of gait and balance are also commonly seen.5 Numerous studies have shown that ET patients have difficulty with tandem walk, in which subjects are required to walk 10 steps in a straight line, with the heel of the leading foot touching the toe of the following foot.6,7,8,9 Early studies examining tandem walk suggested that balance impairment during this test might be because of advanced age of ET patients.7,9 However, recent case studies have challenged this notion.6,10,11 Furthermore, quantitative analysis of gait with more sophisticated instrumentation has allowed for an examination of physiological parameters that are not obvious on clinical examination.12,13,14,15,16,17,18,19 These quantitative studies indicate that ET patients have impairments such as reduced gait speed, impaired dynamic balance, and increased step-to-step variability.
Despite recent advances in characterizing balance and gait dysfunction in ET, a number of questions remain unanswered: (1) How common are balance and gait impairments in ET? (2) Are balance problems in ET simply the result of advanced age? (3) Are the balance and gait impairments similar to those seen in cerebellar ataxia? (4) What are the functional consequences of these impairments? (5) Are some ET patients more susceptible to balance and gait impairments? (6) Does intervention improve balance and gait in ET? The purpose of this article was to conduct a comprehensive review of studies on balance and gait impairments in ET. We classified studies based on the methodology used to examine balance and gait impairments. We used the following two categories: clinical assessment of balance and mobility and quantitative analysis of balance and gait. The implications of these findings on clinical practice and directions for future research are also discussed.
Methods
Studies of interest
The purpose of this article was to examine balance and gait impairments in ET. We included observational studies that were descriptive, case-control studies, and cohort studies. More specifically, we included studies that examined:
Patients diagnosed with ET, alone or in comparison with healthy control subjects. We examined each paper to confirm that ET was defined as a tremor disorder characterized by action tremor in the upper limbs.1 Of all the patients included in the studies reviewed, only one paper20 reported including 5 ET cases with isolated head tremor.
ET patients with a range of tremor severity (from mild to severe)
ET patients who lived at home rather than at an institution.
Either balance or gait dysfunction using clinical bedside tests, performance-based clinical assessments, quantitative testing, or a combination of several of these.
We excluded descriptive case studies that did not report on clinical or quantitative assessment of balance and gait.
Search methods
We searched the following databases: PubMed, MEDLINE, CINAHL, Google Scholar, ISI Web of Knowledge, and EBSCO. In each of the databases, we used the following medical subject headings (MeSH):
“Essential Tremor” AND (“gait” OR “gait ataxia” OR “gait deficits” OR “gait impairments”) AND (“balance” OR “posture” OR balance dysfunction”).
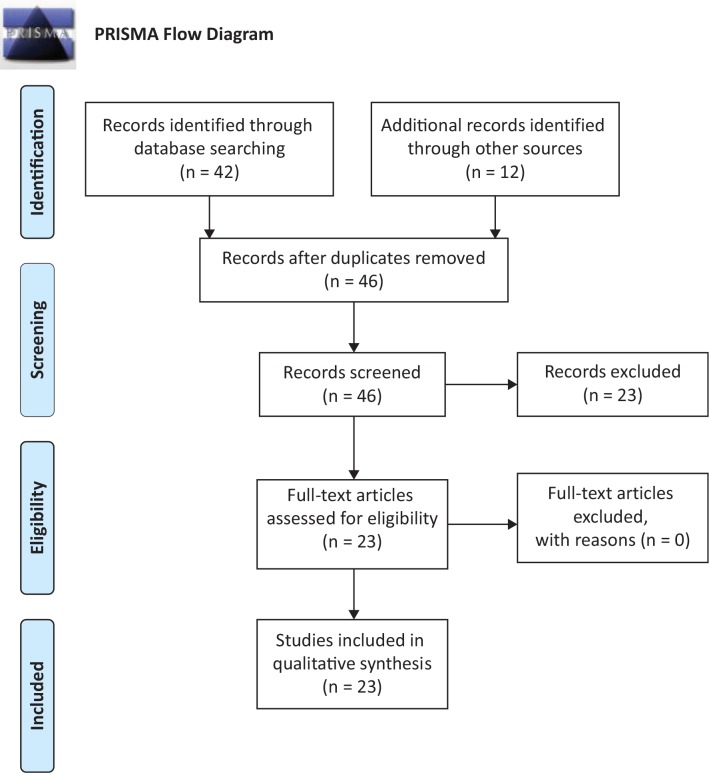
We restricted our search to articles published in English. No restrictions were placed on the date of publication, although we did not find any papers before 1990. We also searched the reference list of relevant articles to identify studies that were missed in the search process. Figure 1 illustrates the flow diagram in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Figure 1.
PRISMA Flow Diagram for Number of Studies That Were Identified, Screened, and Included in This Review
Two-step study selection process
Studies were selected through a two-step process: first, we read the abstract of selected articles that conformed to our inclusion and exclusion criteria (see the “Studies of interest” section). If the abstract was appropriate, we read the paper and examined the references to identify studies that we may have missed. Second, we classified articles into the following two categories defined by methodology: (1) clinical assessment of balance and mobility and (2) quantitative analysis of balance and gait.
Search results
General overview
Our initial search through PubMed yielded 42 articles. An additional 12 articles were identified from other sources (CINAHL, Embase, and Google Scholar). After reviewing the titles and abstracts of all 54 articles, we excluded 8 duplicate articles. Following this, we excluded 23 additional articles because (1) they were not related to ET or (2) they did not examine gait and balance. The remaining 23 articles were selected for the review, as seen in Figure 1 (PRISMA flow diagram). We did not find any additional articles in the reference list of the selected articles. Our search identified studies that used clinical assessments and those that used quantitative assessments. Each of these assessments is dealt with in the following sections. For some assessments, we link scores or score ranges with observable clinical outcomes in ET in order to provide context to aid in the interpretation of the literature review that follows. From each study, we abstracted the following data: first author and year, number of ET patients and control subjects (if any), methodology, primary outcome measure, and results.
Clinical assessment of balance and gait
Activities-specific balance confidence (ABC) scale: This measures self-confidence in performing a variety of functional tasks without falling.21 The original version of the test had 16 items, although recently a shorter 6-item version has been tested in patients with ET.11 Each item is scored between 0 (no confidence) and 100%, and the scores of all items are averaged to provide an overall confidence score (0–100%).
Tandem walk: In this test, patients are asked to walk 10 steps on a straight path, with the heel of the leading foot touching the toe of the following foot. The number of missteps, defined as the number of steps away from the straight-line path, is the primary outcome reported.7,8,9,22 One study examined when the first misstep occurred during tandem walk.6
Berg balance scale (BBS): This performance-based assessment consists of 14 functional tests of balance. Individual items examine balance in sitting and standing, transfers from sit to stand, to more complex items such as alternate stepping, tandem stance, and one-leg stance.23 Each item is scored on a five-point ordinal scale, with a total score of 56 (higher scores indicate better balance). In patients with ET, a score less than 41 is associated with a higher risk for falls.11,20,24
Dynamic gait index (DGI): This is a performance-based assessment of gait, balance, and falls risk in older adults.25,26 The DGI examines a patient’s ability to modify his/her gait in response to changing task demands. The test examines walking on level surface, walking with head turns, walking around and over obstacles, and climbing stairs. Items are scored on a four-point ordinal scale, with a total score of 24 (higher scores indicate better gait and balance). A score below 19 is associated with a higher risk for falls in ET.24
Timed up and go (TUG): This is a performance-based assessment that tests mobility and falls risk in older adults.27 The TUG measures the time a patient takes to stand up from a seated position, walk a distance of 3 m, turn, walk back to the chair, and sit down at a comfortable pace. ET patients who take longer than 14 seconds to complete the TUG are at a higher risk for falls.20,24
Quantitative assessment of balance and gait
Posturography: Quantitative assessment of posture involves the use of a force platform that measures the center of pressure (COP). Typically, subjects are asked to stand on the force platform with their eyes open or closed.12,20 Some studies have examined reactive postural control in response to movement of the force platform.14 Posturography allows for the measurement of the area or path of the COP, which are indicators of the fidelity of the postural system.
Spatiotemporal gait analysis: A number of studies have measured spatiotemporal measures as subjects walked across an instrumented mat.15,17,28 The GAITRite mat is a 4.6-m-long mat embedded with pressure sensors and switches that register the location and timing of each footfall on the mat. The footfalls enable the computation of spatiotemporal measures such as gait speed, cadence (step frequency), step or stride length, step or stride time, stance and swing time, time spent in single and double support, and heel-to-heel base of support.29
Motion analysis: A few studies measured gait using three-dimensional motion analysis systems that use infrared or ultrasound technology.14,18,19 The systems record the movement of markers, placed at various joint axes of rotations, over time and compute kinematic (spatial) measures such as gait speed, stride length, cadence, step width, step height, stance and swing time, and time spent in single and double support.
Results
How common are balance and gait impairments in ET?
Prevalence of balance and gait impairments has primarily been assessed by studies that administered clinical tests of balance and gait. Ten studies examined balance and gait impairments with the tandem walk test (Table 1), eight of which included healthy control subjects in addition to ET patients. The combined sample size across the 10 studies included 784 ET patients and 467 control subjects. As shown in Table 1, five studies reported the number of subjects with abnormality on tandem walk test.7,8,9,10,22 In three studies, the criterion for abnormal tandem walk was presence of two or more missteps during the 10-step tandem walk.7,9,10 Tandem walk abnormality was seen in 50% of ET patients in two studies,7,9 and in 30% of ET patients in the third study.10 Two additional studies detailed the number of ET patients with a range of tandem missteps (from 0 to 10 missteps).8,22 For these studies, we counted the number of ET patients with two or more tandem missteps. The prevalence of tandem walk abnormality in ET patients was reported to be 30% in one study8 and 52% in the other.22 When we combined the results of the five studies (N = 379 ET patients), tandem walk abnormality was seen in 42% (N = 159) of ET patients and 22% (N = 33 out of 144) of controls. The odds ratio for tandem walk abnormality from the combined data was 7.03, indicating that ET patients have seven times higher odds of having tandem walk abnormality compared with healthy controls. These results indicate that balance problems are common in ET patients, occurring in nearly 42% of cases. In addition, the severity of tandem walk abnormality (measured by the occurrence of the first misstep) was greater in ET patients compared with controls.6 The severity of balance impairment was greater for older ET patients, indicating that balance may worsen with age in ET patients.
Table 1.
Clinical Assessment of Balance and Mobility in Essential Tremor
| First Author | ET (n) Control (n) | Mean Age (Years) | Method | Main Results |
|---|---|---|---|---|
| Singer et al.9 | 36 30 |
69.9; 69.2 |
Tandem walk (≥ 2 missteps); tremor score; |
ET patients had more tandem missteps than controls; 50% of ET patients had tandem walk abnormality; ET patients with missteps were older; tremor severity was not associated with tandem missteps |
| Hubble et al.7 | 60; 60 |
68; 68 |
Tandem walk (≥ 2 missteps); tremor score |
ET patients had more missteps during tandem walk; 50% of ET patients had tandem walk abnormality; age was a significant predictor of missteps |
| Lim et al.10 | 41; 44 |
60.9; 58.9 |
Tandem walk (≥ 2 missteps) | 29% of ET patients had tandem walk abnormality; 70% older ET patients (> 70 years) had tandem walk abnormality |
| Louis et al.8 | 122 | 77.1 | NE; tandem walk (≥ 2 missteps) | Tandem missteps were correlated with age, age of tremor onset, and the presence of neck and voice tremors; 30% of ET patients had tandem walk abnormality. Tandem missteps increased with cranial tremor score |
| Louis and Rao22 | 120 | 71.3 | Tandem walk (≥ 2 missteps); TICS | Tandem missteps correlated with age, cranial tremor, and TICS score; tandem walk abnormality was seen in 52% ET patients |
| Parisi et al.20 | 16 ET head tremor, 14 ET no head tremor; 28 control |
59.4; 57.1; 58.4 |
Clinical tests of balance (TUG, ABC and DGI); tremor score | ET patients with head tremor performed worse on clinical tests of balance and mobility except the Berg balance scale |
| Louis et al.11 | 59; 82 |
71.2; 71.6 |
NE; clinical tests of balance (ABC-6; BBS; falls, near falls) | Balance confidence was low in ET, particularly patients with head tremor; near falls highest in ET with head tremor (40% had >5 near falls) in comparison with controls (13% had > 5 near falls) |
| Cinar et al.6 | 90; 50 |
61.4; 60.9 |
Tandem walk; tremor score | First misstep occurred earlier in ET and was negatively correlated with age, and tremor score |
| Louis and Rao41 | 126 ET; 77 PD; 46 Dystonia 173 controls |
75.7; 74.4; 73.2; 74.1 |
NE; clinical tests of balance (ABC-6; falls, near falls) | Balance confidence, number of falls, and near falls were lowest in PD, followed by ET. Need for walking aids was greatest in PD, followed by ET cases |
| Louis et al.42 | 100 | 80.5 | NE, tandem walk, ABC; physical activity, MoCA | Lower physical activity associated with age, tandem missteps, MoCA score, and higher tremor score; tandem missteps, tremor score, and MoCA score were independent predictors of physical activity |
Abbreviations: ABC-6, Activities of Balance Confidence Scale; BBS, Berg Balance Scale; DGI, Dynamic Gait Index; ET, Essential Tremor; MoCA, Montreal Cognitive Assessment; NE, Neurological Examination; PD, Parkinson’s Disease; TICS, Telephone Interview of Cognitive Status; TUG, Timed Up and Go.
There are no clearly defined criteria for determining abnormality during the tandem walk test in the healthy elderly. However, in examining five studies from Table 1 (clinical assessment of tandem walk)7,8,9,10,22 and four studies from Table 2 (quantitative assessment of tandem walk),13,14,15,19 the average number of missteps in healthy controls was 1.1 (range of 0.4–2), whereas the number of average missteps for ET patients was 2.3 (range of 0.6–4.5). Given these data, it seems appropriate to define tandem walk abnormality based on observation of two or more missteps.
Table 2.
Quantitative Analysis of (a) Balance and (b) Gait in ET
| First Author, Year | ET (n) Control (n) | Age (Years) | Method | Outcomes | Results |
|---|---|---|---|---|---|
| (a) Studies on quantitative assessment of balance (listed chronologically) | |||||
| Bove et al.12 | 19 ET; 19 control |
64.1 59.9 |
QP (Quiet stance, cognitive and motor dual task, eyes open and closed) | 1. Center of pressure (COP) area; 2. COP path; 3. COP x displacement; 4. COP y displacement | COP path was greater in ET with head tremor; COP area and path increased with eyes closed and during dual cognitive or motor task in both groups |
| Parisi et al.20 | 16 ET head tremor, 14 ET no head tremor; 28 control |
59.4 57.1 58.4 |
QP (stance eyes open and closed) | 1. Tremor score; 2. Falls; 3. Near falls; 4. COP displacement; 5. COP speed | There were no significant differences in COP sway and speed between ET and controls |
| Hoscovcova et al.13 | 30 ET 25 control |
55.8 53.0 |
Tremor score; clinical tests of balance (ABC, FAB); QP (normal and tandem stance) | 1. Tremor rating scale; 2. ICARS; 3. ABC scale; 4. Fullerton advanced balance scale; 5. Acceleration amplitude; 6. Acceleration frequency; 7. Stride length; 8. Cycle time; 9. Support base; 10. Swing time; 11. Stance time; 12. Velocity; 13. Tandem missteps; 14. Sway path; 15. Sway path; 16. COP area | No differences between ET and controls during normal gait; during tandem gait, ET patients had lower velocity and more missteps; COP area was higher in ET during tandem stance; no differences between groups on clinical tests (ABC and FAB) |
| (b) Studies on quantitative assessment of gait (listed chronologically) | |||||
| Stolze et al.19 | 25 ET; 8 Cerebellar; 21 control |
50.3 52.5 52.7 |
QG (preferred speed); TW; tremor score | 1. Total tremor score; 2. Gait speed; 3. Stride length; 4. Cadence; 5. Step width; 6. Foot angle; 7. Step height; 8. Stance time; 9. Swing time; 10. Double support time; 11. CoV; 12. Tandem missteps | Preferred walk: ET patients walked with greater step width; Tandem walk: ET patients had wider step width and more missteps than controls; Step width and tandem missteps were associated with intention tremor |
| Kronenbuerger et al.14 | 25 ET no DBS; 12 ET DBS; 25 control |
46.3 64.2 46.3 |
QG (preferred and tandem gait on treadmill); QP; | 1. Stride length; 2. Cadence; 3. Stance phase; 4. Number of missteps; 5. Sway area; 6. Falls; | ET had shorter stride length and more missteps; ET had greater sway when vision was absent/sway referenced and platform tilt was sway referenced; DBS did not improve gait and posture |
| Rao et al.15 | 104 ET; 40 control |
86 84.1 |
NE; QG (preferred and tandem walk on GAITRITE) |
1. Gait Speed; 2. Step length; 3. Cadence; 4. Stride time; 5. Double support % 6. Step time difference; 7. Step width; 8. CoV swing length; 9. CoV stride length; 10. Support base; 11. Tandem missteps | In preferred walk, ET had slow speed and cadence, increased double support percent, and increased asymmetry. In tandem walk, ET had more missteps. Gait impairments were worse for ET than controls across age |
| Louis et al.33 | 4 ET | 38–79 | NE; QG (preferred walk on GAITRITE); clinical tests of balance (ABC, POMA) | 1. ABC-6 score; 2. Tinetti POMA score; 3. Gait speed; 4. Percent time in double support; 5. Step time difference; 6. CoV swing time | ABC score and Tinetti POMA score were lower and tandem missteps were higher in ET; gait speed was lower, percent double support, step time difference and CoV swing time were higher |
| Rao et al.17 | 151 ET; 62 control |
84.4 79.6 |
NE; QG (dual task gait on GaitRITE) | 1. Gait speed; 2. Stride length; 3. Cadence; 4. Stride time; 5. Double support time; 6. Step time difference; 7. Step width; 8. CoV Stride length; 9. CoV stride length | Gait most impaired in ET with low cognitive scores (LCS) – less impaired in ET cases with higher cognitive scores (HCS); cognitive motor interference was greatest for ET LCS for double support time, step time difference and CoV stride time |
| Fernandez et al.34 | 24 ET 31 PD; 38 control |
68 68 68 |
QG (force plate) | 1. COP displacement; 2. COP velocity; First step length; 3. First step time; first step speed; | COP displacement in AP direction reduced in ET; length of the first step was reduced in ET |
| Roemmich et al.18 | 31 ET 11 Control |
66.5 63.6 |
QG (preferred walk on treadmill) | Mean and CV for 1. Stride length; 2. Stride time; 3. Step length; 4. Step time; 5. Step width; 6. α (slope of linear least-squares fit) | During preferred walk, ET had slower gait speed and increased variability; during speed matching, ET had higher step width variability; gait variability was associated with midline tremors |
| Rao et al.28 | 132 ET 48 Control |
83.7 79.5 |
NE; ABC; QG (preferred speed GAITRite) | 1. ABC-6 score; 2. Number of falls; 3. Gait speed; 4. Cadence; 5. Step length; 6. Step time difference; 7. Double support %; 8. CoV stride length; 9. CoV stride time | ET with low cognitive scores (LCS) had lower ABC scores and higher number of falls; gait measures correlated with balance confidence and falls; gait speed and ABC-6 score were significant predictors of falls |
| Rao and Louis 16 | 155 ET 60 Control |
81.9 80.1) |
QG (in time with metronome on GAITRite); Tremor score | 1. Cadence; 2. Step time; 3. Cadence error; 4. Cadence SD; 5. Cranial tremor score | Cadence was lower in ET; cadence error (accuracy) and cadence SD (precision) were similar in ET and controls; cadence and cadence error were correlated with cranial tremor score |
Abbreviations: ABC, Activities of Balance Confidence; CoV, Coefficient of Variation; DBS, Deep Brain Stimulation; ET, Essential Tremor; FAB, Fullerton Advanced Balance Assessment; ICARS, International Cooperative Ataxia Rating Scale; NE, Neurological Examination; PD, Parkinson’s Disease; POMA, Performance-Oriented Mobility Assessment; QG, Quantitative Gait; QP, Quantitative Posturography; TUG, Timed Up and Go.
Table 3.
Studies on the effect of intervention on Balance and Gait in Essential Tremor
| First Author, Year | ET (n) Control (n) | Age (Years) | Intervention | Assessment | Outcomes | Results |
|---|---|---|---|---|---|---|
| Ondo et al.53 | 13 ET | 72.8 | Thalamic stimulation | QP (sensory organization test) | 1. Time to onset of compensatory movements; 2. Amplitude of compensatory movements |
Performance on the sensory organization test was worse with DBS off and improved with DBS; |
| Fasano et al.51 | 11 ET 10 control |
69.8; 67.3 |
Bilateral thalamic stimulation | NE; QG (standard and tandem walk on treadmill) |
1.Tremor rating scale; 2. Intention tremor score; 3. Spiral score; 4. Postural tremor score; 5. Overground tandem gait velocity; 6. Overground tandem number missteps; 7. Ataxia score; 8. Swing duration CV; 9. Range of motion |
1. Total tremor score, intention tremor, postural tremor, and spiral tremor, was reduced with thalamic stimulation; 2. Supra therapeutic stimulation improved tremor except during upper limb spirals; 3. Number of missteps was reduced on thalamic stimulation, but was higher than healthy controls; 3. Supratherapeutic stimulation increased number of missteps; 4. With assisted tandem gait on treadmill lower limb kinematics were highly variable; 5. Thalamic stimulation improved ataxia ratio and variability |
| Fasano et al.52 | 11 ET 10 control |
69.8; 67.3 |
Bilateral thalamic stimulation | NE; QG (standard and tandem walk on treadmill) |
1. ICARS score; 2. Gait speed; 3. Stride length; 4. Swing duration; 5. Double support time; 6. Step width; 7. Step height; 8. Ataxia score; 9. Swing duration CV; 10. Range of motion |
1. Thalamic stimulation reduced intention and postural tremor; 2. Joint ROM in ET patients with normal kinematics was similar to controls; 3. ET patients with impaired kinematics (longer disease duration and greater intention tremor) had higher variability in joint movement; 4. Thalamic stimulation reduced joint variability in ET with impaired kinematics |
| Hwynn et al.56 | 38 ET | 67.1 | Thalamic stimulation (unilateral and bilateral) | NE; QG and falls assessment |
1. Fahn–Tolosa–Marin tremor rating scale; 2. Upper extremity scores (rest tremor, postural tremor, kinetic tremor, drawing spirals, pouring water into cup); 3. Gait and falls assessment |
1. About 70% of patients with unilateral thalamic stimulation and 55% with bilateral thalamic stimulation reported worsened gait; 2. Patients with worsened gait had poor baseline tremor scores |
| Earhart et al.24 | 13 ET; 13 control |
61.6; 63.2 |
Bilateral thalamic stimulation | NE; QG (preferred and tandem walk) |
1. ABC Scale; 2. Spatiotemporal gait measured with GAITRite mat; 3. BBS; 4. TUG |
1. ET patients demonstrated tremor reduction with stimulation; 2. During standard and tandem walk, ET patients walked with slower speed, lower cadence, and higher double support during ON and OFF stimulation compared with controls; 3. ET patients performed worse on clinical tests of balance compared with controls; 4. There were no differences with DBS on and off |
| Ulanowski et al.47 | 1 ET | 61 | Physical therapy (balance and functional movement training) for 14 sessions over 8 weeks | Standardized clinical assessment | 1. BBS; 2. FGA, 3. Five-times-sit-to-stand test 4.10-m walk test (10MWT). |
After 8 weeks of therapy, the patient had clinically meaningful changes in the five-times-sit-to-stand test, FGA, and BBS. The improvement reduced fall risk |
Abbreviations: ABC, Activities of Balance Confidence; BBS, Berg Balance Scale; CV, Coefficient of Variation; ET, Essential Tremor; FGA, Functional Gait Assessment; ICARS, International Cooperative Ataxia Rating Scale; NE, Neurological Examination; QG, Quantitative Gait; QP, Quantitative Posturography; TUG, Timed Up and Go.
Most of the studies highlighted in Table 1 tested middle-aged older subjects with advanced ET referred to a tertiary medical center. It is not known whether the prevalence of balance problems is similar in younger ET patients, or ET patients seen at primary/community health centers or ascertained directly from the population. The prevalence in these samples might be lower than in those cited above. Future work needs to examine ET patients at primary health centers and from the population to obtain a more comprehensive and global estimate of the prevalence of balance problems in this disease.
Are balance and gait problems in ET simply related to advanced age?
If balance problems in ET are simply a function of advanced age, then we would expect healthy older subjects to demonstrate balance problems similar to those seen in ET patients. In general, balance (measured by tandem walk abnormality) worsens with age in ET patients, as reported in a number of studies (Table 1).7,8,9,10,22 We can address the influence of age on balance problems by examining the data on prevalence and severity of balance and gait problems by age. Two studies examined prevalence data for tandem walk abnormality in healthy controls below the age of 70 in comparison with those above the age of 70.9,10 In these two studies, the prevalence of tandem abnormality in healthy control subjects increased from 14 (<70 years) to 42% (> 70 years)9 and from 8 (<70 years) to 22% (>70 years).10 These results indicate that balance worsens with age in the healthy elderly. For ET patients, the change in prevalence of tandem walk abnormality with age is more significant: ET patients below 70 years had a prevalence of 14% in one study9 and 8% in another study,10 which was similar to that seen in healthy controls. In older ET patients, the prevalence of tandem abnormality increased to 70% in both studies.9,10 The prevalence data show that balance impairment is much more common in older ET patients than in healthy controls of a comparable age, indicating that ET has an impact on balance impairment above and beyond the effect of age.
Of the 10 papers on tandem walk (Table 1), eight papers addressed whether duration of ET was related to balance impairments. Of these, only one paper9 reported that duration of ET, in addition to age, was correlated with balance impairments. In contrast, seven papers6,7,8,11,22,41,42 did not find a significant correlation of balance impairment with the duration of ET, and two papers10,20 did not report on this relationship.
Quantitative gait and balance data allow us to examine the severity of balance and gait problems in ET patients and control subjects as a function of age. Two studies with large sample sizes (>100 subjects) have shown that while spatiotemporal gait parameters (speed, cadence, and double support time) worsened with age in control subjects, the deficit in the gait parameters in ET patients was more than that seen in controls independent of age.15,17 These data support the notion that ET patients have a gait and balance disorder higher than that seen in healthy aging. Among studies on quantitative gait and balance, only one study12 (N = 19 ET cases) reported a correlation of duration of ET with gait impairments.
Are the balance and gait impairments related to those seen in patients with primary diseases of the cerebellum such as spinocerebellar ataxia?
Quantitative assessment of balance (using posturographic measurement of the COP with the subject standing on a force platform) indicates that patients with cerebellar ataxia demonstrate increased postural sway (measured by the magnitude of excursion of the COP) in addition to oscillations at 3–5 Hz in the frequency domain, which are characteristic of cerebellar tremor.30 In addition, the sense of the visual vertical axis may be deviated in patients with cerebellar ataxia.30 Gait impairments in cerebellar ataxia (measured by quantitative analysis) are characterized by reduced speed and cadence (step frequency), shorter stride length, increased step width and time spent on both feet, and increased variability of stride length and stride time.31 Balance impairments (measured by the tandem walk test) in cerebellar ataxia include a wide support base and greater number of missteps compared with age-matched controls.32
Three studies used quantitative posturography to examine balance in patients with ET (12, 13, 24, Table 2a). All three studies used a case-control design, with a total of 79 ET patients and 72 control subjects.12,13,20 The average age of the subjects (~60 years) was younger than that reported in studies examining tandem walk in ET. Quantitative assessment of balance involved measurement of the COP with subjects standing on a force plate with eyes open or closed, while standing in a normal stance or in a tandem stance. Results of these studies indicated that there were no significant differences in the excursion of the COP (marker of balance control) between ET patients and control subjects. The only exception occurred in ET patients with head tremor, who demonstrated greater excursion in the path of the COP.12 Given the limited evidence on quantitative balance impairments, additional studies are needed to establish whether ET leads to impairments in balance and posture and whether these impairments are comparable to those seen in cerebellar ataxia. In addition, since all three studies in Table 2(a) recruited younger ET patients, future work is needed with older advanced ET patients to examine the spectrum of balance impairments.
Nine studies conducted quantitative examination of gait in ET patients (N = 662) and controls (N = 305), as shown in Table 2(b). Of these nine studies, eight had a case-control design, and one was a case series.33 Three studies used motion analysis to quantify gait,14,18,19 one study used a force plate to assess gait initiation,34 and the remaining five studies used the GAITRite mat to measure spatiotemporal features.15,16,17,28,33 Early studies on quantitative gait assessment reported that ET patients had subtle impairments, such as increased step width and shorter stride, while walking under normal conditions, that is, at a self-selected speed.14,19 Gait impairments became more pronounced under complex conditions, such as tandem walk. Results from these two studies suggest that ET does not lead to significant impairments in gait under normal conditions. Moreover, both studies had a small sample size (n = 25 ET patients in each study).14,19 Recent work from our group (N = 104 ET patients)15 demonstrated that ET patients have a gait disorder characterized by slow gait speed (as a result of lower step frequency), increased step asymmetry, and impaired dynamic balance (indicated by increased time spent in double support). These results have been replicated by other studies (Rao 2013, n = 151 ET patients; Roemmich 2013, n = 11 ET patients; Rao 2016, n = 155 ET patients).16,17,18 Reduced step length is obvious not only during steady-state gait but also during gait initiation, likely due to impairment in preparatory postural adjustments.34 In addition, recent work has demonstrated that ET patients’ gait is characterized by increased stride-to-stride variability,18,33 which is an important predictor of falls risk in the elderly.35,36
The accumulation of evidence of gait impairments in ET challenges traditional notions that ET is associated with mild gait impairments that can be observed primarily in laboratory situations. As shown in Tables 1 and 2, ET results in significant impairments in gait speed, asymmetry, dynamic balance, and variability, which lead to functional consequences of increased falls risk. The impairments seen in ET are qualitatively similar to what is reported in cerebellar ataxia.32,37,38 Classic cerebellar ataxic gait is characterized by slow gait speed, short step length, wide base of support, deviations in the walking path, and increased step-to-step variability.32,37,38,39 Increased step-to-step variability, in particular, may be due to poor timing and scaling of joint movement.39,40 Based on the results of our review, gait impairments in ET do not include a wide base of support and deviations in the walking path, both considered key characteristics of ataxic gait. Thus, gait impairments in ET share several features with cerebellar ataxia, but it is important to note that all features are not shared, and the presentation in ET is not as severe.
What are the functional consequences of balance and gait impairments?
The previous sections have highlighted that ET patients demonstrate balance and gait impairments when assessed by clinical or quantitative examination. In order to establish prognostic significance, one needs to examine whether balance and gait impairments are related to functional limitations, which impact quality of life. It is also important to examine in greater detail the extent to which balance and gait impairments predispose ET patients to increased risk of falls and near falls. Standardized clinical assessments such as the BBS,11,20,24 TUG,20,24 and DGI25,26 have been used to assess functional balance and mobility. In addition, studies have examined self-perceived balance confidence in patients using the ABC scale.
ET patients with balance impairments perform worse in functional tasks, such as rising up from a chair, transferring from one chair to another, picking up objects from the floor, reaching out for objects placed beyond arm’s length, etc.11,20 Moreover, ET patients with balance and gait impairments reported lower confidence in their balance while performing functional tasks.11,20 ET patients with balance impairment (more tandem missteps) have a greater number of near falls as compared with controls.11,41 The number of near falls was higher for ET patients with both upper limb and head tremor.11 Lower balance confidence, and falls were associated with reduction in self-reported physical activity.42 These results indicate that balance impairments not only limit functional activity, but may lead to reduced physical activity. The functional importance of balance impairment was highlighted in a recent study, which reported that impairment on the tandem walk test was a significant predictor of mortality in ET patients.43 Given the association between balance impairment, falls risk, and mortality, it is extremely important to systematically examine markers of falls risk for early identification of people at a high risk of falls and related injuries.
Are some ET patients more susceptible to balance and gait impairments?
An additional issue is whether some ET patients are more susceptible to balance and gait impairments than others. In the elderly population, older age and presence of cognitive deficits are independent predictors of falls risk.44 Older patients with ET have greater balance and gait impairments, which suggest that age is an important predictor in addition to the presence of ET.6,8,9,10,15,22
In addition to age, many studies have reported that ET patients with tremor in midline structures have more pronounced balance problems than ET patients without midline tremor.8,11,20,22 The association between midline tremor and gait impairment has also been reported.16,18 Step frequency or cadence was lower in ET patients with midline tremor.16 In addition, gait variability is also associated with midline tremor.18 Thus, the presence of midline tremor may be a predictor of balance and gait impairments. The presence of midline tremors, as a result of cerebellar dysfunction, increases the risk of falls in patients with ET. Tandem missteps were associated with tremors in midline structures, particularly the neck.8,20,22 No such association was reported for tandem missteps with tremor of the extremities. Since tremors of midline structures may be a sign of cerebellar vermis dysfunction,45,46 the association between midline tremor and tandem walk difficulty may reflect a shared pathology (cerebellar regulation of midline structures).
Finally, ET patients with cognitive impairments have greater balance and gait dysfunction.17,22,28 ET patients with lower scores on a cognitive test demonstrated gait impairments such as increased double support time, step asymmetry, and variability. Furthermore, ET patients with cognitive dysfunction are at a greater risk of near falls and falls.28
Does intervention improve balance and gait in ET patients?
To date there has only been one case study on the effect of physical therapy or exercise intervention on balance and gait in patients with ET.47 This case study included a 61-year-old male patient with ET who had undergone prolonged thalamic stimulation. The patient received 14 sessions of an individualized exercise program that consisted of balance training and functional movement training over 8 weeks. Posttest data demonstrated improvements in most of the outcomes of balance and mobility.47 There are a few studies on the effects of exercise (occupational therapy) in improving upper limb function in ET.48,49,50 These studies implemented resistance training49,50 or manual dexterity training48 but did not examine balance and gait. Given the prevalence of balance and gait impairments in ET, and given their effect on functional limitations, exercise (physical and occupational therapy) intervention studies are needed.
Thalamic stimulation has been used as a surgical intervention to reduce postural and kinetic tremor in ET.51,52 The effect of thalamic stimulation on balance and gait impairments is unclear. A few studies reported that stimulation of thalamic nuclei marginally improved postural control,53 substantially improved lower limb kinematics during gait,51,52 and improved the quality of life in the short term and long term.54,55 However, even with therapeutic stimulation intensity, gait in ET patients was worse than that of controls.51 When supra-therapeutic stimulation was used, balance and gait worsened.51,52 Other studies reported that thalamic stimulation resulted in either no improvement in balance and gait24 or resulted in worsening of gait.56 It is unclear how some studies report improvement while others do not – it is possible that thalamic stimulation improves joint coordination in patients with impaired joint kinematic profiles52 but has limited impact on balance and gait. The other possibility is that the studies mentioned above may have used different stimulation intensities. This is confirmed by the fact that improvement in gait was seen at optimal intensities, whereas worsening was seen at increased stimulation intensity.51
Discussion
There has been an increased interest in understanding balance and gait impairments in ET in the past two decades. While early studies involved testing balance and gait with simple bedside clinical tests (e.g., tandem walk), more recent studies have examined motor physiological parameters of balance and gait using quantitative assessment of posture and gait. The evidence reviewed in this article is consistent with the view that ET results in a variety of balance and gait impairments that may have an impact on the day-to-day functioning of patients. ET patients demonstrate balance impairments in quiet stance and during walking. In quiet stance, the impairment can be seen as increased postural sway, particularly when patients are asked to stand with eyes closed. During a simple bedside test (tandem walk), ET patients have a higher number of missteps compared with matched controls. On quantitative gait analysis, slower gait speed (as a result of low cadence and short step length), impaired dynamic balance (increased time spent on both feet), asymmetry (in step time), and increased variability are commonly seen.
Balance and gait impairments are not rare in ET. Indeed, they are fairly common in ET. Contrary to the traditional view, evidence from a number of studies indicates that slightly less than half (i.e., 42%) of ET patients have difficulty completing the tandem walk. As such, patients with ET have a greater number of missteps (steps away from forward linear progression). Prevalence data from quantitative gait analysis are not available. Future work needs to examine the prevalence of balance and gait dysfunction comprehensively using a large-sample cross-sectional design. In addition, it would be of value to examine longitudinal changes in balance and gait to determine the rate of change in ET patients. Balance and gait impairments result in difficulty performing functional tasks, as measured by performance-based tests of balance and mobility (e.g., BBS, Tinetti Performance-Oriented Mobility Assessment, and TUG Test). Balance and gait impairment also result in reduction in self-confidence in performing functional tasks, and increased risk for falls. Clinical assessment should include the assessment of balance confidence and, if indicated, referral to a physical therapist for more detailed assessment of performance-based balance and mobility deficits. Currently, the precise prevalence of near falls and falls is not known; therefore, future studies with large number of subjects are needed to examine this prevalence.
Studies of the pathological basis of ET implicate the involvement of neural pathways that connect the cerebellum to the thalamus and cerebral cortex. Neuroimaging (Magnetic resonance imaging (MRI) and Positron Emission Tomography (PET)) studies have consistently reported changes in the cerebellum in ET patients.57,58 Postmortem examination of the cerebellum revealed loss of Purkinje cells as well as a host of other changes in the ET cerebellum.59,60 To date, however, the association between neuroimaging findings and balance and gait disorder in ET patients has not been examined. As discussed earlier in this article, ET patients having midline tremors present with greater balance and gait impairments. These results have been used to suggest that balance and gait impairments may result from vermal cerebellar pathology, since midline tremors are seen in ET patients with vermal cerebellar pathology. Future work needs to systematically examine the association between cerebellar pathology and gait and balance impairments in ET patients.
Our review indicates that ET patients who are older, have tremor in midline structures and have cognitive deficits are more susceptible to near falls and falls. The presence of one or more of these factors on clinical examination should prompt a more detailed assessment of balance and gait. Additional research is needed to develop predictive models of falls risk in ET. This will help in identifying ET patients who may benefit from rehabilitation intervention (physical and occupational therapies). Given the limited evidence on the effect of exercise and physical activity interventions, future work needs to examine the feasibility and effectiveness of exercise in improving function and reducing falls risk.
Strengths of this study included its comprehensive nature, the multiplicity of questions addressed, the high clinical relevance of several of these questions, and the attempts both to summarize current data and frame areas for future scholarship. It should be noted that many of the studies had ascertained patients from treatment centers and/or from specialty settings, and it is possible that these studies might have sampled a more severe subset of ET patients with more marked involvement of gait and balance. As we have pointed out, the need for population-based studies is apparent. Furthermore, although controlled data are of greatest value, the literature on gait and ataxia in ET is still a modest one; hence, all data are of some value at this stage. For this reason, we decided not to exclude studies without controls.
In summary, accumulating evidence suggests that balance and gait impairments are common in ET patients and occur to a greater extent compared with controls. Thus, they represent a disease-associated feature. These impairments, which are qualitatively similar to those seen in spinocerebellar ataxias, are not merely subclinical but result in difficulty performing functional tasks and increase falls risk. A subset of patients is more susceptible to balance and gait impairments. The full spectrum of impairments and their pathomechanistic basis remains to be characterized.
Acknowledgments
N/A.
Footnotes
Citation: Rao AK, Louis ED. Ataxic Gait in Essential Tremor: A Disease-Associated Feature?. Tremor Other Hyperkinet Mov. 2019; 9. doi: 10.7916/d8-28jq-8t52
Editor: Ruth H. Walker, Mount Sinai School of Medicine, USA
Funding: AR is supported by the US National Science Foundation (grant no. SCH-1838725). EDL is supported by the National Institutes of Health (grant nos. R01 NS086736 and R01 NS094607).
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
Ethics Statement: This article did not involve experimental investigation of human subjects and thus did not need to obtain informed consent. All primary research reviewed in this article included investigation that was approved by a formal ethics review committee. The study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki and the reporting guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
References
- 1.Bhatia KP, Bain P, Bajaj N, et al. . Consensus statement on the classification of tremors. Mov Disord 2018;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elble RJ, Deuschl G. An update on essential tremor. Curr Neurol Neurosci Rep 2009;9(4):273–277. [DOI] [PubMed] [Google Scholar]
- 3.Benito-Leon J. Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology 2008;31(3):191–192. doi: 10.1159/000154933 [DOI] [PubMed] [Google Scholar]
- 4.Louis ED, Benito-Leon J, Vega-Quiroga S, et al. . Cognitive and motor functional activity in non-demented community-dwelling essential tremor cases. J Neurol Neurosurg Psychiatr 2010;81(9):997–1001. doi: [pii] 10.1136/jnnp.2009.202838 [DOI] [PubMed] [Google Scholar]
- 5.Arkadir D, Louis ED. The balance and gait disorder of essential tremor: what does this mean for patients? Ther Adv Neurol Disord 2013;6(4):229–236. doi: 10.1177/1756285612471415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinar N, Sahin S, Okluoglu Onay T, et al. . Balance in essential tremor during tandem gait: is the first mis-step an important finding? J Clin Neurosci 2013;20(10):1433–1437. doi: 10.1016/j.jocn.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 7.Hubble JP, Busenbark KL, Pahwa R, et al. . Clinical expression of essential tremor: effects of gender and age. Mov Disord 1997;12(6):969–972. doi: 10.1002/mds.870120620 [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord 2010;25(11):1633–1638. doi: 10.1002/mds.23144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord 1994;9(2):193–196. doi: 10.1002/mds.870090212 [DOI] [PubMed] [Google Scholar]
- 10.Lim ES, Seo MW, Woo SR, et al. . Relationship between essential tremor and cerebellar dysfunction according to age. J Clin Neurol 2005;1(1):76–80. doi: 10.3988/jcn.2005.1.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis ED, Rao AK, Gerbin M. Functional correlates of gait and balance difficulty in essential tremor: balance confidence, near misses and falls. Gait Posture 2012;35(1):43–47. doi: 10.1016/j.gaitpost.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bove M, Marinelli L, Avanzino L, et al. . Posturographic analysis of balance control in patients with essential tremor. Mov Disord 2006;21(2):192–198. doi: 10.1002/mds.20696 [DOI] [PubMed] [Google Scholar]
- 13.Hoskovcova M, Ulmanova O, Sprdlik O, et al. . Disorders of balance and gait in essential tremor are associated with midline tremor and age. Cerebellum 2013;12(1):27–34. doi: 10.1007/s12311-012-0384-4 [DOI] [PubMed] [Google Scholar]
- 14.Kronenbuerger M, Konczak J, Ziegler W, et al. . Balance and motor speech impairment in essential tremor. Cerebellum 2009;8(3):389–398. doi: 10.1007/s12311-009-0111-y [DOI] [PubMed] [Google Scholar]
- 15.Rao AK, Gillman A, Louis ED. Quantitative gait analysis in essential tremor reveals impairments that are maintained into advanced age. Gait Posture 2011;34(1):65–70. doi: [pii] 10.1016/j.gaitpost.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao AK, Louis ED. Timing control of gait: a study of essential tremor patients vs. age-matched controls. Cerebellum Ataxias 2016;3:5. doi: 10.1186/s40673-016-0043-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao AK, Uddin J, Gillman A, et al. . Cognitive motor interference during dual-task gait in essential tremor. Gait Posture 2013;38(3):403–409. doi: 10.1016/j.gaitpost.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roemmich RT, Zeilman PR, Vaillancourt DE, et al. . Gait variability magnitude but not structure is altered in essential tremor. J Biomech 2013;46(15):2682–2687. doi: 10.1016/j.jbiomech.2013.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stolze H, Petersen G, Raethjen J, et al. . The gait disorder of advanced essential tremor. Brain 2001;124(Pt 11):2278–2286. [DOI] [PubMed] [Google Scholar]
- 20.Parisi SL, Heroux ME, Culham EG, et al. . Functional mobility and postural control in essential tremor. Arch Phys Med Rehabil 2006;87(10):1357–1364. doi: 10.1016/j.apmr.2006.07.255 [DOI] [PubMed] [Google Scholar]
- 21.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol 1995;50A(1):M28–M34. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Rao AK. Tandem gait performance in essential tremor patients correlates with cognitive function. Cerebellum Ataxias 2015;1(1):1–6. doi: 10.1186/s40673-014-0019-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med 1995;27(1):27–36. [PubMed] [Google Scholar]
- 24.Earhart GM, Clark BR, Tabbal SD, et al. . Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Disord 2009;24(3):386–391. doi: 10.1002/mds.22356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu YP, Fritz SL, Light KE, et al. . Use of item response analysis to investigate measurement properties and clinical validity of data for the dynamic gait index. Phys Ther 2006;86(6):778–787. [PubMed] [Google Scholar]
- 26.Shumway-Cook AW, M.H. Motor control: theory and applications. Baltimore, MD: Williams and Wilkins; 1995. [Google Scholar]
- 27.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 28.Rao AK, Gilman A, Louis ED. Balance confidence and falls in nondemented essential tremor patients: the role of cognition. Arch Phys Med Rehabil 2014;95(10):1832–1837. doi: 10.1016/j.apmr.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonough AL, Batavia M, Chen FC, et al. . The validity and reliability of the GAITRite system's measurements: a preliminary evaluation. Arch Phys Med Rehabil 2001;82(3):419–425. doi: [pii] 10.1053/apmr.2001.19778 [DOI] [PubMed] [Google Scholar]
- 30.Marquer A, Barbieri G, Perennou D. The assessment and treatment of postural disorders in cerebellar ataxia: a systematic review. Ann Phys Rehabil Med 2014;57(2):67–78. doi: 10.1016/j.rehab.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 31.Buckley E, Mazza C, McNeill A. A systematic review of the gait characteristics associated with Cerebellar Ataxia. Gait Posture 2018;60:154–163. doi: 10.1016/j.gaitpost.2017.11.024 [DOI] [PubMed] [Google Scholar]
- 32.Stolze H, Klebe S, Petersen G, et al. . Typical features of cerebellar ataxic gait. J Neurol Neurosurg Psychiatr 2002;73(3):310–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis ED, Galecki M, Rao AK. Four essential tremor cases with moderately impaired gait: how impaired can gait be in this disease? Tremor Other Hyperkinet Mov (N Y) 2013;3. doi: 10.7916/D8QV3K7G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez KM, Roemmich RT, Stegemoller EL, et al. . Gait initiation impairments in both Essential Tremor and Parkinson's disease. Gait Posture 2013;38(4):956–961. doi: 10.1016/j.gaitpost.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893 [DOI] [PubMed] [Google Scholar]
- 36.Kressig RW, Herrmann FR, Grandjean R, et al. . Gait variability while dual-tasking: fall predictor in older inpatients? Aging Clin Exp Res 2008;20(2):123–130. [DOI] [PubMed] [Google Scholar]
- 37.Palliyath S, Hallett M, Thomas SL, et al. . Gait in patients with cerebellar ataxia. Mov Disord 1998;13(6):958–964. doi: 10.1002/mds.870130616 [DOI] [PubMed] [Google Scholar]
- 38.Thach WT, Bastian AJ. Role of the cerebellum in the control and adaptation of gait in health and disease. Prog Brain Res 2004;143:353–366. [DOI] [PubMed] [Google Scholar]
- 39.Bastian AJ, Martin TA, Keating JG, et al. . Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 1996;76(1):492–509. doi: 10.1152/jn.1996.76.1.492 [DOI] [PubMed] [Google Scholar]
- 40.Earhart GM, Bastian AJ. Selection and coordination of human locomotor forms following cerebellar damage. J Neurophysiol 2001;85(2):759–769. doi: 10.1152/jn.2001.85.2.759 [DOI] [PubMed] [Google Scholar]
- 41.Louis ED, Rao AK. Functional aspects of gait in essential tremor: a comparison with age-matched Parkinson's disease cases, dystonia cases, and controls. Tremor Other Hyperkinet Mov (N Y) 2015;5. doi: 10.7916/D8B27T7J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louis ED, Collins K, Rohl B, et al. . Self-reported physical activity in essential tremor: relationship with tremor, balance, and cognitive function. J Neurol Sci 2016;366:240–245. doi: 10.1016/j.jns.2016.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zubair A, Cersonsky TEK, Kellner S, Huey ED, Cosentino S, Louis ED. What predicts mortality in essential tremor? A prospective, longitudinal study of elders. Frontiers Neurol 2018;9:1077. doi: 10.3389/fneur.2018.01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604 [DOI] [PubMed] [Google Scholar]
- 45.Maurice-Williams RS. Mechanism of production of gait unsteadiness by tumours in the posterior fossa. J Neurol Neurosurg Psychiatr 1975;38(2):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umemura K, Ishizaki H, Matsuoka I, et al. . Analysis of body sway in patients with cerebellar lesions. Acta Otolaryngol Suppl 1989;468:253–261. [DOI] [PubMed] [Google Scholar]
- 47.Ulanowski EA, Danzl MM, Sims KM. Physical therapy for a patient with essential tremor and prolonged deep brain stimulation: a case report. Tremor Other Hyperkinet Mov (N Y) 2017;7. doi: 10.7916/D8X92H0G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Budini F, Lowery MM, Hutchinson M, et al. . Dexterity training improves manual precision in patients affected by essential tremor. Arch Phys Med Rehabil 2014;95(4):705–710. doi: 10.1016/j.apmr.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 49.Kavanagh JJ, Wedderburn-Bisshop J, Keogh JW. Resistance training reduces force tremor and improves manual dexterity in older individuals with essential tremor. J Mot Behav 2016;48(1):20–30. doi: 10.1080/00222895.2015.1028583 [DOI] [PubMed] [Google Scholar]
- 50.Sequeira G, Keogh JW, Kavanagh JJ. Resistance training can improve fine manual dexterity in essential tremor patients: a preliminary study. Arch Phys Med Rehabil 2012;93(8):1466–1468. doi: 10.1016/j.apmr.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 51.Fasano A, Herzog J, Raethjen J, et al. . Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain 2010;133(Pt 12):3635–3648. doi: 10.1093/brain/awq267 [DOI] [PubMed] [Google Scholar]
- 52.Fasano A, Herzog J, Raethjen J, et al. . Lower limb joints kinematics in essential tremor and the effect of thalamic stimulation. Gait Posture 2012;36(2):187–193. doi: 10.1016/j.gaitpost.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 53.Ondo WG, Almaguer M, Cohen H. Computerized posturography balance assessment of patients with bilateral ventralis intermedius nuclei deep brain stimulation. Mov Disord 2006;21(12):2243–2247. doi: 10.1002/mds.21165 [DOI] [PubMed] [Google Scholar]
- 54.Nazzaro JM, Pahwa R, Lyons KE. Long-term benefits in quality of life after unilateral thalamic deep brain stimulation for essential tremor. J Neurosurg 2012;117(1):156–161. doi: 10.3171/2012.3.JNS112316 [DOI] [PubMed] [Google Scholar]
- 55.Pahwa R, Lyons KL, Wilkinson SB, et al. . Bilateral thalamic stimulation for the treatment of essential tremor. Neurology 1999;53(7):1447–1450. [DOI] [PubMed] [Google Scholar]
- 56.Hwynn N, Hass CJ, Zeilman P, et al. . Steady or not following thalamic deep brain stimulation for essential tremor. J Neurol 2011;258(9):1643–1648. doi: 10.1007/s00415-011-5986-0 [DOI] [PubMed] [Google Scholar]
- 57.Bhalsing KS, Saini J, Pal PK. Understanding the pathophysiology of essential tremor through advanced neuroimaging: a review. J Neurol Sci 2013;335(1–2):9–13. doi: 10.1016/j.jns.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 58.Louis ED, Huang CC, Dyke JP, et al. . Neuroimaging studies of essential tremor: how well do these studies support/refute the neurodegenerative hypothesis? Tremor Other Hyperkinet Mov (N Y) 2014;4:235. doi: 10.7916/D8DF6PB8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol 2010;9(6):613–622. doi: 10.1016/S1474-4422(10)70090-9 [DOI] [PubMed] [Google Scholar]
- 60.Louis ED. Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum 2016;15(3):235–242. doi: 10.1007/s12311-015-0692-6 [DOI] [PubMed] [Google Scholar]