Abstract
Background
Salidroside, a natural dietary isothiocyanate, has been widely studied for its multiple effects, including promoting proliferation, anti-inflammation, and anti-apoptosis. In the present study, these effects of Salidroside were explored to assess whether it could prevent osteoarthritis (OA) in vitro.
Material/Methods
The cytotoxic and proliferating effects of Salidroside on chondrocytes were detected by use of the Cell Counting Kit 8 assay. The expression levels of proteins were detected by Western blot. The cell apoptosis level was assessed by flow cytometry, and the levels of ROS, NO, caspase 3, and caspase 9 were assessed to evaluate the level of apoptosis. The expression level of pro-inflammatory factors was detected by ELISA.
Results
Our results demonstrated that Salidroside promotes chondrocytes proliferation, inhibits IL-1β-induced apoptosis and inflammation, and scavenges reactive oxygen species (ROS) and NO of chondrocytes. Salidroside upregulates the level of Bcl-2 and downregulates the level of Bax. Salidroside also inhibits the production of caspase 3/9 and suppresses the phosphorylation of PI3K and AKT.
Conclusions
Our results suggest that Salidroside prevents OA by its powerful pro-proliferating, anti-phlogistic, and anti-apoptotic effects by inhibiting PI3K/AKT.
MeSH Keywords: Apoptosis, Chondrocytes, Phosphatidylinositol 3-Kinases
Background
Osteoarthritis (OA) is defined as damaged articular cartilage and synovial inflammation [1]. Chondrocytes are the only resident cells in articular cartilage involved in the pathological process of OA through degeneration, inflammation, necroptosis, and apoptosis. Previous studies have reported that apoptosis plays a critical role in the pathological process of chondrocytes, and the inhibition of chondrocytes apoptosis is a potential therapeutic target for OA prevention [2,3].
Interleukin-1β (IL-1β) is a pro-inflammatory cytokine secreted by chondrocytes. It has been reported that patients with OA have elevated levels of IL-1β in their cartilage [4]. IL-1β accelerates the progression of OA by inducing inflammatory cytokine production, extracellular matrix loss, and chondrocyte apoptosis [5,6]. Chondrocyte apoptosis is closely related to increased ROS and NO production when it is exposed to IL-1β [7,8]. Previous studies have shown that the overproduction of reactive oxygen species (NOS) and nitric oxide (NO) in pathological conditions induces chondrocyte apoptosis. PI3K/AKT takes part in the inflammatory response and is activated in OA progression [9]. Accumulating studies have reported that inhibition of the PI3K/AKT pathway can alleviate the inflammatory response [10], reducing levels of pro-inflammatory cytokines, then further reducing ROS production and NO release. Therefore, inactivation of the PI3K/AKT pathway is thought to help delay OA progression.
Salidroside, the major constituent of a Chinese medicinal herb, Rhodiola rosea L, has been reported to suppress apoptosis in many diseases [11]. Its anti-inflammation effect works on Adriamycin-induced focal segmental glomerulosclerosis through suppressing the PI3K/AKT pathway [12]. Considering its various beneficial effects, Salidroside is thought to protect chondrocytes. In this study, the effects of Salidroside on the proliferation, inflammation, and apoptosis of chondrocytes was investigated.
Material and Methods
Isolation and culture of chondrocytes
Chondrocytes were isolated following a previously described method. Briefly, joint tissues of Sprague-Dawley rats were cut into pieces smaller than 1 mm and digested with 0.25 mg/mL of trypsin, then incubated with 2 mg/mL of collagenase II for 8 h. The undigested tissues were filtered out with a 180-μm cell filter. The suspensions were centrifuged at 1500 rpm for 10 min. After that, cells were stained with trypan blue to evaluate viability. Chondrocytes with viability greater than 85% were maintained in DMEM with 10% FBS (100 U/mL penicillin and 0.1 mg/mL streptomycin). Cells were cultured at 37°C with 5% CO2. All rats were purchased from Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). All experiments were approved by the Ethics Committee of Tongji Medical College.
Cell Counting Kit 8 (CCK8) assay
CCK8 assay was used to evaluate the cytotoxic and proliferating effects of Salidroside on chondrocytes. After culturing for 24, 48, and 72 h, CCK8 reagent was added to each well. The OD value was detected with a microplate reader at 450 nm.
Flow cytometric analysis of apoptosis
Cells were cultured in 6-well plates and treated with PBS, IL-1β, and Salidroside+IL-1β for 24 h, then the cells were collected and resuspended in 200 μL of binding buffer (BD Biosciences, San Diego, CA, USA). After that, 5 μL Annexin V-FITC was added to the cells and they were incubated for 15 min at 37°C in the dark, then we added 10 μL PI and incubated cells on ice for 10 min in the dark. The population of Annexin-V-positive cells was analyzed by flow cytometry using the green and red fluorescence channels and the percentages of apoptotic cells were calculated.
Western blotting analysis
The cells were harvested and lysed with IPH buffer and protease-inhibitor, then the proteins were denatured by boiling in SDS buffer and were loaded on 12% SDS-polyacrylamide gels (Bio Rad). Separated proteins were transferred onto 0.2-μM nitrocellulose membranes by turbo blotting for 7 min at 2.5 A and 25 V using the Bio Rad system. Unspecific protein binding was blocked by incubation in 5% non-fat milk in TBS-Tween 0.05% for 1 h at room temperature or overnight at 4°C. Membranes were subsequently incubated with anti-Cyclin D1 (Abcam, USA, 1: 1000), anti-Cyclin D3 (CST, USA, 1: 1000), anti-Bax (CST, USA, 1: 2000), anti-Bcl2 (Abcam, USA, 1: 2000), anti-PI3K (Abcam, USA, 1: 2000), anti-p-PI3K (Abcam, USA, 1: 500), anti-Akt (CST, USA, 1: 2000), anti-p-Akt (CST, USA, 1: 1000), or anti-GAPDH (Abcam, USA, 1: 10 000). Then, membranes were washed in TBS-Tween and incubated with HRP-conjugated secondary antibody. Signals were detected with SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific) and a LAS 4000 imager (GE Healthcare).
qRT-PCR
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the mRNA level of pro-inflammatory cytokines. Briefly, after cells were treated with PBS, IL-1β, or IL-1β+Salidroside, total RNA was extracted from the treated cells after 6 h with TRIzol (Invitrogen, Carlsbad, USA). The complementary DNA (cDNA) synthesis was conducted with an M-MLV Reverse Transcriptase kit (ELK Biotechnology). Quantitative PCR was performed on StepOne™ real-time PCR. The mRNA level was calculated using the 2–ddCt method. The sequences of primers for qRT-PCR were as follows:
interleukin (IL)-6, forward, 5′-AGCGATGATGCACTGTCAGA-3′ and reverse, 5′-GGAACTCCAGAAGACCAGAGC-3′;
Tumor necrosis factor (TNF)-α, forward, 5′-GCGCCCAGCCTTCCTTAC-3′ and reverse, 5′-GCCCCGGCCTTCCAAATAAATAC-3′;
Bax, forward, 5′-TGGTTGCCCTCTTCTA-3′ and reverse, 5′-CACCCTGGTCTTGGAT-3′;
Bcl-2, forward, 5′-CACAGAGGGGCTACGAGT-3 0 and reverse, 5′-CAGGCTGGAAGGAGAAGA-3′;
GAPDH, forward, 5′-CAAGTTCAACGGCACAG-3′ and reverse 5′-CCAGTAGAC TCCACGAC AT-3′.
ELISA
After cells were treated with PBS (control), IL-1β, Salidroside+IL-1β for 24h, the supernatants were collected to detect the level of IL-6, TNF-α using an ELISA kit.
Measurement of intracellular reactive oxygen species (ROS) and nitric oxide (NO) production
The levels of ROS and NO were measured using an ROS assay kit and a nitrate/nitrite colorimetric assay kit of Griess reaction following the manufactures’ instructions. Both kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Caspase 3/9 activity assay
Caspase colorimetric assay kits (Nanjing Jiancheng Bioengineering Institute) were used to detect the activities of caspase 3 and caspase 9. Briefly, cells were collected and lysed. Then, the absorbance was measured with a microplate reader (405 nm).
Statistical analysis
All data were expressed as mean ± standard deviation (SD). The significance level between the groups was compared using the t test and one-way analyses of variance (ANOVA). P<0.05 was set as statistically significant.
Results
Cytotoxicity of Salidroside on chondrocytes
The cytotoxic effect of Salidroside on cells was assessed by CCK8 assay. Chondrocytes were treated with various concentrations (0, 50, 100, and 200 μmol/L) of Salidroside for 24, 48, and 72 h. We found that 50 and 100 μmol/L of Salidroside significantly increased the OD value of cells, suggesting the increased proliferation of cells at these concentrations. However, 200 μmol/L of Salidroside had an obvious adverse effect on the OD value of chondrocytes after 48 and 72 h treatment. (Figure 1A).
Figure 1.
(A) The cytotoxicity of Salidroside on chondrocytes was assessed by CCK8 assay. The concentrations of Salidroside were 0, 50, 100, and 200 μmol/ml. (B, C) The proteins that regulated cell cycle were detected by Western blot. (D) The levels of Cyclin D1 and Cyclin D3 were detected by qRT-PCR. * P<0.05, **** p<0.0001.
After cells were treated with PBS, 50 μmol/L of Salidroside, and 100 μmol/L of Salidroside, the expression levels of cyclin D1 and cyclin D3 in regulating the cell cycle were also increased after cells were treated with 50 μmol/L or 100 μmol/L of Salidroside (Figure 1B–1D). We found that 50 and 100 μmol/L of Salidroside had a positive effect on promoting chondrocyte proliferation, and we used 100 μmol/L of Salidroside in the following experiments.
Effect of Salidroside on IL-1β-induced inflammatory cytokines
The level of IL-6 and TNF-α were evaluated with ELISA assay and qRT-PCR after cells were treated with PBS, IL-1β, and Salidroside+IL-1β. The result was consistent with previous studies showing IL-1β induces pro-inflammatory cytokines in chondrocytes, while its negative effect is alleviated by adding Salidroside, suggesting Salidroside has an anti-inflammatory effect (Figure 2A, 2B).
Figure 2.
Salidroside inhibited IL-1β-induced pro-inflammatory cytokines. (A) ELISA was used to detected the level of IL-6 and TNF-α level after cells were treated with PBS (control), IL-1β, or IL-1β+Salidroside for 24 h. (B) qRT-PCR was used to evaluated the level of IL-6 and TNF-α. **** p<0.0001.
Effect of Salidroside on IL-1β-induced apoptosis
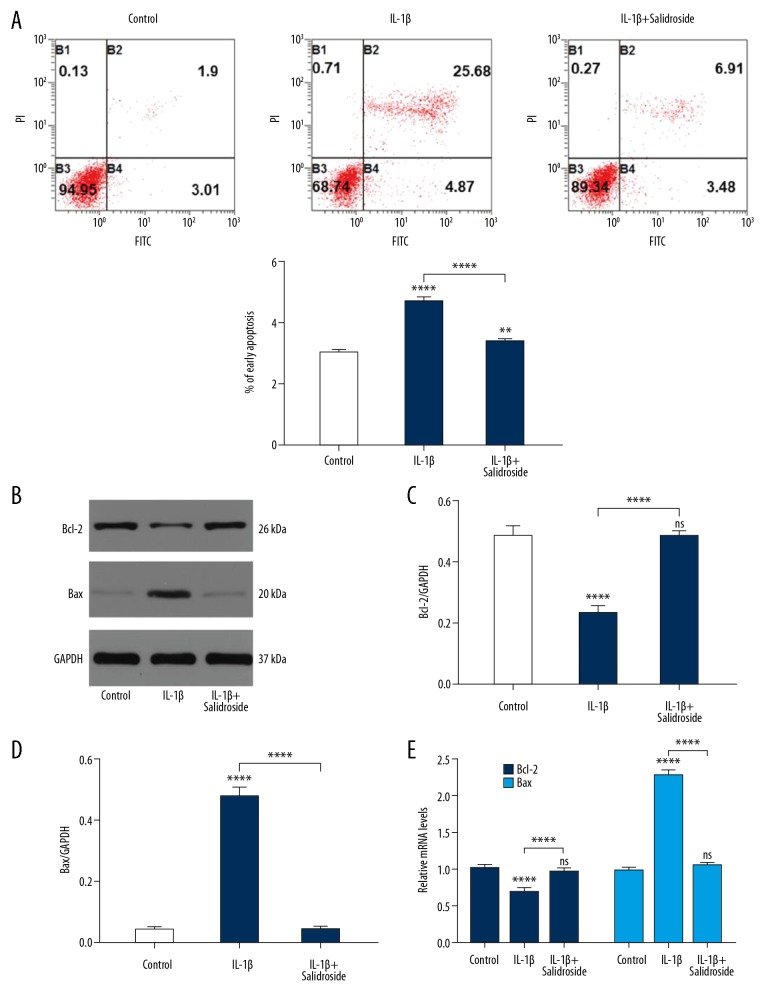
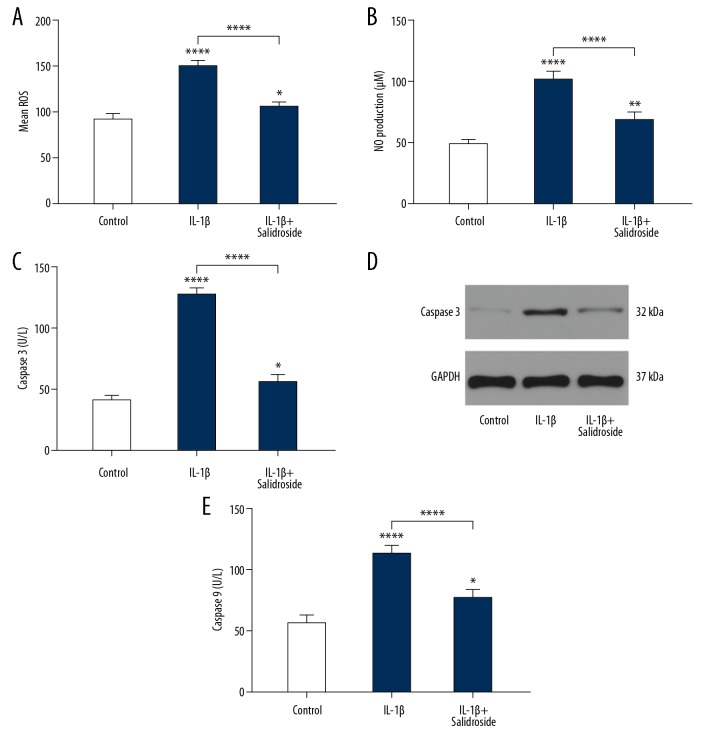
To explore the effect of Salidroside on the apoptosis of chondrocytes, flow cytometry was used to detect the ratio of apoptosis, showing that the apoptosis levels were obviously lower in the Salidroside+IL-1β group than in the IL-1β group (Figure 3A). The levels of Bax and Bcl-2 were detected by Western blot analysis and qRT-PCR. As shown in Figure 3B–3E, IL-1β significantly induced Bax expression and reduced Bcl-2 expression compared to the control group. After adding Salidroside to the IL-1β-treated cells, the apoptosis level of chondrocytes was significantly inhibited, suggesting that Salidroside has an anti-apoptotic effect on chondrocytes. In addition, the expression levels of ROS and NO in apoptosis were also detected, showing that IL-1β increased the levels of ROS and NO, and this was suppressed by adding Salidroside (Figure 4A, 4B). To assess if the IL-1β damages chondrocytes through mitochondria apoptosis pathway, the expression levels of caspase 3 and caspase 9 were detected, showing that IL-1β-induced mitochondria apoptosis can be reversed by Salidroside (Figure 4C–4E).
Figure 3.
Salidroside inhibited IL-1β-induced cell apoptosis. (A) Flow cytometry was used to measure the percentage of cell apoptosis. B1: cells undergoing necrosis; B2: cells in later apoptosis; B3: living cells; B4: cells in early apoptosis. (B–D) The pro-apoptotic protein level and anti-apoptotic protein level were detected by Western blot. (E) qRT-PCR was used to assess the pro-apoptotic protein level and anti-apoptotic protein level. Data are shown as mean ±SD. ** P<0.01, **** p<0.0001.
Figure 4.
Salidroside suppressed IL-1β-induced ROS, NO, and Caspase3/9. (A, B) The ROS and NO levels of the IL-1β group were significantly higher than in the control group, showing Salidroside significantly suppressed the levels of ROS and NO. (C, D) ELISA and WB result showed that salidroside inhibits the IL-1β-induced high level of caspase 3. (E) ELISA data showed that caspase 9 level was decreased after adding salidroside to IL-1β-treated cells.
Involvement of the PI3K/AKT pathway in the apoptosis inhibition induced by Salidroside
To investigate the underlying mechanism of the protective effect of Salidroside on chondrocytes, the expression level of PI3K/AKT was detected. IL-1β treatment significantly increased the phosphorylation level of p-AKT and p-PI3K, and this effect was significantly suppressed by Salidroside (Figure 5A), indicating that Salidroside inhibits the PI3K/AKT signaling pathway.
Figure 5.
The effect of Salidroside on the phosphorylation of PI3K and AKT. (A) Salidroside suppressed the IL-1β-induced the phosphorylation of PI3K and AKT, (B) Salidroside promoted cell proliferation and inhibited apoptosis via inhibition of pro-apoptotic protein and induction of anti-apoptosis and proliferative-related proteins.
Discussion
Previous studies have reported that the progressive cartilage loss of patients with OA is related to the imbalance of anti-apoptotic and pro-apoptotic activities [13]. IL-1β levels are significantly higher in cartilage of patients with OA, which plays a key role in inducing cell apoptosis by increasing ROS production and NO release. It has been reported that IL-1β induces cell apoptosis in various cells, including intervertebral disc cells and chondrocytes [14,15]. In contrast, Salidroside has an anti-apoptotic effect on various type of cells [16]. The present study is the first to assess the proliferative and anti-apoptotic effect of Salidroside on chondrocytes.
Salidroside treatment promotes chondrocyte proliferation in a dose-dependent manner. However, 200 μmol/L Salidroside has a negative effect on the proliferation of chondrocytes, suggesting that a moderate dosage of Salidroside is beneficial for chondrocytes. To determine whether Salidroside protects against OA, chondrocytes were pretreated with Salidroside and then stimulated with IL-1β. We found that IL-1 significantly increased the level of pro-inflammatory cytokines. Pro-inflammatory cytokines play an important role in the pathologic process of osteoarthritis. These cytokines interact with each other and have a synergistic effect on apoptosis activation [15]. However, adding Salidroside to IL-1β treated cells can reverse the IL-1β induced inflammatory response.
When apoptosis occurs, the levels of Bax and Bcl-2 change correspondingly [17]. The upregulated level of Bax further induces apoptosis. Contrary to Bax, Bcl-2 has a significant anti-apoptotic effect. Our present results suggest that Salidroside alleviates the anti-apoptotic effect of IL-1β by decreasing the ratio of Bax to Bcl-2. Lia et al. reported that Salidroside attenuates cardiac apoptosis through the mitochondrial apoptosis pathway [18]. When mitochondrial apoptosis occurs, the levels of caspase 3 and caspase 9 increase [19]. IL-1 induces apoptosis by affecting mitochondrial function, which is supported by the increased level of caspase 3/9. IL-1β also significantly increases production of ROS and NO, which are closely related to apoptosis [20]. In the present study, we observed that the levels of caspase 3/9, NO, and ROS in Salidroside+IL-1β-treated chondrocytes were lower than those in IL-1β-treated cells, suggesting that Salidroside blocks IL-1β-induced apoptosis.
It is well established that the PI3K/AKT pathway regulates inflammation and cell survival [21,22]. Activation of the PI3K/AKT pathway has been reported to induce inflammation and apoptosis of chondrocyte [23]. In the present study, we been found that cells treated with IL-1β had significantly increased levels of p-PI3K and p-AKT. Previous studies have reported that Salidroside has a negative effect on PI3K/AKT activation [12]. Our data are consistent with previous studies reporting that Salidroside blocks the IL-1 β-induced PI3K/AKT activation. As the PI3K/AKT pathway is inactivated, the pro-inflammatory cytokines and apoptosis levels decrease. It should be noted that many signaling pathways regulate the apoptosis process of chondrocytes. Further research is needed to assess the effect of Salidroside on other signaling pathways.
Conclusions
Our results indicate that the optimal concentration of Salidroside to treat chondrocytes is 0 to 100 μmol/L. Salidroside prevents IL-1-induced inflammatory action and apoptosis of chondrocytes by inhibiting the PI3K/AKT signaling pathway (Figure 5B). In vivo and in vitro studies are needed to define the detailed molecular mechanism by which Salidroside prevents OA. Despite its preliminary character, this study validates that Salidroside is a promising bioactive substance in OA prevention.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–87. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Zhou X, Jiang L, Fan G, et al. Role of the ciRS-7/miR-7 axis in the regulation of proliferation, apoptosis and inflammation of chondrocytes induced by IL-1beta. Int Immunopharmacol. 2019;71:233–40. doi: 10.1016/j.intimp.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 3.Behrendt P, Preusse-Prange A, Kluter T, et al. IL-10 reduces apoptosis and extracellular matrix degradation after injurious compression of mature articular cartilage. Osteoarthritis Cartilage. 2016;24(11):1981–88. doi: 10.1016/j.joca.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Hugle T, Geurts J, Nuesch C, et al. Aging and osteoarthritis: An inevitable encounter? J Aging Res. 2012;2012 doi: 10.1155/2012/950192. 950192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng W, Tao Z, Chen C, et al. Plumbagin prevents IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes and prevents the progression of osteoarthritis in mice. Inflammation. 2017;40(3):849–60. doi: 10.1007/s10753-017-0530-8. [DOI] [PubMed] [Google Scholar]
- 6.Xue H, Tu Y, Ma T, et al. miR-93-5p attenuates IL-1beta-induced chondrocyte apoptosis and cartilage degradation in osteoarthritis partially by targeting TCF4. Bone. 2019;123:129–36. doi: 10.1016/j.bone.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Mi B, Wang J, Liu Y, et al. Icariin activates autophagy via down-regulation of the NF-kappaB signaling-mediated apoptosis in chondrocytes. Front Pharmacol. 2018;9:605. doi: 10.3389/fphar.2018.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crivelli B, Bari E, Perteghella S, et al. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur J Pharm Biopharm. 2019;137:37–45. doi: 10.1016/j.ejpb.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Xie H, Chen C, et al. Inhibiting the PI3K/AKT/NF-kappaB signal pathway with nobiletin for attenuating the development of osteoarthritis: In vitro and in vivo studies. Food Funct. 2019;10(4):2161–75. doi: 10.1039/c8fo01786g. [DOI] [PubMed] [Google Scholar]
- 10.Qi W, Lin C, Fan K, et al. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem Biol Interact. 2019;306:19–28. doi: 10.1016/j.cbi.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Ding X, Wang W, Chen J, et al. Salidroside protects inner ear hair cells and spiral ganglion neurons from manganese exposure by regulating ROS levels and inhibiting apoptosis. Toxicol Lett. 2019;310:51–60. doi: 10.1016/j.toxlet.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, He L. Salidroside attenuates adriamycin-induced focal segmental glomerulosclerosis by inhibiting the hypoxia-inducible factor-1alpha expression through phosphatidylinositol 3-kinase/protein kinase B pathway. Nephron. 2019;142(3):243–52. doi: 10.1159/000497821. [DOI] [PubMed] [Google Scholar]
- 13.Miao G, Zang X, Hou H, et al. Bax targeted by miR-29a regulates chondrocyte apoptosis in osteoarthritis. Biomed Res Int. 2019;2019 doi: 10.1155/2019/1434538. 1434538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang CC, Zhou JS, Hu JG, et al. Effects of IGF-1 on IL-1beta-induced apoptosis in rabbit nucleus pulposus cells in vitro. Mol Med Rep. 2013;7(2):441–44. doi: 10.3892/mmr.2012.1238. [DOI] [PubMed] [Google Scholar]
- 15.Zhou RP, Dai BB, Xie YY, et al. Interleukin-1beta and tumor necrosis factor-alpha augment acidosis-induced rat articular chondrocyte apoptosis via nuclear factor-kappaB-dependent upregulation of ASIC1a channel. Biochim Biophys Acta Mol Basis Dis. 2018;1864(1):162–77. doi: 10.1016/j.bbadis.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, Ju J, Fang Y, et al. Salidroside protected against MPP(+) -induced Parkinson’s disease in PC12 cells by inhibiting inflammation, oxidative stress and cell apoptosis. Biotechnol Appl Biochem. 2019;66(2):247–53. doi: 10.1002/bab.1719. [DOI] [PubMed] [Google Scholar]
- 17.Chi Q, Luan Y, Zhang Y, et al. The regulatory effects of miR-138-5p on selenium deficiency-induced chondrocyte apoptosis are mediated by targeting SelM. Metallomics. 2019;11(4):845–57. doi: 10.1039/c9mt00006b. [DOI] [PubMed] [Google Scholar]
- 18.Lai MC, Lin JG, Pai PY, et al. Protective effect of Salidroside on cardiac apoptosis in mice with chronic intermittent hypoxia. Int J Cardiol. 2014;174(3):565–73. doi: 10.1016/j.ijcard.2014.04.132. [DOI] [PubMed] [Google Scholar]
- 19.Zhong T, Zhang J, Han X, et al. 3,3′,4,4′,5-Pentachlorobiphenyl influences mitochondrial apoptosis pathway in granulosa cells. J Cell Biochem. 2019;120(9):15337–46. doi: 10.1002/jcb.28801. [DOI] [PubMed] [Google Scholar]
- 20.Yu X, Lan P, Hou X, et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1beta production via suppressing the NF-kappaB pathway and ROS production. J Hepatol. 2017;66(4):693–702. doi: 10.1016/j.jhep.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Lu H, Yao J, et al. GABARAP promotes bone marrow mesenchymal stem cells-based the osteoarthritis cartilage regeneration through the inhibition of PI3K/AKT/mTOR signaling pathway. J Cell Physiol. 2019 doi: 10.1002/jcp.28705. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Luo G, Zhou J, Li G, et al. Ferruginol diterpenoid selectively inhibits human thyroid cancer growth by inducing mitochondrial dependent apoptosis, endogenous reactive oxygen species (ROS) production, mitochondrial membrane potential loss and suppression of mitogen-activated protein kinase (MAPK) and PI3K/AKT signaling pathways. Med Sci Monit. 2019;25:2935–42. doi: 10.12659/MSM.914348. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Li D, Ni S, Miao KS, et al. PI3K/Akt and caspase pathways mediate oxidative stress-induced chondrocyte apoptosis. Cell Stress Chaperones. 2019;24(1):195–202. doi: 10.1007/s12192-018-0956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]