ABSTRACT
Francisella tularensis is a Gram-negative, intracellular bacterium causing the zoonosis tularemia. This highly infectious microorganism is considered a potential biological threat agent. Humans are usually infected through direct contact with the animal reservoir and tick bites. However, tularemia cases also occur after contact with a contaminated hydro-telluric environment. Water-borne tularemia outbreaks and sporadic cases have occurred worldwide in the last decades, with specific clinical and epidemiological traits. These infections represent a major public health and military challenge. Human contaminations have occurred through consumption or use of F. tularensis-contaminated water, and various aquatic activities such as swimming, canyoning and fishing. In addition, in Sweden and Finland, mosquitoes are primary vectors of tularemia due to infection of mosquito larvae in contaminated aquatic environments. The mechanisms of F. tularensis survival in water may include the formation of biofilms, interactions with free-living amoebae, and the transition to a ‘viable but nonculturable' state, but the relative contribution of these possible mechanisms remains unknown. Many new aquatic species of Francisella have been characterized in recent years. F. tularensis likely shares with these species an ability of long-term survival in the aquatic environment, which has to be considered in terms of tularemia surveillance and control.
KEYWORDS: Tularemia, Francisella tularensis, Francisella species, waterborne diseases, aquatic environment, mosquitoes, amoeba, bacterial biofilms
GRAPHICAL ABSTRACT
Introduction
Francisella tularensis is a small, Gram-negative coccobacillus and the causative agent of the zoonosis tularemia [1]. Because it is highly infectious and can be spread by aerosols, this microorganism is classified in the category A of potential agents of biological threat by the US Centers for Disease Control and Prevention (CDC) [2]. The species F. tularensis is classically divided into four subspecies: F. tularensis subsp. tularensis (Type A strains), F. tularensis subsp. holarctica (Type B strains), F. tularensis subsp. mediasiatica, and F. tularensis subsp. novicida, which may be considered a different species (F. novicida) because it is an aquatic bacterium of low virulence in humans [3,4]. Type A and type B strains of F. tularensis are the two etiological agents of tularemia. The former subspecies is mainly localized in North America, although it has been occasionally detected in arthropods in Slovakia and Austria [5]. The latter one is found throughout the Northern Hemisphere [1], but has recently been detected in Australia [6].
F. tularensis has multiple reservoirs. Firstly, this bacterium can infect a multitude of animal species, including lagomorphs and small rodents, which are the primary sources of human infections [1,7,8]. Secondly, Ixodidae ticks are vectors but also a probable reservoir of F. tularensis owing to the transstadial transmission of this pathogen in these arthropods [1,8]. Mosquitoes and deer flies can also transmit F. tularensis to humans and animals in specific areas, but are not considered long-term reservoirs of this pathogen [1,8]. Finally, a hydro-telluric reservoir of F. tularensis is suggested by numerous studies but has not been extensively characterized [1,8]. Water-borne tularemia was first described in the 1930s in the USSR[9]. Human infection with F. tularensis may occur from these different reservoirs, and the clinical presentation of tularemia is primarily related to the mode of infection. Six major clinical forms of tularemia are classically recognized. The most frequent route of contamination is through the skin, through contact with an infected animal (especially hares in hunters) or arthropod bites. The ulceroglandular form of tularemia combines a skin ulcer at the site of F. tularensis inoculation and regional lymphadenopathy. The glandular form corresponds to regional lymphadenopathy without any visible skin lesion. Infection with F. tularensis through the conjunctiva (e.g. hand to eye contamination) or the oral route (ingestion of contaminated water or food) correspond to the oculoglandular and oropharyngeal forms, respectively. The involvement of lungs through inhalation of infected aerosols or hematogenous spread of bacteria corresponds to the pneumonic form. Finally, whatever the portal of entry of bacteria, severe sepsis often associated with confusion and F. tularensis bacteremia corresponds to the typhoidal form. The present review summarizes the literature data on human tularemia cases infected from aquatic sources, detection of F. tularensis in aquatic environments, and potential mechanisms of F. tularensis survival in water environments.
Search strategy and selection criteria
Data on tularemia cases related to aquatic sources, on the presence of Francisella species in water environments, and the mechanisms of survival of these bacteria in water environments were collected from the English literature in the PubMed database. Only articles published in the last two decades (1998–2018) were included. They were extracted using the keywords ‘tularemia' or ‘Francisella' and ‘case report’ or ‘water' or ‘mosquito' or ‘biofilms' or ‘amoeba.' In addition, data on other aquatic Francisella species were added, including F. novivida, F. philomiragia, F. halioticida, F. hispaniensis, F. noatunensis, F. salina, F. frigiditurris, Allofrancisella (formerly Francisella) gangzhouensis, F. marina, F. ulginis and F. endociliophora.
Tularemia of aquatic sources
Tularemia and drinking water
In the last 20 years, tularemia cases linked to drinking water have been reported in Turkey, Kosovo, Bulgaria, Georgia, Macedonia, Norway, Sweden, Italy, and Germany (Table 1).
Table 1. Tularemia outbreaks and sporadic cases related to drinking water.
| Country | Year of occurrence | Number of cases | Clinical forms (n) | Source of infection (water detection of F. tularensis) | F. tularensis subsp. involved | Reference |
|---|---|---|---|---|---|---|
| Bulgaria | 1997–2005 | 285 | OP (275), UG (6), OG (4) | Drinking water mainly from private wells (culture, pos) | holarctica | [40] |
| Georgia | 2006 | 26 | OP (21), GL (5) | Using water from the community water supply (culture, pos) | NS | [41] |
| Germany | 2007 | 1 | OP | Drinking surface water (NS) | holarctica | [49] |
| Italy | 1988 | 24 | OP (12), OG (1), AS (11) | Drinking water (NS) | NS | [48] |
| Kosovo | 1999–2000 | > 200 | OP | Drinking water or eating food (Ag, neg) | NS | [39] |
| 2001–2002 | > 200 | OP | Drinking water or eating food (Ag, neg) | NS | [38] | |
| Macedonia | 2015 | 13 | NS | Drinking water (NS) | NS | [42] |
| Norway | 1997 | 8 | OP or UG | Drinking water from wells (one well with a lemming carcass PCR-positive for F. tularensis) (PCR, pos) | NS | [43] |
| 2006 | 9 | OP (5), GL (3), UN (1) | Drinking water from private wells or eating snow (PCR, pos) | NS | [44] | |
| 2011 | 39 | OP (21), UG or GL (10), PN (2), TY (2), AS (3), UN (1) | Drinking water from private wells or stream (PCR, pos) | NS | [45] | |
| Sweden | NS | 1 | OP | Drinking water from a well (NS) | holarctica | [46] |
| 2013 | 6 | OP | Drinking water from a private well (culture, pos) | NS | [47] | |
| Turkey | 1988–1998 | 205 | OP (83%), OG (8%), UN (9%) | Drinking water (NS) | NS | [14] |
| NS | 1 | OP | Drinking water from a well (culture, neg) | NS | [29] | |
| 2001 | 14 | OP | Drinking village pipe water (culture, neg) | NS | [35] | |
| 2005 | 5 | OP | Drinking natural spring water (PCR, neg) | NS | [30] | |
| 2005 | 10 | OP (7), AS (3) | Drinking spring water (culture, neg and PCR, pos) | NS | [20] | |
| 2004–2005 | 54 | OP | Drinking water (PCR, neg) | NS | [34] | |
| 2004–2005 | 39 | OP | Drinking water (NS) | NS | [36] | |
| NS | 2 | OP | Drinking water (NS) | NS | [28] | |
| 2005 | 11 | OP (8), OG (3) | Drinking village fountain water (PCR, pos) | NS | [19] | |
| 2004–2005 | 145 | OP | Drinking spring water (NS) | holarctica | [13] | |
| 2005 | 70 | OP mostly | Drinking natural spring water (culture, neg) | NS | [33] | |
| 2004 | 86 | OP mostly | Drinking water from a rivulet (PCR, pos) | NS | [17] | |
| 2004–2005 | 135 | OP | Drinking natural spring water (PCR, neg) | NS | [11] | |
| 2000 | 22 | OP (19), UG (3) | Drinking spring water (PCR positive for Francisella spp.) | NS | [18] | |
| 2005–2006 | 58 | OP | Drinking natural spring water (PCR, pos) | holarctica | [16] | |
| NS | 1 | OG | Drinking and washing face with spring water (NS) | NS | [26] | |
| NS | 3 | OP | Drinking spring water (NS) | holarctica | [27] | |
| NS | 4 | OP | Drinking water (NS) | NS | [23] | |
| 2011 | 2 | PN (with bacteremia) | Drinking water (culture, pos) | holarctica | [24] | |
| 2010 | 4 | OP (3), OP and OG (1) | Drinking (3) or exposure (1) to natural spring water (NS) | NS | [25] | |
| 2009–2011 | 139 | OP (74%), GL (15.8%), OG (5%) | Drinking spring water (PCR, pos) | NS | [15] | |
| NS | 2 | OP (with abdominal lymphadenopathy) | Drinking natural spring water (NS) | NS | [21] | |
| NS | 3 | OP | Drinking natural spring water (NS) | NS | [22] | |
| 2010–2012 | 110 | OP or GL | Drinking water (PCR, pos) | NS | [12] | |
| 2013 | 55 | OP | Drinking tap water contaminated by surface water (culture, neg) | NS | [31] | |
| 2008, 2009 and 2012 | 89, 54 and 35 | OP | Drinking water (culture, pos) | NS | [32] |
(NS) Not specified; Clinical forms: ulceroglandular (UG), glandular (GL), oropharyngeal (OP), oculoglandular (OG), pneumonic (PN), typhoidal (TY), asymptomatic (AS), and unknown form (UN); (Ag) F. tularensis antigen detection; (pos) positive, (neg) negative.
In Turkey, tularemia reemerged in 1988 in the form of water-borne outbreaks of oropharyngeal tularemia cases [10]. Between 1988 and 2018, 28 tularemia outbreaks and non-outbreak tularemia cases linked to consumption of contaminated water were reported in this country [11–36]. The Turkish outbreaks were frequently of large scale, involving more than one hundred people for some of them [11–15]. Patients were almost exclusively suffering from the oropharyngeal form of tularemia, in accordance with the oral route of contamination with F. tularensis. In addition, cases were observed in the whole population, with a male/female sex ratio close to 1 or even lower, and both children and adults involved [11–19,31,33–35]. This is in sharp contrast to countries where infections usually occur through contact with animals or tick bites, and therefore predominate in middle-aged men due to more frequent occupational or leisure exposures [8]. Less frequently, exposure to contaminated water resulted in glandular [12,15], oculoglandular [14,15,19,25,26], and pneumonic [24] forms of tularemia. In some reports, Francisella species or more specifically F. tularensis were detected by PCR or culture in the suspected water sources [12,15–20,24,32]. Moreover, the same F. tularensis genotypes were detected concomitantly from water sources and related tularemia patients in some studies [24,32,37], confirming the epidemiological link between drinking water and human infection. In the majority of cases, the source of contamination was spring water, water from the community water supplies or water from wells. These water sources were not or inadequately disinfected. Contamination of the water by infected animal carcasses or excreta was the most likely hypothesis [10]. Although F. tularensis subsp. holarctica was identified only for some of these outbreaks, only type B strains have been associated with human infections in Europe and Asia.
In Kosovo, two huge oropharyngeal tularemia outbreaks occurred in 1999–2000 and 2001–2002, each one involving more than two hundred patients [38,39]. It was not possible to establish if the primary source of infection was contaminated water or food, but it can be assumed that both were involved in the human transmission of F. tularensis [38,39]. These outbreaks were likely a collateral damage of the Kosovo war, which ended in 1999 and left the country with bad sanitary conditions. Indeed, people reported that mice and rats invaded their villages during this period, which may correlate with contamination of water wells and cellars with F. tularensis. This bacterium was detected in a field mouse recovered from a water well in an affected village [39].
A long-lasting outbreak occurred between 1997 and 2005 in Bulgaria, involving 285 people [40]. F. tularensis was isolated from water samples collected from private wells. Thus, the route of human contamination was likely water consumption. The majority of patients suffered from the oropharyngeal form of tularemia [40]. Similarly, in Georgia, a tularemia outbreak involving 26 patients occurred in 2006 [41], with a predominance of oropharyngeal cases. The water source was the community water supply from which F. tularensis was isolated [41]. Finally, a small outbreak involving 13 patients was described in 2015 in Macedonia [42].
Water-borne tularemia cases are also reported in Scandinavia. In Norway, three small outbreaks, involving eight to 39 people, linked to the consumption of contaminated water were reported over 20 years [43–45]. Most of the patients suffered from oropharyngeal tularemia [43–45], although ulceroglandular, glandular, pneumonic and typhoidal forms were also observed [43–45]. The source of these infections was water collected from private water wells, as confirmed by PCR-detection of F. tularensis DNA [43–45]. F. tularensis-infected rodent carcasses probably contaminated these water wells. In one report, a lemming carcass retrieved from the contaminated water well was PCR-positive for F. tularensis [43]. The involved F. tularensis subspecies was not identified, but only type B strains are found in this country. In Sweden, a small outbreak and sporadic cases of oropharyngeal tularemia were also associated with the consumption of contaminated water from private water wells [46,47].
Tularemia cases related to F. tularensis-contaminated water consumption have been occasionally observed in other countries. In Italy, in 1988, a tularemia outbreak linked to the use of water collected from an aqueduct involved 24 people living in the same village, most of which developed oropharyngeal tularemia [48]. More recently, a German oropharyngeal case of tularemia was suspected to be associated with drinking of non-purified surface water during a holiday in Bavaria [49].
Tularemia and mosquitoes
In Sweden and Finland, tularemia is mainly transmitted through mosquito bites. Large tularemia outbreaks, frequently involving more than one hundred people, have occurred almost annually in Sweden during the last two decades [46,50–54]. These outbreaks mainly occurred in central Sweden (counties of Örebro, Stockholm, Södermanland, Västmanland, Värmland, and Dalarna), in wet and woody areas of the Boreal Forest, during the late summer period, because of the mosquito vector habitat and seasonality [46,50–54]. A large scale epidemiological study in Sweden over 29 years identified that tularemia incidence was positively correlated with the presence of lakes and rivers [55]. Similarly, outbreak modeling demonstrated that tularemia in Sweden is concentrated in a few high-risk regions, with a high incidence in summer likely related to the population dynamics of the mosquito vector [56]. Due to this specific mode of transmission, the ulceroglandular form of tularemia is predominant in Sweden. In addition, cases occur in the exposed population regardless of age, but more frequently in men than women probably because of more outdoor activities in men [46,50,51,53]. Swedish mosquitoes collected in Örebro were PCR-positive for F. tularensis subsp. holarctica, still arguing their role in tularemia transmission in this country [57].
Tularemia is also predominantly a mosquito-borne disease in Finland, where several tularemia outbreaks [58] or sporadic cases [59,60] have been linked with this mode of transmission. Here again, tularemia is a seasonal disease occurring during late summer, and ulceroglandular tularemia is the primary clinical presentation [58,61].
The potential role of mosquitoes in the transmission of F. tularensis to humans has been occasionally reported in other countries, such as Germany [62]. All these reports involved F. tularensis subsp. holarctica as they occurred in Europe. Table 2 summarizes the literature reports of mosquito-borne tularemia cases. Interestingly, F. tularensis DNA was detected in Alaskan mosquitoes, suggesting that tularemia could be a mosquito-borne disease in this U.S. state [63].
Table 2. Tularemia outbreaks and sporadic cases related to mosquito bites.
| Country | Year of occurrence | Number of cases | Clinical forms | F. tularensis subsp. involved | Reference |
|---|---|---|---|---|---|
| Finland | 2000 | 1 | UG | NS | [59] |
| 2007 | 50 | UG mostly | holarctica | [58] | |
| 2010 | 1 | UG | NS | [60] | |
| Germany | NS | 1 | UG | holarctica | [62] |
| Sweden | 1981, 1995, 1999, 2000, 2002, 2003, and 2004 | 349 | UG mostly | holarctica | [50] |
| 2000–2004 | 278 | UG | holarctica | [46,51] | |
| 2000 | 105 | UG mostly | NS | [52] | |
| 2003 | 475 | NS | NS | [53] | |
| 2006 | 90 | NS | NS | [54] |
(NS) Not specified; Clinical forms: ulceroglandular (UG).
Tularemia of other aquatic sources
All around the world, human contamination with F. tularensis has occasionally occurred through other types of water exposure (Table 3).
Table 3. Tularemia outbreaks and sporadic cases related to other aquatic sources.
| Country | Year of occurrence | Number of cases | Clinical forms (n) | Source of infection | F. tularensis subsp. involved | Reference |
|---|---|---|---|---|---|---|
| Canada | NS | 1 | UG | Suspicion of finger injury while cleaning freshwater fishes | NS | [70] |
| Finland | NS | 1 | PN | Near-drowning accident | NS | [65] |
| France | NS | 1 | PN with bacteremia | Near-drowning accident while fishing in a river | holarctica | [64] |
| 2008–2014 | 3 | Otomastoiditis | Canyoneering in the same river | holarctica | [68] | |
| Spain | 1998 | 19 | UG and GL | Crayfish fishing in a river | holarctica | [69] |
| Turkey | 2007 | 3 | OP | Swimming in the same lake | NS | [67] |
| 2010 | 1 | GL | Swimming in freshwater | NS | [66] | |
| USA | 2000–2006 (Martha’s Vineyard island) | 59 | PN (38), UG or GL (9), TY (2), OP (1) | Water environmental source likely | tularensis | [73,74] |
| 2016 | 1 | UG | Finger injury while fishing in a freshwater lake. | holarctica | [71] |
(NS) Not specified; Clinical forms: ulceroglandular (UG), glandular (GL), oropharyngeal (OP), pneumonic (PN), and typhoidal (TY).
Tularemia cases were reported after near-drowning accidents in France [64] and Finland [65]. For these two cases, patients developed a pneumonic form of the disease after inhalation of contaminated water. For the French case, contamination occurred after inhalation of freshwater and was caused by F. tularensis subsp. holarctica. The involved subspecies and water salinity were not specified for the Finnish case. Four tularemia cases reported in Turkey were related to swimming activities [66,67]. Three of these cases were oropharyngeal forms that developed after swimming in the same lake [67]. The fourth patient suffered from a glandular form after swimming in a natural aquatic environment in a valley [66]. Otomastoiditis caused by F. tularensis subsp. holarctica were reported in three patients (two from France, one from Germany) after canyoning in the same river in France, between 2009 and 2014 [68]. Otitis likely occurred after exposure to F. tularensis contaminated water, although the source was not specifically identified [68].
Tularemia cases also occurred after handling F. tularensis-contaminated aquatic animals [69–71]. A small tularemia outbreak involving 19 patients occurred in Spain after crayfish fishing [69]. The patients developed a glandular or ulceroglandular form of the disease through skin injuries while catching or cleaning red swamp crayfishes sinned in the same river. Cases were grouped over three weeks after which fishing was prohibited [69]. The source of contamination was confirmed by PCR-detection of F. tularensis DNA in one crayfish and two water samples from the river [69]. In Canada, a patient developed ulceroglandular tularemia after a finger injury while cleaning pike and pickerel, which are freshwater fishes [70]. Similarly, in the USA, a patient contracted an ulceroglandular tularemia due to F. tularensis subsp. holarctica after a finger injury while fishing on a freshwater lake [71].
The particular ecology of Martha’s Vineyard (Massachusetts), an island in the east coast of the USA should be highlighted. Two outbreaks of pneumonic tularemia occurred on this island, the first in 1978 [72] and the second from 2000 to 2006 [73,74]. F. tularensis subsp. tularensis was isolated from one dead patient and rabbits [73,75]. According to a case–control study, landscaping activities, especially lawn mowing and brush-cutting, were the most likely sources of exposure to F. tularensis through aerosols from environmental material [73,76]. However, the environmental reservoir of F. tularensis could not be characterized. Skunks and raccoons were found to be seropositive for F. tularensis, but other animal species tested had not or very rarely been infected with this pathogen [75]. A low prevalence of infection and a high degree of Francisella genetic diversity in ticks argued against the role of these arthropods as a source of human contamination [77]. In contrast, the role of the aquatic environment as a long-term reservoir of F. tularensis was strongly suspected. PCR detection of Francisella sp. DNA was frequently reported from brackish-water samples collected on the island [78].
The role of brackish water as a potential reservoir of F. tularensis is supported by a more recent study in Nunavik territory (Canada) demonstrating that tularemia seroprevalence in the human population was positively correlated with residence near the coast [79].
Other Francisella species and aquatic reservoir
The genus Francisella includes many other species with a proven or probable aquatic habitat. Genetic analyses have suggested that the ancestral Francisella species originated in a marine habitat [80]. Here, we review water-borne human infections caused by Francisella species other than F. tularensis (Table 4). Some of these species are opportunistic pathogens mainly causing diseases in immunocompromised patients exposed to an aquatic environment. Among them, infections caused by F. philomiragia or F. novicida have been most frequently reported.
Table 4. Human infections caused by Francisella species other than F. tularensis related to aquatic sources.
| Country | Year of occurrence | Number of cases | Clinical forms | Source of infection | F. tularensis subsp. involved | Reference |
|---|---|---|---|---|---|---|
| Australia | NS | 1 | UG | Cut in the toe in brackish water | F. novicida-like | [99] |
| NS | 1 | NS, bacteremia | Cut with a fishhook while fishing | F. hispaniensis | [105] | |
| Canada | NS | 1 | OP and PN | Skin abrasion from a saltwater crab | F. philomiragia | [84] |
| Spain | NS | 1 | OP | Holiday activities in the Mediterranean sea | F. philomiragia | [83] |
| Turkey | NS | 1 | TY | Swimming in the sea and taking mud baths | F. philomiragia | [81] |
| USA | 1977–1985 | 5 | PN, bacteremia | Near-drowning accident in saltwater or brackish water | F. philomiragia | [86] |
| 1995 | 1 | NS, bacteremia | Use of water from a private well for food preparation and bathing | F. novicida-like | [100] | |
| NS | 1 | NS, bacteremia | Working in the brackish water of Assawoman Bay in Maryland | F. philomiragia | [85] | |
| NS | 1 | TY | Practice of jet-ski in a bay in the Atlantic Ocean | F. philomiragia | [82] | |
| NS | 1 | NS, bacteremia | Near-drowning accident while surfing in the Atlantic Ocean | F. novicida | [97] | |
| 2001 | 1 | NS | Exposure to hot spring water near a salt-lake | F. novicida | [89] | |
| NS | 1 | UG | Suspicion of infection through contact of an open wound in brackish water | Species close to F. halioticida | [113] | |
| 2011 | 3 | NS, bacteremia | Consumption of ice from ice machines | F. novicida | [98] |
(NS) Not specified; Clinical forms: ulceroglandular (UG), glandular (GL), oropharyngeal (OP), oculoglandular (OG), pneumonic (PN), and typhoidal (TY).
Less than 20 human infections with F. philomiragia have been published in the English literature [81–88]. These infections occurred in healthy individuals that have survived a near-drowning accident [86] or in immunocompromised patients (especially people suffering from chronic granulomatous disease) after exposure to an aquatic environment [81–86]. Interestingly, human contamination usually occurred after exposure to salt- or brackish-water [81–86]. Infections with F. philomiragia most often occurred during recreational activities in sea, ocean or bay connected to the ocean [81–83,85,86]. A young patient was contaminated after a skin lesion caused by a saltwater crab [84]. In a review of 14 cases, Wenger et al. showed that most F. philomiragia infections occurred in patients living within 50 miles of a salt-water coastline [86], again suggesting a clear association between salt-water exposure and F. philomiragia infections.
The geographical distribution of F. philomiragia is probably wide as human infections with this species have been described in the USA [82,85,86], Canada [84], Europe [83] and Turkey [81]. Frequent detection of F. philomiragia in water samples by culture or PCR indicates that the aquatic environment is likely the primary reservoir of this bacterium [78,89–93]. In two studies, in Norway [92] and the USA [78], F. philomiragia was only found in salt- or brackish-water but not in fresh-water, suggesting that water salinity is a major element in the natural life cycle of F. philomiragia. However, this species was also isolated from spring water near a salt lake in the USA [89] and cooling towers in China [91], reflecting its distribution in different aquatic reservoirs. F. philomiragia DNA was also detected in ballast water from cargo traveling all around the world [90], which might be a mode of diffusion of this species at the global scale. Although the aquatic reservoir appears to be predominant, F. philomiragia was isolated in a sick muskrat and in brackish water surrounding it [94] suggesting that a mammal reservoir may exist for this bacterium. F. philomiragia was also detected in Dermacentor ticks [95].
F. novicida is also a rare human pathogen. A dozen cases have been published in the English literature so far [96]. For all cases with an identified mode of contamination, an aquatic source was involved [89,97–100]. In the USA, a case of F. novicida bacteremia occurred after a near-drowning accident in the Atlantic Ocean [97], another case after exposure to hot spring water near a salt lake [89], and the last one after exposure to water from a private water well [100]. A striking outbreak of F. novicida bacteremia occurred among inmates in the USA after consumption of ice from ice machines from which F. novicida DNA was detected by PCR [98]. Finally, in Australia, a patient developed an ulceroglandular form of infection with F. novicida after cutting himself in brackish water [99]. Most of the patients suffering from F. novicida infection were immunocompromised or had underlying health conditions [98,100]. The identification of F. novicida has never been reported in animals or arthropods [96]. The only known reservoir of this bacterium is the aquatic environment as attested by its repeated isolation from water samples [78,89,101,102]. As for F. philomiragia, salinity seems to impact F. novicida survival in water, as this bacterium was detected by culture or PCR only in sea-water [101,102], brackish-water [78] and spring water near a salt lake [89].
Other Francisella species have been rarely associated with human infections originating from aquatic sources. F. hispaniensis was first isolated in 2003 from a Spanish patient suffering from bacteremia [103,104]. The source of contamination was not identified [103]. However, a few years later, F. hispaniensis was isolated in the blood of an Australian immunocompromised patient after he cut himself with a fishhook while fishing [105].
The genus Francisella also includes species that are pathogenic for marine animals. F. noatunensis subsp. orientalis and Francisella noatunensis subsp. noatunensis are widely described as warm- and cold-water fish pathogens, responsible for ‘piscine francisellosis.’ This disease causes high morbidity and mortality in many fish species worldwide and is responsible for economic losses in aquaculture [106–109]. F. noatunensis does not seem to be pathogenic in humans [106]. F. halioticida can infect Haliotis mollusks (abalones) [110,111] and F. marina sp. nov. was recently identified as causing disease in Spotted Rose Snapper fishes [112]. Interestingly, a novel Francisella species very close to F. halioticida was isolated in the USA, in a diabetic patient, from an infected skin wound developed after contact with brackish water [113]. Finally, other Francisella species are endosymbionts of marine ciliates such as F. endociliophora [114].
Over the past ten years, new Francisella species have been isolated from the aquatic environment. F. salina and F. uliginis were isolated from sea-water in the USA [101,102]. Water from cooling towers also seems to be a reservoir of Francisella species as attested by recent isolation from these air conditioning systems of F. frigiditurris in the USA [102] and F. guangzhouensis in China and Germany [91,115–117]. These two latter species have been transferred to the new genus Allofrancisella [115].
Table 5 summaries studies dealing with the detection of Francisella species in water samples, either using culture, PCR or both methods. It shows the broad spectrum of Francisella species found in aquatic reservoirs. Regarding PCR methods, it is important to notice that Francisella species that are not yet characterized may not be PCR-amplified from water samples due to the use of inadequate primers [78]. Even if there is PCR amplification with a new species, it may not be accurately identified as a novel species due to a lack of resolution within the utilized amplicon. Full identification of a novel species requires whole genome sequencing.
Table 5. Detection of Francisella species in water samples.
| Country | Year of sampling | Type of water samples (n) | Testing methods | Findings (n samples) | Reference |
|---|---|---|---|---|---|
| China | 2008 | Cooling towers (NS) | Culture and strain identification by fatty acid analysis, and 16S rRNA, 23S rRNAs, recA, rpoA, rpoB, rpoD, rpoH, groEL, dnaK, gyrB, sdhA, and fopA genes sequencing | F. guangzhouensis (4) | [117] |
| 2009–2011 | Cooling towers (312) | Culture and strain identification by 16S rRNA, rpoB and sdhA genes sequencing | Francisella strains phylogenetically close to F. philomiragia (1) or F. guangzhouensis (8) | [91] | |
| 2008 and after | Cooling towers (NS) | Culture and strain identification by mass spectrometry, fatty acid analysis, and 16S rRNA, rpoB, mdhA, and sdhA genes sequencing | Francisella strains phylogenetically close to F. guangzhouensis (5). Description of Allofrancisella inopinata gen. nov., sp. nov. and A. frigidaquae sp. nov.; transfer of F. guangzhouensis to A. guangzhouensis comb. nov. | [115] | |
| Germany | 2005–2006 | NS (28) | PCR targeting 16S rRNA and fopA genes | F. tularensis (1) | [132] |
| 2012 | Cooling tower (NS) | Culture and strain identification by 16S rRNA, fopA, gyrA, rpoA, groEL, sdhA, and dnaK genes sequencing | Francisella strain phylogenetically close to F. guangzhouensis (1) | [116] | |
| Netherlands | 2013–2017 | Surface water collected from areas with reported human or hare tularemia cases (127) or unrelated to recent tularemia cases (339) | PCR targeting ISFtu2 and fopA genes | F. tularensis in 88% of the case-related samples and in 10% of the randomly collected samples | [128] |
| Norway | 2010 | Seawater (149) or freshwater (64) | PCR sequencing of 16S rRNA; and for positive samples sdhA and purCD PCR | Francisella sp. in seawater (38) but not in freshwater samples. F. philomiragia-related species mostly. | [92] |
| Sweden | 2003– 2005 | Surface water (341) | PCR sequencing of lpnA, 16S rDNA, lpnA, and FtM19InDel | F. tularensis (108), mainly subsp. holarctica, rarely subsp. mediasiatica | [131] |
| Turkey | 2008 - 2009 | Rivers, spring waters or village fountains in tularemia-endemic areas (154) | Culture and strains identification by 16S rRNA gene sequencing; and PCR targeting ISFtu2 | F. tularensis subsp. holarctica isolation (4); or F. tularensis positive PCR ISFtu2 (17) | [130] |
| Ukraine | 1941– 2008 | NS (NS) | Culture | F. tularensis (393) | [129] |
| USA | 2003 | NS (23) | PCR sequencing of 16S rDNA; and for positive samples ISFtu2, 23 kDa, tul4, fopA and sdhA PCR | F. philomiragia (1) | [93] |
| NS | Seawater (NS) | Culture (CHAB-PACCV medium) and strains identification by PCR sequencing of 16S rRNA and sdhA genes | F. philomiragia-like (2) and F. novicida-like (1). The two F. philomiragia-like strains were latter characterized as new Francisella species by Challacombe et al.: F. salina and F. uliginis. | [101,102] | |
| 2005– 2007 (Martha’s Vineyard island) | Fresh-water (35) or brackish-water (42) | Culture and PCR targeting 16S rRNA gene; for positive samples sdhA, tul4, ISFtu2, and fopA PCR. | No positive fresh-water samples. Francisella DNA detected by PCR in brackish-water samples (19). Mainly F. philomiragia; few F. novicida- or F. tularensis-like strains. F. philomiragia grown from one brackish-water sample. | [78] | |
| NS | Hot or cold spring waters near a salt lake (NS) | Culture and strain identification by fatty acid analyses, ribotyping and 16S rRNA gene sequencing | F. philomiragia and F. novicida (NS) | [89] | |
| NS | Cooling tower (NS) | NS | F. frigiditurris (NS) | [102] | |
| Cargo ships* | 2007–2008 | Ballast water from 5 general cargo ships (NS) | PCR sequencing of 16S rRNA gene | F. philomiragia and F. noatunensis in ballast water from 4 cargo ships | [90] |
(NS) Not specified; * Cargo ships from Columbia, Republic of the Congo, USA, Canada, and Iran.
Mechanisms of F. tularensis survival in water environments
The high frequency of water-borne tularemia cases implies the persistence of F. tularensis in the aquatic environment. However, the mechanisms of F. tularensis survival in this environment have not been elucidated so far.
Long-term survival of F. tularensis in water
Experimental studies suggest long-term survival of F. tularensis in various water environments. Several authors described the survival of F. tularensis in water microcosm from 1 to 70 days [118–120]. F. tularensis survival seems to be influenced by both water temperature [118] and salinity [119]. Gilbert and Rose observed that F. tularensis subsp. holarctica remains cultivable after a stay in water for one day at 5°C or 25°C, but up to 28 days at 8°C [118]. Berrada and Telford showed that both type A and type B strains of F. tularensis remain cultivable after a stay of 8–10 days in fresh-water, but 30–42 days in brackish-water, both at 21°C [119]. In another study, F. tularensis subsp. holarctica remained cultivable after a stay in fresh water at 8°C up to 70 days [120].
Interestingly, when F. tularensis became uncultivable on agar plates, bacteria could still be detected and were metabolically active in water [118,120]. This phenomenon was observed by Gilbert and Rose [118] and Forsman et al. [120] for F. tularensis subsp. holarctica. The latter authors described the persistence of metabolic activity for F. tularensis in water up to 140 days [120]. This state is defined as ‘viable but non-culturable' (VBNC) and could be responsible for long-term survival of bacteria in the water environment. The VBNC state has been defined as a state from which bacterial cells cannot be cultured but maintain a metabolic activity and cellular integrity [118]. In addition, the VBNC state may be reversible, as bacteria may become cultivable under certain conditions. This reversion in the ability to grow on acellular media is called ‘resuscitation' of VBNC bacteria. The VBNC state has been described for a wide range of bacteria such as Vibrio sp. [121,122], Campylobacter sp. [123], Escherichia coli [122] and Legionella pneumophila [124]. Depending on the bacterial species, the infectious nature and pathogenic potential of VBNC cells are variable, as well as the ways of their resuscitation. Forsman et al. reported that F. tularensis VBNC cells were no longer virulent in mice, and could not be resuscitated [120]. Finally, a recent study demonstrated that F. tularensis subsp. holarctica possesses a mechanosensitive channel that protects this bacterium from hypo-osmotic shock when it is released from an infected animal to water [125].
Long-term survival in water has also been reported for other Francisella species. Berrada and Telford showed that F. novicida (like F. tularensis) remains cultivable after a stay of up to 30–42 days in brackish-water, at 21°C [119]. The fish-pathogen Francisella species are able to survive in water in the absence of a suitable fish host. Indeed, F. noatunensis subsp. orientalis remains cultivable after a stay up to 2 days in freshwater and up to 3 days in seawater [126]. F. noatunensis subsp. noatunensis remains cultivable after up to 12 days in freshwater and up to 50 days in seawater [127]. Interestingly, F. noatunensis was also able to enter in a VBNC state after a period of stay in the water, and these VBNC were not pathogenic to cods [127].
Long-term survival of F. tularensis in natural aquatic environments is suggested by a number of studies detecting this bacterium by culture or PCR in environmental water samples [12,16,17,19,20,32,40,41,43,44,47,78,128–132]. Not surprisingly, long-term detection of F. tularensis in water environments was reported in countries were water-borne tularemia cases are frequent and predominant. In Turkey, two environmental studies identified F. tularensis subsp. holarctica in water samples collected from the aquatic environment or from village water supply systems highlighting the role of different water sources as common and persistent reservoirs of F. tularensis in this country [32,130]. In Sweden, F. tularensis subsp. holarctica was PCR-detected in water samples collected during outbreak and non-outbreak periods, in tularemia endemic areas [131]. However, F. tularensis was also detected from water samples in countries where tularemia is not or rarely a water-borne disease [128,129,132]. Hightower et al. isolated F. tularensis strains from water in Ukraine and considered that the aquatic environment was the third main F. tularensis reservoir after arthropods and mammals in this country [129]. In Germany, following the reemergence of tularemia in 2004, an ecological study in outbreak areas found a river water sample PCR-positive for F. tularensis suggesting a natural aquatic reservoir for this bacterium [132]. In the Netherland, tularemia also re-emerged in 2011 leading to the surveillance of F. tularensis prevalence in the environment [128]. Surface water samples were PCR-positive for F. tularensis subsp. holarctica in 10% of the randomly collected samples and 88% of the samples collected in areas where tularemia cases among hares or humans had been reported [128]. These studies show the almost constant presence of F. tularensis in randomly collected water samples at different time periods. This observation can only be explained by repeated contaminations of the aquatic environment, particularly from the animal reservoir during epizootics, but also by the persistence of these bacteria in the water environment during non-epizootic periods.
Table 5 summarizes studies dealing with F. tularensis detection in water environments. PCR was more effective than culture for detection of F. tularensis in water samples, possibly because of a low bacterial inoculum, the encroachment of F. tularensis by other bacterial species, or a VBNC state of bacteria. However, PCR results should be interpreted with caution since it has been shown that F. tularensis specific primers (such as those targeting fopA or tul4 genes) may also amplify DNA from Francisella species other than the tularemia agents [78].
Survival in biofilms
An essential mechanism for survival and persistence of bacteria in the water environment is biofilm formation. Biofilms are defined as naturally formed adherent communities of bacteria within an extracellular polymeric matrix [133]. A number of bacterial species, such as Vibrio cholerae [134], Legionella pneumophila [135], Helicobacter pylori [136] and Pseudomonas aeruginosa [137] form biofilms to promote their survival under environmental water conditions. In vitro studies have demonstrated that both F. tularensis subsp. holarctica [138,139] and F. tularensis subsp. tularensis [139] can form biofilms. The aquatic Francisella species, F. novicida [139–141] and F. philomiragia [142] have also been capable of biofilm formation experimentally. F. novicida was also demonstrated to be able to form biofilm in chitin surface, the second most abundant biopolymer in nature, providing the structure of arthropods, insects, and fungi [139]. F. philomiragia was shown to form more biofilm at 25°C than at 37°C, which is compatible with its natural aquatic reservoir [142]. The fish pathogen F. noatunensis was also demonstrated to form biofilm in vivo. [143]. Until now, biofilm formation has not been associated with virulence in Francisella species [133]. Thus, biofilm formation is most likely a way of environmental survival and persistence in these species [133]. To our knowledge, Francisella biofilm has never been described in natural environmental water microcosm.
Survival in amoebae
Free-living amoebae are ubiquitous organisms in soil and water environments. Several human pathogens, such as L. pneumophila and some Mycobacterium species resist phagocytosis and digestion by the free-living amoebae and may survive in water environment inside amoebae [144]. Moreover, these bacteria may survive for long periods in amoeba cysts [144]. This mode of survival may apply to Francisella species, especially F. tularensis. Multiple experimental studies have focused on the interaction between Francisella species and several amoeba species [145–154]. Berdal et al. demonstrated that F. tularensis could penetrate in the amoeba Acanthamoeba castellanii and be released from it [152]. Then, other authors described the multiplication of F. tularensis subsp. tularensis in A. castellannii 24 h post-infection [150]. F. novicida and F. philomiragia were also able to multiply within Hartmanella vermiformis and A. castellanii [142,146,148,150,151]. F. noatunensis was also able to infect and replicate within the amoeba Dictostelium discoideum [149]. Francisella cells were localized within vacuoles in amoeba trophozoites [146] but were also able to survive in amoebal cysts for several weeks [150]. This latter finding suggested that amoeba could be a long-term reservoir of Francisella spp. in water environments. Other authors described an enhanced survival of F. tularensis subsp. tularensis, F. tularensis subsp. holarctica and F. novicida in co-culture with amoebae such as A. castellanii, A. polyphaga, Vermamoeba vermiformis or Ochromonas danica [145,147,153]. These authors described the presence of Francisella sp. inside and outside the amoeba in the co-culture model, suggesting that bacterial survival could be related to intra-amoebal replication, to favorable interaction between extracellular bacteria and amoeba, or both [145,147,153]. Interestingly, Gustafsson et al. demonstrated that growth supernatant of A. palestinensis (without the presence of amoebae) increased multiplication of F. tularensis [154]. After five days in co-culture with amoeba, F. tularensis subsp. holarctica was also shown to enter in a VBNC state [147]. In contrast to L. pneumophila, passage through amoebae did not increase Francisella novicida virulence [148]. Despite some disagreements about interaction mechanisms, all these reports argue that Francisella spp. are resistant to free-living amoeba and that protists may contribute to the survival of Francisella sp. in the water environment. However, to our knowledge, Francisella species have never been detected within amoebae in environmental water samples, in contrast to L. pneumophila and Mycobacterium spp. [155].
Survival in mosquito larvae
Mosquito larvae may also represent a long-term F. tularensis reservoir in the aquatic environment. It has been shown that these larvae can ingest F. tularensis subsp. holarctica that are present in water and ingested bacteria survive throughout the different maturation stages of these arthropods up to adult mosquitoes [57,63,138,156,157]. The fact that mosquito-borne tularemia cases have occurred over years in Sweden and Finland should be considered a further evidence of the existence of a long-term aquatic reservoir of F. tularensis. However, it should be notified that mosquitoes could also be infected at the adult stage after a blood meal on an infected host [57]; in this case tularaemia is not water-borne.
Discussion
Although water-borne tularemia was first described in the 1930s [9], this route of human contamination has been largely underestimated. Tularemia cases linked to the aquatic reservoir are common and can occur as large epidemics. Thus, tularemia is a major public health problem in countries where water-borne tularemia cases predominate. These cases may occur through consumption of F. tularensis-contaminated drinking water, such as in Turkey [11–36] and its neighboring countries [38–42], and in Norway [43–45] where large-scale tularemia outbreaks caused by F. tularensis subsp. holarctica are regularly reported. Drinking water as a source of human infections with type B strains of F. tularensis have also been occasionally encountered in Sweden [46,47] and central Europe [48,49]. Human infections have occurred after consumption of contaminated water from the community water supplies, especially in countries with bad sanitary conditions, old water networks and inappropriate water treatment. Consumption of unsanitized surface water or well water was also a source of contamination. Consequently, it is of primary interest for practitioners to keep in mind that tularemia may correspond to sub-acute or chronic pharyngitis associated with cervical lymphadenopathy, especially in patients living or traveling in countries where water-borne tularemia cases are frequent. In addition, medical questioning regarding tularemia exposure should include a statement about unsanitized water consumption. Until now, this mode of human contamination has been observed in restricted areas and only linked to F. tularensis subsp. holarctica. However, similar cases could occur throughout the Northern hemisphere where type B strains are encountered. F. tularensis subsp. tularensis has also been associated with the water environment [72–74], and could also cause tularemia cases related to drinking water.
Mosquito-borne tularemia is also related to contamination of the aquatic environment by F. tularensis. Tularemia outbreaks caused by F. tularensis subsp. holarctica in Sweden [46,50–54] and Finland [58–60] are primarily related to mosquito bites. Because of skin inoculation of bacteria, the ulceroglandular form of tularemia predominates. Infections usually occur during the warm season at the time of maximum activity of mosquitoes. Scandinavian practitioners are now particularly aware of this situation and usually diagnose tularemia early in the course of the disease. In contrast, this mode of transmission is most often unknown by physicians in other parts of the word, and mosquito-borne tularemia cases could be missed in patients returning from Scandinavian countries. Also, potential transmission of tularemia through mosquito bites outside Scandinavia has not been thoroughly evaluated. A probable autochtonous mosquito-borne tularemia case was reported in Germany [62]. In Alaska, mosquitoes tested positive for F. tularensis DNA [63] arguing that these arthropods could also transmit tularemia to humans in this US state. In this time of global warming, mosquito vectors could spread to new geographic areas, leading to a rise in mosquito-borne tularemia cases [158].
Finally, tularemia cases may occur through other types of aquatic exposure such as near drowning accident [64,65], swimming [66,67], canyoning [68] and fishing activities [69–71], due to penetration of F. tularensis through the skin, conjunctiva, or digestive and respiratory tracts. These cases have been reported in Europe [64,65,68,69], Turkey [66,67] and North America [70,71], suggesting a wide aquatic distribution of F. tularensis. Type B strains of F. tularensis were likely involved in all these cases, except one case occurring in Canada for which type A and type B strains could be involved [70]. These data suggest that F. tularensis subsp. holarctica could be more frequently associated with aquatic reservoirs than F. tularensis subsp. tularensis There is currently no explanation regarding the preferential association of type B strains with water. Williamson et al. demonstrated that type B strains can resist to hypoosmotic shock when released into water [125]. It would be interesting to compare the osmotic shock resistance of type A versus type B strains. Noticeably, almost all human contaminations occurred after contact with fresh-water, suggesting that F. tularensis may better survive in such aquatic environment. F. tularensis subsp. tularensis may also infect humans through water exposure, as attested by the particular ecology of the Martha’s Vineyard island in the USA [72–74]. In this case, brackish-water was considered as a potential reservoir of F. tularensis [78]. Altogether, available data indicate that human contamination with F. tularensis may occur from a wide diversity of aquatic sources and activities.
Francisella species other than F. tularensis are primarily considered aquatic bacteria. F. philomiragia and F. novicida have been occasionally involved in human infections, especially in immunocompromised patients, but also in specific situations such as near-drowning accident [81–86,89,97–100]. Infections caused by these species were frequently associated with exposure to brackish-water or salt-water [81–86,89,97,99]. In line with this observation, F. philomiragia and F. novicida have been frequently isolated from brackish- and salt-water samples [78,89,90,92,101,102]. In recent years, novel Francisella species have been detected in environmental or clinical samples, the majority of them being associated with an aquatic reservoir. Among them, F. hispaniensis [105] and F. halioticida [113] are rare human pathogens. These findings strongly suggest that the Francisella species are mainly aquatic bacteria.
Several mechanisms could be involved in the survival of F. tularensis in aquatic environments. Experimental and epidemiological studies have demonstrated that both F. tularensis subsp. tularensis and F. tularensis subsp. holarctica can survive for long periods in water microcosms [32,78,118–120,125,128–132]. Interestingly, after a long stay in water, F. tularensis has been shown experimentally to evolve to a VBNC state [118,120], which could account for long-term survival of this bacterium in water. Both type A and type B strains of F. tularensis can form biofilms in vitro [138,139], another potential survival mechanism of these bacteria in aquatic environments. Experimentally, both subspecies are able to multiply in amoebae or at least interact with these protozoa to enhance their survival [145,147,150,152,153]. Finally, F. tularensis subsp. holarctica is also able to infect mosquito larvae in vitro and survive during larvae maturation up to the adult stage [57,63,138,156,157]. It should be mentioned, however, that all these mechanisms have been evaluated experimentally, but not yet confirmed in natural water environments. It is very likely that all these mechanisms exist and are entangled. Infected animals and animal carcasses may contaminate water environments, in which protozoa, mosquito larvae and biofilm communities may become contaminated with F. tularensis and serve as reservoirs for this bacterium.
In conclusion, our goal was to demonstrate that F. tularensis is likely able to survive for prolonged periods in various aquatic environments, which likely constitute a significant reservoir for this bacterium. Figure 1 summarizes the probable tularemia aquatic cycle as it can be pictured from current literature data. In terms of public health, it is important to remember that providing people with access to safe drinking water via treatment of municipal and private sources remains a priority. Better characterization and control of the aquatic reservoir of F. tularensis would also be of tremendous importance following a bioterrorist attack. Following a bioterrorist attack, F. tularensis could survive for months in the environment, leading to a high number of secondary tularemia cases. Water and mosquito reservoirs should be monitored in the overall tularemia surveillance, in addition to the wildlife reservoir.
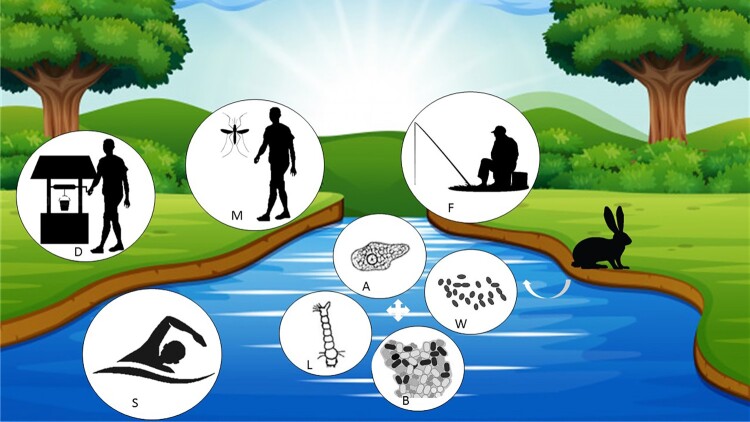
Figure 1.
Potential aquatic sources of human infections with Francisella tularensis. Francisella tularensis is released into water from animals. The bacterium is able to survive in water (W), in mosquito larvae (L), in biofilms (B), or in cooperation with amoeba (A). Human can be contaminated from the aquatic reservoir by drinking contaminated water (D), after a mosquito bite (M), or during swimming (S) and fishing (F) activities.
Funding Statement
This work was supported by the Direction Générale de l’Armement (DGA, ANR-17-ASTR-0024).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Sjöstedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci. 2007;1105:1–29. [DOI] [PubMed] [Google Scholar]
- 2.Dennis DT, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. [DOI] [PubMed] [Google Scholar]
- 3.Johansson A, et al. Objections to the transfer of Francisella novicida to the subspecies rank of Francisella tularensis. Int J Syst Evol Microbiol. 2010;60:1717–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busse H-J, et al. Objections to the transfer of Francisella novicida to the subspecies rank of Francisella tularensis - response to Johansson et al. Int J Syst Evol Microbiol. 2010;60:1718–1720. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri RR, et al. Genome sequencing shows that European isolates of Francisella tularensis subspecies tularensis are almost identical to US laboratory strain Schu S4. PloS One. 2007;2:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson J, et al. Francisella tularensis subspecies holarctica, Tasmania, Australia, 2011. Emerg Infect Dis 2012;18:1484–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gyuranecz M, et al. Investigation of the ecology of Francisella tularensis during an inter-epizootic period. Vector Borne Zoonotic Dis Larchmt N. 2011;11:1031–1035. [DOI] [PubMed] [Google Scholar]
- 8.Maurin M, Gyuranecz M.. Tularaemia: clinical aspects in Europe. Lancet Infect Dis. 2016;16:113–124. [DOI] [PubMed] [Google Scholar]
- 9.Karpoff SP, Antonoff NI.. The spread of tularemia through water, as a New Factor in its Epidemiology. J Bacteriol. 1936;32:243–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akalin H, Helvaci S, Gedikoğlu S.. Re-emergence of tularemia in Turkey. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2009;13:547–551. [DOI] [PubMed] [Google Scholar]
- 11.Willke A, et al. An outbreak of oropharyngeal tularaemia linked to natural spring water. J Med Microbiol. 2009;58:112–116. [DOI] [PubMed] [Google Scholar]
- 12.Balci E, et al. Tularemia outbreaks in Kayseri, Turkey: an evaluation of the effect of climate change and climate variability on tularemia outbreaks. J Infect Public Health. 2014;7:125–132. [DOI] [PubMed] [Google Scholar]
- 13.Meric M, et al. Evaluation of clinical, laboratory, and therapeutic features of 145 tularemia cases: the role of quinolones in oropharyngeal tularemia. APMIS Acta Pathol Microbiol Immunol Scand. 2008;116:66–73. [DOI] [PubMed] [Google Scholar]
- 14.Helvaci S, Gedikoğlu S, Akalin H, et al. Tularemia in Bursa, Turkey: 205 cases in ten years. Eur J Epidemiol. 2000;16:271–276. [DOI] [PubMed] [Google Scholar]
- 15.Ulu-Kilic A, Gulen G, Sezen F, et al. Tularemia in central Anatolia. Infection. 2013;41:391–399. [DOI] [PubMed] [Google Scholar]
- 16.Meric M, Sayan M, Dundar D, et al. Tularaemia outbreaks in Sakarya, Turkey: case-control and environmental studies. Singapore Med J. 2010;51:655–659. [PubMed] [Google Scholar]
- 17.Leblebicioglu H, et al. Outbreak of tularemia: a case-control study and environmental investigation in Turkey. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2008;12:265–269. [DOI] [PubMed] [Google Scholar]
- 18.Sencan I, et al. An outbreak of oropharyngeal tularemia with cervical adenopathy predominantly in the left side. Yonsei Med J. 2009;50:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozdemir D, et al. Comparison of the 2000 and 2005 outbreaks of tularemia in the Duzce region of Turkey. Jpn J Infect Dis. 2007;60:51–52. [PubMed] [Google Scholar]
- 20.Gürcan S, et al. Tularemia re-emerging in European part of Turkey after 60 years. Jpn J Infect Dis. 2006;59:391–393. [PubMed] [Google Scholar]
- 21.Gülhan B, Tezer H, Kanık-Yüksek S, et al. A rare cause of abdominal lymphadenopathy–tularemia: report of two pediatric cases. Turk J Pediatr. 2014;56:192–195. [PubMed] [Google Scholar]
- 22.Yilmaz GR, et al. Tularemia during pregnancy: three cases. Vector Borne Zoonotic Dis Larchmt N. 2014;14:171–173. [DOI] [PubMed] [Google Scholar]
- 23.Turhan V, Berber U, Haholu A, et al. Differential diagnosis of cervical lymphadenitis mimicking malignancy due to tularemia: our experiences. Indian J Pathol Microbiol. 2013;56:252–257. [DOI] [PubMed] [Google Scholar]
- 24.Karagöz S, et al. Francisella tularensis bacteremia: report of two cases and review of the literature. New Microbiol. 2013;36:315–323. [PubMed] [Google Scholar]
- 25.Yeşilyurt M, Kiliç S, Çelebі B, et al. Tularemia during pregnancy: report of four cases. Scand J Infect Dis. 2013;45:324–328. [DOI] [PubMed] [Google Scholar]
- 26.Bilgul M, Mücahit Y, Ertaban DA, et al. A patient with cervical swelling. Intern Med Tokyo Jpn. 2011;50:2253–2254. [DOI] [PubMed] [Google Scholar]
- 27.Ugur KS, et al. Three cases of oropharyngeal tularemia in Turkey. Auris Nasus Larynx. 2011;38:532–537. [DOI] [PubMed] [Google Scholar]
- 28.Kandemir B, Erayman I, Bitirgen M, et al. Tularemia presenting with tonsillopharyngitis and cervical lymphadenitis: report of two cases. Scand J Infect Dis. 2007;39:620–622. [DOI] [PubMed] [Google Scholar]
- 29.Arikan OK, Koç C, Bozdoğan O.. Tularemia presenting as tonsillopharyngitis and cervical lymphadenitis: a case report and review of the literature. Eur Arch Oto-Rhino-Laryngol. 2003;260:298–300. [DOI] [PubMed] [Google Scholar]
- 30.Karadenizli A, Gurcan S, Kolayli F, et al. Outbreak of tularaemia in Golcuk, Turkey in 2005: report of 5 cases and an overview of the literature from Turkey. Scand J Infect Dis 2005;37:712–716. [DOI] [PubMed] [Google Scholar]
- 31.Aktas D, et al. Oropharyngeal tularemia outbreak associated with drinking contaminated tap water, Turkey, July-September 2013. Emerg Infect Dis 2015;21:2194–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karadenizli A, et al. Genomic analyses of Francisella tularensis strains confirm disease transmission from drinking water sources, Turkey, 2008, 2009 and 2012. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2015;20(21). pii: 21136. [DOI] [PubMed] [Google Scholar]
- 33.Celebi S, Hacimustafaoglu M, Gedikoglu S.. Tularemia in children. Indian J Pediatr. 2008;75:1129–1132. [DOI] [PubMed] [Google Scholar]
- 34.Celebi G, et al. Tularemia, a reemerging disease in northwest Turkey: epidemiological investigation and evaluation of treatment responses. Jpn J Infect Dis. 2006;59:229–234. [PubMed] [Google Scholar]
- 35.Gürcan S, Otkun MT, Otkun M, et al. An outbreak of tularemia in Western Black Sea region of Turkey. Yonsei Med J. 2004;45:17–22. [DOI] [PubMed] [Google Scholar]
- 36.Sahin M, Atabay HI, Bicakci Z, et al. Outbreaks of tularemia in Turkey. Kobe J Med Sci. 2007;53:37–42. [PubMed] [Google Scholar]
- 37.Kilic S, et al. Water as source of Francisella tularensis infection in humans, Turkey. Emerg Infect Dis. 2015;21:2213–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunow R, Kalaveshi A, Kühn A, et al. Surveillance of tularaemia in Kosovo, 2001 to 2010. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2012;17(28). pii: 20217. [DOI] [PubMed] [Google Scholar]
- 39.Reintjes R, et al. Tularemia outbreak investigation in Kosovo: case control and environmental studies. Emerg Infect Dis. 2002;8:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantardjiev T, et al. Tularemia outbreak, Bulgaria, 1997–2005. Emerg Infect Dis. 2006;12:678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chitadze N, et al. Water-borne outbreak of oropharyngeal and glandular tularemia in Georgia: investigation and follow-up. Infection. 2009;37:514–521. [DOI] [PubMed] [Google Scholar]
- 42.Hristovski KD, Pacemska-Atanasova T, Olson LW, et al. Potential health implications of water resources depletion and sewage discharges in the Republic of Macedonia. J Water Health. 2016;14:682–691. [DOI] [PubMed] [Google Scholar]
- 43.Berdal BP, et al. Field detection of Francisella tularensis. Scand J Infect Dis. 2000;32:287–291. [DOI] [PubMed] [Google Scholar]
- 44.Brantsaeter AB, Krogh T, Radtke A, et al. Tularaemia outbreak in northern Norway. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2007;12:E070329.2. [DOI] [PubMed] [Google Scholar]
- 45.Larssen KW, et al. Outbreak of tularaemia in central Norway, January to March 2011. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2011;16(13). pii: 19828. [PubMed] [Google Scholar]
- 46.Eliasson H, Bäck E.. Tularaemia in an emergent area in Sweden: an analysis of 234 cases in five years. Scand J Infect Dis. 2007;39:880–889. [DOI] [PubMed] [Google Scholar]
- 47.Lindhusen Lindhé E, Hjertqvist M, Wahab T.. Outbreak of tularaemia connected to a contaminated well in the Västra Götaland region in Sweden. Zoonoses Public Health. 2018;65:142–146. [DOI] [PubMed] [Google Scholar]
- 48.Mignani E, Palmieri F, Fontana M, et al. Italian epidemic of waterborne tularaemia. Lancet Lond Engl. 1988;2:1423. [DOI] [PubMed] [Google Scholar]
- 49.Dlugaiczyk J, et al. Oropharyngeal tularemia–a differential diagnosis of tonsillopharyngitis and cervical lymphadenitis. Wien Klin Wochenschr. 2010;122:110–114. [DOI] [PubMed] [Google Scholar]
- 50.Rydén P, et al. Outbreaks of tularemia in a boreal forest region depends on mosquito prevalence. J Infect Dis. 2012;205:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Svensson K, et al. Landscape epidemiology of tularemia outbreaks in Sweden. Emerg Infect Dis. 2009;15:1937–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eliasson H, et al. The 2000 tularemia outbreak: a case-control study of risk factors in disease-endemic and emergent areas, Sweden. Emerg Infect Dis. 2002;8:956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Payne L, Arneborn M, Tegnell A, et al. Endemic tularemia, Sweden, 2003. Emerg Infect Dis 2005;11:1440–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wik O. Large tularaemia outbreak in Varmland, central Sweden, 2006. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2006;11:E060921.1. [DOI] [PubMed] [Google Scholar]
- 55.Desvars A, et al. Epidemiology and ecology of tularemia in Sweden, 1984–2012. Emerg Infect Dis 2015;21:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desvars-Larrive A, et al. High-risk regions and outbreak modelling of tularemia in humans. Epidemiol Infect. 2017;145:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thelaus J, et al. Francisella tularensis subspecies holarctica occurs in Swedish mosquitoes, persists through the developmental stages of laboratory-infected mosquitoes and is transmissible during blood feeding. Microb Ecol. 2014;67:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jounio U, Renko M, Uhari M.. An outbreak of holarctica-type tularemia in pediatric patients. Pediatr Infect Dis J. 2010;29:160–162. [DOI] [PubMed] [Google Scholar]
- 59.Karhukorpi EK, Karhukorpi J.. Rapid laboratory diagnosis of ulceroglandular tularemia with polymerase chain reaction. Scand J Infect Dis. 2001;33:383–385. [DOI] [PubMed] [Google Scholar]
- 60.Ylipalosaari P, Ala-Kokko TI, Tuominen H, et al. Guillain-Barré syndrome and ulceroglandular tularemia. Infection. 2013;41:881–883. [DOI] [PubMed] [Google Scholar]
- 61.Rossow H, et al. Incidence and seroprevalence of tularaemia in Finland, 1995 to 2013: regional epidemics with cyclic pattern. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2015;20:21209. [DOI] [PubMed] [Google Scholar]
- 62.Hanke CA, et al. Ulceroglandular tularemia in a toddler in Germany after a mosquito bite. Eur J Pediatr. 2009;168:937–940. [DOI] [PubMed] [Google Scholar]
- 63.Triebenbach AN, et al. Detection of Francisella tularensis in Alaskan mosquitoes (Diptera: Culicidae) and assessment of a laboratory model for transmission. J Med Entomol. 2010;47:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ughetto E, et al. An original case of Francisella tularensis subsp. holarctica bacteremia after a near-drowning accident. Infect Dis Lond Engl. 2015;47:588–590. [DOI] [PubMed] [Google Scholar]
- 65.Väyrynen SA, et al. Pneumonic tularaemia: experience of 58 cases from 2000 to 2012 in Northern Finland. Infect Dis Lond Engl. 2017;49:758–764. [DOI] [PubMed] [Google Scholar]
- 66.Bayhan-Taş GI, Tanir G, Celebi B.. Two cases of glandular tularemia from Turkey. Turk J Pediatr. 2012;54:203–206. [PubMed] [Google Scholar]
- 67.Peker E, Ayaydin A, Duran N.. Familial tularaemia. Indian J Med Microbiol 2009;27:272–275. [DOI] [PubMed] [Google Scholar]
- 68.Guerpillon B, et al. Keep an Ear Out for Francisella tularensis: Otomastoiditis cases after Canyoneering. Front Med. 2016;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anda P, et al. Waterborne outbreak of tularemia associated with crayfish fishing. Emerg Infect Dis. 2001;7:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jassal DS, Targownik L, Thottingal P, et al. Photo quiz. Diagnosis: tularemia. Clin Infect Dis 1999;29(275):420–421. [DOI] [PubMed] [Google Scholar]
- 71.Whitten T, et al. Notes from the field: Francisella tularensis type B infection from a fish Hook injury - Minnesota, 2016. MMWR Morb Mortal Wkly Rep 2017;66:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teutsch SM, et al. Pneumonic tularemia on Martha’s Vineyard. N Engl J Med. 1979;301:826–828. [DOI] [PubMed] [Google Scholar]
- 73.Feldman KA, et al. An outbreak of primary pneumonic tularemia on Martha’s Vineyard. N Engl J Med. 2001;345:1601–1606. [DOI] [PubMed] [Google Scholar]
- 74.Matyas BT, Nieder HS, Telford SR.. Pneumonic tularemia on Martha’s Vineyard: clinical, epidemiologic, and ecological characteristics. Ann N Y Acad Sci. 2007;1105:351–377. [DOI] [PubMed] [Google Scholar]
- 75.Berrada ZL, Goethert HK, Telford SR.. Raccoons and skunks as sentinels for enzootic tularemia. Emerg Infect Dis. 2006;12:1019–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feldman KA, et al. Tularemia on Martha’s Vineyard: seroprevalence and occupational risk. Emerg Infect Dis. 2003;9:350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goethert HK, Shani I, Telford SR.. Genotypic diversity of Francisella tularensis infecting Dermacentor variabilis ticks on Martha’s Vineyard, Massachusetts. J Clin Microbiol. 2004;42:4968–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berrada ZL, Telford SR.. Diversity of Francisella species in environmental samples from Martha’s Vineyard, Massachusetts. Microb Ecol. 2010;59:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Messier V, et al. Seroprevalence of seven zoonotic infections in Nunavik, Quebec (Canada). Zoonoses Public Health. 2012;59:107–117. [DOI] [PubMed] [Google Scholar]
- 80.Sjödin A, et al. Genome characterisation of the genus Francisella reveals insight into similar evolutionary paths in pathogens of mammals and fish. BMC Genomics. 2012;13:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friis-Møller A, Lemming LE, Valerius NH, et al. Problems in identification of Francisella philomiragia associated with fatal bacteremia in a patient with chronic granulomatous disease. J Clin Microbiol. 2004;42:1840–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Polack FP, Harrington SM, Winkelstein JA, et al. Recurrent Francisella philomiragia sepsis in chronic granulomatous disease. Pediatr Infect Dis J. 1998;17:442–443. [DOI] [PubMed] [Google Scholar]
- 83.Robles-Marhuenda A, et al. Francisella philomiragia: Think of chronic granulomatous disease. J Clin Immunol. 2018;38:257–259. [DOI] [PubMed] [Google Scholar]
- 84.Mailman TL, Schmidt MH.. Francisella philomiragia adenitis and pulmonary nodules in a child with chronic granulomatous disease. Can J Infect Dis Med Microbiol J Can Mal Infect Microbiol Medicale. 2005;16:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sicherer SH, Asturias EJ, Winkelstein JA, et al. Francisella philomiragia sepsis in chronic granulomatous disease. Pediatr Infect Dis J. 1997;16:420–422. [DOI] [PubMed] [Google Scholar]
- 86.Wenger JD, et al. Infection caused by Francisella philomiragia (formerly Yersinia philomiragia). A newly recognized human pathogen. Ann Intern Med. 1989;110:888–892. [DOI] [PubMed] [Google Scholar]
- 87.Kreitmann L, et al. Disseminated infection caused by Francisella philomiragia, France, 2014. Emerg Infect Dis 2015;21:2260–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Relich RF, et al. Francisella philomiragia bacteremia in a patient with acute respiratory insufficiency and acute-on-chronic kidney disease. J Clin Microbiol. 2015;53:3947–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whitehouse CA, Kesterson KE, Duncan DD, et al. Identification and characterization of Francisella species from natural warm springs in Utah, USA. Lett Appl Microbiol. 2012;54:313–324. [DOI] [PubMed] [Google Scholar]
- 90.Brinkmeyer R. Diversity of bacteria in ships ballast water as revealed by next generation DNA sequencing. Mar Pollut Bull. 2016;107:277–285. [DOI] [PubMed] [Google Scholar]
- 91.Gu Q, et al. Characterization of Francisella species isolated from the cooling water of an air conditioning system. Braz J Microbiol Publ Braz Soc Microbiol. 2015;46:921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duodu S, Larsson P, Sjödin A, et al. The distribution of Francisella-like bacteria associated with coastal waters in Norway. Microb Ecol. 2012;64:370–377. [DOI] [PubMed] [Google Scholar]
- 93.Barns SM, Grow CC, Okinaka RT, et al. Detection of diverse new Francisella-like bacteria in environmental samples. Appl Environ Microbiol. 2005;71:5494–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jensen WI, Owen CR, Jellison WL.. Yersinia philomiragia sp. nov., a new member of the Pasteurella group of bacteria, naturally pathogenic for the muskrat (Ondatra zibethica). J Bacteriol. 1969;100:1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bonnet S, et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector Borne Zoonotic Dis Larchmt N. 2013;13:226–236. [DOI] [PubMed] [Google Scholar]
- 96.Kingry LC, Petersen JM.. Comparative review of Francisella tularensis and Francisella novicida. Front Cell Infect Microbiol. 2014;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brett M, et al. Francisella novicida bacteremia after a near-drowning accident. J Clin Microbiol. 2012;50:2826–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brett ME, et al. Outbreak of Francisella novicida bacteremia among inmates at a louisiana correctional facility. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59:826–833. [DOI] [PubMed] [Google Scholar]
- 99.Whipp MJ, et al. Characterization of a novicida-like subspecies of Francisella tularensis isolated in Australia. J Med Microbiol. 2003;52:839–842. [DOI] [PubMed] [Google Scholar]
- 100.Clarridge JE, et al. Characterization of two unusual clinically significant Francisella strains. J Clin Microbiol. 1996;34:1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petersen JM, et al. Direct isolation of Francisella spp. from environmental samples. Lett Appl Microbiol. 2009;48:663–667. [DOI] [PubMed] [Google Scholar]
- 102.Challacombe JF, et al. Whole-genome relationships among Francisella bacteria of diverse origins define new species and provide specific regions for detection. Appl Environ Microbiol. 2017;83:e02589–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Escudero R, et al. A possible novel Francisella genomic species isolated from blood and urine of a patient with severe illness. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2010;16:1026–1030. [DOI] [PubMed] [Google Scholar]
- 104.Huber B, et al. Description of Francisella hispaniensis sp. nov., isolated from human blood, reclassification of Francisella novicida (Larson et al. 1955) Olsufiev et al. 1959 as Francisella tularensis subsp. novicida comb. nov. and emended description of the genus Francisella. Int J Syst Evol Microbiol. 2010;60:1887–1896. [DOI] [PubMed] [Google Scholar]
- 105.Aravena-Román M, Merritt A, Inglis TJJ.. First case of Francisella bacteraemia in Western Australia. New Microbes New Infect. 2015;8:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Colquhoun DJ, Duodu S.. Francisella infections in farmed and wild aquatic organisms. Vet Res. 2011;42:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ottem KF, Nylund A, Karlsbakk E, et al. Elevation of Francisella philomiragia subsp. noatunensis Mikalsen et al. (2007) to Francisella noatunensis comb. nov. [syn. Francisella piscicida Ottem et al. (2008) syn. nov]. and characterization of Francisella noatunensis subsp. orientalis subsp. nov., two important fish pathogens. J Appl Microbiol 2009;106:1231–1243. [DOI] [PubMed] [Google Scholar]
- 108.Mikalsen J, Olsen AB, Tengs T, et al. Francisella philomiragia subsp. noatunensis subsp. nov., isolated from farmed Atlantic cod (Gadus morhua L.). Int J Syst Evol Microbiol. 2007;57:1960–1965. [DOI] [PubMed] [Google Scholar]
- 109.Ottem KF, et al. New species in the genus Francisella (Gammaproteobacteria; Francisellaceae); Francisella piscicida sp. nov. isolated from cod (Gadus morhua). Arch Microbiol. 2007;188:547–550. [DOI] [PubMed] [Google Scholar]
- 110.Brevik OJ, Ottem KF, Kamaishi T, et al. Francisella halioticida sp. nov., a pathogen of farmed giant abalone (Haliotis gigantea) in Japan. J Appl Microbiol. 2011;111:1044–1056. [DOI] [PubMed] [Google Scholar]
- 111.Meyer GR, Lowe GJ, Gilmore SR, et al. Disease and mortality among Yesso scallops Patinopecten yessoensis putatively caused by infection with Francisella halioticida. Dis Aquat Organ. 2017;125:79–84. [DOI] [PubMed] [Google Scholar]
- 112.Soto E, et al. Francisella marina sp. nov., Etiologic agent of Systemic disease in cultured spotted rose snapper (Lutjanus guttatus) in Central America. Appl Environ Microbiol. 2018;84(16). pii: e00144–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Respicio-Kingry LB, et al. Cutaneous infection caused by a novel Francisella sp. J Clin Microbiol. 2013;51:3456–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sjödin A, et al. Complete genome sequence of Francisella endociliophora strain FSC1006, isolated from a laboratory culture of the marine ciliate Euplotes raikovi. Genome Announc. 2014;2(6):e01227–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qu P-H, et al. Allofrancisella inopinata gen. nov., sp. nov. and Allofrancisella frigidaquae sp. nov., isolated from water-cooling systems, and transfer of Francisella guangzhouensis Qu et al. 2013 to the new genus as Allofrancisella guangzhouensis comb. nov. Int J Syst Evol Microbiol 2016;66:4832–4838. [DOI] [PubMed] [Google Scholar]
- 116.Rydzewski K, et al. Genome sequence and phenotypic analysis of a first German Francisella sp. isolate (W12–1067) not belonging to the species Francisella tularensis. BMC Microbiol. 2014;14:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qu P-H, et al. Francisella guangzhouensis sp. nov., isolated from air-conditioning systems. Int J Syst Evol Microbiol. 2013;63:3628–3635. [DOI] [PubMed] [Google Scholar]
- 118.Gilbert SE, Rose LJ.. Survival and persistence of nonspore-forming biothreat agents in water. Lett Appl Microbiol. 2012;55:189–194. [DOI] [PubMed] [Google Scholar]
- 119.Berrada ZL, Telford Iii SR.. Survival of Francisella tularensis type A in brackish-water. Arch Microbiol. 2011;193:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Forsman M, Henningson EW, Larsson E, et al. Francisella tularensis does not manifest virulence in viable but non-culturable state. FEMS Microbiol Ecol. 2000;31:217–224. [DOI] [PubMed] [Google Scholar]
- 121.Oliver JD. The viable but non-culturable state in the human pathogen Vibrio vulnificus. FEMS Microbiol Lett. 1995;133:203–208. [DOI] [PubMed] [Google Scholar]
- 122.Xu HS, et al. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8:313–323. [DOI] [PubMed] [Google Scholar]
- 123.Rollins DM, Colwell RR.. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steinert M, Emödy L, Amann R, et al. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:2047–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williamson DR, et al. A Single mechanosensitive channel protects Francisella tularensis subsp. holarctica from hypoosmotic shock and promotes survival in the aquatic environment. Appl Environ Microbiol. 2018;84(5). pii: e02203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soto E, Revan F.. Culturability and persistence of Francisella noatunensis subsp. orientalis (syn. Francisella asiatica) in sea- and freshwater microcosms. Microb Ecol. 2012;63:398–404. [DOI] [PubMed] [Google Scholar]
- 127.Duodu S, Colquhoun D.. Monitoring the survival of fish-pathogenic Francisella in water microcosms. FEMS Microbiol Ecol. 2010;74:534–541. [DOI] [PubMed] [Google Scholar]
- 128.Janse I, van der Plaats RQJ, de Roda Husman AM, et al. Environmental surveillance of zoonotic Francisella tularensis in the Netherlands. Front Cell Infect Microbiol. 2018;8:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hightower J, et al. Historical distribution and host-vector diversity of Francisella tularensis, the causative agent of tularemia, in Ukraine. Parasit Vectors. 2014;7:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simşek H, Taner M, Karadenizli A, et al. Identification of Francisella tularensis by both culture and real-time TaqMan PCR methods from environmental water specimens in outbreak areas where tularemia cases were not previously reported. Eur J Clin Microbiol Infect Dis. 2012;31:2353–2357. [DOI] [PubMed] [Google Scholar]
- 131.Broman T, et al. Molecular detection of persistent Francisella tularensis subspecies holarctica in natural waters. Int J Microbiol. 2011;2011. pii: 851946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaysser P, et al. Re-emergence of tularemia in Germany: presence of Francisella tularensis in different rodent species in endemic areas. BMC Infect Dis. 2008;8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Hoek ML. Biofilms: an advancement in our understanding of Francisella species. Virulence. 2013;4:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huq A, Whitehouse CA, Grim CJ, et al. Biofilms in water, its role and impact in human disease transmission. Curr Opin Biotechnol. 2008;19:244–247. [DOI] [PubMed] [Google Scholar]
- 135.Lau HY, Ashbolt NJ.. The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water. J Appl Microbiol. 2009;107:368–378. [DOI] [PubMed] [Google Scholar]
- 136.Percival SL, Thomas JG.. Transmission of Helicobacter pylori and the role of water and biofilms. J Water Health. 2009;7:469–477. [DOI] [PubMed] [Google Scholar]
- 137.Costerton JW. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol. 2001;9:50–52. [DOI] [PubMed] [Google Scholar]
- 138.Mahajan UV, Gravgaard J, Turnbull M, et al. Larval exposure to Francisella tularensis LVS affects fitness of the mosquito Culex quinquefasciatus. FEMS Microbiol Ecol. 2011;78:520–530. [DOI] [PubMed] [Google Scholar]
- 139.Margolis JJ, et al. Contributions of Francisella tularensis subsp. novicida chitinases and Sec secretion system to biofilm formation on chitin. Appl Environ Microbiol. 2010;76:596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Durham-Colleran MW, Verhoeven AB, van Hoek ML.. Francisella novicida forms in vitro biofilms mediated by an orphan response regulator. Microb Ecol. 2010;59:457–465. [DOI] [PubMed] [Google Scholar]
- 141.Dean RE, Ireland PM, Jordan JE, et al. Rela regulates virulence and intracellular survival of Francisella novicida. Microbiol Read Engl. 2009;155:4104–4113. [DOI] [PubMed] [Google Scholar]
- 142.Verhoeven AB, Durham-Colleran MW, Pierson T, et al. Francisella philomiragia biofilm formation and interaction with the aquatic protist Acanthamoeba castellanii. Biol Bull. 2010;219:178–188. [DOI] [PubMed] [Google Scholar]
- 143.Soto E, Halliday-Simmonds I, Francis S, et al. Biofilm formation of Francisella noatunensis subsp. orientalis. Vet Microbiol. 2015;181:313–317. [DOI] [PubMed] [Google Scholar]
- 144.Greub G, Raoult D.. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17:413–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Buse HY, Schaefer Iii FW, Rice EW.. Enhanced survival but not amplification of Francisella spp. in the presence of free-living amoebae. Acta Microbiol Immunol Hung. 2017;64:17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Santic M, et al. Intra-Vacuolar Proliferation of F. novicida within H. vermiformis. Front Microbiol. 2011;2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thelaus J, et al. Influence of nutrient status and grazing pressure on the fate of Francisella tularensis in lake water. FEMS Microbiol Ecol. 2009;67:69–80. [DOI] [PubMed] [Google Scholar]
- 148.Ozanic M, et al. F. novicida-infected A. castellanii does Not enhance bacterial virulence in mice. Front Cell Infect Microbiol. 2016;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lampe EO, et al. Dissection of Francisella-host Cell interactions in Dictyostelium discoideum. Appl Environ Microbiol. 2015;82:1586–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.El-Etr SH, et al. Francisella tularensis type A strains cause the rapid encystment of Acanthamoeba castellanii and survive in amoebal cysts for three weeks postinfection. Appl Environ Microbiol. 2009;75:7488–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lauriano CM, et al. Mgla regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proc Natl Acad Sci U S A. 2004;101:4246–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Berdal BP, Mehl R, Meidell NK, et al. Field investigations of tularemia in Norway. FEMS Immunol Med Microbiol. 1996;13:191–195. [DOI] [PubMed] [Google Scholar]
- 153.Abd H, Johansson T, Golovliov I, et al. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl Environ Microbiol. 2003;69:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gustafsson K. Growth and survival of four strains of Francisella tularensis in a rich medium preconditioned with Acanthamoeba palestinensis. Can J Microbiol. 1989;35:1100–1104. [DOI] [PubMed] [Google Scholar]
- 155.Thomas V, Herrera-Rimann K, Blanc DS, et al. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl Environ Microbiol. 2006;72:2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bäckman S, Näslund J, Forsman M, et al. Transmission of tularemia from a water source by transstadial maintenance in a mosquito vector. Sci Rep. 2015;5:7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lundström JO, et al. Transstadial transmission of Francisella tularensis holarctica in mosquitoes, Sweden. Emerg Infect Dis. 2011;17:794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Andersen LK, Davis MDP.. Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the International Society of Dermatology Climate Change Task Force. Int J Dermatol. 2017;56:252–259. [DOI] [PubMed] [Google Scholar]