Abstract
17β-Hydroxysteroid dehydrogenase type 1 (17β-HSD1) is a key enzyme in the biosynthesis of 17β-estradiol. Novel estrone-based compounds bearing various 15β-oxa-linked substituents and hydroxy, methoxy, benzyloxy, and sulfamate groups in position C3 as potential 17β-HSD1 inhibitors have been synthesized. In addition, in vitro inhibitory potentials measured in the presence of excess amount of NADPH or NADH were investigated. We observed substantial inhibitory potentials for several derivatives (IC50 < 1 µM) and increased binding affinities compared to unsubstituted core molecules. Binding and inhibition were found to be cofactor-dependent for some of the compounds and we propose structural explanations for this phenomenon. Our results may contribute to the development of new 17β-HSD1 inhibitors, potential drug candidates for antiestrogen therapy of hormone-dependent gynecological cancers.
Keywords: Michael addition, substituted 15β-alkoxy-estrone derivatives, 17β-HSD1, estrogen biosynthesis, NADPH and NADH
Introduction
The 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1, EC 1.1.1.62) catalyzes the conversion of estrone (1) to highly active estrogen 17β-estradiol (2) (Scheme 1). Human 17β-HSD1 is expressed in tissues of female reproductive organs (such as placenta, ovarian follicles, mammary gland and uterus)1. The expression of 17β-HSD1 was shown to be elevated and to have prognostic significance in gynecological malignancies, e.g. in hormone-dependent breast cancer2–4. The enzyme is involved in progression of these diseases due to the increase of local 17β-estradiol (2) levels. Inhibition of the enzyme is able to control estrogen actions at the pre-receptor level; therefore, a suppression of the 17β-HSD1 activity has a significant therapeutic potential5. Numerous 17β-HSD1 inhibitors based on either steroidal or non-steroidal structures have been developed, but none of them have been introduced to the medical practice, so far6–11.
Scheme 1.

Transformation of estrone to 17β-estradiol catalyzed by 17β-HSD1.
Extensive earlier studies indicated that attachment of C15 substituents on the estrone scaffold might be a successful way for the synthesis of inhibitors against 17β-HSD112,13. In addition to improving binding affinity to 17β-HSD1, appropriate side chains may confer selectivity towards 17β-HSD type 27,10,14. 17β-HSD type 2 catalyzes inactivation of 17β-estradiol (2) to estrone (1) and is considered to be an important enzyme for the control of proliferation of breast cancer cells15. C15 substituents may also suppress inherent estrogenicity of the estrone core9,10,12,16. These features of C15 derivatives make this substitution strategy particularly attractive for the development of estrone-based 17β-HSD1 inhibitors.
It was shown that neither the presence of the phenolic hydroxyl group nor the hydrogen bonding of the C3 function is essential to the effective 17β-HSD1 binding of estrone derivatives17. This tolerance provides further options for the modulation of enzyme inhibition and other biological effects of the candidate compounds. Accordingly, several 3-methoxy analogues were tested as 17β-HSD1 inhibitors12,13 presumably that they exert reduced estrogenicity compared to estrone possessing phenolic hydroxy group18. Estrone 3-sulfamate analogues in this series tend to show moderate 17β-HSD1 inhibition13. However, the sulfamate moiety may lead to an inhibitory effect against steroid sulfatase (STS), another enzyme playing a central role in 17β-estradiol biosynthesis. Such a dual inhibitory effect was recently proposed to be beneficial as it should result in a stronger suppression of estrogen biosynthesis compared to selective inhibition of 17β-HSD119. Steroidal sulfamates may be delivered to the tumour by the carbonic anhydrase II, and evolve targeted actions20.
In this paper, we report the synthesis and chemical characterization of new substituted 15β-alkoxy estrone derivatives. We also aimed to investigation 17β-HSD1 inhibitory potentials of these compounds, including comparison of their inhibitor potentials measured in the presence of NADPH or NADH.
Experimental
General
Melting points (mp) were determined on a Kofler block and are uncorrected. Specific rotations were measured in CHCl3, or MeOH (c 1) at 25 C with a POLAMAT-A (Zeiss-Jena) polarimeter and are given in units of 10−1 deg cm2 g−1. Elementary analysis data were determined with a PerkinElmer CHN analyzer model 2400. Reactions were monitored by TLC on Kieselgel-G (Merck Si 254 F) layers (0.25 mm thick); solvent systems (ss): (A) (ethyl acetate/CH2Cl2 (1:1 v/v), (B) acetone/toluene/hexane (30:35:35 v/v), (C) ethyl acetate/CH2Cl2 (5:95 v/v), (D) ethyl acetate. The spots were detected by spraying with 5% phosphomolybdic acid in 50% aqueous H3PO4. The Rf values were determined for the spots observed by illumination at 254 and 365 nm. Flash chromatography: silica gel 60, 40–63 μm. All solvents were distilled immediately prior to use. NMR spectra were recorded on a Bruker DRX 500 instrument at 500 (1H NMR) or 125 MHz (13 C NMR). Chemical shifts are reported in ppm (δ scale), and coupling constants (J) in Hz. For the determination of multiplicities, the J-MOD pulse sequence was used.
Materials for enzyme experiments
Radiolabelled steroids [6,7-3H(N)]estrone (S. A. = 52 Ci/mmol), was obtained from American Radiolabeled Chemicals (St. Louis, MO, USA). Non-radioactive estrone, 3-methyl-O-estrone, 3-benzyl-O-estrone and estrone-3-sulfamate (EMATE) standards and other chemicals and solvents of analytical grade purity were purchased from Sigma (St. Louis, MO, USA), from Fluka (Buchs, Switzerland) or Merck (Darmstadt, Germany).
Human placenta microsomal fraction and cytosol were produced and applied with the permission of the Human Investigation Review Board of the University of Szeged.
General procedure for the synthesis of 3-methoxy-, and 3-benzyloxy-15β-alkoxyestra-1,3,5(10)-trien-17-ones (8‒12)
A solution of 623 or 712 (2 mmol) in CH2Cl2 (10 ml) and ethane-1,2-diol, propane-1,3-diol or butane-1,4-diol (15 ml), containing 5% aqueous sodium hydroxide (1 ml) was stirred at room temperature for 6 h. The reaction mixture, after the addition of CH2Cl2 (50 ml), was diluted with water (100 ml). The organic phase was separated, washed with water, dried over Na2SO4, and evaporated in vacuo. The residual product was purified by flash chromatography using ethyl acetate/CH2Cl2 in different proportions.
3-Methoxy-15β-(2’-hydroxy)ethoxy-estra-1,3,5(10)-trien-17-one (8)
Compound 6 (565 mg, 2 mmol) and ethane-1,2-diol (15 ml) were used for the synthesis as described in general procedure. The crude product was chromatographed on silica gel with dichloromethane/hexane (1:1 v/v) to yield pure 8 (580 mg, 84%). Mp: 139‒140 °C; Rf = 0.55 (ss B); [α]D20 + 54 (c 1 in CHCl3). Found: C, 73.45; H, 7.98. C21H28O4 (344.45) requires: C,73.23; H, 8.19%. 1H NMR (δ, ppm, CDCl3): 1.16 (s, 3H, 18-H3), 2.92 (m, 2H, 6-H2), 3.42 and 3.64 (2xm, 2x1H, linker H2), 3.72 (m, 2H, linker OCH2), 3.77 (s, 3H, 3-OCH3), 4.22 (t, 1 H, J = 5.3 Hz, 15-H), 6.64 (d, 1H, J = 2.2 Hz, 4-H), 6.71 (dd, 1H, J = 8.6 Hz, J = 2.2 Hz, 2-H), 7.19 (d, 1H, J = 8.6 Hz, 1-H).13C NMR (δ, ppm, CDCl3):17.5 (C-18), 25.6, 26.2, 29.4, 32.5, 34.8, 43.1, 44.0, 47.2 (C-13), 54.4, 55.1 (3-OCH3), 61.9 (CH2-OH), 70.7 (linker CH2), 75.1 (C-15), 111.4 (C-2), 113.8 (C-4), 126.0 (C-1), 132.0 (C-10), 137.6 (C-5), 157.5 (C-3), 219.4 (C-17).
3-Methoxy-15β-(3’-hydroxy)propoxy-estra-1,3,5(10)-trien-17-one (9)
Compound 6 (565 mg, 2 mmol) and propane-1,3-diol (15 ml) were used for the synthesis as described in general procedure. The crude product was chromatographed on silica gel with ethyl acetate/CH2Cl2 (1:99 v/v) to yield pure 9 (575 mg, 80%). Mp: 83‒84 °C; Rf = 0.50 (ss B); [α]D20 + 48 (c 1 in CHCl3). Found: C, 73.92; H, 8.26%. C22H30O4 requires: C, 73.71; H, 8.44%. 1H NMR (δ, ppm, CDCl3): 1.15 (s, 3H, 18-H3), 2.94 (m, 2H, 6-H2), 3.42 (m, 1H) and 3.74 (m, 3H): 2xlinker H2, 3.77 (s, 3H, 3-OCH3), 4.17 (t, 1 H, J = 5.3 Hz, 15-H), 6.64 (d, 1H, J = 2.2 Hz, 4-H), 6.71 (dd, 1H, J = 8.6 Hz, J = 2.2 Hz, 2-H), 7.19 (d, 1H, J = 8.6 Hz, 1-H).13C NMR (δ, ppm, CDCl3):17.4 (C-18), 25.7, 26.2, 29.4, 32.3, 32.6, 34.9, 42.8, 44.1, 47.2 (C-13), 54.2, 55.1 (3-OCH3), 61.5 (CH2-OH), 68.3 (linker CH2), 75.1 (C-15), 111.4 (C-2), 113.8 (C-4), 126.1 (C-1), 131.9 (C-10), 137.7 (C-5), 157.5 (C-3), 219.5 (C-17).
3-Benzyloxy-15β-(2’-hydroxy)ethoxy-estra-1,3,5(10)-trien-17-one (10)
Compound 7 (717 mg, 2 mmol) and ethane-1,2-diol (15 ml) were used for the synthesis as described in general procedure. The crude product was chromatographed on silica gel with ethyl acetate/CH2Cl2 (30:70 v/v) to yield pure 10 (690 mg, 82%). Mp: 102‒104 °C; Rf = 0.45 (ss B); [α]D20 + 73 (c 1 in CHCl3). Found: C, 76.95; 7.84. C27H32O4 (420.54) requires: C, 77.11; H, 7.67%. 1H NMR (δ, ppm, CDCl3): 1.17 (s, 3H, 18-H3), 2.91 (m, 2H, 6-H2), 3.42 and 3.65 (2xm, 2x1H, linker H2), 3.73 (m, 2H, linker OCH2), 4.23 (t, 1 H, J = 5.7 Hz, 15-H), 5.04 (s, 2H, Bn-H2), 6.74 (d, 1H, J = 2.2 Hz, 4-H), 6.79 (dd, 1H, J = 8.6 Hz, J = 2.2 Hz, 2-H), 7.19 (d, 1H, J = 8.6 Hz, 1-H), 7.32 (t, 1H, J = 7.3 Hz, 4-H of Bn), 7.39 (t, 2H, J = 7.3 Hz, 3-H and 5-H of Bn), 7.45 (d, 2H, J = 7.3 Hz, 2-H and 6-H of Bn).13C NMR (δ, ppm, CDCl3): 17.6 (C-18), 25.7, 26.2, 29.5, 32.7, 34.9, 43.1, 44.2, 47.2 (C-13), 54.5, 62.0 (CH2-OH), 70.0 (linker CH2), 70.8 (Bn-CH2), 75.2 (C-15), 112.4 (C-2), 114.9 (C-4), 126.1 (C-1), 127.4 (2 C: C-2 and C-6 of Bn), 127.8 (C-4 of Bn), 128.5 (2 C: C-3 and C-5 of Bn), 132.4 (C-10), 137.2 (C-1 of Bn), 137.7 (C-5), 156.9 (C-3), 219.1 (C-17).
3-Benzyloxy-15β-(3’-hydroxy)propoxy-estra-1,3,5(10)-trien-17-one (11)
Compound 7 (717 mg, 2 mmol) and propane-1,3-diol (15 ml) were used for the synthesis as described in general procedure. The crude product was chromatographed on silica gel with ethyl acetate/CH2Cl2 (30:70 v/v) to yield pure 11 (742 mg, 78%). Mp: 144‒146 °C; Rf = 0.35 (ss B); [α]D25 + 88 (c 1 in CHCl3). Found: C, 77.54; H, 8.02. C28H34O4 (434.57) requires: C, 77.39; H, 7.89%. 1H NMR (δ, ppm, CDCl3): 1.15 (s, 3H, 18-H3), 2.94 (m, 2H, 6-H2), 3.36 (t, 2H, J = 6.0 Hz, linker H2), 3.72 (m, 2H, linker H2), 4.17 (t, 1 H, J = 6.5 Hz, 15-H), 5.03 (s, 2H, H2 of Bn), 6.73 (d, 1H, J = 3.0 Hz, 4-H), 6.78 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.18 (d, 1H, J = 10.5 Hz, 1-H), 7.37 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 26.2, 26.6, 30.0, 32.8, 33.2, 35.4, 43.3 (linker CH2), 44.7, 47.7 (C-13), 54.8, 62.1 (CH2-OH), 68.9 (OCH2), 70.4 (Bn-CH2), 75.7 (C-15), 112.8 (C-2), 115.4 (C-4), 126.6 (C-1), 127.9 (C-2 and -6 of Bn), 128.3 (C-4 of Bn), 129.0 (C-3and C-5 of Bn), 132.8 (C-10), 138.0 (C-1 of Bn), 138.3 (C-5), 157.4 (C-3), 219.7 (C-17)
3-Benzyloxy-15β-(4’-hydroxy)butoxy-estra-1,3,5(10)-trien-17-one (12)
Compound 7 (717 mg, 2 mmol) and butane-1,4-diol (15 ml) were used for the synthesis as described in general procedure. The crude product was chromatographed on silica gel with ethyl acetate/CH2Cl2 (1:1 v/v) to yield pure 12 (720 mg, 80%). Mp: 131‒130 °C; Rf = 0.30 (ss B); [α]D25 + 58 (c 1 in CHCl3). Found: C, 77.48; H, 9.15. C29H36O4 (448.59) requires: C, 77.64; H, 8.09%. 1H NMR (δ, ppm, CDCl3): 1.16 (s, 3H, 18-H3), 3.34 (m, 2H, linker H2, 3.64 (m, 2H, O-CH2), 4.14 (t, 1 H, J = 6.5 Hz, 15-H), 5.03 (s, 2H, H2 of Bn), 6.73 (d, 1H, J = 3.0 Hz, 4-H), 6.78 (dd, 1H, J = 10.5 Hz, 2-H), J = 3.0 Hz, 2H), 7.18 (d, 1H, J = 10.5 Hz, 1-H), 7.36 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 26.2, 26.8, 27.0, 30.0, 33.2, 35.3, 41.9, 43.7 (linker CH2), 44.7, 47.7 (C-13), 55.0, 63.0 (CH2OH), 69.9 (OCH2), 70.5 (CH2 of Bn), 75.2 (C-15), 112.8 (C-2), 115.4 (C-4), 126.6 (C-1), 126.6 (C-2 and C-6 of Bn), 127.8 (C-4 of Bn), 128.3 (C-3 and C-5 of Bn), 132.9 (C-10), 138.0 (C-1 of Bn), 138.3 (C-5), 157.3 (C-3), 220.2 (C-17).
3-Methoxy-15β-(carboxyl)methoxy-estra-1,3,5(10)-trien-17-one (13)
Compound 8 was dissolved in acetone (15 ml). Jones reagent (1 ml) was added while cooling with ice. The mixture was diluted with ice-water, and the precipitate was filtered off, washed with water and dried. The crude product was dissolved in CH2Cl2 and was chromatographed on silica gel with ethyl acetate/CH2Cl2 (25:75 v/v), yielding pure 13 (285 mg, 39%). Mp: 142‒144 °C; Rf = 0.32 (ss B); [α]D25 + 42 (c 1 CHCl3). Found C, 70.54; H, 7.43. C21H26O5 (358.43) requires: C, 70.73; H, 7.31%. 1H NMR (δ, ppm, DMSO-d6): 1.08 (s, 3H, 18-H3), 2.82 (m, 2H, 6-H2), 3.69 (s, 3H, 3-OCH3), 4.05 (m, 2H, O-CH2), 4.29 (t, 1 H, J = 5.5 Hz, 15-H), 6.63 (s, 1H, 4-H), 6.68 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.17 (d, 1H, J = 8.5 Hz, 1-H), 12.60 (brs, 1H, OH). 13C NMR (δ, ppm, DMSO-d6): 17.2 (C-18), 25.3, 25.4, 29.1, 32.4, 34.7, 42.5 (C-13), 43.7, 46.5, 53.2, 54.8 (3-OCH3), 65.8 (O-CH2), 74.9 (C-15), 111.4 (C-2), 113.5 (C-4), 126.0 (C-1), 131.8 (C-10), 137.4 (C-5), 157.1 (C-3), 171.9 (COOH), 218.8 (C-17).
3-Methoxy-15β-(2’-carboxyl)ethoxy-estra-1,3,5(10)-trien-17-one (14)
Compound 9 was dissolved in acetone (15 ml). Jones reagent (1 ml) was added during cooling with ice. The mixture was diluted with ice-water, and extracted with CH2Cl2. The organic phase was evaporated to dryness and subjected to chromatographic separation on silica gel in ethyl acetate/CH2Cl2 (1:1 v/v), yielding pure 14 (346 mg, 46%). Mp: 150‒152 °C; Rf = 0.30 (ss B); [α]D25 + 46 (c 1 in CHCl3). Found: C, 71.15; H, 7.32. C22H28O5 (372.46) requires: C, 70.94; H, 7.58%. 1H NMR (δ, ppm, CDCl3): 1.14 (s, 3H, 18-H3), 2.91 (m, 2H, 6-H2), 3.59 (m, 1H, 14-H), 3.80 (s, 4H, 2x linker H2), 4.21 (t, 1 H, J = 6.5 Hz, 15-H), 6.66 (s, 1H, 4-H), 6.73 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.21 (d, 1H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 26.2, 26.6, 29.9, 33.1, 35.2, 35.5, 43.3, 44.6 (linker CH2), 47.7 (C-13), 54.9, 55.6 (3-OCH3), 65.0 (linker CH2), 75.4 (C-15), 111.7 (C-2), 114.5 (C-4), 126.6 (C-1), 132.6 (C-10), 138.3 (C-5), 158.0 (C-3), 177.2 (COOH), 220.1 (C-17).
3-Benzyloxy-15β-(2’-carboxyl)ethoxy-estra-1,3,5(10)-trien-17-one (15)
Compound 11 (435 mg, 1 mmol) was dissolved in acetone (10 ml) and Jones reagent (1 ml) was added while cooling with ice. The mixture was diluted with ice-water, the precipitate separating out was filtered, dried and recrystallized from CH2Cl2/hexane, yielding 15 (342 mg, 76%). Mp: 184‒186 °C; Rf = 0.30 (ss B); [α]D20 + 98 (c 1 in CHCl3). (Found: C, 74.86; H, 7.35. C28H32O5 (448.55) requires: C, 74.79; H, 7.19%). 1H NMR (δ, ppm, CDCl3): 1.15 (s, 3H, 18-H3), 2.93 (m, 2H, 6-H2), 3.60 (m, 1H, O-CH2), 3.81 (m, 1H, O-CH2), 4.21 (t, 1H, J = 6.5 Hz, 15-H), 5.06 (s, 2H, Bn-H2), 6.75 (s, 1H, 4-H), 6.80 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.21 (d, 1H, J = 10.5 Hz, 1-H), 7.40 (m, -5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 17.8 (C-18), 26.2, 26.6, 29.9, 33.2, 35.2, 35.5, 43.3 (linker CH2), 44.6, 47.7 (C-13), 54.9, 65.0 (O-CH2), 70.4 (Bn-CH2), 75.4 (C-15), 112.6 (C-2), 115.5 (C-4), 126.6 (C-1), 127.9 (C-2 and C-6 of Bn), 128.3 (C-4 of Bn), 129.0 (C-3 and C-5 of Bn), 132.9 (C-10), 138.0 (C-1 of Bn), 138.4 (C-5), 157.3 (C-3), 177.3 (COOH), 220.1 (C-17).
3-Hydroxy-15β-(2’-carboxyl)ethoxy-estra-1,3,5(10)-trien-17-one (16)
Compound 15 (448 mg, 1 mmol) was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (300 mg, 10%) was hydrogenated under 5 bar for 6 h at room temperature. The solution was filtered off, the residue was crystallized from methanol to yield 16 (320 mg, 89%). Mp: 200‒202 °C; Rf = 0.2 (ss B); [α]D25 + 29 (c 1 in CHCl3). Found C, 70.18; H, 7.45. C21H26O5 requires: C, 70.37; H, 7.31%. 1H NMR (δ, ppm, DMSO): 0.78 (s, 3H, 18-H3), 2.52 (m, 2H, 6-H2), 3.18 (m, 6H, 2x linker H2), 3.90 (t, 1 H, J = 6.5 Hz, 15-H), 6.24 (d, 1H, J = 2.5 Hz, 4-H), 6.29 (dd, 1H, J = 10.5 Hz, J = 2.5 Hz, 2-H), 6.81 (d, 1H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, DMSO-d6): 17.6 (C-18), 25.8, 26.0, 29.3, 32.7, 35.1, 35.4, 43.1 (linker CH2), 43.9, 46.9 (C-13), 53.6, 65.1 (linker CH2), 74.6 (C-15), 113.1 (C-2), 115.4 (C-4), 126.2 (C-1), 130.6 (C-10), 137.6 (C-5), 155.4 (C-3), 173.1 (COOH), 219.3 (C-17).
3-Benzyloxy-15β-(3’-carboxyl)propoxy-estra-1,3,5(10)-trien-17-one (17)
Compound 12 (448 mg, 1 mmol) was dissolved in acetone (10 ml) and Jones reagent (1 ml) was added while cooling with ice. The mixture was diluted with ice-water, the precipitate separating out was filtered, dried, and crystallized from acetone/hexane to yield 17 (390 mg, 84%). Mp: 138‒140 °C; Rf = 0.25 (ss B); [α]D25 + 57 (c 1 in CHCl3). Found: C, 75.22; H, 7.67. C29H34O5 (462.58) requires: C, 70.30; H, 7.41%. 1H NMR (δ, ppm, CDCl3): 1.14 (s, 3H, 18-H3), 3.29 (m, 1H, O-CH2), 3.56 (m, 1H, O-CH2), 4.12 (t, 1 H, J = 6.5 Hz, 15-H), 5.02 (s, 2H, Bn-H2), 6.73 (d, 1H, J = 3.0 Hz, 4-H), 6.77 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.18 (d, 1H, J = 10.5 Hz, 1-H), 7.36 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 25.5, 26.2, 26.8, 29.9, 31.3, 33.1, 35.3, 43.5 (linker CH2), 44.7, 47.7 (C-13), 55.0, 68.6 (O-CH2), 70.4 (Bn-CH2), 75.3 (C-15), 112.8 (C-2), 115.4 (C-4), 126.6 (C-1), 127.8 (C-2 and C-6 of Bn), 128.3 (C-4 of Bn), 129.0 (C-3 and C-5 of Bn), 132.9 (C-10), 137.7 (C-1 of Bn), 138.3 (C-5), 157.3 (C-3), 179.5 (COOH), 220.2 (C-17).
3-Methoxy-15β-(2’-cyano)ethoxy-estra-1,3,5(10)-trien-17-one (18)
Compound 6 (282 mg, 1 mmol) was dissolved in CH2Cl2 (10 ml) and 3-hydroxypropionylnitrile (10 ml), containing 5% aqueous NaOH (1 ml), was stirred at room temperature for 8 h. CH2Cl2 (50 ml) was added to the reaction mixture and then it was diluted with water (100 ml). The organic phase was separated, washed with water, dried over Na2SO4, and evaporated in vacuo. The residual product was purified by flash chromatography using CH2Cl2 to yield 18 (305 mg, 86%). Mp: 197‒200 °C; Rf = 0.50 (ss B); [α]D25 + 63 (c 1 in CHCl3). Found C, 74.92; H, 7.55. C22H27NO3 (353.45) requires C, 74.76; H, 7.70%. 1H NMR (δ, ppm, CDCl3): 1.17 (s, 3H, 18-H3), 2.60 (s 2H, linker H2), 3.00 (m, 2H, 6-H2), 3.65 (s, 3H, 3OCH3), 3.77 (s, 2’H, linker H2), 16-H2), 4.23 (t, 1 H, J = 6.4 Hz, 15-H), 6.65 (s, 1H, 4-H), 6.71 (d, 1H, J = 10.5 Hz, 2-H), 7.19 (d, 1H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 18.2 (C-18), 20.0, 26.4, 27.1, 30.1, 33.4, 35.4, 43.5, 44.9 (linker CH2), 47.9 (C-13), 55.1 (3-OCH3), 55.9, 65.0 (O-CH2), 72.2 (C-15), 112.2 (C-2), 114.6 (C-4), 118.3 (CN), 126.8 (C-1), 132.7 (C-10), 138.5 (C-5), 158.3 (C-3), 219.2 (C-17).
3-Benzyloxy-15β-(2’-cyano)ethoxy-estra-1,3,5(10)-trien-17-one (19)
Compound 7 (358 mg, 1 mmol) dissolved in CH2Cl2 (10 ml) and 3-hydroxypropionytrile (10 ml), containing 5% aqueous NaOH (1 ml), was stirred at room temperature for 8 h. After adding CH2Cl2 (50 ml) to the reaction mixture, the organic phase was separated, washed with water, dried over Na2SO4, and evaporated in vacuo. The residual product was purified by flash chromatography using ethyl acetate/CH2Cl2 (2.5/97.5 v/v) to yield 19 (346 mg, 80%). Mp: 183‒185 °C; Rf = 0.45 (ss B); [α]D25 + 54 (c 1 in CHCl3). Found C, 78.52; H, 7.42. C28H31NO3 (429.55) requires: C, 78.29; H, 7.27%. 1H NMR (δ, ppm, CDCl3): 1.20 (s, 3H, 18-H3), 3.54 (m, 1H, O-CH2), 3.78 (m, 1H, O-CH2), 4.27 (t, 1 H, J = 6.5 Hz, 15-H), 5.07 (s, 2H, Bn-H2), 6.78 (s, 1H, 4-H), 6.81 (d, 1H, J = 11.0 Hz, 2-H), 7.22 (d, 1H, J = 11.0 Hz, 1-H), 7.41 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 19.8, 26.2, 26.8, 29.9, 33.2, 35.2, 43.3 (CN), 44.7, 47.7 (C-13), 54.9, 64.8 (O-CH2), 70.4 (Bn-CH2), 76.0 (C-15), 112.8 (C-2), 115.4 (C-4), 118.1 (CN), 126.6 (C-1), 127.9 (C-2 and C-6-of Bn), 128.3 (C-4-of Bn), 129.0 (C-3 and C-5 of Bn), 132.7 (C-10), 137.7 (C-1 of Bn), 138.4 (C-5), 157.4 (C-3), 219.1 (C-17).
3-Hydroxy-15β-(2’-cyano)ethoxy-estra-1,3,5(10)-trien-17-one (20)
Compound 19 (430 mg, 1 mmol) was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (300 mg, 10%) was hydrogenated under 5 bar pressure for 6 h, at room temperature. The reaction mixture was filtered off, evaporated in vacuo and crystallized from CH2Cl2/hexane to yield 20 (286 mg, 84%). Mp: 221‒223 °C; Rf = 0.25 (ss A); [α]D25 + 37 (c 1 in MeOH). Found C, 74.62; H, 7.35. C21H25O3N (339.43) requires: C, 74.31; H, 7.42%. 1H NMR (δ, ppm, DMSO-d6): 1.07 (s, 3H, 18-H3), 2.76 (m, 2H, 6-H2), 3.33 (s, 3H, CN-H2), 15-H), 3.70 (m, 1H, O-CH2), 4.04 (m, 1H, O-CH2), 4.21 (t, 1 H, J = 6.5 Hz, 15-H), 6.47 (d, 1H, J = 3.0 Hz, 4-H), 6.53 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.05 (d, 1H, J = 10.5 Hz, 1-H), 9.00 (brs, 1H, 3-OH). 13C NMR (δ, ppm, DMSO-d6): 17.2 (C-18), 18.5, 25.4, 25.7, 28.9, 32.3, 34.7, 42.7 (linker CH2), 43.5, 46.5 (C-13), 53.1, 63.7 (O-CH2), 74.5 (C-15), 112.7 (C-2), 115.0 (C-4), 119.2 (CN), 125.9 (C-1), 130.1 (C-10), 137.2 (C-5), 155.0 (C-3), 218.6 (C-17).
3-Sulfamoyloxy-15β-(2’-cyano)ethoxy-estra-1,3,5(10)-trien-17-one (21)
Compound 20 (340 mg, 1 mmol) was dissolved in dimethylformamide (20 ml), and 575 mg (5 mmol) sulfamoylchloride was added dropwise during cooling with ice. The reaction mixture was allowed to stand 6 h and then poured onto ice (300 g). The precipitate was filtered off and dried. The product was crystallized from ethyl acetate to yield 21 (360 mg, 86%). Mp: 78‒80 °C; Rf = 0.30 (ss A); [α]D25 + 52 (c 1 in CHCl3). Found: C, 60 55; H, 6.42. C21H26N2O5S (418.51) requires: C, 60.27; H, 6.26%. 1H NMR (δ, ppm, CDCl3): 1.10 (s, 3H, 18-H3), 3.45 (m, 1H, O-CH2), 3.70 (m, 1H, O-CH2), 4.19 (t, 1 H, J = 7.0 Hz, 15-H), 5.25 (s, 2H, NH2), 6.99 (d, 1H, J = 3.0 Hz, 4-H), 7.02 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.23 (t, 1 H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 17.9 (C-18), 19.8, 26.0, 26.4, 29.6, 33.0, 34.7, 43.2 (linker CH2), 44.7, 47.6 (C-13), 54.8, 64.7 (OCH2), 75.9 (C-15), 118.3 (CN), 119.5 (C-2), 122.5 (C-4), 127.0 (C-1), 139.3 (C-10), 139.4 (C-5), 148.5 (C-3), 219.3 (C-17).
3-Benzyloxy-15β-(2’-methoxy-2’-oxoethoxy)-estra-1,3,5(10)-trien-17-one (22)
Compound 7 (717 mg, 2 mmol) in CH2Cl2 (10 ml) and ethane-1,2 diol (15 ml), containing 5% aqueous sodium hydroxide (1 ml) was stirred at room temperature for 6 h. The reaction mixture was diluted with water (100 ml). The organic phase was separated, washed with water, dried and evaporated in vacuo. The crude 3-benzyloxy-15β-(2’-hydroxy)ethoxy-estra-1,3,5(10)-trien-17-one was dissolved in acetone (15 ml) and Jones reagent (1 ml) was added cooling with ice. The mixture was diluted with ice-water, the precipitate was filtered off, and dried. The crude 3-benzyloxy-15β-(carboxyl)methoxy-estra-1,3,5(10)-trien-17-one was dissolved in tetrahydrofurane (10 ml) and diethyl ether containing 1% diazomethane (50 ml) was added during cooling with ice. After standing 6 h, the solution was evaporated and the residue was chromatographed on silica gel with ethyl acetate/CH2Cl2 (2.5/97.5 v/v) to yield 22 (265 mg, 29%). Mp: 101‒103 °C; Rf = 0.55 (ss C); [α]D25 + 71 (c 1 in CHCl3). Found: C, 75.12; H, 7.35. C28H32O5 (448.55) requires: C, 74.97; H, 7.19%. 1H NMR (δ, ppm, CDCl3): 1.21 (s, 3H, 18-H3), 2.93 (m, 2H, 6-H2), 3.76 (s, 3H, COOCH3), 4.10 (m, 2H, O-CH2), 4.36 (t, 1 H, J = 6.5 Hz, 15-H), 5.05 (s, 2H, Bn-H2), 6.99 (s, 1H, 4-H), 6.76 (d, 1H, J = 3.5 Hz, 2-H), 7.21 (d, 1H, J = 10.5 Hz, 1-H), 7.38 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 16.9 (C-18), 25.2, 25.6, 29.0, 32.3, 34.3, 42.4, 43.9, 46.8 (C-13), 51.2, 54.1 (OCH3), 66.0 (O-CH2), 69.5 (Bn-CH2), 75.2 (C-15), 111.8 (C-2), 114.5 (C-4), 125.6 (C-1), 126.9 (C-2 and C-6 of Bn), 127.3 (C-4-of Bn), 128.0 (C-3 and C-5 of Bn), 131.9 (C-10), 136.9 (C-1’), 137.5 (C-5), 156.4 (C-3), 170.2 (C = O), 218.4 (C-17).
3-Methoxy-15β-(3’-methoxy-3’-oxopropoxy)-estra-1,3,5(10)-trien-17-one (23)
Compound 14 (373 mg, 1 mmol) was dissolved in tetrahydrofuran (10 ml) and diethylether containing 1% diazomethane (50 ml) was added during cooling with ice. After standing 6 h, the solution was evaporated and the residue was crystallized from MeOH, to yield 23 (370 mg, 95%). Mp: 95‒97 °C; Rf = 0.58 (ss C); [α]D25 + 64 (c 1 in CHCl3). Found: C, 71.62; H, 8.04; C23H30O5 (386.48) requires: C, 71.48; H, 7.82%. 1H NMR (δ, ppm, CDCl3): 1.13 (s, 3H, 18-H3), 2.40 (m, 2H, linker H2), 2.90 (m, 2H, 6-H2), 3.59 (m, 2H, linker H2) 3.69 (s, 3H, 3-OCH3), 3.80 (s, 3H, COOCH3), 4.20 (t, 1 H, J = 6.5 Hz, 15-H), 6.68 (s, 1H, 4-H), 6.74 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.21 (d, 1H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 17.8 (C-18), 26.2, 26.6, 30.0, 33.2, 35.2, 35.6, 43.3, 44.6 (linker CH2), 47.7 (C-13), 52.1, 54.9, 55.6 (3-OCH3), 65.3 (O-CH2), 75.3 (C-15), 111.9 (C-2), 114.3 (C-4), 126.6 (C-1), 132.6 (C-10), 138.3 (C-5), 158.1 (C-3), 172.4 (C = O), 219.9 (C-17).
3-Hydroxy-15β-(3’-methoxy-3’-oxopropoxy)-estra-1,3,5(10)-trien-17-one (24)
Compound 15 (448 mg, 1 mmol) was dissolved in tetrahydrofuran (10 ml) and diethyl ether containing 1% diazomethane (50 ml) was added during cooling with ice. After standing 6 h, the solution was evaporated and the residue was crystallized from MeOH, to yield crude 3-benzyloxy-15β-(3’-methoxy-3’-oxopropoxy)-estra-1,3,5(10)-trien-17-one. This compound was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (300 mg, 10%) was hydrogenated under 5 bar pressure for 6 h, at room temperature. The reaction mixture was filtered off, evaporated in vacuo and the residue was chromatographed on silica gel with ethyl acetate/CH2Cl2 (10:90 v/v) to yield 24 (196 mg, 52%). Mp: 140‒142 °C; Rf = 0.25 (ss A); [α]D20 + 51 (c 1 in CHCl3). Found: C, 71.08; H, 7.76. C22H28O5 (372.45) requires: C, 70.94; H, 7.58%. 1H NMR (δ, ppm, DMSO-d6): 0.99 (s, 3H, 18-H3), 2.75 (m, 2H, 6-H2), 3.34 (s, 4H, 2 x linker H2), 3.58 (s, 3H, COO-H3), 4.13 (t, 1 H, J = 6.5 Hz, 15-H), 6.48 (s, 1H, 4-H), 6.52 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.04 (d, 1H, J = 10.5 Hz, 1-H), 8.99 (brs, 1H, 3-OH). 13C NMR (δ, ppm, DMSO-d6): 18.0 (C-18), 26.3, 26.5, 29.9, 33.2, 35.6, 35.6, 43.4 (linker CH2), 44.4, 47.4 (C-13), 52.1, 54.0, 65.3 (O-CH2), 75.2 (C-15), 113.6 (C-2), 115.9 (C-4), 126.8 (C-1), 131.0 (C-10), 138.0 (C-5), 155.9 (C-3), 172.6 (C = O), 219.7 (C-17).
General procedure for the synthesis of 3-hydroxy-, and 3-benzyloxy-15β-(carboxamido)alkoxy-estra-1,3,5(10)-trienes with ammonium hydroxide, morpholine or N-cyclohexyl,N-methylamine (25‒33)
To the solution of the corresponding 3-hydroxy-, or 3-benzyloxy-15β-(carboxyl)alkoxy-estra-1,3,5(10)-triene (1 mmol) in CH2Cl2 (20 ml) 0.2 ml (2 mmol) oxalyl chloride was added dropwise while cooling in ice under continuous stirring. The solution was allowed to stand at room temperature for 2 h. After evaporation in vacuo the residue was dissolved in CH2Cl2 (20 ml) and 4 mmol of the corresponding amine component was added while cooling in ice under continuous stirring. After 1 h, water (100 ml) was added and extracted with CH2Cl2 (2x 50 ml). The organic phase was washed with water, dried and evaporated. The residual material was chromatographed on a silica gel column with ethyl acetate/CH2Cl2 in different concentrations.
3-Benzyloxy-15β-(3’-amino-3-oxopropoxy)-estra-1,3,5,(10)-trien-17-one (25)
Compound 15 (448 mg, 1 mol) was used for synthesis as described in general procedure. The amine component was ammonium hydroxide solution (20 ml). The crude product was chromatographed on silica gel with ethyl acetate/CH2Cl2 (1:1 v/v) to yield 22 (238 mg, 53%). Mp: 183‒185 °C; Rf = 0.55 (ss C); [α]D25 + 61 (c 1 in CHCl3). Found: C, 75.36; H, 7.26. C28H33NO4 (447.57) requires: C, 75.14; H, 7.43%. 1H NMR (δ, ppm, CDCl3): 1.26 (s, 3H, 18-H3), 2.30 (m, 2H, linker H2), 2.96 (m, 2H, 6-H2),3.60 (m, 2H, linker H2), 4.43 (t, 1 H, J = 6.0 Hz, 15-H), 5.07 (s, 2H, Bn-H2), 6.78 (s, 1H, 4-H), 6.83 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.24 (d, 1H, J = 10.5 Hz, 1-H), 7.38 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 15.5 (C-18), 22.0, 23.9, 24.2, 27.8, 30.9, 33.2, 33.8, 40.9, 41.9 (linker CH2), 45.9 (C-13), 50.1, 51.8, 64.7 (linker CH2), 73.9 (C-15), 112.2 (C-2), 114.6 (C-2), 125.8 (C-1), 123.0 (C-10), 126.8 (C-2 and C-6 of Bn), 127.1 (C-4 of Bn), 129.0 (C-3 and C-5 of Bn), 137.4 (C-5), 155.8 (C-3), 171.8 (C = O), 220.1 (C-17).
3-Hydroxy-15β-(3’-amino-3’-oxopropoxy)-estra-1,3,5(10)-trien-17-one (26)
Compound 25 (448 mg, 1 mmol) was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (300 mg, 10%) was hydrogenated under 5 bar pressure for 6 h, at room temperature. The reaction mixture was filtered off and evaporated in vacuo. The residue was chromatographed on silica gel column with ethyl acetate/CH2Cl2 (1:1 v/v) to yield 26 (308 mg, 86%). Mp: 124‒126 °C; Rf = 0.20 (ss A); [α]D25 + 54 (c 1 in MeOH). Found: C, 70.38; H, 7.85. C21H27NO4 (357.44) requires: C, 70.56; H, 7.61%. 1H NMR (δ, ppm, DMSO-d6): 1.03 (s, 3H, 18-H3), 2.77 (m, 2H, 6-H2), 3.33 (s, 2H, linker H2), 3.47 (m, 1H, O-CH2), 3.68 (m, 1H, O-CH2), 4.11 (t, 1 H, J = 6.5 Hz, 15-H), 6.47 (d, 1H, J = 3.5 Hz, 4-H), 6.52 (dd, 1H, J = 10.5 Hz, J = 3.5 Hz, 2-H), 6.78 (brs, 1H, NH2), 7.04 (d, 1H, J = 10.5 Hz, 1-H), 7.22 (brs, 1H, NH2), 7.90 (brs, 1H, 3-OH). 13C NMR (δ, ppm, DMSO-d6): 16.5 (C-18), 24.7, 24.9, 28.3, 31.7, 34.0, 35.3, 42.0, 42.8 (linker CH2), 45.8 (C-13), 52.5, 64.4 (linker CH2), 73.5 (C-15), 112.0 (C-2), 114.2 (C-2), 125.1 (C-1), 129.5 (C-10), 136.5 (C-5), 154.3 (C-3), 171.6 (C = O), 218.3 (C-17).
3-Benzyloxy-15β-(2’-morpholino-2’-oxoethoxy-estra-1,3,5(10)-trien-17-one (27)
3-Benzyloxy-15β-(carboxyl)methoxy-estra-1,3,5(10)-triene (434 mg, 1 mmol) was used for the synthesis as described in general procedure. The crude steroidal carbonyl chloride was dissolved in CH2Cl2 (20 ml), and morpholine (0.35 ml, 4 mmol) was added dropwise during cooling with ice under continuous stirring. After 1 h, water (100 ml) was added, and extracted with CH2Cl2 (2 x 50 ml). The organic solution was washed with water, dried, and evaporated. The residual material was chromatographed on silica gel column with ethyl acetate/CH2Cl2 (25:75 v/v) to yield 27 (432 mg, 85%). Mp: 121‒123 °C; Rf = 0.45 (ss D); [α]D25 + 37 (c 1 in MeOH). Found: C, 74.14; H, 7.53. C31H37NO5 (503.63) requires: C, 73.93; H, 7.41%. 1H NMR (δ, ppm, CDCl3): 1.16 (s, 3H, 18-H3), 3.58 (m, 8H, 4x morpholine H2), 4.08 (d, 1H, J = 13.0 Hz, O-CH2), 4.20 (d, 1H, J = 13.0 Hz, O-CH2), 4.35 (t, 1 H, J = 5.5 Hz, 15-H), 5.04 (s, 2H, Bn-H2), 6.75 (s, 1H, 4-H), 6.80 (dd, 1H, J = 8.5 Hz, J = 2.5 Hz, 2-H), 7.20 (d, 1H, J = 8.5 Hz, 1-H), 7.32 (t, 1H, J = 7.5 Hz, Bn 4-H), 7.38 (t, 2H, J = 7.5 Hz, Bn 3- and 5-H), 7.43 (d, 2H, J = 7.5 Hz, Bn-2- and 6-H). 13C NMR (δ, ppm, CDCl3): 17.5 (C-18), 25.6, 26.3, 29.5, 32.7, 34.8, 42.1, 43.0, 44.3, 45.9 (C-13), 47.2, 54.4, 66.7 (morpholine CH2), 66.7 (morpholine CH2), 69.1 (O-CH2), 69.9 (Bn-CH2), 76.0 (C-15), 112.4 (C-2), 114.8 (C-4), 126.2 (C-1), 127.4 (C-2 and C-6 of Bn), 127.8 (C-4 of Bn), 128.5 (C-3 and C-5 of Bn), 132.1 (C-10), 137.1 (C-1 of Bn), 137.5 (C-5), 156.9 (C-3), 167.5 (C = O), 218.7 (C-17).
3-Hydroxy-15β-(carboxmorpholydo)methoxy-2’-morpholino-2’oxoethoxy)-estra-1,3,5(10)-trien-17-one (28)
Compound 27 (503 mg, 1 mmol) was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (448 mg, 10%) was hydrogenated under 5 bar pressure for 6 h at room temperature. The reaction mixture was filtered off, evaporated in vacuo, and the residue was crystallized from MeOH to yield 28 (350 mg, 69%). Mp: 215‒220 °C; Rf = 0.40 (ss D); [α]D25 + 54 (c 1 in MeOH). Found: C, 73.07; H, 7.92. C25H33NO4 (411.53) requires: C, 72.96; H, 8.08%. 1H NMR (δ, ppm, CDCl3): 1.14 (s, 3H, 18-H3), 3.51 (s, 2H, linker H2), 3.58 (m, 2H, 2x H2 of morpholine), 3.60 (m 2H, 2x H2 of morpholine), 4.14 (t, 1 H, J = 6.5 Hz, 15-H), 6.60 (d, 1H J = 2.5 Hz, 4-H), 6.65 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.12 (d, 1H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 26.0, 26.2, 26.7, 29.9, 30.1, 33.1, 35.4, 42.5, 43.7, 44.5, 46.4, 47.7 (C-13), 54.9, 66.6 (linker CH2), 67.3 (linker CH2), 75.2 (C-15), 113.4 (C-2), 115.8 (C-4), 126.7 (C-1), 132.1 (C-10), 138.2 (C-5), 154.2 (C-1), 172.0 (linker C = O), 220.5 (C-17).
3-Benzyloxy-15β-(3’-morpholino-3’oxopropoxy)-estra-1,3,5(10)-trien-17-one (29)
Compound 15 (448 mg, 1 mmol) was used for synthesis as described in general procedure. The crude steroidal carbonyl chloride was dissolved in CH2Cl2 (20 ml), and morpholine (0.35 ml, 4 mmol) was added dropwise during cooling with ice under continuous stirring. After 1 h, water (100 ml) was added, and extracted with CH2Cl2 (2x 50 ml). The organic solution was washed with water, dried, and evaporated. The residual material was chromatographed on silica gel column with ethyl acetate/CH2Cl2 (2.5:97.5 v/v) to yield 29 (372 mg, 72%). Mp: 128‒130 °C; Rf = 0.42 (ss D); [α]D25 + 37 (c 1 in CHCl3). Found: C, 74.46; H, 7.72. C32H39NO5 (517.66) requires: C, 74.24; H, 7.59%. 1H NMR (δ, ppm, CDCl3): 1.12 (s, 3H, 18-H3), 2.89 (m, 2H, 6-H2), 3.51(m, 2H, linker H2), 4.20 (t, 1H, J = 6.5 Hz, 15-H), 5.04 (s, 2H, Bn-H2), 6.74 (d, 1H, J = 3.5 Hz, 4-H), 6.79 (dd, 1H, J = 10.5 Hz, J = 3.5 Hz, 2-H), 7.19 (d, 1H, J = 10.5 Hz, 1-H), 7.31 (t, 1 H, J = 8.5 Hz, Bn 4-H), 7.38 (t, 2H, J = 8.5 Hz, Bn 3- and 5-H), 7.43 (d, 2H, J = 8.5 Hz, Bn 2- and 6-H). 13C NMR (δ, ppm, CDCl3): 17.9 (C-18), 26.2, 26.6, 30.0, 33.1, 33.7, 35.4, 42.4 (morpholine CH2), 43.5 (linker CH2), 44.6, 46.5 (morpholine CH2), 47.7 (C-13), 54.9, 66.2 (O-CH2), 67.1 (morpholine CH2), 67.3 (morpholine CH2), 70.4 (Bn-CH2), 75.5 (C-15), 112.8 (C-2), 115.4 (C-4), 126.6 (C-1), 127.8 (C-2 and C-6 of Bn), 128.3 (C-4 of Bn), 129.0 (C-3 and C-5 of Bn), 132.9, 137.9 (C-1’), 138.2 (C-5), 157.3 (C-3), 169.9 (C = O), 219.9 (C-17).
3-Sulfamoyloxy-15β-(3’-morpholino-3’-oxopropoxy)-estra-1,3,5(10)-trien-17-one (30)
Compound 29 (517 mg, 1 mmol) was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (300 mg, 10%) was hydrogenated under 5 bar pressure for 6 h, at room temperature. The reaction mixture was filtered off and evaporated in vacuo. The residue was dissolved in dimethylformamide (20 ml), and 575 mg (5 mmol) sulfamoyl chloride was added dropwise during cooling with ice. The reaction mixture was allowed to stand 6 h and then poured onto ice (300 g). The precipitate separated out was filtered off, and subjected to chromatographic separation on silica gel column with ethyl acetate/CH2Cl2 (1:1 v/v) to yield 30 (240 mg, 45%). Mp: 104‒108 °C; Rf = 0.35 (ss D); [α]D25 + 17 (c 1 in CHCl3). Found: C, 59.43; H, 6.54. C25H34N2O7S (506.61) requires: C, 59.27; H, 6.76%. 1H NMR (δ, ppm, CDCl3): 1.12 (s, 3H, 18-H3), 2.74 (m, 2H, linker CH2), 4.46 (m, 4H, 2x H2 of morpholine), 3.56 (m, 2H, O-CH2), 3.61 (m, 4H, 2x H2 of morpholine), 3.82 (t, 1 H, J = 6.5 Hz, 15-H), 5.42 (s, 2H, NH2), 6.78 (d, 1H, J = 3.5 Hz, 4-H), 7.20 (dd, 1H, J = 10.0 Hz, J = 3.5 Hz, 2-H), 7.30 (t, 1 H, J = 10.0 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 17.8 (C-18), 26.1, 26.7, 30.5, 33.4, 33.9, 35.5, 42.2 (morpholine CH2), 43.4 (linker CH2), 45.1, 46.7 (morpholine CH2), 47.5 (C-13), 55.8, 64.3 (O-CH2), 66.7 (morpholine CH2), 67.5 (morpholine CH2), 75.7 (C-15), 112.5 (C-2), 115.1 (C-4), 126.8 (C-1), 133.0, 140.5 (C-5), 159.1 (C-3), 170.9 (C = O), 220.2 (C-17).
3-Benzyloxy-15β-(4’-morpholino-4’oxobutoxy)-estra-1,3,5(10)-trien-17-one (31)
Compound 17 (462 mg, 1 mmol) was used for the synthesis as described in general procedure. The crude steroidal carbonyl chloride was dissolved in CH2Cl2 (20 ml), and morpholine (0.35 ml, 4 mmol) was added dropwise during cooling with ice under continuous stirring. After 1 h, water (100 ml) was added, and extracted with CH2Cl2 (2 x 50 ml). The organic solution was washed with water, dried and evaporated in vacuo. The residual material was chromatographed on silica gel column with ethyl acetate/CH2Cl2 (30:70 v/v) to yield 31 (810 mg, 76%). Mp: 135‒138 °C; Rf = 0.42 (ss D); [α]D25 + 20 (c 1 in CHCl3). Found: C, 74.87; H, 7.43. C33H41NO5 (531.68) requires: C, 74.55; H, 7.77%. 1H NMR (δ, ppm, CDCl3): 1.14 (s, 3H, 18-H3), 1.91 (m, 4H, 2 x morpholine H2), 2.37 (m, 4H, 2 x morpholine H2), 3.36 (t, 2H, J = 6.5 Hz, O-CH2), 4.15 (t, 1 H, J = 6.5 Hz, 15-H), 5.04 (s, 2H, Bn-H2), 6.73 (d, 1H, J = 3.5 Hz, 4-H), 6.79 (dd, 1H, J = 10.5 Hz, J = 3.5 Hz, 2-H), 7.20 (d, 1H, J = 10.5 Hz, 1-H), 7.36 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 26.0, 26.2, 26.7, 30.0, 33.1, 34.7, 35.4, 42.3 (morpholine CH2), 43.7 (linker CH2), 44.6, 46.3 (morpholine CH2), 47.7 (C-13), 54.9, 67.0 (morpholine CH2), 67.3 (morpholine CH2), 69.0 (O-CH2), 70.4 (Bn-CH2), 75.1 (C-15), 112.8 (C-2), 115.4 (C-4), 126.6 (C-1), 127.8 (C-2 and C-6 of Bn), 128.3 (C-4’), 129.0 (C-3 and C-5 of Bn), 132.9 (C-10), 137.9 (C-1 of Bn), 138.1 (C-5), 157.4 (C-3), 171.6 (C = O), 220.0 (C-17).
3-Hydroxy-15β-(4’-morpholino-4’-oxobutoxy)-estra-1,3,5(10)-trien-17-one (32)
Compound 31 (531 mg, 1 mmol) was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (300 mg, 10%) was hydrogenated under 5 bar pressure for 6 h, at room temperature. The reaction mixture was filtered off, evaporated in vacuo and crystallized from MeOH to yield 32 (390 mg, 88%). Mp: 80‒84 °C; Rf = 0.35 (ss D); [α]D25 + 46 (c 1 in CHCl3). Found: C, 70.58; H, 8.12. C26H35NO5 (441.57) requires: C, 70.72; H, 7.99%. 1H NMR (δ, ppm, CDCl3): 1.14 (s, 3H, 18-H3), 3.33 (t, 2H, J = 6.0 Hz, linker H2), 3.48 (m, 4H, 2x morpholine H2), 361 (m, 4H, 2x morpholine H2), 4.14 (t, 1 H, J = 6.5 Hz, 15-H), 6.60 (d, 1H J = 2.5 Hz, 4-H), 6.65 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.12 (d, 1H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 26.0, 26.2, 26.7, 29.9, 30.1, 33.1, 35.4, 42.5, 43.7, 44.5, 46.4, 47.7 (C-13), 54.9, 66.6 (linker CH2), 67.3 (linker CH2), 69.0 (linker CH2), 75.2 (C-15), 113.4 (C-2), 115.8 (C-4), 126.7 (C-1), 132.1 (C-10), 138.2 (C-5), 154.2 (C-1), 172.0 (linker C = O), 220.5 (C-17).
3-Methoxy-15β-(2’N-cyclohexyl,N-methyl-2’oxoethoxy)-estra-1,3,5(10)-trien-17-one (33)
Starting from 3-methoxy-15β-(carboxyl)methoxy-estra-1,3,5(10)-trien-17-one (13) 358 mg (1 mmol) was used for synthesis as described in general procedure. The crude steroidal carbonyl chloride was dissolved in CH2Cl2 (20 ml), and N-cyclohexyl, N-methylamine (4.5 ml, 4 mmol) was added dropwise during cooling with ice under continuous stirring. After 1 h, water (100 ml) was added, and extracted with CH2Cl2 (2 x 50 ml). The organic solution was washed with water, dried, and evaporated. The residual material was chromatographed on silica gel column with ethyl acetate/CH2Cl2 (25:75 v/v) to yield 33 (385 mg, 84%). Mp: 53‒58 °C (foam); Rf = 0.50 (ss B); [α]D25 + 61 (c 1 in CHCl3). Found: C, 73.92; H, 8.82. C28H39NO4 (453.61) requires: C, 74.14; H, 8.67%. 1H NMR (δ, ppm, CDCl3): 1.18 (s, 3H, 18-H3), 3.30 (s, 3H, N-CH3), 3.77 (s, 3H, 3-OCH3), 4.12 (m, 2H, O-CH2), 4.39 (m, 1H, 15-H), 6.65 (s, 1H, 4-H), 6.71 (d, 1H, J = 11.0 Hz, 2-H), 7.19 (d, 1H, J = 11.0 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 17.9 (C-18), 25.8, 26.0, 26.2, 27.6, 29.3, 29.9, 30.0, 31.3, 33.2, 35.4, 43.5, 43.8 (C-13), 44.8, 47.7, 53.0, 55.0, 55.6, 69.9 (O-CH2), 75.7 (C-15), 112.0 (C-2), 114.3 (C-4), 126.6 (C-1), 132.5 (C-10), 138.1 (C-5), 158.1 (C-3), 166.3 (C = O), 219.5 (C-17).
General procedure for the synthesis of 3-benzyloxy-15β-(carbamoyloxy)alkoxy-estra-1,3,5(10)-trien-17-one (34‒40)
To the solution of 3-benzyloxy-15β-(2’-hydroxy)ethoxy-estra-1,3,5(10)-trien-17-one (10) or 3-benzyloxy-15β-(3’-hydroxy)propoxy-estra-1,3,5(10)-trien-17-one (11) mmol) in CH2Cl2 (30 ml) containing 0.2 ml triethylamine, 4 mmol of the corresponding alkyl or aryl isocyanate was added under continuous stirring. After the addition of the reagent the reaction mixture was heated at reflux for 1 h, poured into water (100 ml), and then extracted with CH2Cl2 (2x 30 ml). The organic phase was washed with NaHCO3 solution, water, dried and evaporated in vacuo. The residual material was chromatographed on a silica gel column with ethyl acetate/CH2Cl2 in different concentrations.
3-Benzyloxy-15β-(2’-cyclohexylcarbamoyloxy)ethoxy-estra-1,3,5(10)-trien-17-one (34)
Compound 10 (420 mg, 1 mmol) was used for the synthesis as described in general procedure. The reagent was cyclohexyl isocyanate (4 mmol). The crude product was chromatographed with ethyl acetate/CH2Cl2 (2.5:97.5 v/v) to yield 34 (430 mg, 78%). Mp: 96‒98 °C; Rf = 0.55 (ss B); [α]D25 + 39 (c 1 in CHCl3). Found: C, 74.65; H, 8.14. C34H43NO5 (545.71) requires: C, 74.83; H, 7.94%. 1H NMR (δ, ppm, CDCl3): 1.16 (s, 3H, 18-H3), 3.65 (m, 2H, 16-H2), 3.68 (m, 1H, OCH2), 4.19 (m, 3H, OCH2), 4.55 (d, 1H, J = 7.5 Hz, 15-H), 5.03 (s, 2H, Bn-H2), 6.73 (d, 1H, J = 3.0 Hz, 4-H), 6.78 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.19 (d, 1H, J = 10.5 Hz, 1-H), 7.37 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 17.9 (C-18), 24.8, 25.2, 25.9, 26.2, 30.0, 33.2, 33.8, 35.2, 43.7 (O-CH2), 44.6, 47.8 (C-13), 50.2, 53.8, 55.0, 64.0, 68.3 (O-CH2), 70.4, 70.5 (Bn-CH2), 75.4 (C-15), 112.7 (C-2), 115.4 (C-4), 126.6 (C-1), 127.8 (C-2 and C-6 of Bn), 127.8 (C-4 of Bn), 129.0 (C-3 and C-5 of Bn), 133.0 (C-10), 137.8 (C-1’), 138.3 (C-5), 157.3 (C-3), 220.0 (C-17).
3-Hydroxy-15β-(2’-cyclohexylcarbamoyloxy)ethoxy-estra -1,3,5(10)-trien-17-one (35)
Compound 34 (545 mg, 1 mmol) was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (300 mg, 10%) was hydrogenated under 5 bar for 6 h, at room temperature. The reaction mixture was filtered off and evaporated in vacuo. The residual material was chromatographed on silica gel column with ethyl acetate/CH2Cl2 (25:75 v/v) to yield 35 (390 mg, 85%). Mp: 95‒96 °C; Rf = 0.40 (ss D); [α]D25 + 51 (c 1 in CHCl3). Found: C, 71.37; H, 8.02. C27H37NO5 (455.60) requires: C, 71.18; H, 8.19%.1H NMR (δ, ppm, CDCl3): 1.12 (s, 3H, 18-H3), 1. 22 (m, 4H, 2x H2 of cyclohexyl), 1. 56 (m, 4H, 2x H2 of cyclohexyl), 3.62 (m, 2H, 16-H2), 3.66 (m, 2H, O-CH2), 4.28 (m, 2H, O-CH2), 4.55 (d, 1H, J = 7.5 Hz, 15-H), 6.61 (d, 1H, J = 3.0 Hz, 4-H), 6.71 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.10 (d, 1H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 17.9 (C-18), 24.6, 25.4, 25.9, 25.9, 26.1, 30.1, 33.3, 33.7, 35.4, 44.1 (O-CH2), 44.8, 48.9 (C-13), 50.4, 54.0, 55.0, 64.2, 68.4 (O-CH2), 70.5, 75.6 (C-15), 112.7 (C-2), 115.5 (C-4), 126.8 (C-1), 133.3 (C-10), 138.6 (C-5), 157.5 (C-3), 220.0 (C-17).
3-Benzyloxy-15β-(2’-t-butylcarbamoyloxy)ethoxy-estra-1,3,5(10)-trien-17-one (36)
Compound 10 (420 mg, 1 mmol) was used for the synthesis as described in general procedure. The reagent was t-butyl isocyanate (4 mmol). The crude product was chromatographed with ethyl acetate/CH2Cl2 (2.5:97.5 v/v) to yield 36 (265 mg, 51%). Mp: 206‒207 °C; Rf = 0.45 (ss B); [α]D25 +8 (c 1 in CHCl3). Found: C, 73.82; H, 8.15. C32H41NO5 (519.67) requires: C, 73.95; H, 7.95%.1H NMR (δ, ppm, CDCl3): %) 1H NMR (δ, ppm, CDCl3): 1.15 (s, 3H, 18-H3), 1.42 (s, 9H, t-Bu), 3.62 (m, 2H, O-CH2), 3.81 (m, 2H, O-CH2), 4.21 (t, 1 H, J = 7.0 Hz, 15-H), 5.12 (s, 2H, Bn-H2), 6.62 (d, 1H, J = 3.5 Hz, 4-H), 6.80 (dd, 1H, J = 11.0 Hz, J = 3.5 Hz, 2-H), 7.19 (d, 1H, J = 11.0 Hz, 1-H), 7.40 (m, 5 H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 13 C NMR (δ, ppm, CDCl3): 18.2 (C-18), 26.0, 26.5, 29.3 (3 x CH3 of t-Bu), 29.6, 33.0, 35.1, 41.9, 43.9 (O-CH2), 44.7, 48.0 (C-13), 55.0, 56.7 (quaterner C of t-Bu), 64.9 (O-CH2), 67.1 (O-CH2), 70.3 (Bn-CH2), 75.3 (C-15), 112.8 (C-2), 115.2 (C-4), 124.4 (C-1), 126.8 (C-2 and C-6 of 3-Bn), 128.1 (C-4 of 3-Bn), 129.5 (C-3 and C-5 of 3-Bn), 133.1 (C-10), 138.0 (C-1 of 3-Bn), 133.5 (C-5), 158.1 (C-3), 220.0 (C-17).
3-Benzyloxy-15β-(2’-phenylcarbamoyloxy)ethoxy-estra-1,3,5(10)-trien-17-one (37)
Compound 10 (420 mg, 1 mmol) was used for the synthesis as described in general procedure. The reagent was phenyl isocyanate (4 mmol). The crude product was chromatographed with ethyl acetate/CH2Cl2 (25:75 v/v) to yield 37 (310 mg, 57%). Mp: 73‒76 °C (foam); Rf = 0.65 (ss B); [α]D25 + 35 (c 1 in CHCl3). Found: C, 75.83; H, 7.12. C34H37NO5 (539.66) requires: C, 75.67; H, 6.91% 1H NMR (δ, ppm, CDCl3): 1.16 (s, 3H, 18-H3), 3.60 (m, 1H, O-CH2), 3.73 (m, 1H, O-CH2), 4.21 (t, 1 H, J = 6.5 Hz, 15-H), 4.30 (m, 2H, O-CH2), 5.02 (s, 2H, Bn-H2), 6.64 (s, 1H, NH), 6.67 (d, 1H, J = 3.0 Hz, 4-H), 6.77 (dd, 1H, J = 10.5 Hz, J = 3.0 Hz, 2-H), 7.06 (t, 1H, J = 9.0 Hz, 4H of N-Ph), 7.17 (d, 1H, J = 10.5 Hz, 1-H), 7.34 (m, 9 H, 5x CH of Bn and 4x CH of N-Ph). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 26.2, 26.7, 29.9, 33.2, 35.2, 41.8, 43.7 (O-CH2), 44.6, 47.8 (C-13), 55.0, 64.7 (O-CH2), 68.1 (O-CH2), 70.4 (Bn-CH2), 75.5 (C-15), 112.8 (C-2), 115.4 (C-4), 121.1 (C-4” of N-Ph), 124.1 (C-1), 126.6 (C-2’ and C-6’), 127.9 (C-4’), 128.3 (C-2 and C-6 of 3Bn), 129.0 (C-3 and C-5 of 3Bn), 129.5 (C-3 and C-5 of N-Ph), 132.9 (C-10), 137.7 (C-1 of 3-Bn), 132.8 (C-5), 154.8 (C-1), 157.3 (C-3), 220.0 (C-17).
3-Benzyloxy-15β-(2’-4’’-chlorophenylcarbamoyloxy)ethoxy-estra-1,3,5(10)-trien-17-one (38)
Compound 10 (420 mg, 1 mmol) was used for the synthesis as described in general procedure. The reagent was 4-chlorophenyl isocyanate (4 mmol). The crude product was chromatographed with ethyl acetate/CH2Cl2 (25:75 v/v) to yield 38 (380 mg, 66%). Mp: 103‒106 °C; Rf = 0.62 (ss B); [α]d25 + 32 (c 1 in CHCl3) . Found: C, 71.38; H, 6.55. C34H35ClNO5 (574.11) requires: C, 71.13; H, 6.32%. 1H NMR (δ, ppm, CDCl3): 1.18 (s, 3H, 18-H3), 3.60 (m, 1H, O-CH2), 3.76 (m, 1H, O-CH2), 4.23 (t, 1 H, J = 7.0 Hz, 15-H), 4.33 (m, 2H, O-CH2), 5.06 (s, 2H, Bn-H2), 6.70 (s, 2H, 4-H, NH), 6.81 (dd, 1H, J = 11.0 Hz, J = 3.0 Hz, 2-H), 7.21 (d, 1H, J = 11.0 Hz, 1-H), 7.37 (m, 9H, 5x CH of 3-Bn and 6’-H, 4x CH of N-Ph). 13C NMR (δ, ppm, CDCl3): 18.0 (C-18), 26.2, 26.7, 27.5, 29.9, 33.2, 35.2, 42.2, 43.7 (O-CH2), 44.6, 47.8 (C-13), 55.0, 62.4, 64.8, 68.1 (O-CH2), 70.4 (Bn-CH2), 75.6 (C-15), 112.8 (C-2), 115.3 (C-4), 118.5 (C-4 of 3-Bn), 126.6 (C-1), 127.9 (C-2 and C-6 of 3-Bn), 128.3 (C-2 and C-6 of N-Ph), 129.0 (C-3 and C-5 of 3-Bn), 129.3 (C-3 and C-5 of N-Ph),129.5 (C-4 of N-Ph), 157.3 (C-3), 160.1 (C = O), 219.9 (C-17).
3-Benzyloxy-15β-(3’-butylcarbamoyloxy)propoxy-estra-1,3,5(10)-trien-17-one (39)
Compound 11 (434 mg, 1 mmol) was used for the synthesis as described in general procedure. The reagent was n-butyl isocyanate (4 mmol). The crude product was chromatographed with ethyl acetate/CH2Cl2 (2.5:97.5 v/v) to yield 39 (460 mg, 86%). Mp: 101‒103 °C; Rf = 0.40 (ss B); [α]D25 + 58 (c 1 in CHCl3). Found: C, 74. 43; H, 8.39. C33H43NO5 (533.70) requires: C, 74.27; H, 8.12%). 1H NMR (δ, ppm, CDCl3): 0.88 (t, 3H, J = 6.5 Hz, (CH2)3-H3), 11.1 (s, 3H, 18-H3, 2.88 (m, 2H, 6-H2), 3.12 (d, 1H, J = 8.0 Hz, NH-CH2), 3.29 (m, 1H, O-CH2), 3.55 (m, 1H, O-CH2), 4.09 (m, 3H, O-CH2, 15-H), 4.57 (brs, 1H, NH), 4.99 (s, 2H, Bn-H2), 6.70 (d, 1H, J = 3.0 Hz, 4-H), 6.74 (dd, 1H, J = 11.0 Hz, J = 3.0 Hz, 2-H), 7.15 (d, 1H, J = 11.0 Hz, 1-H), 7.33 (m, 5H, 5x CH of Bn). 13C NMR (δ, ppm, CDCl3): 13.6 (CH2)3-CH3), 17.4 (C-18), 19.7, 25.6, 26.2, 29.4, 29.6, 31.9, 32.6, 34.7, 40.6, 43.0, 44.1, 47.1 (C-13), 54.4, 61.7 (O-CH2), 65.7 (O-CH2), 69.8 (Bn-CH2), 74.6 (C-15), 112.2 (C-2), 114.8 (C-4), 126.0 (C-1), 127.3 (C-2 and C-6 of Bn), 127.7 (C-4 of Bn), 128.4 (C-3 and C-5 of Bn), 132.4 (C-10), 137.2 (C-1 of Bn), 137.7 (C-5), 156.4 (C = O), 156.8 (C-3), 219.5 (C-17).
3-Hydroxy-15β-(3’-n-butylcarbamoyloxy)propoxy-estra-1,3,5(10)-trien-17-one (40)
Compound 39 (533 mg, 1 mmol) was dissolved in ethyl acetate (30 ml) and the solution containing Pd/C (300 mg, 10%) was hydrogenated under 5 bar for 6 h, at room temperature. The reaction mixture was filtered off and evaporated in vacuo. The residual material was chromatographed on silica gel column with ethyl acetate/CH2Cl2 (25:75 v/v) to yield 40 (290 mg, 65%). Mp: 65‒70 °C (foam); Rf = 0.42 (ss D); [α]D25 + 63 (c 1 in CHCl3). Found: C, 70.27; H, 8.63. C26H37NO5 (443.58) requires: C, 70.40; H, 8.41%. 1H NMR (δ, ppm, CDCl3): 0.91 (s, 3H, J = 6.5 Hz, NH-(CH2)2-H3), 1.15 (s, 3H, 18-H3), 3.13 (m, 2H, 6-H2), 3.20 (t, 2H, J = 6.5 Hz, N-H2) , 3.33 (t 2H, J = 6.0 Hz, linker H2) 4.75 (s, 1H, 15-H), 6.17 (s, 1H, 4-H), 6.64 (dd, 1H, J = 10.5 Hz, J = 3.5 Hz, 2-H), 7.11 (d, 1H, J = 10.5 Hz, 1-H). 13C NMR (δ, ppm, CDCl3): 11.6 (NH-(CH2)2-CH3), 13.9, 18.0 (C-18), 23.6, 26.2, 26.7, 29.8, 30.1, 33.1, 35.3, 43.2 (C-13), 43.6 (linker CH2), 44.6, 47.8 (linker CH2), 55.0, 62.5 (linker CH2), 66.2 (linker CH2), 75.2 (C-15), 113.3 (C-2), 115.8 (C-4), 126.6 (C-1), 132.1 (C-10), 138.4 (C-5), 154.5 (C-3), 220.8 (C-17).
Measurement of inhibition of 17β-HSD1
Our previously published methods were used for the measurement of 17β-HSD1 inhibition21,22. In brief, human placental cytosol was incubated as enzyme source with 1 μM [3H]-labelled estrone substrate at 37 °C. The cofactor, either NADH or NADPH, was used in an excess concentration of 100 μM. The buffer medium consisted of 0.1 M HEPES (pH = 7.3), 1 mM EDTA, and 1 mM dithiotreitol. The substrate was added to the incubate in 10 μl of a 25 v/v% propylene glycol in HEPES buffer solution, whereas test compounds were applied in 10 μl of dimethyl sulfoxide solution.
After an incubation time of 2.5 min, the enzymatic reaction was stopped and the product 17β-estradiol was isolated by TLC. Radioactivity of the 17β-estradiol (2) formed was measured by means of liquid scintillation counting. Test compounds were usually applied in 10 μM concentration, whereas concentrations of 0.1–50 µM were used during determination of IC50 values. The inhibitor effect was assessed with relative conversion results calculated in comparison to non-inhibited controls (100%). IC50 results were calculated by using unweighted iterative least squares logistic curve fitting by means of the “absolute IC50 calculation” function of the GraphPad Prism 4.0 (GraphPad Software, Inc., San Diego, CA, USA). The IC50 of unlabelled estrone (1) was measured as reference. The relative inhibitory potential (RIP) values of the test compounds were calculated by using reference IC50 data measured with the corresponding cofactor: RIP = IC50 of test compound/IC50 of unlabelled estrone (1).
Results and discussion
Synthetic studies
To prepare novel substituted 15β-alkoxy steroids, 3-methoxy-estra-1,3,5(10),15-tetraen-17-one (6), and 3-benzyloxy-estra-1,3,5(10),15-tetraen-17-one (7) were chosen as starting compounds12,23. The synthetic strategy for the preparation of the different type of compounds is illustrated in Scheme 2.
Scheme 2.
Reagents and conditions: (i) and (ii)12,23; (iii) CH2Cl2, hydroxyl alcohol, NaOH; (iv) acetone, Jones reagent; (v) CH2Cl2, hydroxyl alkylnitrile, NaOH; (vi) CH2Cl2, TEA, hydroxyl alkyl-, or arylisocyanate; (vii) THF, diethylether, CH2N2; (viii) CH2Cl2, oxalyl chloride, amines.
Treatment of Δ15-17-one compound 6 with aqueous potassium hydroxyde in methanol afforded 3,15β-dimethoxy-estra-1,3,5(10)-trien-17-one via 1,4 addition in practically quantitative yield. On the basis of an earlier observation from W. S. Johnson and W. F. Johns23, we extended this 1,4-addition process to 1,2-ethanediol, 1,3-propanediol and 1,4-butanediol to receive from compound 6 the corresponding 3-methoxy-15β-(2’-hydroxy)ethoxy-, and 3-methoxy-15β-(3’-hydroxy)propoxy-estra-1,3,5(10)-triene-17-ones (8 and 9), from compound 7 the 3-benzyloxy-15β-(2’-hydroxy)ethoxy-, 3-benzyloxy-15β-(3’-hydroxy)propoxy-, and 3-benzyloxy-15β-(4’-hydroxy)butoxy-estra-1,3,5(10)-trien-17-ones (10‒12). The addition of different nucleophiles is highly stereospecific, giving 15β substituted estranes in all cases12,23. Jones oxidation of these compounds (8‒12) furnished the corresponding 3-methoxy-, and 3-benzyloxy-15β-(carboxyl)-alkoxy-estra-1,3,5(10)-trien-17-one derivatives (13‒17). The 1,4-addition process of compounds 6 and 7 with 3-hydroxypropionitrile afforded the corresponding 3-methoxy-, and 3-benzyloxy-15β-(2’-cyano)ethoxy-estra-1,3,5(10)-trien-17-ones (18, 19). Cleavage of the 3-benzyloxy group of 19 yielded 20, which reacted with sulfamoyl chloride to yield 21. Esterification of 15β-(carboxyl)alkoxy derivatives by diazomethane yielded the corresponding methyl esters 22–24. The 15β-(carboxyl)alkoxy compounds were reacted with oxalyl chloride to give carboxylic acid chloride which, upon reaction with ammonium hydroxide, morpholine or N-cyclohexyl, N-methylamine yielded the corresponding carboxamides 25‒33. The 3-benzyloxy-15β-(2’-hydroxy)ethoxy- and 3-benzyloxy-15β-(3’-hydroxy)propoxy-estra-1,3,5(10)-triene-17-ones (10 and 11) reacted with different alkyl- and aryl isocyanates to furnish alkyl- and aryl urethane derivatives 34‒40.
Inhibitory potentials of the C15 estrone derivatives towards 17β-HSD1
Ligand-based approach and the role of reference compounds
Experimental testing and biochemical analysis of the inhibition of novel compounds provide a feasible way for the development of inhibitors against 17β-HSD1. This ligand-based approach may also give valuable information on the molecular basis of substrate binding and catalytic mechanisms24.
In our present experiments, thirty substituted 15β-alkoxy estrone derivatives possessing hydroxy, methoxy, benzyloxy and sulfamate groups in position C3 were investigated. In vitro conversion of estrone (1) to 17β-estradiol (2) was measured and cofactors, either NADPH or NADH were present in excess amounts in the incubations. Inhibitory effects were evaluated first by relative conversion measured at 10 μM test concentration. For more potent inhibitors IC50 values were determined. Inhibitory potentials and binding affinities were evaluated in comparison to those of natural substrate estrone (1) and the parent unsubstituted core molecules of the inhibitors, including 3-methoxy- and 3-benzyloxy-estrone (3 and 4) and estrone-3-sulfamate (5) (EMATE). Comparative evaluation of inhibitory potentials measured with different cofactors was performed on the basis of RIP parameters which were calculated in comparison to substrate estrone (Table 1).
Table 1.
Reference parameters of in vitro 17β-HSD1 inhibition of the unsubstituted compounds.
 |
IC50: concentration which decreases the enzyme activity to 50%.
Relative conversions (Rel. conv., control incubation with no inhibition is 100%) measured in the presence of 10 μM concentration of the compound tested. Mean ± SD, n = 3.
RIP: relative inhibitory potential compared to estrone; SD: standard deviation.
17β-HSD1 inhibition results of the test compounds
Results obtained with NADPH
In the NADPH supplemented incubations compounds 18, 20, and 40 were found to be the most potent inhibitors (Table 2). Their IC50 values are below 1 µM (0.42–0.64 μM), indicating inhibitory effects similar to those of the unsubstituted parent compounds estrone (1) and 3-methyl-O-estrone (3) (0.63 and 0.77 μM, respectively). Compounds 11, 21, 22, and 25 proved to be effective inhibitors displaying IC50 close to 1 µM (0.78–1.5 μM). Three compounds of this group (11, 22, 25) are derivatives of 3-benzyl-O-estrone, and they exert substantially stronger inhibition in comparison to the unsubstituted core itself. The IC50 value was found to be 2.7 µM for another 3-benzyloxy compound (9), and that shows a somewhat weaker effect, but still an improved inhibition when compared to the parent molecule (4). IC50 values of 23, 24, and 26 were found also in the micromolar range (2.2–5.1 µM). These results, however, indicate decreased potentials compared to the unsubstituted estrone (1) and 3-methyl-O-estrone (3) cores. Relative conversions measured for the 10 µM test concentration of the other compounds in the presence of NADPH were higher than 50%. These results mean IC50 values higher than 10 µM and reveal a weak inhibitory effect against the 17β-HSD1.
Table 2.
In vitro 17β-HSD1 inhibition of the C15 derivatized test compounds.
 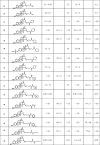
|
IC50: concentration which decreases the enzyme activity to 50%.
Relative conversions (Rel. conv., control incubation with no inhibition is 100%) measured in the presence of 10 μM concentration of the compound tested. Mean ± SD, n = 3.
RIP: relative inhibitory potential compared to estrone; SD: standard deviation.
Results obtained with NADH
In experiments performed with NADH in excess, an outstanding inhibitory potential was measured for 3-hydroxy compound (35) (IC50 = 0.38 µM). Two other 3-hydroxy compounds (20 and 40) were found to be somewhat less potent, and their IC50 values (3.2 and 1.4 µM, respectively) reflect similar effects as the unsubstituted estrone. The 3-benzyloxy compound (17) and the 3-sulfamate compound (21) also displayed medium strengths with IC50 values 3.5 µM and 4.0 µM, respectively. The result of 17 indicates an improved inhibition compared to the basic molecule 3-benzyl-O-estrone (4). Compounds 24, 28, and 33 displayed moderate inhibitions with IC50 values between 5.6 and 7.0 µM. Other compounds exerted weak inhibition when NADH was applied as cofactor. In these cases, relative conversions were not suppressed below 50% at the 10 µM test concentration (IC50>10 µM).
Results with NADPH versus NADH
Results measured with NADPH or NADH showed different tendencies for some of the test compounds when the inhibitory effects of the C15 derivatives were compared to those of their unsubstituted core compounds. Using the RIP values, derivative 35 exerted similar effect in the presence of NADPH than estrone (1) (RIP = 0.66 and 1.0, respectively), but this compound had a strong 5-fold increase in inhibitory potential, when NADH was applied (RIP = 0.19). Derivative 18 also displayed a maintained effect with NADPH (RIP = 0.89), but showed diminished potential with NADH (RIP = 10) in comparison to its unsubstituted core 3-methyl-O-estrone (3). The latter displayed RIP values 1.2 with NADPH and 2.1 with NADH). C15 substituents increased the inhibitory potentials in the case of 3-benzyloxy compounds 11, 22, and 25 when NADPH was applied (RIP = 1.2–2.4), but maintained effects were measured for these derivatives in the presence of NADH (RIP > 5.0). Compound 17 had an opposite behavior, that is, it showed improved inhibition with NADH (RIP = 1.8) compared to the unsubstituted core 3-benzyl-O-estrone (4) ((RIP (NADPH) > 15 and RIP (NADH) > 5)). On the other hand, 20 and 40 showed retained potentials with both cofactors compared to their unsubstituted core estrone (1) with RIP parameters close to 1 (0.70–1.6). For some other compounds derivatized at C15, however, decreased inhibitory effects could be observed when either NADPH or NADH was applied.
Biomedical evaluation of the inhibitory potentials obtained with NADPH
Literature background
Estrane-based inhibitors, as C15-substituted derivatives are assumed to occupy the substrate binding site of 17β-HSD1. Side chains are capable of establishing further contacts to the enzyme than the substrate molecule itself and, in this way, they may modulate binding affinity and inhibitory potential this way5,7,9,25,26. Messinger et al. analysed the X-ray structure of the 17β-HSD127 and identified a hole in the proximity of the enzyme’s active site, which is composed of flexible amino acids Ser222, Leu219 and Met193 as well as Tyr218, Leu96, and Gly19812. The hole shows its opening towards the environment of C15 of the steroidal backbone, and thought to be able to accommodate side chains with appropriate length, spacer unit, and capping group. This finding inspired the Messinger group to synthesize numerous C15-substituted estrone derivatives as presumed 17β-HSD1 inhibitors and they have identified several compounds with high potential, displaying IC50 values in the low nanomolar range12,13.
Further studies also established that 17β-HSD1 accomplished complex processes in ligand binding sites11,28. That is, the enzyme protein could change its conformation depending on the inhibitor molecule offered12. These mechanisms indicate that a very small change in inhibitor structure can make large differences in the course of binding to the enzyme12. Inhibitor studies, therefore, may give ambiguous picture with regard to the binding properties of the compounds and complete structure–activity relations could be revealed sometimes scarcely9,11,12.
17β-HSD1 can use either NADPH or NADH as hydride donor for the estrone (1) to 17β-estradiol (2) transformation. NADPH, in vivo, seems to be the prevailing cofactor in the process29,30. It is, therefore, reasonable to make biomedical evaluation of inhibitor candidates according to their potentials exerted in NADPH supplemented medium22.
Discussion of test compounds
Among the test compounds, we investigated four 15β-(2’-cyano)ethoxy derivatives. Three of them, the 3-methoxy-, 3-hydroxy-, and 3-sulfamate compounds (18, 20, and 21, respectively) proved to be potent inhibitors. These results indicate that 15β-(2’-cyano)ethoxy substituent can be a beneficial side chain concerning 17β-HSD1 inhibitory effect of estrone derivatives possessing different functionalities in their C3 position.
Four 3-benzyloxyestrone derivatives exerted substantial inhibitory effects against 17β-HSD1. Two compounds with 15β-(3’-hydroxy)propoxy-, and 15β-(4’-hydroxy)butoxy side chain (11 and 12 respectively), as well as the 15β-(1’-methoxycarbonyl)methoxy derivative 22 and 15β-(2’-carbonylamido)ethoxy derivative 25 were found to be potent inhibitors. Potentials observed for these compounds are interesting, since very few effective 3-benzyl-O-estrone derivatives have been published in the literature9,13,21.
Some of the compounds tested display structural similarities to C15 derivatized estrone-based compounds published earlier as 17β-HSD1 inhibitor candidates12,13 and interesting conclusions can be arrived at by comparison our inhibitors with their counterparts. In previous studies 15β-propanolyl and 15β-pentanolyl substituents were found to be beneficial substituents on estrone concerning 17β-HSD1 inhibition12,13. In our case, the corresponding 15β-(3’-hydroxy)propoxy- and 15β-(4’hydroxy)butoxy-substituted 3-benzyl-O-estrone 11 and 12 also displayed considerable inhibitions. We found that the 3-methyl-O-estrone compound bearing a C15 substituent with a cyclohexyl capping group 33 was a poor inhibitor. In Messingers’ experiments, however, its non-oxa analogue exerted potent inhibition12,13. A related cyclohexyl derivative of estrone 35, on the other hand, showed also a strong inhibitory effect in our tests. The Messinger group found efficient 17β-HSD1 inhibitors of estrone and 3-methyl-O-estrone compounds that bore 15β-substituents containing a morpholino capping group and long chain with 3–5 methylene units12,13. In comparison, 15β-oxy-coupled morpholino compounds in our tests displayed only weak inhibitions. The comparison of oxa-coupled derivatives with earlier non-oxa analogues indicates the importance of the C15 linker unit in the 17β-HSD1 inhibitory effect of estrone derivatives. Benchmarking of recent results against earlier data is not easy, since different studies may be performed with different methodologies and reference inhibition parameters may be missing in previous studies. Our best inhibitors, nevertheless, may be estimated to be equipotent with some of related earlier C15 derivatives of estrone compounds and non-oxa analogues studied by Messinger et al12,13.
Our investigations reveal that C15 substituents of the tested estrogen derivatives have decisive influence in the binding to 17β-HSD1. These results further indicate that remote fragments on position C3 also determine affinity of the investigated inhibitor molecules. C15 substituents of the test compounds show high variety in their chemical nature. Compounds found to be potent inhibitors also possess diverse side chains and it seems difficult to identify chain length, capping groups or spacer units which can be definitely beneficial in 17β-HSD1 binding. C15 substituents of the compounds can be, however, regarded as side chains both long and flexible. Considering these common features, we may assume that binding may be promoted by accommodation of these side chains in the binding hole of 17β-HSD1 that exists in proximity of C15 positions of ring D as described by Messinger et al12.
Our experiments identified several potent 17β-HSD1 inhibitors among 15β-derivatized estrone-based compounds tested. The results demonstrate definitive influence of C15 substituents as well as crucial role of different functionalities in position C3. Structural diversity of the test compounds makes difficult to give complete structure–activity relationship conclusions, nevertheless, several interesting observations can be established from the inhibition data set.
Cofactor dependence of the 17β-HSD1 inhibition, a comparative evaluation
Literature background
Both cofactor molecules, NADPH and NADH, bind to 17β-HSD1 in an extended conformation, with the nicotinamide moiety pointing towards the active site of the enzyme. Nicotinamide is relatively flexible in the complex and the major interactions between cofactor and enzyme occur at the adenine dinucleotide phosphate part30. Most of these interactions are common for both NADPH and NADH25,27,31. A major difference between the two cofactors is, however, that the 2′-phosphate group of NADPH is stabilized mainly through hydrogen bonds with residues Ser11 and Arg3727,31, whereas the free hydroxyl groups on adenosine ribose of non-phosphorylated cofactor NADH may form hydrogen bonds with Ser11, but not with Arg3731. When a cofactor binds to 17β-HSD1, structural changes are induced in the area of the substrate binding site as well. An otherwise disordered loop, which are composed of residues 189–200, may adopt a specific conformation to accommodate more space for the cofactor in the active centre25,27. Structural differences between the holo form and the apo form of the enzyme may modify interactions of ligands bound in the substrate binding site. For instance, side chain of a potent inhibitor 3-[3',17'β-dihydroxyestra-1',3',5'(10')-trien-16'β-methyl]-3-benzamide occupies a different position in the ternary inhibitor complex, which is different from that in the binary complex25. Differences in binding of substrate or inhibitor ligands may also occur when different cofactors are complexed. The NADPH-bound holoenzyme exerts higher affinity to the substrate estrone than the NADH-bound complex29,30. In an earlier study we observed highly different inhibitory potential and binding affinity of various D-secoestrones depending on which cofactor (either NADPH or NADH) was applied in the in vitro experiments22.
Discussion of test compounds, NADPH versus NADH
In our experiments, substantial inhibitory potentials and increased binding affinities were observed in presence of both cofactors. However, some of the compounds exerted different inhibitory potentials towards 17β-HSD1 complexed to NADPH- or NADH-complexed 17β-HSD1. Substrate estrone displays different IC50 values depending on the cofactor applied and RIP parameters reflect more reliably the cofactor-dependent differences in the inhibitor binding.
RIP parameters demonstrate 3–8 fold stronger binding with NADH than with NADPH for estrone derivatized with a 15β-(1’-morpholinocarbonyl)methoxy- side chain (28), for 3-methoxy-estrone and estrone derivatized with 15β-(1’-N-methyl,cyclohexylamino carbonyl)methoxy- and 15β-(2’-cyclohexylcarbamoyloxy)ethoxy chains (33 and 35), as well for 3-benzyl-O-estrone derivative possessing a 15β-(3’-carboxylic)propoxy substituent (17). Binding of 3-benzyl-O-estrone compounds substituted with a 15β-(3’-hydroxy)propoxy, 15β-(1’-methoxycarbonyl)methoxy, or a 15β-(2’-aminocarbonyl)ethoxy side chain (11, 22, and 25) and 3-methyl-O-estrone bearing a 15β-(2’-cyano)methoxy side chain (18) display similar (2–11 fold) cofactor preference in terms of RIP values, but these derivatives favour binding to the NADPH bound enzyme.
Cofactor-dependent affinities of some of the investigated compounds indicate that binding capabilities of the binding hole may be different depending on the nature of cofactor the enzyme complexed with. The binding hole suitable to accommodate C15 side chains is known to be formed by amino acids Leu96, Met193, Gly198, Tyr218, Leu219, and Ser22212. Residues Met193 and Gly198 are also constituents of the disordered loop (amino acids 189–200) which adopt a specific conformation following cofactor binding25,27. The presence or absence of 2′-phosphate in the cofactors causes differences in the structure of the holoenzymes. Furthermore, the area of the substrate binding site may also be affected in a different way in these complexes25,27,31. We may also assume that binding of NADPH or NADH modifies the conformation of the loop of residues 189–200 differently. Joint residues Met193 and Gly198 of the two structural elements transmit this difference from the loop to the binding hole, and these processes induce different positioning and binding capabilities of the binding hole in the holoenzyme variants. Hosting certain C15 side chains, therefore, might be, therefore, favoured or unfavoured in the binding hole altered differently upon binding of phosphorylated or unphosphorylated cofactors.
In our earlier investigation, potent 17β-HSD1 inhibitory effects of ring D modified seco-oxime and seco-alcohol estrones were identified22. In that case we assumed that polar functionalities of short side chains of compounds studied might establish hydrophilic interactions or hydrogen bonds towards suitable amino acids of the enzyme present in close proximity of ring D region. Binding affinity of certain compounds in that series also displayed strong cofactor dependence and this phenomenon indicated that complexation with NADPH or NADH furnished different conformations to enzyme residues involved in the interactions.
In our present study we identified another group of compounds, C15 derivatized estrones, which may exert cofactor-dependent inhibition towards the 17β-HSD1. Structural features and binding mechanisms of side chains in the region of ring D differ to a great extent for those compounds investigated in our earlier report and for those presented in this study. Cofactor dependence of inhibitor binding, however, could be observed in both series. These results suggest that binding of the phosphorylated or the unphosphorylated cofactor may exert different influence on more areas and structural elements of the substrate binding site.
Early studies assigned NADH as a catalytic cofactor of 17β-HSD1 and numerous in vitro inhibition tests have been performed with this recognition. (See corresponding references in our earlier work22.) Later, however, it became accepted that NADPH might be the prevalent partner of 17β-HSD1 in its main in vivo function in the catalysis of the estrone–17β-estradiol conversion22,29,30. Our present results obtained with C15 estrone derivatives support that in vitro potentials obtained with the two cofactors may differ substantially for certain inhibitor compounds. Data measured in the presence of NADPH are more relevant in inhibitor optimization and in lead selection, but NADH results could be valuable in understanding of the mechanism the inhibition of 17β-HSD1.
Conclusions
17β-HSD1 inhibitory potential of 15β-oxa-coupled estrone derivatives possessing hydroxy, methoxy, benzyloxy and sulfamate functionalities in position C3 has been investigated. Thirty inhibitor candidates were tested via in vitro radioincubations. We found several potent 17β-HSD1 inhibitors and the results demonstrated that potent inhibitory effect could be achieved with both various C15 substituents, and different C3 functional groups. We identified four 3- benzyloxyestrone derivatives (11, 22, 25), which exerted substantial inhibitory effect. We also found that 15β-(2’-cyano)ethoxy was a beneficial substituent of compounds bearing different functionalities in position C3 (18, 19, 20, and 21). A comparison with earlier non-oxa analogues indicates that beyond the effect of capping groups and spacer units, there might be a strong influence of the presence or absence of oxygen in the 15β linker on the inhibitory potential.
Some of the compounds displayed considerable difference in binding affinities towards 17β-HSD1 complexed with NADPH or NADH. It is reasonable to assume that side chains of the potent compounds can be accommodated in the binding hole of 17β-HSD1 existing in proximity of the C15 position of ring D of the steroidal ligands12. This binding hole shares Met193 and Gly198 with a loop element which is known to adopt a specific conformation upon cofactor binding25,27. We suppose that conformation of this loop may be different in NADPH- or NADH-complexed 17β-HSD1. Structural differences can be forwarded by the joint amino acids inducing different positioning and binding capabilities of the binding hole. Further structural investigations (e.g., molecular docking studies) may confirm mechanisms involved in binding of our inhibitor compounds and different binding affinities of the test compounds exerted towards the holoenzyme variants.
Our investigations provide valuable data on binding processes of the enzyme and may contribute to the development of new 17β-HSD1 inhibitors, as novel drug molecules acting on enzyme level.
Funding Statement
The work of Anita Kiss was supported by a Ph.D. Fellowship of the Talentum Fund of Richter Gedeon Plc. Financial support by the National Research, Development and Innovation Office-NKFIH through Project [GINOP-2.3.2–15-2016–00038] is gratefully acknowledged. This research was supported by the Hungarian Scientific Research Fund [OTKA K113150, OTKA SNN 124329].
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Martel C, Rhéaume E, Takahashi M, et al. Distribution of 17β-hydroxysteroid dehydrogenase gene expression and activity in rat and human tissues. J Steroid Biochem Mol Biol 1992;41:597–603. [DOI] [PubMed] [Google Scholar]
- 2.Sasano H, Frost AR, Saitoh R, et al. Aromatase and 17β-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab 1996;81:4042–6. [DOI] [PubMed] [Google Scholar]
- 3.Gunnarsson C, Jerevall PL, Hammar K, et al. Amplification of HSD17B1 has prognostic significance in postmenopausal breast cancer. Breast Cancer Res Treat 2008;108:35–41. [DOI] [PubMed] [Google Scholar]
- 4.Lin SX, Chen J, Mazumdar M, et al. Molecular therapy of breast cancer: progress and future directions. Nat Rev Endocrinol 2010;6:485–93. [DOI] [PubMed] [Google Scholar]
- 5.Marchais-Oberwinkler S, Henn C, Möller G, et al. 17β-Hydroxysteroid dehydrogenases (17β-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development. J Steroid Biochem Mol Biol 2011;125:66–82. [DOI] [PubMed] [Google Scholar]
- 6.Penning TM. 17β-Hydroxysteroid dehydrogenase: inhibitors and inhibitor design. Endocr Relat Cancer 1996;3:41–56. [Google Scholar]
- 7.(a) Poirier D. Inhibitors of 17β-hydroxysteroid dehydrogenases. Curr Med Chem. 2003;10:453–77. (b) Poirier D. 17β-Hydroxysteroid dehydrogenase inhibitors: a patent review. Expert Opin Ther Pat. 2010;20: 1123–45.12570693 [Google Scholar]
- 8.Day JM, Tutill HJ, Purohit A, Reed MJ. Design and validation of specific inhibitors of 17β-hydroxysteroid dehydrogenases for therapeutic application in breast and prostate cancer, and in endometriosis. Endocr Relat Cancer 2008;15:665–92. [DOI] [PubMed] [Google Scholar]
- 9.Brozic P, Lanisnik Risner T, Gobec S. Inhibitors of 17β-hydroxysteroid dehydrogenase type 1. Curr Med Chem. 2008;15:137–50. [DOI] [PubMed] [Google Scholar]
- 10.Lin SX, Poirier D, Adamski J. A challenge for medicinal chemistry by the 17β-hydroxysteroid dehydrogenase superfamily: an integrated biological function and inhibition study. Curr Top Med Chem 2013;13:1164–71. [DOI] [PubMed] [Google Scholar]
- 11.Salah M, Abdelsamie AS, Frotscher M. Inhibitors of 17β-hydroxysteroid dehydrogenase type 1, 2 and 14: structures, biological activities and future challenges. Mol Cell Endocrinol 2019;489:66–81. [DOI] [PubMed] [Google Scholar]
- 12.Messinger J, Husen B, Koskimies P, et al. Estrone C15 derivatives-a new class of 17β-hydroxysteroid dehydrogenase type 1 inhibitors. Mol Cell Endocrinol 2009;301:216–24. [DOI] [PubMed] [Google Scholar]
- 13.(a) Messinger J, Thole H-H, Husen B, et al. Novel 17β-hydroxysteroid dehydrogenase type I inhibitors. 2005;Patent WO05047303.; (b) Messinger J, Thole H-H, Husen B, et al. 17β-HSD1 and STS inhibitors. 2006; Patent WO2006/125800.; (c) Messinger J, Husen B, Schoen U, et al. Substituted estratrien derivatives as 17β HSD inhibitors. 2008; Patent WO2008/065100.; (d) Messinger J, Schoen U, Husen B, et al. Therapeutically active triazoles and their use. 2008; Patent WO2008/034796.; (e) Messinger J, Schoen U, Thole H-H, et al. Therapeutically active triazoles and their use. 2008; Patent US 20080146531.; (f) Hirvelae L, Kangas L, Koskimies P, et al. Therapeutically active estratrienthiazole derivatives as inhibitors of 17β-hydroxysteroid dehydrogenase, type 1. 2014; Patent WO 2014207311 A1.
- 14.(a) Lawrence HR, Vicker N, Allan GM, et al. Novel and potent 17β-hydroxysteroid dehydrogenase type 1 inhibitors. J Med Chem 2005;48:2759–62. [DOI] [PubMed] [Google Scholar]; (b) Allan GM, Bubert C, Vicker N, et al. Novel, potent inhibitors of 17beta-hydroxysteroid dehydrogenase type 1 . Mol Cell Endocrinol 2006;248:204–7. [DOI] [PubMed] [Google Scholar]
- 15.(a) Moeller G, Adamski J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol 2009;301:7–19. [DOI] [PubMed] [Google Scholar]; (b) Aka JA, Mazumdar M, Chen CQ, et al. 17beta-hydroxysteroid dehydrogenase type 1, stimulates breast cancer by dihydrotestosterone inactivation in addition to estradiol production. Mol Endocrinol. 2010;24:832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang CY, Chen J, Yin DC, Lin SX. The contribution of 17β-hydroxysteroid dehydrogenase type 1 to the estradiol-estrone ratio in estrogen-sensitive breast cancer cells. PLoS One 2012;7:e29835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poirier D, Mérand Y, Labrie C, Labrie F. D-ring alkylamine derivatives of estradiol: effect on er-binding affinity and antiestrogenic activity. Bioorg Med Chem Lett 1996;6:2537–42. [Google Scholar]
- 17.(a) Langer LJ, Alexander JA, Engel LL. Human placental estradiol-17 beta dehydrogenase. II. Kinetics and substrate specificities. J Biol Chem 1959;234:2609–14. [PubMed] [Google Scholar]; (b) Sweet F, Boyd J, Medina O, et al. Hydrogen bonding in steroidogenesis: studies on new heterocyclic analogs of estrone that inhibit human estradiol 17β-dehydrogenase. Biochem Biophys Res Commun 1991;180:1057–63. [DOI] [PubMed] [Google Scholar]
- 18.Kanamarlapudi NR, Warren JC. Synthesis and study of the estrogenic activity of 2,4-bis(bromomethyl)estradiol-17 beta 3-methyl ether. J Biol Chem 1975;250:6484–7. [PubMed] [Google Scholar]
- 19.Salah M, Abdelsamie AS, Frotscher M. First dual inhibitors of steroid sulfatase (STS) and 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1): designed multiple ligands as novel potential therapeutics for estrogen-dependent diseases. J Med Chem 2017;60:4086–92. [DOI] [PubMed] [Google Scholar]
- 20.Vicker N, Lawrence HR, Allan GM, et al. Focused libraries of 16‐substituted estrone derivatives and modified E‐ring steroids: inhibitors of 17ß‐hydroxysteroid dehydrogenase type 1. Chem Med Chem 2006;1:464–81. [DOI] [PubMed] [Google Scholar]
- 21.Szabó J, Bacsa I, Wölfling J, et al. Synthesis and in vitro pharmacological evaluation of N-[(1-benzyl-1,2,3-triazol-4-yl)methyl]-carboxamides on d-secoestrone scaffolds. J Enzyme Inhib Med Chem 2016;31:574–9. [DOI] [PubMed] [Google Scholar]
- 22.Herman BE, Szabó J, Bacsa I, et al. Comparative investigation of the in vitro inhibitory potencies of 13-epimeric estrones and D-secoestrones towards 17β-hydroxysteroid dehydrogenase type 1. J Enzyme Inhib Med Chem 2016;31:61–9. [DOI] [PubMed] [Google Scholar]
- 23.(a) Johnson WS, Johns WF. 14-Isoestrone methyl ether and its identity with totally synthetic material 14-isoestrone methyl ether and its identity with totally synthetic material. J Am Chem Soc 1955;79:2005–9. [Google Scholar]; (b) Cantrall EW, Littell R., Bernstein S.. The synthesis of C-15β-substituted estra-1,3,5(10)-trienes.II. J Org Chem 1964;29:64–8. [Google Scholar]
- 24.Hong Y, Chen S. Aromatase, estrone sulfatase, and 17β-hydroxysteroid dehydrogenase: structure-function studies and inhibitor development. Mol Cell Endocrinol 2011;340:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazumdar M, Fournier D, Zhu DW, et al. Binary and ternary crystal structure analyses of a novel inhibitor with 17β-HSD type 1: a lead compound for breast cancer therapy. Biochem J 2009;424:357–66. [DOI] [PubMed] [Google Scholar]
- 26.Srungboonmee K, Songtawee N, Monnor T, et al. Probing the origins of 17β-hydroxysteroid dehydrogenase type 1 inhibitory activity via QSAR and molecular docking. Eur J Med Chem 2015;96:231–7. [DOI] [PubMed] [Google Scholar]
- 27.Breton R, Housset D, Mazza C, Fontecilla-Camps JC. The structure of a complex of human 17β-hydroxysteroid dehydrogenase with estradiol and NADP + identifies two principal targets for the design of inhibitors. Structure 1996;4:905–15. [DOI] [PubMed] [Google Scholar]
- 28.Thomas MP, Potter BV. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol 2013;137:27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin JZ, Lin SX. Human estrogenic 17beta-hydroxysteroid dehydrogenase: predominance of estrone reduction and its induction by NADPH. Biochem Biophys Res Commun 1999;259:489–93. [DOI] [PubMed] [Google Scholar]
- 30.Huang YW, Pineau I, Chang HJ, et al. Critical residues for specificity toward NADH or NADPH in human estrogenic 17β-hydroxysteroid dehydrogenase: site-directed mutagenesis designed from the three-dimensional structure of the enzyme. Mol Endocrinol 2001;15:2010–20. [DOI] [PubMed] [Google Scholar]
- 31.Mazza C, Breton R, Housset D, Fontecilla-Camps JC. Unusual charge stabilization of NADP + in 17β-hydroxysteroid dehydrogenase. J Biol Chem 1998;273:8145–52. [DOI] [PubMed] [Google Scholar]



