ABSTRACT
Mushrooms are cherished as sources of food, nutrients and medicine. Inadequate data on the identity and medicinal properties of many wild Nigerian mushrooms has limited their utilization. This work was carried out to identify and authenticate a puffball mushroom using molecular tools and investigate its antidiabetic properties. Taxonomic guides were employed in morphological identifying the mushroom as Lycoperdon umbrinum, methanol extract of fruiting bodies was evaluated for antidiabetic activity using in vitro α-amylase assay and in vivo activity in the alloxan-induced diabetic rat model. The macro fungus was identified using Internal Transcribed Spacers (ITS) sequence analysis after which sequences generated were compared using the basic local alignment search tool (BLAST) at NCBI GenBank. In the acute in vivo test, the 400 mg/kg dose showed the best activity with percentage reduction in blood glucose 29.3%, compared with 5 mg/kg glibenclamide at 15%. The in vitro assay established that the extract possessed potent activity with IC50 of 0.46 µg/mL compared to its DCM, butanol fractions and acarbose (IC50 5.3 µg/mL, 5.6 µg/mL, 45 µg/mL) respectively. BLAST analysis revealed the mushroom (accession number, KRO78278.1) to show 98% identity to Calvatia gigantea. The study established the identity of this mushroom and confirmed its antidiabetic activity.
KEYWORDS: Diabetes mellitus, antidiabetic, alpha amylase, cytoxicity, antioxidant, Calvatia gigantea, MTT
Introduction
Various studies have shown that some mushrooms are consumed as food and may have potential to lower elevated blood sugar (Valverde et al. 2015; Zhang et al. 2016). Mushrooms have a long history of having been employed by humans as medicines for thousands of years (Ferreira et al. 2010; Valverde et al. 2015; Zhang et al. 2016). Because they have been demonstrated to affect one or more target functions in the body, leading to either an improved state of health and well-being and/or reduction of risk of disease, they are referred to as functional foods (Guillamon et al., 2010, Wang et al. 2017, Vamanu, 2018). Of the 14,000 to 15,000 species of mushrooms in the world around 700 of them have known medicinal properties (Lurie et al. 2009; Li et al. 2018). Edible mushrooms have higher protein contents and minerals and contain less fat but are rich in vitamins B, D, K and sometimes vitamins A and C (Wang, Zhang et al. 2014, Huang, Cai et al. 2016). Several authors have indicated that edible mushrooms are highly nutritional and compared favourably with meat, egg and milk. Furthermore, chemical analysis shows that more than one third of the iron in the mushrooms is in available form and they are increasingly being recognised as one of the important food items for their significant roles in human health, nutrition and diseases (Kumari, Reddy et al. 2011, Zeng, Suwandi et al. 2012, Teke, Kinge et al. 2018)
Many medicinal mushrooms have been found to be suitable for diabetic and heart patients due to low starch and low cholesterol content. Several mushroom species have been reported to be effective for both the control of blood glucose levels and the modification of the course of diabetic complications (Wasser 2011, 2014; D DE et al. 2012; Wu and Xu 2015; Alam et al. 2016; Friedman 2016; Zhang et al. 2016). This is because they are known to contain bioactive components that help with the proper functioning of metabolic organs such as the liver, pancreas and other endocrinal glands, thereby promoting formation of insulin and related hormones which ensure healthy metabolic functioning (Calvo et al. 2016, Amandip Kaur et al. 2015). Mushrooms contain polysaccharides such as beta glucans which can restore the function of pancreatic tissues eventually triggering increased insulin output by β – cells, thus leading to decrease blood glucose levels. Beta glucans have been shown to improve the sensitivity of peripheral tissues to insulin (Rondanelli et al. 2009; Rop et al. 2009; D DE et al. 2012; Chen et al. 2013; Sari et al. 2017)
In Nigeria puffball mushrooms popularly known as ‘Isu or Iso aparo in Yoruba language are part of culinary delicacies with several of the species of puffball mushrooms consumed locally (Oso 1977). Puffball are known especially for their nutritive component and a lot of them have also been reported for biological activities including blood glucose lowering activities (Kivrak et al. 2014; Lee et al. 2017, 2018; Ye et al. 2017). However, accurate identification of these local mushrooms is lacking. The need for unambiguous identification of mushrooms is essential if they are to be maximally utilised as food alternatives and as sources of antidiabetic molecules. Traditional methods of identification of mushrooms based on morphology have been wholly inadequate. Thus, newer DNA sequence methods of identification have offered better discrimination of mushroom species (Jo et al. 2014; Lallawmsanga et al. 2016; Sugawara et al. 2016). Molecular markers, especially DNA markers have been found to be quick and reliable in establishing the identities of mushroom collected from the wild and are helpful in taxonomy (Jo et al. 2014; Khaund and Joshi 2014, 2016; Gawlikowski et al. 2015; Karun and Sridhar 2017). Consequently, this work was carried out to identify and authenticate a puffball mushroom collected in Ibadan, Nigeria, using molecular tools and in addition, to investigating its antidiabetic properties.
Materials and methods
Collection of mushroom
The puff ball mushroom was collected from the University of Ibadan botanical garden. The mushroom fruit body was dried and maintained in a desiccator until needed for use. The morphological identification was carried out by mycologist from the Department of Botany and Microbiology, University of Ibadan, where voucher specimens were deposited.
Extraction of DNA from mushroom sample
Total genomic DNA was extracted from collected mushroom using a plant/fungi DNA isolation kit (NORGEN BIOTEK CORP) according to manufacturer’s instructions. Briefly, the purified genomic DNA from DNA extraction was stored in buffer at – 20°C until required.
PCR amplification of the ITS region
The entire region of the rDNA of the mushroom sample denoted LUB approximately 670 bp was amplified by PCR using primers ITS 1 (TCCGTAGGTGAACCTGCGG) and ITS 4 (TCCTCCGCTTATTGATATGC). The reaction mix was made up of a total volume of 25 μl, composed of 23 μl of Taq polymerase “Ready to Go” mixture (Pharmacia, Sweden) with 0.2 μl of each primer (100 pM) and 2 μl of DNA template solution. The following thermocycling conditions was used for PCR reaction on a GenAmp PCR System 2400, Perkin-Elmer, USA: 30 cycles of denaturation at 95°C for 30 s; annealing at 50°C for 1 min; and extension at 72°C for 1 min. The amplification products were gel purified and electrophoresed on ethidium-stained agarose gel (0.7%). DNA sequencing was performed using the same primer pair as used previously (ITS 1and ITS 4) in an Applied Biosystem DNA Analyzer (USA).
Sequence alignment
Alignments were performed using Clustal W (Thompson et al., 1997). The aligned sequences were corrected manually. DNA sequence data were analysed to provide pairwise percentage sequence divergence. The data obtained from the sequence alignment were used to plot a tree diagram using Molecular Evolutionary Genetics Analysis (MEGA 4 Software). The bootstraps varied from 75 to 100. The Neighbor Joining (NJ) method was used (Tamura et al. 2013)
Preparation of mushroom extract
The powdered mushroom (2.5 kg) was macerated exhaustively in absolute methanol with periodic stirring at room temperature for 72 h. The mixture was filtered using muslin filter attached to a suction pump. The filtrate was concentrated in vacuo at 40°C and stored in the refrigerator at 4°C until needed for analysis.
Cytotoxicity assays
Brine shrimp assay
This assay was carried out using method McLaughlin (1998). Eggs of Artemia salina (brine shrimp) were obtained from the Department of Pharmacognosy, University of Ibadan. They were hatched in natural sea water obtained from Suntan Beach, Badagry, Lagos, Nigeria and incubated for 48 h in 3.8 g/L sea water. 3 mg of extract was made up to 1 mg/mL in sea water and diluted in ten-fold serial dilutions ending with five concentrations. A suspension of nauplii containing 10–15 shrimp (100 µL) was added to each well, covered and incubated at room temperature (25–29°C) for 24 h. After 24 h, plates were examined under binocular stereomicroscope and the number of dead (non-motile) nauplli in each well were counted. This experiment was done in triplicate. Analysis of data was performed using GraphPad prism to determine the lethal concentration.
MTT colorimeter assay
The MTT colorimetric assay was used to evaluate the reduction of viability of cell cultures in the presence and absence of the extracts. The ability of the mushroom extract to be cytotoxic was measured using the tetrazolium dye (MTT), which is metabolized by mitochondrial enzymes of viable (surviving) cells to an insoluble, colored formazan product. The level of metabolism that occurs in the individual well of the 96-well microtitre plate is dependent on the number of healthy viable cells present (Mosmann 1983).
In vivo animal study
For animal experiments male Wistar albino rats weighing 150–200 g were obtained commercially and maintained in the animal house at the Physiology Department University of Ibadan. Animals were fed with standard pellet and water given ad libitum and acclimatised for three weeks before used for experiments. This study was conducted according to the principles of the Declaration of Helsinki.
Diabetes mellitus was induced intraperitoneally in the experimental rats by the administration of diabetogenic dose of alloxan monohydrate (120 mg/kg body weight). The animals were kept under observation and after 72 h blood glucose was measured by using a One-touch glucometer. The diabetic rats (glucose level > 200 mg/dl) were separated and divided into six different groups of six animals per group.
Experimental design
Rats were divided into six groups comprising of six animals per group.
Group A: diabetic induced rats treated with 100 mg/kg extract
Group B: diabetic induced rats treated with standard drug glibenclamide
Group C: diabetic induced rats treated with 200 mg/kg of extract
Group D: diabetic induced rats treated with 400 mg/kg of extract
Group E: diabetic induced untreated rats
Group F: normalglyceamic rats administered water and food ad libitum)
Alpha amylase inhibitory assay
The α-amylase inhibitory activities of crude and partitioned fraction were determined based on the modified method of using colorimetric assay (Bernfeld 1955; Aiyer 2005) and acarbose was the reference compound.
Statistical analysis
The results were expressed as mean ± standard error of the mean (SEM). The statistical significance of the differences was analysed by one-way ANOVA. P < 0.05 was considered significant.
Results and discussion
The rise in the prevalence of diabetes mellitus and its associated health cost has increased interest in alternative therapies (MEZQUITA RAYA and REYES GARCIA 2014; Coda et al. 2018). Mushrooms have long been associated with humans and provide profound biological and economic benefits. Historical records have provided information on the consumption of wild mushrooms by man for their taste and pleasing flavour (Patel and Goyal 2012; Valverde et al. 2015; Ullah et al. 2017). Morphology and physiological parameter such as appearance, colour, dimension, spores and form of the fungus on media, with other environmental growth preferences is not sufficient for the identification of macrofungi. However, molecular based tools offer better alternatives for accurate identification (Akram et al. 2012; Al-Habib et al. 2014; Wang et al. 2017) of wild medicinal and edible mushrooms, which will help in proper documentation and effective exploration. We have used the Internal transcribed spacer (ITS) sequence for the molecular identification of Calvatia gigantea. BLAST analysis result from GenBank showed that the ITS sequence of the mushroom sample (LUB) matched Calvatia gigantea with accession number KRO78278.1 (Figure 1). This contradicts the earlier morphological identification as a Lycoperdon species. This observation may be attributable to both Calvatia and Lycoperdon species belonging to the puffball family hence the possibility of substituting one for the another when morphology is sole means of identification.
Figure 1.
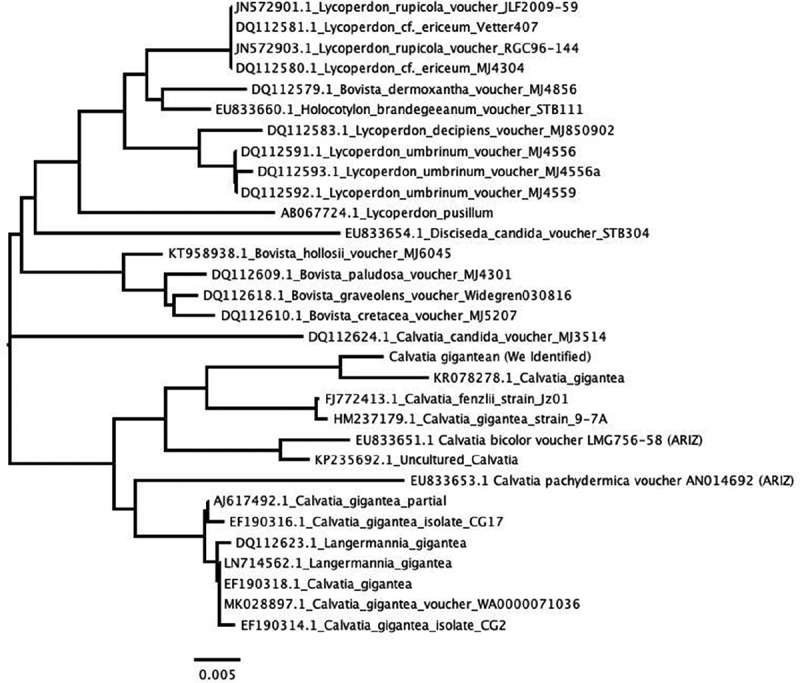
Phylogenetic analysis based on rDNA sequence of Calvatia gigantea obtained in this study and reference sequences from GenBank.
The Brine shrimp lethality assay is considered as a rapid preliminary assay for screening for the presence of biochemical activity of natural products and was used to determine the toxicity of the crude extract (Meyer et al 1982). This test is based on the lethality of the crude extract against Artemia salina nauplli based on the classification of cytoxicity of extract on brine shrimp according to methods established by Meyer and colleagues, C. gigantia was considered not cytotoxic having an LC50 > 1000µg/mL (Table 1). This was further confirmed by an MTT colorimeter assay against Vero cells that shows C. gigantea nontoxic with CC50 of 337.4, as compared to cyclophosphamide with CC50 of 8.71. Most species of the Calvatia genus generally known as giant puffballs are known to be edible, they are used as sources of traditionally nutritional food especially before spore maturation (Eroglu et al. 2016; Lee et al. 2017, 2018), hence their non toxicity in our assays further establish their edibility.
Table 1.
In vitro Brine Shrimp lethality activity and cytotoxic activity Calvatia gigantea extract on Vero Cell Line.
| CC50 µg/ml |
LC50 µg/ml |
|
|---|---|---|
| Test | Vero cell line (MTT assay) | Brine shrimp |
| Calvatia gigantea | >1000 | 1255.45 |
| Cyclophosphamide | 8.7 ± 0.2 | – |
The acute in vivo antidiabetic assay model, designed to assess the effect of the extracts on diabetic rats over a short period of time revealed that the 400 mg/kg of the extract at the 30th minute significantly reduced the blood glucose level from 330 mg/dL to 235 mg/dL that is by 28%. As reported by Priyanka et al., 2010 a 25% reduction in blood glucose levels is considered a significant hypoglycemic effect, this assertion has also been confirmed by other authors (Sathya et al. 2008; Murthy et al. 2013; Xing et al. 2015). Furthermore, at the end of the 90th minute, there was a significant reduction of glucose level for all the doses of extract comparable to the standard glibenclamide (Figure 2). For the chronic model (7 days) in vivo assay, on day 3 there was a reduction in the blood glucose level for all the doses tested but this was more significant for glibenclamide (30%). At the end of the experiment, there was a significant reduction in the glucose level of all the groups, but this was more significant is the 200 mg/kg group (Figure 3).
Figure 2.
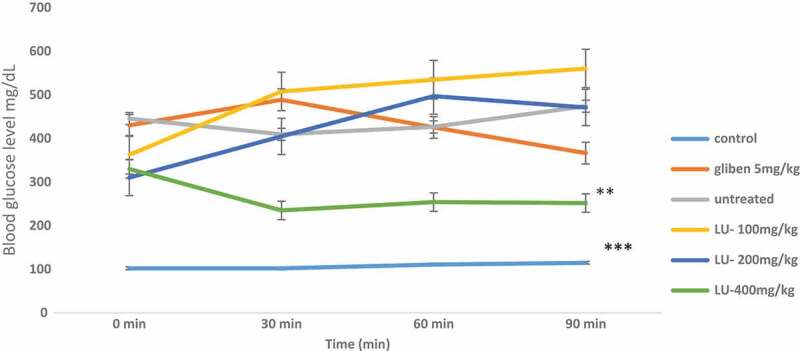
Effect of Calvatia gigantea on alloxan- induced diabetic rats within 90 min.
Key: LU 100mg/kg = Calvatia gigantea extract 100mg/kg; LU 200mg/kg = Calvatia gigantea extract 200mg/kg; LU 300mg/kg = Calvatia gigantea extract 300mg/kg, *** = P < 0.0001, ** = P < 0.05
Figure 3.
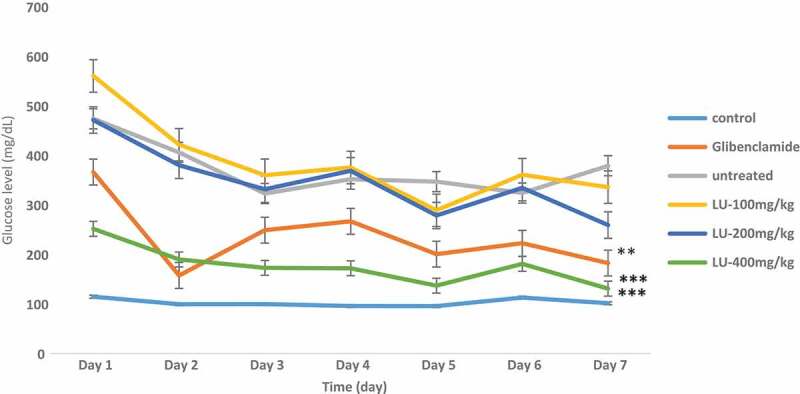
Effect of a Calvatiagiganteacrude extract on fasting glucose level in alloxan – induced diabetic rats from day 1 to day 7.
Key: LU 100mg/kg = Calvatia gigantean extract 100mg/kg; LU 200mg/kg = Calvatia gigantean extract 200mg/kg; LU 300mg/kg = Calvatia gigantean extract 300mg/kg. *** = P < 0.0007, ** = P < 005
The in vitro alpha amylase inhibitory assay indicated that the mushroom was 90 times more active than acarbose the standard drug. C. gigantea could inhibit 50% of the enzyme at 0.46 µg/mL while acarbose inhibited at 45 µg/mL (Figure 4). All the fractions (dichloromethane, ethyl acetate, butanol and aqueous) of C. gigantea possessed better α-amylase inhibitory activity than acarbose (IC50 0f 5.3 µg/mL, 6.5 µg/mL, 5.7 µg/mL and 6.2 µg/mL respectively) except for the n-hexane fraction with an IC50 of 163 µg/mL (Figure 5). These findings suggest that C. gigantea might be a promising source of medicine and has the potential to be a health food and food supplementary product. In addition, the extract and its fractions may be explored for the development of natural product-based pharmaceutics.
Figure 4.
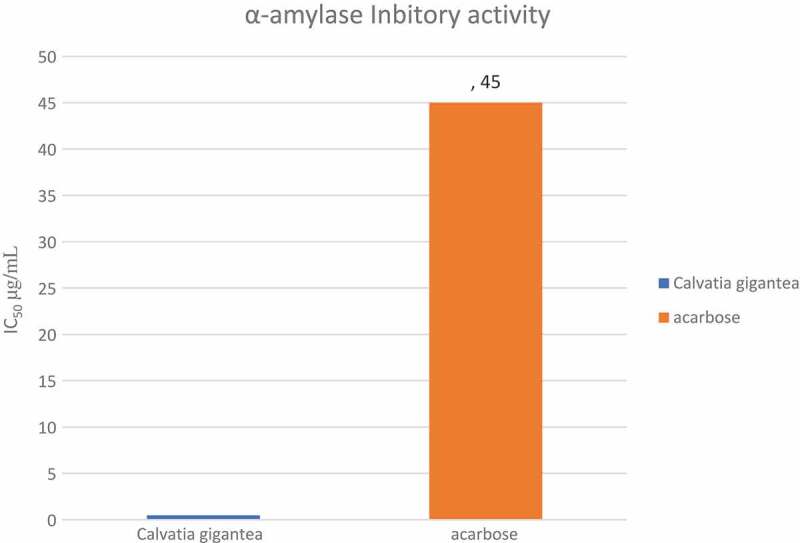
IC50 valuesof crude extract of a Calvatia gigantea and acarbose in α-amylase inhibitory assay.
Figure 5.
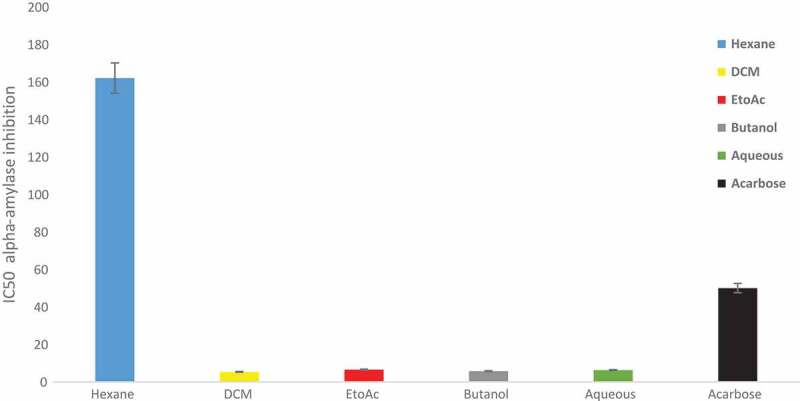
α-amylase inhibitory assay IC50 values of hexane, dichloromethane, ethylacetate, butanol and aqueous fractions of Calvatia gigantea.
Conclusion and recommendation
The in vitro alpha amylase inhibitory assay established that the extract possessed potent antidiabetic activity. However, the observed reduction in antidiabetic activity upon fractionation of the extract demonstrates that the antidiabetic activity may reside in the whole plant and not in the fractions. The significant inhibition of the enzyme α-amylase and the in vivo antidiabetic activity exhibited by the mushroom investigated underscores the fact that the fungi could potentially serve as a functional food, as well as a drug lead.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aiyer PV. 2005. Amylases and their applications. African journal of biotechnology. 4:1525–1529. [Google Scholar]
- Akram K, Ahn JJ, Kwon JH.. 2012. Identification and characterization of gamma-irradiated dried Lentinus edodes using ESR, SEM, and FTIR analyses. J Food Sci. 77:C690–6. [DOI] [PubMed] [Google Scholar]
- Alam F, Islam MA, Kamal MA, Gan SH. 2016. Updates on managing type 2 diabetes mellitus with natural products: towards antidiabetic drug development. Curr Med Chem. 25:5395–5431. [DOI] [PubMed] [Google Scholar]
- Al-Habib MN, Holliday JC, Tura D. 2014. The pale brittle stem mushroom, Psathyrella candolleana (higher Basidiomycetes): an indigenous medicinal mushroom new to Iraq. Int J Med Mushrooms. 16:617–622. [DOI] [PubMed] [Google Scholar]
- Bernfeld P. 1955. α-and β-amylases. J Methods in Enzymol. 1:149–158. [Google Scholar]
- Calvo MS, Mehrotra A, Beelman RB, Nadkarni G, Wang L, Cai W, Goh BC, Kalaras MD, Uribarri J. 2016. A retrospective study in adults with metabolic syndrome: diabetic risk factor response to daily consumption of agaricus bisporus (White Button Mushrooms). Plant Foods Hum Nutr. 71:245–251. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang XD, Jiang Z. 2013. The application of fungal beta-glucans for the treatment of colon cancer. Anticancer Agents Med Chem. 13:725–730. [DOI] [PubMed] [Google Scholar]
- Coda A, Sculley D, Santos D, Girones X, Acharya S. 2018. Exploring the effectiveness of smart technologies in the management of type 2 diabetes mellitus. J Diabetes Sci Technol. 12:199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva, DD, Rapior S, Hyde KD, Bahkali AH. 2012. Medicinal mushrooms in prevention and control of diabetes mellitus. J Fungal Diversity, 56:1–29. [Google Scholar]
- Eroglu C, Secme M, Atmaca P, Kaygusuz O, Gezer K, Bagci G, Dodurga Y. 2016. Extract of Calvatia gigantea inhibits proliferation of A549 human lung cancer cells. Cytotechnology. 68:2075–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira IC, Vaz JA, Vasconcelos MH, Martins A. 2010. Compounds from wild mushrooms with antitumor potential. Anticancer Agents Med Chem. 10:424–436. [DOI] [PubMed] [Google Scholar]
- Friedman M. 2016. Mushroom polysaccharides: chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods. 5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlikowski T, Romek M, Satora L. 2015. Edible mushroom-related poisoning: A study on circumstances of mushroom collection, transport, and storage. Hum Exp Toxicol. 34:718–724. [DOI] [PubMed] [Google Scholar]
- Guillamon E, Garcia-Lafuente A, Lozano M, D'arrigo M, Rostagno M. A, Villares A, Martinez, JA. 2010. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia, 81:715–23. [DOI] [PubMed]
- Huang G, C W, X B. 2016. Vitamin D2, Ergosterol, and Vitamin B2 Content in Commercially Dried Mushrooms Marketed in China and Increased Vitamin D2 Content Following UV-C Irradiation. Int J Vitam Nutr Res, 1–10 [DOI] [PubMed]
- Jo WS, Hossain MA, Park SC. 2014. Toxicological profiles of poisonous, edible, and medicinal mushrooms. Mycobiology. 42:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karun NC, Sridhar KR. 2017. Edible wild mushrooms of the Western Ghats: data on the ethnic knowledge. Data Brief. 14:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur AMANDIP, Dhingra GURPAULSINGH, Shri RICHA. 2015. Antidiabetic potential of mushrooms. Asian J Pharm Clin Res. 5:111–125. [Google Scholar]
- Khaund P, Joshi SR. 2014. DNA barcoding of wild edible mushrooms consumed by the ethnic tribes of India. Gene. 550:123–130. [DOI] [PubMed] [Google Scholar]
- Khaund P, Joshi SR. 2016. Lentinula edodes based GIS mapping, biometabolites and antiinflamatory activity of wild edible mushrooms from tropical ‘sacred grove‘ forests of Meghalaya, India. Rev Biol Trop. 64:247–257. [DOI] [PubMed] [Google Scholar]
- Kivrak I, Kivrak S, Harmandar M. 2014. Free amino acid profiling in the giant puffball mushroom (Calvatia gigantea) using UPLC-MS/MS. Food Chem. 158:88–92. [DOI] [PubMed] [Google Scholar]
- Kumari D, Reddy MS, Upadhyay, RC. 2011. Nutritional composition and antioxidant activities of 18 different wild Cantharellus mushrooms of northwestern Himalayas. Food Sci Technol Int, 17:557–67. [DOI] [PubMed]
- Lallawmsanga, Passari AK, Mishra VK, Leo VV, Singh BP, VALLIAMMAI MEYYAPPAN G, Gupta VK, Uthandi S, Upadhyay RC. 2016. Antimicrobial potential, identification and phylogenetic affiliation of wild mushrooms from two sub-tropical semi-evergreen indian forest ecosystems. PLoS One. 11:e0166368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee D, Lee JC, Kang KS, Ryoo R, Park HJ, Kim KH. 2018. Bioactivity-guided isolation of anti-inflammatory constituents of the rare mushroom calvatia nipponica in lps-stimulated RAW264.7 Macrophages. Chem Biodivers. 15:e1800203. [DOI] [PubMed] [Google Scholar]
- Lee S, Park JY, Lee D, Seok S, Kwon YJ, Jang TS, Kang KS, Kim KH. 2017. Chemical constituents from the rare mushroom Calvatia nipponica inhibit the promotion of angiogenesis in HUVECs. Bioorg Med Chem Lett. 27:4122–4127. [DOI] [PubMed] [Google Scholar]
- Li H, Wu S, Ma X, Chen W, Zhang J, Duan S, Gao Y, Kui L, Huang W, Wu P, et al. 2018. The genome sequences of 90 mushrooms. Sci Rep. 8:9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie Y, Wasser SP, Taha M, Shehade H, Nijim J, Hoffmann Y, Basis F, Vardi M, Lavon O, Suaed S, et al. 2009. Mushroom poisoning from species of genus Inocybe (fiber head mushroom): a case series with exact species identification. Clin Toxicol (Phila). 47:562–565. [DOI] [PubMed] [Google Scholar]
- Mclaughlin J. 1998. The use of biologicalassays to evaluate botanicals. Drug Information Journal. 32:513–524. [Google Scholar]
- Meyer, BN, Ferrigni, NR, Putnam, JE, Jacobsen, LB, Nichols, DE & Mclaughlin, JL. 1982. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med, 45, 31–4. [PubMed] [Google Scholar]
- Mezquita Raya P, Reyes Garcia R. 2014. Is treatment with liraglutide efficient?. Endocrinol Nutr. 61:202–208. [DOI] [PubMed] [Google Scholar]
- Mosmann TJJOIM. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. [DOI] [PubMed] [Google Scholar]
- Murthy TE, Kommineni MK, Mayuren C. 2013. Influence of losartan on the hypoglycemic activity of glimepiride in normal and diabetic rats. Ther Adv Endocrinol Metab. 4:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oso B. 1977. Mushrooms in Yoruba mythology and medicinal practices. J Econ Bot. 31:367–371. [Google Scholar]
- Patel S, Goyal A. 2012. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M, Opizzi A, Monteferrario F. 2009. [The biological activity of beta-glucans]. Minerva Med. 100:237–245. [PubMed] [Google Scholar]
- Rop O, Mlcek J, Jurikova T. 2009. Beta-glucans in higher fungi and their health effects. Nutr Rev. 67:624–631. [DOI] [PubMed] [Google Scholar]
- Sari M, Prange A, Lelley JI, Hambitzer R. 2017. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 216:45–51. [DOI] [PubMed] [Google Scholar]
- Sathya S, Kokilavani R, Gurusamy K. 2008. Hypoglycemic effect of Gymnema sylvestre (retz.,) R.Br leaf in normal and alloxan induced diabetic rats. Anc Sci Life. 28:12–14. [PMC free article] [PubMed] [Google Scholar]
- Sugawara R, Yamada S, Tu Z, Sugawara A, Suzuki K, Hoshiba T, Eisaka S, Yamaguchi A. 2016. Rapid and reliable species identification of wild mushrooms by matrix assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). Anal Chim Acta. 934:163–169. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar SJMB & EVOLUTION . 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teke NA, Kinge TR, Bechem E, Nji TM, Ndam LM, Mih AM 2018. Ethnomycological study in the Kilum-Ijim mountain forest, Northwest Region, Cameroon. J Ethnobiol Ethnomed, 14, 25.. [DOI] [PMC free article] [PubMed]
- Thompson JD, Gibson TJ, Plewnaik F, Jeanmougin F, Higgins, DGJNAR. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools; 25:4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah TS, Firdous SS, Mehmood A, Shaheen H, Dar M. 2017. Ethnomycological and nutritional analyses of some wild edible mushrooms from Western Himalayas, Azad Jammu and Kashmir (Pakistan). Int J Med Mushrooms. 19:949–955. [DOI] [PubMed] [Google Scholar]
- Valverde ME, Hernandez-Perez T, Paredes-Lopez O. 2015. Edible mushrooms: improving human health and promoting quality life. Int J Microbiol. 2015:376387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamanu EJCPD. 2018. Complementary functional strategy for modulation of human gutmicrobiota. 24:4144–4149 [DOI] [PubMed] [Google Scholar]
- Wang S, Liu Y, Xu J. 2017. Comparison of Different Drying Methods for recovery of mushroom DNA. Sci Rep. 7:3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Zhang, J, Wu, LH, Zhao, YL, Li, T, Li, JQ, Wang, YZ, Liu, HG. 2014. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. FoodChem, 151:279–85 [DOI] [PubMed]
- Wasser SP. 2011. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl Microbiol Biotechnol. 89:1323–1332. [DOI] [PubMed] [Google Scholar]
- Wasser SP. 2014. Medicinal mushroom science: current perspectives, advances, evidences, and challenges. Biomed J. 37:345–356. [DOI] [PubMed] [Google Scholar]
- Wu T, Xu B. 2015. Antidiabetic and antioxidant activities of eight medicinal mushroom species from China. Int J Med Mushrooms. 17:129–140. [DOI] [PubMed] [Google Scholar]
- Xing R, He X, Liu S, Yu H, Qin Y, Chen X, Li K, Li R, Li P. 2015. Antidiabetic activity of differently regioselective chitosan sulfates in alloxan-induced diabetic rats. Mar Drugs. 13:3072–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Liu K, Zeng Q, Zeng Q. 2017. Antimicrobial activity of puffball (Bovistella radicata) and separation of bioactive compounds. AMB Express. 7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Suwandi J, Fuller J, Doronila A, Ng, K. 2012. Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci Technol Int, 18:367–79 [DOI] [PubMed]
- Zhang JJ, Li Y, Zhou T, Xu DP, Zhang P, Li S, Li HB. 2016. Bioactivities and health benefits of mushrooms mainly from China. Molecules. 21, 938. [DOI] [PMC free article] [PubMed] [Google Scholar]


