Abstract
Breast cancer therapy involves a multidisciplinary approach com-prising surgery, radiotherapy, neoadjuvant and adjuvant therapy. Effective therapy of breast cancer requires maximum therapeutic efficacy, with mini-mal undesirable effects to ensure a good quality of life for patients. The carefully selected combination of therapeutic interventions provides patients with the opportunity to derive maximum benefit from therapy while minimiz-ing or eliminating recurrence, resistance and toxic effects, as well as ensuring that patients have a good quality of life. This review discusses therapeutic op-tions for breast cancer treatments and various combinations that had been previously exploited. The review will also give an insight into the potential application of the nanotechnology platform for co-delivery of therapeutics in breast cancer therapy.
Keywords: Breast cancer, chemotherapy, combination therapy, early breast cancer, metastatic breast cancer, nanotechnology, radiotherapy, surgery
1. INTRODUCTION
Breast cancer (BC) is the most commonly occurring cancer in women and represents the leading cause of death associated with cancer among females globally [1, 2]. Like most cancers, BC is a heterogeneous disease with different molecular subtypes. The major subclasses identified by genetic profiling are: Basal-like, Luminal-A, Luminal-B, human epidermal growth factor 2 (HER2)-positive/HER2-enriched/HER2-overexpressing BC and normal-like tumors [3-7] (Fig. 1). However, there would be subpopulations of tumors within the main subtypes expressing molecular features of another subtype. This molecular classification of BC closely resembles the clinical classifications of BC which are based on proliferation markers, histological grade, estrogen and progesterone receptors expression (ER and PgR respectively), as well as the overexpression of HER2 [8]. The Luminal (A and B) subtypes are typically hormone receptor positive and form the majority (90-95%) of hormone receptor (HR) positive (ER or PgR receptor positive), HER2-negative tumors [4, 7]. Molecular subtype of BC may impact prognosis and may influence decision making in the therapeutic management of BC [4, 9, 10]. The prognostic importance of the intrinsic subtypes was evaluated in a large group (1730) of patients from the UK and Canada who had received different adjuvant treatments except for trastuzumab.
Fig. (1).
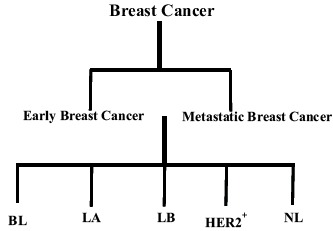
Classification of breast cancer according to molecular subclasses.
Key: BL = Basal-like; LA = Luminal-A; LB = Luminal-B; HER2+ = Human epidermal growth factor 2 (HER2)-positive/HER2-enriched/HER2-overexpressing BC; NL = Normal-like tumors.
The results obtained showed that intrinsic subtype of BC is an independent prognostic variable, irrespective of tumor size and nodal status. Moreover, HER2-positive/Luminal A tumors showed a similar outcome to HER2-negative/Luminal A tumors [11]. Amongst the molecular subtypes of BC, HER2-positive and basal-like subtypes are associated with aggressive disease and poor outcomes [6, 7, 12] and Luminal B tumors show remarkably worse prognosis than Luminal A tumors, which have frequently shown better outcomes than the other subtypes [4, 6]. Also, in spite of Basal-like disease showing worse outcome than Luminal B tumors at 5-year follow-up, 10-year follow-up does not reflect this, as survival curves for Basal-like disease and Luminal B disease cross at 10-year follow-up [4]. Regarding therapeutic decisions, the molecular subtype of BC would influence decisions such as the choice of adjuvant therapy to be employed. Thus, patients with HR-positive BC would be expected to derive the most benefit from adjuvant endocrine therapy. Moreover, endocrine therapy reduced the risk of death in patients with HR-positive BC in contrast to patients with ER or PgR receptor negative BC [13]. Similarly, the advent of immunotherapy with biotherapeutic agents such as the monoclonal antibody, trastuzumab, has resulted in profoundly increased survival with HER2-positive BC, which is otherwise associated with poor prognosis [14]. Basal-like BC on the other hand, which is negative for the HRs (ER or PgR receptors) and HER2 does not present a therapeutic target and so would not be expected to derive benefit from endocrine or molecularly targeted therapy [6, 7].
This review will discuss the use of combination therapy in the clinical management of breast cancer and give an insight into the potential application of the nanotechnology platform for co-delivery of therapeutics in breast cancer therapy. However, it is not intended to be an exhaustive review on the subject.
2. BREAST CANCER THERAPY
Therapy of breast cancer involves a multimodal strategy with a combination of neoadjuvant chemotherapy, surgery of operable tumors, radiotherapy and adjuvant chemotherapy and/ or endocrine therapy [15, 16] (Fig. 2). In locally advanced and inoperable BC, the conventional therapeutic approach is the use of neoadjuvant therapy. Systemic neoadjuvant therapy may help in shrinking tumors and make otherwise inoperable tumors operable [17, 18].
Fig. (2).
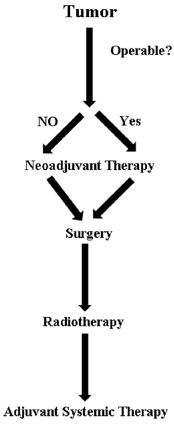
Schematic representation of treatment strategies for breast cancer.
Currently, neoadjuvant chemotherapy is extensively used in early-stage breast cancer (EBC) and locally advanced BC as it helps to provide greater chances for breast-conserving surgery (BCS) by downstaging the disease and to determine tumor response to therapy [19, 20]. BC surgery is achieved primarily by lumpectomy followed by whole-breast irradiation or mastectomy [15]. Adjuvant therapy for BC may involve local irradiation, systemic therapy with cytotoxic agents, molecular targeted agents or any combination of these [15, 16, 21, 22].
In EBC where the disease is detectable only in the breast and local lymph nodes (in women with lymph node-positive disease), cancer can be removed by surgery [23, 24]. The disease is potentially curable in such cases and when it is only locally advanced, with no detectable distant metastasis [1].
BCS is recognized as a suitable treatment option for many women with EBC and can eliminate all detected macroscopic disease. BCS compares favorably to total mastectomy regarding overall survival and local recurrence rates, while allowing patients to keep their breasts without excessive disfiguration [25, 26]. BCS is usually followed by whole breast irradiation which may or may not involve further (boost) treatment to the tumor bed [27]. Data from a meta-analysis of individual patient data (over 10,000 women) in 17 randomized trials carried out by the Early Breast Cancer Trialists’ Collaborative Group (EBCTG) have shown that the addition of radiotherapy to BCS improved both long term overall survival and local regional control, reducing local recurrence [23, 26]. Although whole breast irradiation is considered to be relatively well tolerated [27-29], it has been linked to some inevitable acute and delayed toxic effects [28, 29]. Accelerated partial breast irradiation (APBI) has been introduced as an alternative to whole breast irradiation in certain patients with favorable EBC [27]. APBI could reduce treatment duration and exposure of normal tissues by delivering hypofractionated doses of radiation to restricted areas.
Moreover, experimental data have shown that most recurrences are located near the lumpectomy bed [30]. However, a sizeable proportion of EBC cases would eventually relapse [15] as undetected deposits of disease may remain which over time may result into the development of clinically fatal disease [15, 23, 31]. Thus, the understanding of EBC as a systemic disease has constituted a major milestone in the advancement of breast cancer research, while also playing a major role in current therapy decision making. Therefore, adjuvant systemic chemotherapy is now a mainstay in the treatment of EBC. Systemic adjuvant treatment is directed mainly at controlling any remaining deposits of disease, reducing recurrence rates, and improving long term survival in BC patients [31]. Advances in chemotherapy involve the introduction and use of new cytotoxic agents, novel treatment strategies and neoadjuvant therapy which have been shown to improve overall treatment outcomes such as overall survival and disease-free survival [26, 32].
In metastatic breast cancer (MBC), the role of surgery for removal of primary tumor is traditionally seen as that of palliation rather than for survival benefits [33]. Systemic chemotherapy with cytotoxic agents or endocrine therapy had been the core treatment strategy for metastatic breast cancer for several decades [34] and it remains a crucial component of treatment regimens [35]. Chemotherapeutic agents are appropriate for most patients with MBC including those with hormone receptor-positive (HR+) disease with extensive visceral involvement, HR+ disease after failure of hormone-directed therapy, HER2-positive disease (systemic chemotherapeutic agents used in combination with HER2-directed therapy [trastuzumab ± pertuzumab]), and HR-negative (HR-)/HER2-negative disease (triple-negative disease) [35]. Nevertheless, there is no generally accepted first-line chemotherapy protocol or approach for the treatment of MBC [36]. Unlike EBC, metastatic breast cancer is considered largely incurable with currently available therapies [1, 37], although there are a few long-term survivors who continue in complete remission after initial treatment [38, 39].
3. THERAPEUTIC COMBINATIONS IN BREAST CANCER
The practice of combining therapeutics dates back to many centuries [40, 41] with traditional Chinese medicine practice of using a combination of herbs in their formulations [40, 42]. Combination chemotherapy (or polychemotherapy) for BC treatment traditionally involved a combination of an alkylating agent (cyclophosphamide) and antimetabolites (methotrexate and 5-fluorouracil) which significantly reduced the risk of recurrence [43]. It is generally accepted that polychemotherapy results in higher response rates than single-agent chemotherapy, although the impact on overall survival is less well documented [44].
The use of combination chemotherapy potentially provides advantages such as chances for better efficacy and dose reduction while increasing or maintaining efficacy, decreased toxicity and reduced or delayed development of drug resistance [40, 41]. Due to these advantages, combination chemotherapy has now become the conventionally applied strategy in clinical practice [45]. More recently, advances in the areas of isolation technology and chemical synthetic capability, as well as omics and cell biology, have also played an important role in increasing the application of drug combinations in modern medical practice [40, 46].
Chemotherapy in BC may involve the use of initial neoadjuvant chemotherapy [47-49] before the initiation of adjuvant chemotherapy subsequent to appropriate surgical therapy and/or radiotherapy [50].
Neoadjuvant chemotherapy can be applied using a combination modality. In phase II neoadjuvant trial, a combination of docetaxel and epirubicin was evaluated for activity and toxicity in women with large, operable or locally advanced (Stage III) breast carcinoma, as well as patients with inflammatory breast carcinoma. The results of the trial showed an observed response rate of 76.7%. More than 25% of the patients experienced clinically significant diarrhoea and 80% experienced grade 4 neutropenia with one-third experiencing febrile neutropenia. The subsequent use of prophylactic hemopoietic growth factor support allowed patients to complete the planned treatment. The investigators concluded that neoadjuvant epirubicin plus docetaxel was active and feasible for patients with breast carcinoma including patients with unfavorable disease presentations such as locally advanced breast carcinoma and inflammatory breast carcinoma [16].
The effects of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide were reported in the National Surgical Adjuvant Breast and Bowel Project Protocol B-27. The study reported that the addition of four cycles of preoperative docetaxel after four cycles of preoperative doxorubicin and cyclophosphamide resulted in a significant increase in clinical and pathologic response rates for operable breast cancer [51]. Also, Earl and co-investigators in the Neo-tAnGo (Phase 3 neoadjuvant trial) study assessed the advantage of adding gemcitabine to accelerated paclitaxel with epirubicin and cyclophosphamide. They also evaluated the effect of sequencing the blocks of epirubicin and cyclophosphamide and paclitaxel (with or without gemcitabine). Their findings showed that the addition of gemcitabine to paclitaxel and epirubicin and cyclophosphamide chemotherapy does not improve pathological complete response (pCR). However, sequencing chemotherapy such that taxanes are received prior to anthracyclines could improve pCR in standard BC neoadjuvant chemotherapy [52].
Furthermore, the German Breast Group investigated (according to selected subtypes), the definition and effect of pCR in over 6,000 patients with primary breast cancer who received anthracycline and taxanes based neoadjuvant chemotherapy in seven randomized trials [53].
Buzdar and colleagues (ACOSOG Z1041 trial) showed that the value of a taxane-first sequence was not shown in patients with HER2-positive disease in the neoadjuvant setting. pCR rates obtained in breast or nodes did not differ between the two regimens in the analysis of anthracyclines followed by taxane plus trastuzumab compared with taxanes plus trastuzumab followed by anthracyclines plus trastuzumab [54]. In another phase II neoadjuvant trial involving 57 patients (9 with triple negative BC), the efficacy and toxicity of docetaxel and carboplatin combination as neoadjuvant therapy for stage II or III breast cancer (BC) were assessed. In the multicentre trial, patients received docetaxel (75 mg/m2) and carboplatin (area under the curve (AUC) of 6) intravenously on day 1 and pegfilgrastim (6mg) subcutaneously on day 2. Treatment cycles were repeated every 14 days for a total of 4 cycles (unless there was progression at any time), prior to definitive breast surgery. A high rate of clinical responses was reported for the regimen. The results showed that 9 of the 57 patients (16%) had pCR, and 4 of the 9 (44%) patients with triple negative BC (TNBC) achieved pCR. The toxicity profile was also similar to other anthracycline-regimens with thrombocytopenia being the only grade 4 toxicity reported in 5% of the subjects. The study concluded that 4 cycles of 2-weekly neoadjuvant carboplatin and docetaxel followed by pegfilgrastim is an active regimen for BC resulting in pCR rates similar to other anthracycline-containing regimens, with an acceptable toxicity profile [24].
Therapeutic combinations in adjuvant therapy of BC (Fig. 3) may take the form of combinations comprising only chemotherapeutic agents (that is at least two chemotherapeutic agents) or combinations of chemotherapeutic agents with hormonal therapy or immunotherapy.
Fig. (3).
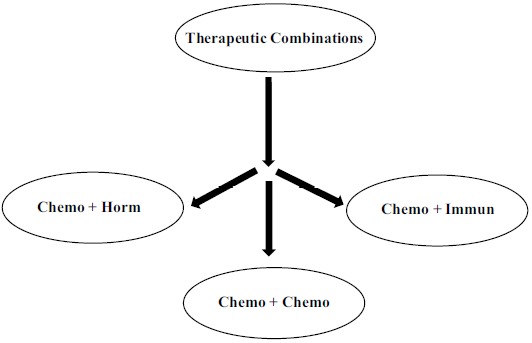
Schematic representation of main therapeutic combinations in BC treatment.
Key: Chemo = chemotherapy; Horm = hormononal therapy; Immun = immunotherapy.
Reports from the Istituto Nazionale Tumori in Milan, Italy, showed improved outcomes (significant reduction in risk of recurrence) with the addition of an alkylating agent (cyclophosphamide) and antimetabolites (methotrexate and 5-fluorouracil) to BC therapy [43]. Subsequently, a National Institute of Health consensus panel in the US in 2001 recommended the use of multiple agents rather than single agents in most women with local breast cancer, irrespective of lymph node, menopausal, or hormone receptor status. This recommendation was made based on the existence of sufficient evidence in favor of polychemotherapy [55].
The first combination adjuvant chemotherapy regimen tested in a prospective clinical trial comprised cyclophosphamide, methotrexate and 5-fuorouracil (5-FU) (CMF regimen). The trial was initiated by the Istituto Nazionale Tumori in Milan, Italy, in 1973. The trial randomized node-positive patients after radical mastectomy to 12 cycles of CMF (cyclophosphamide: 100 mg/m2 orally on days 1-14; methotrexate: 40 mg/m2 IV on days 1 and 8; and 5-fluorouracil: 600 mg/m2 IV on days 1 and 8) administered every 28 days versus no additional treatment. The 342-month follow-up updated report showed an improved disease-free survival (DFS; hazard ratio (HR), 0.71; P = 0.005) and overall survival (OS; HR, 0.79; P = 0.04) for CMF compared with the control (no additional treatment) population [43].
In an overview of randomized trials of adjuvant polychemotherapy among women with EBC published by the EBCTCG in 1998, their analysis showed that the CMF regimen with or without other drugs had been evaluated in as many as 28 trials [56]. Also, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-20 trial found that the addition of the CMF combination to tamoxifen improved 5-year DFS (HR, 0.65; P = 0.001) and OS (HR, 0.64; P = 0.03) for patients with ER-positive, lymph node-negative disease at lower risk for recurrence [57].
Doxorubicin containing chemotherapy combinations have also been tested in clinical trials. An example of the initial trials to assess doxorubicin was the NSABP B-11 trial which evaluated melphalan and 5-fluorouracil with or without doxorubicin in 697 (non-tamoxifen responsive) patients, and found an improved 5-year DFS (HR, 0.65; P = 0.007) as well as a trend for improved OS (HR, 0.74; P = 0.08) for patients that received doxorubicin [58].
Early chemotherapy combinations also included cyclophosphamide. For instance, in the NSABP B-15 trial, patients (2,194) with node-positive disease were randomized to doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) (AC; every 3 weeks for four cycles) given over 12 weeks versus the conventional CMF regimen for six cycles over 24 weeks [59]. Other drug combinations that were employed in the earlier chemotherapy combinations include: 5-FU, adriamycin (doxorubicin) and cyclophosphamide (FAC) [60]; 5-FU 500 mg/m2, epirubicin 50 mg/m2, cyclophosphamide 500 mg/m2 (FEC50) [61]; 5-FU 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2 (FEC100) [62]. Combination regimens involving the use of platinum-based drugs such as cisplatin were also evaluated. Cisplatin has been evaluated in combination with Adriamycin (doxorubicin) and cyclophosphamide [63], 5-FU and etoposide [64], and other agents such as vinblastine [65], epirubicin [66], methotrexate, cyclophosphamide, mitomycin C and vincristine [67].
The taxanes form an important class of cytotoxic agents commonly used in chemotherapy combinations for the treatment of BC. They have emerged as some of the most active chemotherapeutic agents introduced in the treatment of breast cancer in the 1990s [68].
The first of the class to be introduced was paclitaxel which entered clinical trials in the early 1980s [69]. Another member of the class, docetaxel was later developed and introduced due to initial scarcity and difficulty in paclitaxel production [45]. Evaluation of paclitaxel in combination with other cytotoxic agents for MBC treatments began with phase I clinical trials of paclitaxel/doxorubicin combinations with The University of Texas M. D. Anderson Cancer Center and the Medicine Branch of the National Cancer Institute (USA) simultaneously initiating phase trials of this combination [70]. The combination was also assessed in Italy by researchers at the Istituto Nazionale Tumori in Milan, Italy. Their combination was based on a bolus dose of doxorubicin with an infusion of paclitaxel over 3 hours. In the trial, doses up to 200 mg/m2 of doxorubicin and 60 mg/m2 of paclitaxel could be given [71].
Combinations of paclitaxel with cisplatin, an agent with less myelosuppressive effects than many other cytotoxic agents used for the treatment of MBC had been employed in clinical practice. A preliminary study showed an overall response rate of 85% with an 11% complete response rate and there was an acceptable tolerance of the therapy [70]. The paclitaxel/cisplatin combination has also been assessed by other investigators at different dose combinations [72, 73]. Combinations of paclitaxel with other agents such as cyclophosphamide, 5-FU and mitoxantrone have also been used in the treatment of MBC [70].
Docetaxel has been used in combination with agents like doxorubicin and this combination is considered to be very effective in the treatment of locally advanced and MBC [74]. In phase III multicentre study, the docetaxel and doxorubicin combination (AT) was compared with a combination of doxorubicin and cyclophosphamide (AC) as first-line chemotherapy for MBC. The study involved 429 patients randomly assigned to receive one of either the AT or AC regimen. 214 patients received doxorubicin (50 mg/m2) plus docetaxel (75 mg/m2) while 215 patients received doxorubicin (60 mg/m2) plus cyclophosphamide (600 mg/m2) on day 1, every 3 weeks for up to eight cycles. The results showed that although there was no difference in overall survival, the overall response rate (59%, with 10% complete response, 49% partial response) in patients that received AT was significantly greater (P = 0.009) than for those that received AC (47%, with 7% complete response, 39% partial response). The toxicity profile was similar in both groups as Grade 3/4 neutropenia was frequent in both groups. However, febrile neutropenia and infections were more frequent for patients that received AT than for those that received AC (33% v 10%, P < 0.001; 8% v 2%, P = 0.01 respectively). Time to progression (TTP; primary endpoint of the study) and time to treatment failure were found to be significantly longer with AT than AC (median TTP, 37.3 v 31.9 weeks; log-rank P = 0.014; median TTF, 25.6 v 23.7 weeks; log-rank P = 0.048). The investigators concluded from the results that AT significantly improves time to progression and overall response rate compared with AC in patients with MBC. However, there was no difference in OS. Thus, AT represents a valid option for the treatment of MBC [75]. A phase II trial of this combination was conducted by the NSABP (NSABP B - 57 trial) at 12 institutions (89 patients) using doxorubicin (60 mg/m2) plus docetaxel (60 mg/m2), given every 21 days. The result of the trial showed that amongst the evaluable patients, there was an overall response rate of 65.7% with 20.9% complete remission, 44.8% partial response and median response duration of 25.9 months. The investigators inferred that AT is an active combination and the administration is feasible with primary ciprofloxacin and secondary colony-stimulating factor prophylaxis [76].
Also, a combination of docetaxel with epirubicin was assessed in phase I/II program by the International Breast Cancer Study Group. In the program, 70 patients with advanced breast cancer received up to eight courses of docetaxel (75 mg/m2) in combination with epirubicin (90 mg/m2) every 3 weeks. From their study, they did not find it necessary to administer G-CSF to patients routinely, although individual patients may require the support. They also pointed out the need for weekly monitoring of patients and dose modification when necessary, to avoid complications [77]. Another phase I/II nonrandomized trial of epirubicin and docetaxel in locally advanced breast cancer evaluated the combination in 2-weekly or 3-weekly schedules (National Cancer Institute of Canada (NCIC) Clinical Trials Group (CTG) - NCIC CTG MA.22). This trial was conducted to determine optimal dosing regimens for docetaxel/epirubicin combination chemotherapy in women with locally advanced breast cancer. In the study, escalating doses of epirubicin and docetaxel were administered to patients in either a standard 3-weekly (Schedule A - docetaxel: 75 mg/m2 IV and epirubicin: 75, 90, 105, or 120 mg/m2 IV; with pegfilgrastim 6 mg primary prophylaxis subcutaneously per cycle on day 2) or dose-dense 2-weekly (Schedule B - docetaxel and epirubicin both administered at doses of 50, 60, and 70 mg/m2 IV; with pegfilgrastim support) regimen. Phase I was used for dose finding for phase II for each dosing schedule. The investigators concluded from their findings that the epirubicin/docetaxel combination chemotherapy can be administered in locally advanced BC patients with acceptable toxicity. Also, the regimen can induce strong reductions in tumor RNA integrity in some patients and these are associated with posttreatment pCRs [78].
Furthermore, the combination of docetaxel with cisplatin was reported to be effective in patients with anthracycline-resistant MBC. This combination was assessed in a phase II trial in 39 patients with MBC resistant to previous anthracycline treatment. Patients received docetaxel (75 mg/m2) followed by cisplatin (60 mg/m2) every 3 weeks for a maximum of 6 cycles or until disease progression. The investigators reported that all 39 patients in the trial were evaluable for safety while 36 were evaluable for efficacy. They found an objective response rate of 31% with 3 complete responses, the median time to disease progression was 7 months, and the median overall survival was 23 months (median follow-up of 41 months). The most frequently observed severe hematologic toxicity during the study was neutropenia which occurred in 39% of the patients. The most common nonhematologic toxicities were asthenia and nausea and no treatment-related death was observed. In this study, the docetaxel and cisplatin combination was found to be active and safe in patients with MBC resistant to anthracyclines [79]. A phase II multicentre randomized study involving 142 patients with at least one measurable lesion compared the efficacy and safety of docetaxel plus epirubicin (ET) with 5-fluorouracil plus epirubicin and cyclophosphamide (FEC) as first-line chemotherapy for metastatic breast cancer. The patients were randomized to receive either docetaxel (75 mg/m2) plus epirubicin (75 mg/m2) or 5-FU (500 mg/m2) plus epirubicin (75 mg/m2) and cyclophosphamide (500 mg/m2) intravenously once every 3 weeks for up to eight cycles. Granulocyte-colony-stimulating factor (G-CSF) was given prophylactically after the first cycle when required and dose reduction, delay and withdrawal were applied when necessary. The intent-to-treat population (n = 142) showed an overall response rate of 59% (95% CI, 47-70%) for ET and 32% (95% CI, 21-43%) for FEC after a median seven and six cycles, respectively. Also, per-protocol analysis (n = 132) revealed an overall response rate of 63.1% (95% CI = 50-78%) and 34.3% (95% CI = 23-47%) for ET and FEC after a median seven and six cycles, respectively. The median time to progression (ITT) was 7.8 months (95% CI, 5.8-9.6 months) for ET and 5.9 months (95% CI, 4.6-7.8 months) for FEC. Median survival (ITT) for ET and FEC were 34 and 28 months respectively, after a median follow-up of 23.8 months. Hematologic toxicity was more common with ET and febrile neutropenia (13 patients; 18.6%) as well as two (possibly study treatment-related) deaths were reported in the ET group. On the other hand, nonhematologic grade 3-4 toxicities were infrequent in both arms. The investigators concluded that both ET and FEC were associated with acceptable toxicity and ET is a highly active first-line therapy for MBC [68].
Mitomycin combinations have also been used in the treatment of BC. Mitomycins have been in clinical use since the 1960s and have demonstrated potency against a broad range of cancers [80]. A study reported the efficacy of low-dose mitomycin and low-dose weekly doxorubicin in MBC patients previously treated with CMF. Forty-six biopsy-proven BC patients previously treated with CMF were entered in the study. Patients received a chemotherapy regimen consisting of doxorubicin (20 mg/m2 IV weekly starting on day 1) and mitomycin (10 mg/m2 IV on day 1). Treatment cycles were repeated every 28 days or later, pending recovery of the neutrophil count (≥ 1500/µl) and the platelet count (≥ 100,000/µl) and the doses of subsequent cycles were determined by the degree of myelosuppression. Forty-four of the 46 patients entered in the study were evaluable. The results revealed an overall response rate of 43% (11% complete remission and 32% partial response), 11.5 months median duration of survival and median duration of response of 8 months for responders (complete response and partial response). Also, toxicity of the regimen was moderate, comprising neutropenia (74%), thrombocytopenia (25%), pneumonitis (11%), and cardiomyopathy (2%). Thus, the low-dose mitomycin and low-dose weekly doxorubicin combination chemotherapy were considered effective for treating patients previously treated with CMF [81]. Another study published in 1990 reported the efficacy and reduced toxicity of a combination of mitomycin C and mitoxantrone in chemotherapy-naive advanced BC patients. The study involved 33 patients with predominantly visceral disease. The patients received a median of two cycles of chemotherapy with a combination of mitomycin C (10 mg/m2) every 6 weeks and mitoxantrone (6 mg/m2) every 3 weeks. Thirty-two of the patients were evaluable and 47% (15 patients) achieved a partial response for a median duration of 7 months. It was noted that the response rate and duration of response for the mitomycin C and mitoxantrone combination might be inferior to those reported for CMF or CAF combination chemotherapy to some extent. However, the combination regimen produced minimal gastrointestinal toxicity and hair loss with acceptable hematological toxicity [82]. Another study reported that a combination of mitomycin C and vinblastine is active and well tolerated in heavily pre-treated BC patients. In the study, 40 previously treated (median of 3 prior regimens) women with measurable disease received mitomycin C (10mg/m2 IV on day 1) and vinblastine (6mg/m2 IV on days 1 and 21) in a 42-day cycle. The combination chemotherapy regimen resulted in 35% partial response (14 patients) with additional 10 patients (25%) having stable disease lasting for 4 months or longer, giving a total clinical benefit of 60%. The median TTP and overall survival durations were 4 months (range: 1.5-23) and 11 months (range, 9-13) respectively, for a median follow-up period of 11 months (range: 2.5-49 months). Also, 1- and 2-year overall survival rates were 39.4% and 15.7% respectively.
The treatment was reportedly well tolerated with an acceptable toxicity profile. Grade 3-4 hematologic and non-hematologic toxicity was reported in 8 (20%) and 3 (7.5%) patients, respectively. In addition, two cases of fatalities (5%) occurred with pulmonary toxicity in women heavily exposed to mitomycin-C (cumulative doses of ≥ 40 mg/m2) and soon after red blood cell transfusion. Thus, the need to take the precaution of avoiding blood transfusion alone with mitomycin-C therapy was pointed out. The investigators concluded that chemotherapy with mitomycin-C and vinblastine is active and well-tolerated in heavily pre-treated BC patients [83].
Furthermore, a retrospective review of the medical records of 48 patients treated with a combination of mitomycin C and methotrexate showed that the combination may be effective in MBC patients pre-treated with anthracycline and taxanes. The patients were given mitomycin C (8 mg/m2 on day 1) and methotrexate (60 mg/m2 on days 1 and 15) in a treatment cycle repeated every 4 weeks. The results showed that although there was no complete response, there was 24% (11) partial response and a median time to progression of 4.8 months. Grade 3 thrombocytopenia was observed in five patients (10%) and other toxicity was mild and manageable [84]. Also, the combination of mitomycin C and capecitabine was evaluated in a phase II study involving 30 patients. The patients were treated with mitomycin C (8 mg/m2 IV bolus on day 1) and capecitabine (1000 mg/m2 twice-daily, orally on days 1-14) in 3-weekly cycles for a total of 6 cycles or until disease progression. Therapy with capecitabine alone was continued beyond six cycles until there was disease progression, unacceptable toxicity, patient refusal, noncompliance to the protocol, physician decision to discontinue treatment or treatment delay >2 weeks (unless there was perceived benefit to the patient). One patient discontinued treatment after the first cycle due to prolonged thrombocytopenia, thus 29 patients were evaluable for response. The overall response rate was 65.5% (95% CI, 48.2-82.8%) and an additional 31% of patients had stable disease. The median TTP was 8.5 months (95% CI, 6.1-10.9). Analysis of the subgroup of patients with prior exposure to (neo) adjuvant chemotherapy (n=18) demonstrated an overall response rate of 61.1% and TTP of 7.3 months. Median follow up of 18.5 months (range 5.7-47) showed that 14 patients (46.7%) were still alive and the median overall survival was 29.8 months (CI 95%, 18.3-41.3). Thrombocytopenia, pneumonitis and hemolytic uremic syndrome were the main adverse events in the study. The data suggest that capecitabine and mitomycin C combination chemotherapy has good antitumor activity as first-line treatment in MBC. However, the regimen is associated with mitomycin C-specific toxicity [36].
Another retrospective review of the medical records of 31 patients with HER2-negative MBC previously treated with anthracycline, taxane, capecitabine, and vinorelbine showed that a mitomycin C and methotrexate chemotherapy combination demonstrated the effectiveness of this combination in this group of patients. Each treatment cycle comprised mitomycin C (8 mg/m2 on day 1) and methotrexate (60 mg/m2 on day 1 and day 15) by IV administration every 4 weeks. Mitomycin C was administered till a cumulative dose of 50 mg/m2 was attained, following which only methotrexate (60 mg/m2 on day 1 and day 15) was repeated until progressive disease or adverse events were noticed.
The study revealed response rate and clinical benefit rate of 9.7 and 19.4% respectively and median TTP and times to failure of 3.9 and 3.7 months respectively. Grade 3 and/or 4 adverse events were observed in 36% patients (grades 3 and 4 thrombocytopenia: 12.9 and 3.2% respectively; grades 3-4 leucopenia and anemia: 12.9 and 9.7% respectively) and 7 patients needed dose reductions due to hematological toxicity. There was no renal toxicity and all toxicities were manageable. The study indicated that mitomycin C and methotrexate combination chemotherapy may be effective and tolerable for heavily pre-treated patients with good performance status [85]. In addition, a case study report showed the efficacy of mitomycin C and methotrexate combination in MBC which was resistant to eribulin, vinorelbine, and bevacizumab with paclitaxel. The patient was started on the treatment with mitomycin C (8 mg/m2 on day 1) and methotrexate (60 mg/m2 on day 1 and day 15) by IV administration every 4 weeks and 3 treatment cycles were completed as scheduled. During sequential mitomycin C/metho-trexate treatment, the progressively increasing levels of tumor markers decreased for the first time after the disease showed resistance to any chemotherapy. The combination regimen provided disease control for 7 months without disease progression. Grade 1 hematuria was observed after 3 treatment cycles but improved spontaneously without treatment. Grade 2 interstitial pneumonia which necessitated skipping treatment occurred in the fourth course. Grades 3 and 4 hematological adverse effects were observed after 5 cycles which necessitated postponement of treatment. Also, the dose of mitomycin C was reduced by 50% in cycles 7 and 8 because of hematological toxicity. The cycles were however completed as scheduled. Thus, this case report suggests that mitomycin C/methotrexate combination may be an effective treatment for MBC patients with progressive disease following aggressive treatment with multiple regimens [37].
Gemcitabine, capecitabine and vinorelbine containing chemotherapy combinations have also been used in the therapy of BC. A phase II randomized trial evaluated lapatinib combination with capecitabine, vinorelbine, or gemcitabine in HER2-positive MBC patients with progression after treatment with a taxane (Latin American Cooperative Oncology Group (LACOG) 0801 Study). The trial enrolled a total of 142 women 18 years and older, with histologically confirmed locally advanced or metastatic invasive HER2-positive adenocarcinoma of the breast. The primary endpoint of the trial was an overall response (percentage of patients experiencing a confirmed complete response or partial response) and secondary endpoints included progression-free survival, overall survival, and duration of response.
The patients were randomized to: LC arm, to receive every 3-week cycles of lapatinib (1250 mg; orally once daily continuously) plus capecitabine (2000 mg/m2/day orally in 2 doses on days 1 to 14); LV arm, to receive lapatinib at the same dose as LC arm, plus vinorelbine (25 mg/m2/day IV on days 1 and 8); or LG arm to receive lapatinib at the same dose as LC and LG arms, plus gemcitabine (1000 mg/m2 on days 1 and 8). The patients were stratified by previous treatment with trastuzumab (yes vs. no), previous taxane therapy in the neoadjuvant or adjuvant setting (yes vs. no), and the presence of liver metastases (yes vs. no). The patients were randomized to study treatment until disease progression or unacceptable toxicity, withdrawal of consent, loss to follow-up, or death. The results showed an overall response rate of 49% (95% CI, 34.8%-63.4%), 56% (95% CI, 40%-70.4%) and 41% (95% CI, 27%-56.8%) in the LC, LV and LG groups, respectively. The median progression-free survival was 9 months in the LC arm and 7 months in the other 2 arms (P = 0.28). The most common grade 3 and 4 adverse events in the LC arm were hand-foot syndrome (18%), diarrhea (6%), and increased alanine aminotransferase/aspartate aminotransferase (4%); while the were neutropenia (36%), diarrhea (9%), and febrile neutropenia (6%) in the LV arm; and neutropenia (47%), alanine aminotransferase/aspartate aminotransferase (13%), and rash (4%) in the LG arm. Also, no new safety signals were detected with these combinations. Thus, these combinations represent potential alternatives to be further explored in the sequence of regimens for patients with HER2-positive BC [86].
Another phase II trial assessed the efficacy and safety of panitumumab in combination with gemcitabine and carboplatin as a treatment for metastatic TNBC in 71 women. The primary end point in the study was progression-free survival while secondary end points included overall response rate, clinical benefit rate, and safety. All patients in the study received IV infusions of gemcitabine (1500 mg/m2), carboplatin (area under the concentration-time curve = 2.5), and panitumumab (6 mg/kg) on day 1 of each 14-day treatment cycle. Doses of study drugs could be reduced, withheld, or delayed as necessary, for patients who experienced toxicities during the study.
In this regard, two dose-level reductions of each study drug were permitted: panitumumab (4.8 and 3.6 mg/kg); gemcitabine (1100 and 800 mg/m2); and carboplatin (area under the concentration-time curve = 2 and 1.5). If more than 2 dose reductions were required, the particular agent was discontinued, and treatment with the remaining study agents could be continued. The results of the trial showed that at a median follow-up time of 11 months, the median progression-free survival was 4.4 months (95% CI, 3.2-5.5 months). Also, only 9% of patients remained progression-free at 1 year. Median overall survival was 11.6 months (95% CI, 8.6-15.2 months) and 24% of patients remained alive at 2 years. The investigators noted that currently available clinical trials results do not encourage the combination of an EGFR inhibitor with chemotherapy in the treatment of patients with metastatic TNBC [87]. Furthermore, an open-label, 3-arm, multinational, randomized phase II (NorCap-CA223) trial involving 152 patients (23 centers in 13 countries), compared the disease control rate (DCR) of first-line all-oral vinorelbine/capecitabine, gemcitabine/paclitaxel and gemcitabine/docetaxel in HER2-negative MBC. The primary objective of the trial was to determine the DCR (total number of patients achieving complete response, partial response, or stable disease sustained for at least 3 months) and the secondary objectives were to assess the safety, efficacy, and quality of life associated with the 3 regimens. The prespecified efficacy end points were DCR in the evaluable population, objective response rate, duration of disease control, stable disease, and response; PFS; time to treatment failure; and OS. The patients were randomized to receive 1 of 3 chemotherapy doublets: oral vinorelbine with oral capecitabine (NORCAP); gemcitabine with paclitaxel (GEMPAC); or gemcitabine with docetaxel (GEMDOC), repeated in every 21-day cycle. In the NORCAP arm, patients received oral vinorelbine (60 mg/m2 on days 1 and 8 of cycle 1), the dose was increased (80 mg/m2 on days 1 and 8) from cycle 2 onward in the absence of Grade 3 or 4 toxicity in cycle 1, in combination with capecitabine (1000 mg/m2 twice daily on days 1 to 14 of each cycle). In the GEMPAC arm, patients received intravenous gemcitabine (1250 mg/m2 on days 1 and 8) in combination with intravenous paclitaxel (175 mg/m2 on day 1). In the GEMDOC arm, intravenous gemcitabine (1000 mg/m2) was given in combination with intravenous docetaxel (75 mg/m2 on day 1). Chemotherapy was continued until disease progression, unacceptable toxicity, or patient refusal. The results showed DCR of 73% (95% CI) in the NORCAP arm (36 of 49 patients), 78% (95% CI) in the GEMPAC arm (39 of 50 patients), and 80% (95% CI) in the GEMDOC arm (40 of 50 patients). Objective response rates were 33% (16 of 49 patients), 24% (12 of 50 patients), and 50% (25 of 50 patients), for the NORCAP, GEMPAC and GEMDOC regimens respectively. Median progression-free survival for the regimens were 7.6, 9.0, and 11.4 months respectively. Median overall survival was 30 to 31 months with all regimens. The most common Grade ≥ 3 adverse event with each regimen was neutropenia (24 patients [50%], 23 patients [46%], and 43 patients [86%], respectively). The most common nonhematological Grade ≥ 3 adverse event was fatigue. The occurrence of Grade 2 alopecia was 72% (36 patients) in the GEMPAC arm, 76% (38 patients) in the GEMDOC and 8% (4 patients) in the NORCAP.
Also, there was no evidence that the NORCAP regimen had a detrimental effect on quality of life. Therefore, it was concluded that all-oral NORCAP is an active first-line chemotherapy regimen which may be offered as an alternative to first-line taxane-based therapy for HER2-negative MBC, especially for patients who wish to avoid alopecia or frequent IV administrations [88]. An open-label, prospective, randomized, controlled Phase III trial (Success-A study) assessed the toxicity profile when gemcitabine is added to adjuvant taxane-based chemotherapy in high-risk EBC patients. The trial involved 271 study centers with a total of 3754 women, of which 3690 patients were evaluated according to the National Cancer Institute (NCI) toxicity criteria (the safety population). BC patients with all molecular subtypes were eligible for the study. However, to be eligible to participate in the study, patients had operable BC with clear surgical margins, metastases to the axillary nodes or were node negative with a high-risk profile. Mastectomy or lumpectomy with sentinel lymph node biopsy, with or without axillary dissection was the surgical treatment employed. Radiotherapy was applied after BCS or at high risk of local recurrence and chemotherapy was not commenced earlier than six weeks after surgery. Initially, all patients received three full cycles of FEC chemotherapy [epirubicin (100 mg/m2); fluorouracil (500 mg/m2); cyclophosphamide (500mg/m2)]. This was followed by either three cycles of full-dose docetaxel (100mg/m2; D) for patients in the FEC-D arm or 3 cycles of gemcitabine (1000 mg/m2 d1, d8) and dose-reduced docetaxel (75 mg/m2, d1) (DG) for patients in the FEC-DG arm. Upon completion of chemotherapy, the patients were further randomized to receive either 2 years of zoledronic acid treatment (4 mg IV every 3 months) or 5 years of zoledronic acid treatment (4 mg IV every 3 months for two years, followed by 4 mg IV every 6 months for the duration of additional three years). During the zoledronic acid treatment period, patients received daily calcium (500 mg p.o.) and vitamin D (400 IE p.o.). Patients with positive HR status of the primary tumor received tamoxifen treatment (20mg p.o.) per day for 2 years after chemotherapy was completed. After chemotherapy, postmenopausal patients with positive HR status were treated with anastrozole 1mg p.o. for additional 3 years while premenopausal patients continued tamoxifen treatment for an additional 3 years. The results showed the safety and toxicity analyses for the 3690 patients (safety population) who were treated with at least one cycle of FEC chemotherapy. There was no difference in neutropenia or febrile neutropenia. However, thrombocytopenia and leukopenia were significantly increased with FEC-DG treatment (2.0 vs. 0.5%, p < 0.001) and (64.1 vs. 58.5%, p < 0.001) respectively. Also, significantly more G-CSF support in cycles 4 to 6 (FEC-DG: 57.8% vs. FEC-D: 36.3%, p < 0.001) was provided with FEC-DG. Significantly more frequent dose reductions > 20% (4 vs. 2.4%) and postponement of treatment cycles (0.9 vs. 0.4%) were also necessary in the FEC-DG arm. Eight deaths occurred during treatment in the FEC-DG arm compared to four in the FEC-D arm. The investigators concluded that addition of gemcitabine increased hematological toxicity and was associated with more dose reductions and postponements of treatment cycles [89].
Another open-label randomized phase 3 trial (tAnGo trial) studied the effect of gemcitabine when included in adjuvant anthracycline and taxanes-containing chemotherapy for EBC. The phase 3 superiority trial included over 3,000 women (18 years or older) recruited from 127 hospitals and clinical centers in UK and Ireland, randomly assigned (1:1) to one of two treatment regimens. One group (control group) received epirubicin, cyclophosphamide, and paclitaxel {four cycles of epirubicin (90 mg/m2) administered IV and cyclophosphamide (600 mg/m2) administered IV on day 1 every 3 weeks, followed by four cycles of paclitaxel (175 mg/m2) as a 3 h infusion on day 1 every 3 weeks}, while the other group received epirubicin, cyclophosphamide and paclitaxel plus gemcitabine (the same chemotherapy regimen as the other group, with the addition of gemcitabine (1250 mg/m2) to the paclitaxel cycles, administered IV as a 0.5 h infusion on days 1 and 8 every 3 weeks). The primary endpoint of the trial was disease-free survival with the aim of detecting 5% differences in 5-year disease-free survival between the treatment groups. The final (10-year follow-up) intent-to-treat analysis showed that there was no significant difference in disease-free survival between the treatment groups (65% in the gemcitabine group vs. 65% in the control group). Also, median disease-free survival was not reached (adjusted hazard ratio 0.97 [95% CI 0.86-1.10], p=0.64). Both regimens were found to be safe, deliverable, and tolerable based on toxicity profile, dose intensity and a detailed safety substudy. The results indicated that addition of gemcitabine to anthracycline and taxane-based adjuvant chemotherapy at the dose and schedule of the trial did not confer a therapeutic advantage in terms of disease-free survival in EBC, although it could result in increased toxicity [90].
Heat shock protein 90 (Hsp90) inhibitors have also been used in combination therapy with other anticancer drugs such as the taxanes, cisplatin, etoposide and trastuzumab. A combination of the Hsp90 inhibitor, 17-allylamino, 17-demethoxygel-danamycin (17-AAG), a geldanamycin analog with trastuzumab produced encouraging results in the treatment of HER2-positive MBC progressing on trastuzumab [91].
The possibility of safely administering tanespi-mycin (17-AAG) in combination with trastuzumab at a dose that inhibits Hsp90 function in vivo in lymphocytes was investigated in a phase I dose escalation study. In the study, twenty-five patients, 18 years or older, with histologic documentation of a nonhematologic malignancy (irrespective of HER-2 expression), evidence of progression during treatment with standard therapy, Karnofsky performance status of at least 70%, negative pregnancy test, 2 weeks’ removal from prior radiation or chemotherapy (6 weeks for nitrosoureas) and with acceptable hematologic profile were enrolled in the study. Patients were assigned to four tanespimycin dose levels: 225 (n = 4), 300 (n = 3), 375 (n = 8), and 450 mg/m2 (n = 10). At the 375 and 450 mg/m2 dose levels, dose-limiting toxicity was seen in one patient in each dose group. In these patients, grade 4 fatigue, as well as grade 2 nausea and anorexia, necessitated a dose delay for more than 2 weeks. At the 450 mg/m2 dose level, there was thrombocytopenia which necessitated a dose delay greater than 2 weeks.
The authors reported that the 17-AAG plus trastuzumab combination was well tolerated and demonstrated antitumor activity in patients with HER-2 BC in which tumors had progressed during treatment with trastuzumab. The data suggest the possibility of inhibiting Hsp90 function in vivo to a degree that would result in inhibition of tumor growth. Based on the data obtained in this study, the investigators conducted a phase II trial to study weekly 17-AAG (450 mg/m2) in combination with trastuzumab for patients with HER-2 positive MBC with progressive disease after one line of trastuzumab-based therapy [92]. In the phase II trial, thirty-one patients (median age of 53 years and a median Karnofsky performance status (KPS) of 90%) were enrolled. All patients received weekly 17-AAG (450mg/m2) intravenously and trastuzumab at a conventional dose, with therapy, continued until disease progression. The primary endpoint was response rate by Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The results showed 22% overall response rate, 59% clinical benefit rate [complete response + partial response + stable disease], 6 months median progression-free survival (95% CI: 4-9) and a median overall survival of 17 months (95% CI: 16-28). The most common toxicities recorded were diarrhea, fatigue, nausea, and headache, mostly of grade 1 severity. The investigators concluded that 17-AAG plus trastuzumab showed significant anticancer activity in patients with HER2-positive, MBC previously progressing on trastuzumab [93].
Combination therapy with monoclonal antibodies such as trastuzumab and pertuzumab has also been reported. A Phase III randomized, double-blind, placebo-controlled trial demonstrated the efficacy of first-line therapy with pertuzumab, trastuzumab, and docetaxel combination therapy in patients with HER2 positive MBC (The Clinical Evaluation of Pertuzumab and Trastuzumab CLEOPATRA trial). Patients 18 years or older, with locally recurrent, unresectable, or centrally confirmed metastatic HER2-positive BC were enrolled in the study. To be eligible for the study, patients had a left ventricular ejection fraction (LVEF) of 50% or more at baseline, an Eastern Cooperative Oncology Group performance status of 0 or 1 and had not received more than one hormonal treatment for metastatic disease. Also, adjuvant or neoadjuvant chemotherapy with or without trastuzumab was permitted. Patients with central nervous system metastases, LVEF less than 50% during or after previous trastuzumab therapy, or who had received other anticancer therapy (except for one previous hormonal regimen) or who had a cumulative exposure of doxorubicin more than 360 mg/m2 of the body-surface area or its equivalent were excluded from the study. The drugs were administered IV in 3-weekly cycles. Pertuzumab (840 mg) or placebo was given on day 1 of cycle 1, followed by 420 mg on day 1 of each subsequent cycle; trastuzumab (8 mg/kg body weight) was administered on day 2 of cycle 1, followed by 6 mg per kilogram on day 1 of the remaining cycles. Pertuzumab or placebo and trastuzumab were administered until disease progression or the manifestation of uncontrollable toxic effects (dose reductions were not permitted). Docetaxel (75 mg/m2) was given on day 2 of cycle 1 and on day 1 of the remaining cycles. A minimum of six cycles was recommended, but fewer cycles were allowed in the event of disease progression or uncontrollable toxic effects.
Also, more cycles were allowed at the discretion of the investigator or patient. In addition, docetaxel could be escalated to 100 mg/m2 if uncontrollable toxic effects did not occur and there could be a 25% reduction in the event of myelosuppression, hepatic dysfunction, or other toxic effects. In this study, primary end point analysis revealed that patients in the pertuzumab, trastuzu-mab, and docetaxel group (pertuzumab group) had a significantly longer median progression-free survival (based on assessment by independent reviewers), compared to those in the placebo, trastuzumab, and docetaxel (control) group (hazard ratio = 0.62, in favor of the pertuzumab group). Furthermore, the second interim analysis of overall survival confirmed the significantly longer survival demonstrated in the pertuzumab group (hazard ratio, 0.66), with similar safety profiles, including cardiac tolerability, across the two groups. The final prespecified overall survival results with a median follow up of 50 months showed a median overall survival of 56.5 months (95% CI, 49.3 to not reached) in the pertuzumab group, compared to 40.8 months (95% CI, 35.8 to 48.3) in the control group, revealing a difference of 15.7 months in median survival between the two groups. Thus, it was concluded that the addition of pertuzumab to trastuzumab and docetaxel significantly improved the median overall survival in patients with HER2-positive MBC [94-97].
4. NANOPARTICLES FOR COMBINATION THERAPY IN BC
Nanoparticles offer several advantages in drug delivery for combination therapy due to their unique characteristics. Some of these characteristics and advantages include: potential for functionalization for enhanced drug-carrying capacity [98], tissue or organ specific transport and delivery [98, 99], reduction in administered dose and toxicity [100]; the ability to carry and deliver multiple classes of diagnostics and therapeutic agents loaded within a nanoparticle, which would then exert their various effects in a controlled manner [98, 101] and reduction in the frequency of administration [100]. The capability of nanoparticles to bear multiple therapeutic agents is of interest (for this review) as it would make it easier to administer drugs in combination without having to increase the frequency of administration. Thus, therapeutic agents belonging to different classes can be combined within the same nanoparticle system to achieve the desired therapeutic goal. Combination therapy using nanoparticle formulations provides certain advantages over combining the free drugs for therapy. The controlled release feature offered by nanoparticle systems can normalize the pharmacokinetics, biodistribution, and stability of drugs that possess very different chemical properties that would independently have produced contrasting pharmacological behaviors. These long-circulating formulations are capable of continuous release of drugs at controlled ratios or permit independent modification of release rates of each drug in ways that would not be achievable with conventional formulations of free drug which are rapidly cleared from the system [102].
Multidrug loaded nanoparticle formulations comprising different classes of therapeutic agents have been developed and studied for BC therapy in preclinical breast cancer models. For instance, polymer-lipid hybrid nanoparticles (PLN) of co-encapsulated doxorubicin and mitomycin C have demonstrated efficacy in models of human BC, including multidrug-resistant cells [103-105]. Also, a multidrug loaded nanoparticle micellar formulation was developed for the delivery of three drugs paclitaxel, rapamycin, and 17-AAG (Triolimus). This nanoparticle-based formulation was evaluated in tumor xenografts including MDA-MB-231 tumor bearing mice. In the study, Triolimus demonstrated the considerably more superior antitumor effect in treated mice compared to paclitaxel micelle treated mice. In the paclitaxel treatment group, all the mice had persistent tumor regrowth by day 33. In the Triolimus treatment group on the other hand, only one (1) of the mice showed persistent tumor regrowth with initial evidence of recurrence by day 45, all other mice in the group remained tumor-free for more than 1 year after starting the treatment [106]. Combination therapy with a co-delivered chemotherapeutic agent and nucleic acid using a nanoparticle system is also a promising strategy for the effective treatment of BC. The in vitro and in vivo antitumor efficacy of pH-responsive hyaluronic acid (HA) ligand modified paclitaxel and DNA loaded nanoparticles solid lipid nanoparticles has been reported [107].
Also, the in vitro and in vivo efficacy of trastu-zumab modified emtansine (DM1) loaded nanoparticles has been demonstrated. The authors concluded that this nanoparticle system holds promise in the therapy of HER2 positive BC [108]. The efficacy of docetaxel-loaded, trastuzumab functionalized nanostructured lipid carriers in BC cell lines has also been reported [109]. There has also been a report of the co-delivery of gemcitabine and gadolinium, a magnetic resonance imaging (MRI) contrast agent, using self-assembled nanoparticles [110]. Other workers have developed trastuzu-mab and diethylene-triamine-pentaacetic acid (DPTA) conjugated iron oxide nanoparticles labelled with thechnetium-99m (99m-Tc) and galium-68 (Ga-68) for imaging of BC. They tested the nanoparticles in female Balb/c mice xenografts of BT-474 cells. They reported high selectivity of the nanoparticles for the BT-474 cells tumor xenograft and concluded that the system represents a promising multi-modal nano-radiopharmaceutical agent for in vivo BC diagnosis by positron emission tomography (PET) and single photon emission computed tomography SPECT imaging [111].
Furthermore, a multicenter, prospective, open-label, single phase II study evaluated neoadjuvant anthracycline-based regimens in combination with nanoparticle albumin-bound paclitaxel (nab-paclitaxel) and trastuzumab for HER-2 positive operable BC. Forty-six (46) patients were enrolled in the study, one of which was excluded from treatment (existence of another malignant disease). The patients (stage I to IIIA BC) were treated with neoadjuvant epirubicin/ cyclophosphamide (EC) or 5-FU/epirubicin/cyclophosphamide every 3 weeks for 4 cycles, followed by nab-paclitaxel (260 mg/m2) plus trastuzumab every 3 weeks for 4 cycles. The pCR rate was the primary endpoint for the study and the secondary endpoints included clinical response rate, disease-free survival, pathologic response rate (defined as pCR or minimal residual invasive disease only in the breast), BCS rate, and safety. Forty-four (44) of the 45 patients treated in the study were evaluated on nab-paclitaxel as there was rapid disease progression on EC therapy in one of the patients. Overall, there was pCR in 49% of the 45 patients treated in the study. Hematologic toxicity was the most common cause of dose delay or reduction, with only one patient requiring dose reduction for nab-paclitaxel due to peripheral neuropathy. The investigators concluded that this combination appears safe and effective for neoadjuvant therapy [19].
Also, a phase I study evaluated the efficacy and safety of combination therapy with nab-paclitaxel, carboplatin and trastuzumab in HER2-overexpre-ssing locally advanced BC. The study enrolled a total of 6 patients and was designed to determine the dose-limiting toxicity (DLT), maximum tolerated dose and recommended dose of the combination treatment in women with HER2-overexpre-ssing locally advanced BC. Nab-paclitaxel was administered at a starting dose of 220 mg/m2 (level 1), escalated to 260 mg/m2 (level 2). Nab-paclitaxel was administered with carboplatin (AUC, 6 mg/ml/min) and tri-weekly trastuzumab. No DLT was observed during the first cycle, however, 4 patients developed grade 4 thrombocytopenia, 2 had grade 4 neutropenia and 3 exhibited a grade 4 decrease in hemoglobin levels. The study concluded that although no DLTs were observed in the first cycle of the treatment, hematological toxicity (anemia) presented difficulties with continuity [112].
The employment of nanoparticle-based drug delivery systems for combination therapy could be potentially beneficial in breast cancer therapy. However, the delivery systems need to be properly designed to maximize the benefits of this platform, while avoiding undesirable effects. A fairly recent review also discussed the use of combined drug delivery approaches in the setting of metastatic breast cancer [113].
In vivo barriers for both drug combinations and nanotechnology used in co-delivery of therapeutics in breast cancer.
Varying biodistribution/pharmacokinetics of combination drugs through cocktail administration has been attributed to their ineffectiveness in the clinic [114]. The problem is being solved by a nanotechnology platform for drug delivery. The unique ability of multifunctional therapeutic nanoparticles to provide site-specific tumor targeting, improve the solubility of anticancer drugs, synchronize the disposition (pharmacokinetics) of encapsulated drugs (drug combination), overcome drug resistance and enhance anticancer activity of therapeutic drugs (concurrent chemotherapy with trastuzumab or pertuzumab is more effective than sequential use of these agents) represents an important innovation in drug delivery [115-122].
The reticuloendothelial system (RES), also termed the mononuclear phagocyte system (MPS), plays key roles in nanoparticle (NP) clearance. Upon entry into organs, NPs are susceptible to resident phagocytic cell-mediated clearance and may inadvertently trigger the secretion of cytokines such as tumor necrosis factor (TNF), interleukins, and interferons, resulting in local inflammation that can cause tissue damage [123, 124]. Modifying the surface chemistry to prevent serum protein absorption/adsorption onto NPs is critical in minimizing their nonspecific uptake into normal tissues, thus reducing the total dosage required for achieving the same therapeutic effects of administered drugs and prevent side effects of the anticancer drugs. Strategies to minimize nonspecific absorption/adsorption of proteins include coating the NP surface using PEGylation to hinder opsonization [125-127]. The polyethylene glycol (PEG) subunits form tight associations with water molecules, generating a hydrating layer that blocks protein absorption/adsorption [128]. In vivo, PEGylated NPs exhibit increased circulatory half-life with an enhanced ability to target tumor tissues [129]. The predominant factor governing renal clearance is the size of the nanoparticles. One strategy for overcoming this challenge is to create NPs that break down into renal-clearable particles by using biodegradable components as exemplified by poly(lactide) and poly-ɛ-caprolactone nanoparticles [117-120]. Another barrier is the blood-brain barrier. NPs are thought to cross the BBB via receptor-mediated endocytosis across endothelial cells, which requires the attachment of targeting ligands, peptides, or receptors to the NPs, or alternatively coating the NPs with various surfactants that allow the specific adsorption of serum proteins needed for receptor-mediated transport [130].
CONCLUSION
Combination therapy offers the potential of improving therapeutic efficacy and efficiency in BC treatment. Various classes of agents have been used in combination chemotherapy regimens over the years. Maximum benefit from therapeutic combinations would emanate from proper identification of molecular subclass of disease to permit the use of agents that would provide the maximum therapeutic advantage. In other words, a rational combination of therapeutic agents based on disease profile would provide the greatest benefit to BC patients. Thus, therapeutics to be employed in combination therapy need to be carefully selected to ensure that patients derive maximum benefit from the therapy. The number of therapeutic agents must be kept to a minimum to avoid unnecessarily increasing the agents to be administered unless there is evidence of additional benefits to be derived from the inclusion of more agents in the treatment regimen. Moreover, the addition of some agents to existing regimens may result in increased toxicity without significant improvement in therapeutic efficacy. The application of nanoparticles in combination treatment may further extend the benefits derivable from such a treatment strategy based on the unique advantages presented by the nanoparticle platform.
ACKNOWLEDGEMENTS
Dr. Emmanuel O. Akala and Dr. Funmilola A. Fisusi (Authors) contributed substantially to the content, drafting and revision of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The work reported here was funded by NIH/NCI: 1SC1CA199810-01 awarded to Emmanuel O. Akala. This work was carried out in facilities supported by NCRR/NIH Grants #1 C06 RR 020608-01 and #1C06 RR 14469-01.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 2.Torre L.A., Siegel R.L., Ward E.M., Jemal A. Global cancer incidence and mortality rates and trends: an update. Cancer Epidemiol. Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Perou C.M., Sorlie T., Eisen M.B., et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Prat A., Pineda E., Adamo B., et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Matta J., Ortiz C., Encarnacion J., Dutil J., Suarez E. Variability in dna repair capacity levels among molecular breast cancer subtypes: triple negative breast cancer shows lowest repair. Int. J. Mol. Sci. 2017;18(7) doi: 10.3390/ijms18071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheang M.C.U., Voduc D., Bajdik C., et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin. Cancer Res. 2008;14(5):1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 7.Leidy J., Khan A., Kandil D. Basal-like breast cancer: update on clinicopathologic, immunohistochemical, and molecular features. Arch. Pathol. Lab. Med. 2014;138(1):37–43. doi: 10.5858/arpa.2012-0439-RA. [DOI] [PubMed] [Google Scholar]
- 8.Lousberg L., Collignon J., Jerusalem G. Resistance to therapy in estrogen receptor positive and human epidermal growth factor 2 positive breast cancers: progress with latest therapeutic strategies. Ther. Adv. Med. Oncol. 2016;8(6):429–449. doi: 10.1177/1758834016665077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van’t Veer L.J., Dai H., van de Vijver M.J., et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 10.Sotiriou C., Neo S-Y., McShane L.M., et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. USA. 2003;100(18):10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prat A., Carey L.A., Adamo B., et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J. Natl. Cancer Inst. 2014;106(8):152. doi: 10.1093/jnci/dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 13.Blok E.J., Derks M.G.M., van der Hoeven J.J.M., van de Velde C.J.H., Kroep J.R. Extended adjuvant endocrine therapy in hormone-receptor positive early breast cancer: current and future evidence. Cancer Treat. Rev. 2015;41(3):271–276. doi: 10.1016/j.ctrv.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Nuciforo P., Thyparambil S., Aura C., et al. High Her2 protein levels correlate with increased survival in breast cancer patients treated with anti-Her2 therapy. Mol. Oncol. 2016;10(1):138–147. doi: 10.1016/j.molonc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew H.K. Adjuvant therapy for breast cancer: who should get what? West. J. Med. 2001;174(4):284–287. doi: 10.1136/ewjm.174.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Matteis A., Nuzzo F., D’Aiuto G., et al. Docetaxel plus epidoxorubicin as neoadjuvant treatment in patients with large operable or locally advanced carcinoma of the breast. Cancer. 2002;94(4):895–901. doi: 10.1002/cncr.20335.abs. [DOI] [PubMed] [Google Scholar]
- 17.Giordano S.H. Update on locally advanced breast cancer. Oncologist. 2003;8(6):521–530. doi: 10.1634/theoncologist.8-6-521. [DOI] [PubMed] [Google Scholar]
- 18.Wang M., Hou L., Chen M., et al. Neoadjuvant chemotherapy creates surgery opportunities for inoperable locally advanced breast cancer. Sci. Rep. 2017;7:44673. doi: 10.1038/srep44673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka S., Iwamoto M., Kimura K., et al. Phase ii study of neoadjuvant anthracycline-based regimens combined with nanoparticle albumin-bound paclitaxel and trastuzumab for human epidermal growth factor receptor 2-positive operable breast cancer. Clin. Breast Cancer. 2015;15(3):191–196. doi: 10.1016/j.clbc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Kummel S., Holtschmidt J., Loibl S. Surgical treatment of primary breast cancer in the neoadjuvant setting. Br. J. Surg. 2014;101(8):912–924. doi: 10.1002/bjs.9545. [DOI] [PubMed] [Google Scholar]
- 21.Neuman H.B., Morrogh M., Gonen M., Van Z.K.J., Morrow M., King T.A. Stage IV breast cancer in the era of targeted therapy: does surgery of the primary tumor matter? Cancer. 2010;116(5):1226–1233. doi: 10.1002/cncr.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennigs A., Riedel F., Marmé F., et al. Changes in chemotherapy usage and outcome of early breast cancer patients in the last decade. Breast Cancer Res. Treat. 2016;160(3):491–499. doi: 10.1007/s10549-016-4016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darby S., McGale P., Correa C., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy V., Pockaj B.A., Allred J.B., et al. A Phase II trial of docetaxel and carboplatin administered every 2 weeks as preoperative therapy for stage Ii or Iii breast cancer: NCCTG study N0338. J. Clin. Oncol. 2013;36(6):540–544. doi: 10.1097/COC.0b013e318256f619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher B., Anderson S., Bryant J., et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin S.A. Surgical management of the breast: breast conservation therapy and mastectomy. Surg. Clin. North Am. 2013;93(2):411–428. doi: 10.1016/j.suc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Shaitelman S.F., Kim L.H. Accelerated partial-breast irradiation: the current state of our knowledge. Oncology. 2013;27(4):329–342. [PubMed] [Google Scholar]
- 28.Hopwood P., Haviland J.S., Sumo G., Mills J., Bliss J.M., Yarnold J.R. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised standardisation of breast radiotherapy (start) trials. Lancet Oncol. 2010;11(3):231–240. doi: 10.1016/S1470-2045(09)70382-1. [DOI] [PubMed] [Google Scholar]
- 29.Sardaro A., Petruzzelli M.F., D’Errico M.P., Grimaldi L., Pili G., Portaluri M. Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother. Oncol. 2012;103(2):133–142. doi: 10.1016/j.radonc.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Rakhra S., Bethke K., Strauss J., et al. Risk factors leading to complications in early-stage breast cancer following breast-conserving surgery and intraoperative radiotherapy. Ann. Surg. Oncol. 2016;24(5):1258–1261. doi: 10.1245/s10434-016-5679-0. [DOI] [PubMed] [Google Scholar]
- 31.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. •••;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 32.Houssami N., Macaskill P., von Minckwitz G., Marinovich M.L., Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur. J. Cancer. 2012;48(18):3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Rashid O.M., Takabe K. Does removal of the primary tumor in metastatic breast cancer improve survival? J Womens Health Wellness. 2014;23(2):184–188. doi: 10.1089/jwh.2013.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hortobagyi G.N. Treatment of breast cancer. N. Engl. J. Med. 1998;339(14):974–984. doi: 10.1056/NEJM199810013391407. [DOI] [PubMed] [Google Scholar]
- 35.Sachdev J.C., Jahanzeb M. Use of cytotoxic chemotherapy in metastatic breast cancer: putting taxanes in perspective. Clin. Breast Cancer. 2016;16(2):73–81. doi: 10.1016/j.clbc.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Vrdoljak E., Boban M., Omrcen T., Hrepic D., Fridl-Vidas V., Boskovic L. Combination of capecitabine and mitomycin C as first-line treatment in patients with metastatic breast cancer. Neoplasma. 2011;58(2):172–178. doi: 10.4149/neo_2011_02_172. [DOI] [PubMed] [Google Scholar]
- 37.Tanabe M. Combination chemotherapy of mitomycin C and methotrexate was effective on metastatic breast cancer resistant to eribulin, vinorelbine, and bevacizumab after anthracycline, taxane, and capecitabine. Case Rep. Oncol. 2016;9(2):422–426. doi: 10.1159/000447770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg P.A., Hortobagyi G.N., Smith T.L., Ziegler L.D., Frye D.K., Buzdar A.U. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1996;14(8):2197–2205. doi: 10.1200/JCO.1996.14.8.2197. [DOI] [PubMed] [Google Scholar]
- 39.Tomiak E., Piccart M., Mignolet F., et al. Characterisation of complete responders to combination chemotherapy for advanced breast cancer: a retrospective eortc breast group study. Eur. J. Cancer. 1996;32a(11):1876–1887. doi: 10.1016/0959-8049(96)00189-x. [DOI] [PubMed] [Google Scholar]
- 40.Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Biochem. Behav. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 41.Foucquier J., Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol. Res. Perspect. 2015;3(3):e00149. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan R., Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol. Ther. 2000;86(2):191–198. doi: 10.1016/s0163-7258(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 43.Bonadonna G., Brusamolino E., Valagussa P., et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N. Engl. J. Med. 1976;294(8):405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 44.Pronzato P., Rondini M. First line chemotherapy of metastatic breast cancer. Ann. Oncol. 2006;17:v165–v8. doi: 10.1093/annonc/mdj974. [DOI] [PubMed] [Google Scholar]
- 45.Anampa J., Makower D., Sparano J.A. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med. 2015;13(1):195. doi: 10.1186/s12916-015-0439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keith C.T., Borisy A.A., Stockwell B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005;4(1):71–78. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 47.Heys S.D., Hutcheon A.W., Sarkar T.K., et al. Neoadjuvant docetaxel in breast cancer: 3-Year Survival Results from the Aberdeen trial. Clin. Breast Cancer. 2002;3:S69–S74. doi: 10.3816/cbc.2002.s.015. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra V., Dorr V.J., Lyss A.P., et al. Neoadjuvant and adjuvant chemotherapy with doxorubicin and docetaxel in locally advanced breast cancer. Clin. Breast Cancer. 2004;5(5):377–384. doi: 10.3816/cbc.2004.n.045. [DOI] [PubMed] [Google Scholar]
- 49.Colleoni M., Goldhirsch A. Neoadjuvant chemotherapy for breast cancer: any progress? Lancet Oncol. 2014;15(2):131–132. doi: 10.1016/S1470-2045(13)70584-9. [DOI] [PubMed] [Google Scholar]
- 50.Kaufmann M., von Minckwitz G., Mamounas E.P., et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann. Surg. Oncol. 2012;19(5):1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 51.Bear H.D., Anderson S., Brown A., et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from national surgical adjuvant breast and bowel project protocol B-27. J. Clin. Oncol. 2003;21(22):4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Earl H.M., Vallier A.L., Hiller L., et al. Effects of the addition of gemcitabine, and paclitaxel-first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high-risk early breast cancer (neo-tango): an open-label, 2x2 factorial randomised phase 3 trial. Lancet Oncol. 2014;15(2):201–212. doi: 10.1016/S1470-2045(13)70554-0. [DOI] [PubMed] [Google Scholar]
- 53.von Minckwitz G., Untch M., Blohmer J.U., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 54.Buzdar A.U., Suman V.J., Meric-Bernstam F., et al. fluorouracil, epirubicin, and cyclophosphamide (fec-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by fec-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(13):1317–1325. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abrams J.S. Adjuvant therapy for breast cancer--results from the USA consensus conference. Breast Cancer. 2001;8(4):298–304. doi: 10.1007/BF02967528. [DOI] [PubMed] [Google Scholar]
- 56.Early breast cancer trialists’ collaborative group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Early breast cancer trialists’ collaborative group. Lancet. 1998;352(9132):930–942. [PubMed] [Google Scholar]
- 57.Fisher B., Dignam J., Wolmark N., et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J. Natl. Cancer Inst. 1997;89(22):1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 58.Fisher B., Redmond C., Wickerham D.L., et al. Doxorubicin-containing regimens for the treatment of stage ii breast cancer: the national surgical adjuvant breast and bowel project experience. J. Clin. Oncol. 1989;7(5):572–582. doi: 10.1200/JCO.1989.7.5.572. [DOI] [PubMed] [Google Scholar]
- 59.Fisher B., Brown A.M., Dimitrov N.V., et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the national surgical adjuvant breast and bowel project B-15. J. Clin. Oncol. 1990;8(9):1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 60.Hortobagyi G.N., Blumenschein G.R., Spanos W., et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer. 1983;51(5):763–768. doi: 10.1002/1097-0142(19830301)51:5<763::aid-cncr2820510502>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 61.French Epirubicin Study Group A prospective randomized phase Iii trial comparing combination chemotherapy with cyclophosphamide, fluorouracil, and either doxorubicin or epirubicin. French epirubicin study group. J. Clin. Oncol. 1988;6(4):679–688. doi: 10.1200/JCO.1988.6.4.679. [DOI] [PubMed] [Google Scholar]
- 62.French adjuvant study group. Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French adjuvant study group 05 randomized trial. J. Clin. Oncol. 2001;19(3):602–611. doi: 10.1200/JCO.2001.19.3.602. [DOI] [PubMed] [Google Scholar]
- 63.Creagan E.T., Green S.J., Ahmann D.L., Ingle J.N., Edmonson J.H., Jr R.F.M. A phase Iii clinical trial comparing the combination cyclophosphamide, adriamycin, cisplatin with cyclophosphamide, 5-fluorouracil, prednisone in patients with advanced breast cancer. J. Clin. Oncol. 1984;2(11):1260–1265. doi: 10.1200/JCO.1984.2.11.1260. [DOI] [PubMed] [Google Scholar]
- 64.Saphner T., Tormey D.C., Albertini M. Continuous infusion 5-fluorouracil with escalating doses of intermittent cisplatin and etoposide. A phase I study. Cancer. 1991;68(11):2359–2362. doi: 10.1002/1097-0142(19911201)68:11<2359::aid-cncr2820681105>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 65.Trump D.L., Ettinger D.S., Doxorubicin M.D.A. vincristine, and cis-diamminedichloroplatinum (Ii) therapy in patients with advanced breast cancer. Med. Pediatr. Oncol. •••;981(9):1–3. doi: 10.1002/mpo.2950090102. [DOI] [PubMed] [Google Scholar]
- 66.Martoni A., Tomasi L., Farabegoli G., et al. A phase II study of 4′-epi-doxorubicin plus cis-platinum in advanced solid tumors. Eur. J. Cancer Clin. Oncol. 1984;20(1):11–17. doi: 10.1016/0277-5379(84)90028-2. [DOI] [PubMed] [Google Scholar]
- 67.Smith I.E., Talbot D.C. Cisplatin and Its analogues in the treatment of advanced breast cancer: a review. Br. J. Cancer. 1992;65(6):787–793. doi: 10.1038/bjc.1992.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonneterre J., Dieras V., Tubiana-Hulin M., et al. Phase II multicentre randomised study of docetaxel plus epirubicin vs. 5-fluorouracil plus epirubicin and cyclophosphamide in metastatic breast cancer. Br. J. Cancer. 2004;91(8):1466–1471. doi: 10.1038/sj.bjc.6602179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donehower R.C., Rowinsky E.K. An overview of experience with taxol* (paclitaxel) in the U.S.A. Cancer Treat. Rev. 1993;19:63–78. doi: 10.1016/0305-7372(93)90049-w. [DOI] [PubMed] [Google Scholar]
- 70.Hortobagyi G.N. Paclitaxel-based combination chemotherapy for breast cancer. Oncology. 1997;11:29–37. [PubMed] [Google Scholar]
- 71.Gianni L., Munzone E., Capri G., et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J. Clin. Oncol. 1995;13(11):2688–2699. doi: 10.1200/JCO.1995.13.11.2688. [DOI] [PubMed] [Google Scholar]
- 72.Wasserheit C., Frazein A., Oratz R., et al. Phase ii trial of paclitaxel and cisplatin in women with advanced breast cancer: an active regimen with limiting neurotoxicity. J. Clin. Oncol. 1996;14(7):1993–1999. doi: 10.1200/JCO.1996.14.7.1993. [DOI] [PubMed] [Google Scholar]
- 73.Gelmon K.A., O’Reilly S.E., Tolcher A.W., et al. Phase I/II trial of biweekly paclitaxel and cisplatin in the treatment of metastatic breast cancer. J. Clin. Oncol. 1996;14(4):1185–1191. doi: 10.1200/JCO.1996.14.4.1185. [DOI] [PubMed] [Google Scholar]
- 74.Esteva F.J., Valero V., Pusztai L., Boehnke-Michaud L., Buzdar A.U., Hortobagyi G.N. Chemotherapy of metastatic breast cancer: what to expect in 2001 and beyond. Oncologist. 2001;6(2):133–146. doi: 10.1634/theoncologist.6-2-133. [DOI] [PubMed] [Google Scholar]
- 75.Nabholtz J-M., Falkson C., Campos D., et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III Trial. J. Clin. Oncol. 2003;21(6):968–975. doi: 10.1200/JCO.2003.04.040. [DOI] [PubMed] [Google Scholar]
- 76.Smith R.E., Anderson S.J., Lembersky B.C., Brown A., Mamounas E.P. Phase ii trial of a doxorubicin/docetaxel doublet for locally advanced and metastatic breast cancer: results from national surgical adjuvant breast and bowel project trial Bp-57. Clin. Breast Cancer. 2004;5(3):208–215. doi: 10.3816/cbc.2004.n.024. [DOI] [PubMed] [Google Scholar]
- 77.Sessa C., Pagani O. Docetaxel and epirubicin in advanced breast cancer. Oncologist. 2001;6:13–16. doi: 10.1634/theoncologist.6-suppl_3-13. [DOI] [PubMed] [Google Scholar]
- 78.Trudeau M.E., Chapman J-A.W., Guo B., et al. A phase I/II trial of epirubicin and docetaxel in locally advanced breast cancer (labc) on 2-weekly or 3-weekly schedules: NCIC CTG MA.22. Springerplus. 2015;4(1):631. doi: 10.1186/s40064-015-1392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park S.H., Cho E.K., Bang S-M., Shin D.B., Lee J.H., Lee Y.D. Docetaxel plus cisplatin is effective for patients with metastatic breast cancer resistant to previous anthracycline treatment: a phase II clinical trial. BMC Cancer. 2005;5:21. doi: 10.1186/1471-2407-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrez J-C. Mitomycins syntheses: a recent update. Beilstein J. Org. Chem. 2009;5:33. doi: 10.3762/bjoc.5.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colozza M., Tonato M., Grignani F., Davis S. Low-dose mitomycin and weekly low-dose doxorubicin combination chemotherapy for patients with metastatic breast carcinoma previously treated with cyclophosphamide, methotrexate, and 5-fluorouracil. Cancer. 1988;62(2):262–265. doi: 10.1002/1097-0142(19880715)62:2<262::aid-cncr2820620206>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 82.Panasci L., Shenouda G., Begin L., Pollak M., Reinke A., Margolese R. Mitomycin C and mitoxantrone chemotherapy for advanced breast cancer: efficacy with minimal gastrointestinal toxicity and alopecia. Cancer Chemother. Pharmacol. 1990;26(6):457–460. doi: 10.1007/BF02994099. [DOI] [PubMed] [Google Scholar]
- 83.Ospovat I., Siegelmann-Danieli N., Grenader T., Hubert A., Hamburger T., Peretz T. Mitomycin C and vinblastine: an active regimen in previously treated breast cancer patients. Tumori. 2009;95(6):683–686. doi: 10.1177/030089160909500607. [DOI] [PubMed] [Google Scholar]
- 84.Tanabe M., Ito Y., Tokudome N., et al. Possible use of combination chemotherapy with mitomycin C and methotrexate for metastatic breast cancer pretreated with anthracycline and taxanes. Breast Cancer. 2009;16(4):301. doi: 10.1007/s12282-009-0093-0. [DOI] [PubMed] [Google Scholar]
- 85.Fukuda T., Tanabe M., Kobayashi K., et al. Combination chemotherapy with mitomycin C and methotrexate is active against metastatic HER2-negative breast cancer even after treatment with anthracycline, taxane, capecitabine, and vinorelbine. Springerplus. 2015;4(1):376. doi: 10.1186/s40064-015-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gómez H.L., Neciosup S., Tosello C., et al. A phase II randomized study of lapatinib combined with capecitabine, vinorelbine, or gemcitabine in patients with HER2-positive metastatic breast cancer with progression after a taxane (latin american cooperative oncology group 0801 study). Clin. Breast Cancer. 2016;16(1):38–44. doi: 10.1016/j.clbc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Yardley D.A., Ward P.J., Daniel B.R., et al. Panitumumab, gemcitabine, and carboplatin as treatment for women with metastatic triple-negative breast cancer: a sarah cannon research institute phase II trial. Clin. Breast Cancer. 2016;16(5):349–355. doi: 10.1016/j.clbc.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Cinieri S., Chan A., Altundag K., et al. Final results of the randomized phase Ii norcap-ca223 trial comparing first-line all-oral versus taxane-based chemotherapy for HER2-negative metastatic breast cancer. Clin. Breast Cancer. 2017;17(2):91–99. doi: 10.1016/j.clbc.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 89.Schroder L., Rack B., Sommer H., et al. Toxicity assessment of a phase Iii study evaluating fec-doc and fec-doc combined with gemcitabine as an adjuvant treatment for high-risk early breast cancer: the success-a trial. Geburtshilfe Frauenheilkd. 2016;76(5):542–550. doi: 10.1055/s-0042-106209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Earl H.M., Hiller L., Howard H.C., et al. Addition of gemcitabine to paclitaxel, epirubicin, and cyclophosphamide adjuvant chemotherapy for women with early-stage breast cancer (tango): final 10-year follow-up of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(6):755–769. doi: 10.1016/S1470-2045(17)30319-4. [DOI] [PubMed] [Google Scholar]
- 91.Lu X., Xiao L., Wang L., Ruden D.M. Hsp90 inhibitors and drug resistance in cancer: the potential benefits of combination therapies of Hsp90 inhibitors and other anti-cancer drugs. Biochem. Pharmacol. 2012;83(8):995–1004. doi: 10.1016/j.bcp.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Modi S., Stopeck A.T., Gordon M.S., et al. Combination of trastuzumab and tanespimycin (17-aag, kos-953) is safe and active in trastuzumab-refractory her-2-overexpressing breast cancer: a phase I dose-escalation study. J. Clin. Oncol. 2007;25(34):5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 93.Modi S., Stopeck A., Linden H., et al. Hsp90 inhibition is effective in breast cancer: a phase ii trial of tanespimycin (17-aag) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin. Cancer Res. 2011;17(15):5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 94.Baselga J., Cortés J., Kim S-B., et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. new england journal of medicine. N. Engl. J. Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swain S.M., Kim S-B., Cortés J., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (Cleopatra study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swain S.M., Ewer M.S., Cortés J., et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in Cleopatra: a randomized, double-blind, placebo-controlled phase III Study. Oncologist. 2013;18(3):257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Swain S.M., Baselga J., Kim S.B. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhaskar S., Tian F., Stoeger T., et al. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010;7(1):3. doi: 10.1186/1743-8977-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drbohlavova J., Chomoucka J., Adam V., et al. Nanocarriers for anticancer drugs-new trends in nanomedicine. Curr. Drug Metab. 2013;14(5):547–564. doi: 10.2174/1389200211314050005. [DOI] [PubMed] [Google Scholar]
- 100.Su H., Wang Y., Gu Y., Bowman L., Zhao J., Ding M. Potential applications and human biosafety of nanomaterials used in nanomedicine. J. Appl. Toxicol. 2018;38(1):3–24. doi: 10.1002/jat.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davis M.E., Chen Z. DM. S. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 102.Ma L., Kohli M., Smith A. Nanoparticles for combination drug therapy. ACS Nano. 2013;7(11):9518–9525. doi: 10.1021/nn405674m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shuhendler A.J., Cheung R.Y., Manias J., Connor A., Rauth A.M., Wu X.Y. A novel doxorubicin-mitomycin c co-encapsulated nanoparticle formulation exhibits anti-cancer synergy in multidrug resistant human breast cancer cells. Breast Cancer Res. Treat. 2009;119(2):255. doi: 10.1007/s10549-008-0271-3. [DOI] [PubMed] [Google Scholar]
- 104.Prasad P., Shuhendler A., Cai P., Rauth A.M., Wu X.Y. Doxorubicin and mitomycin c co-loaded polymer-lipid hybrid nanoparticles inhibit growth of sensitive and multidrug resistant human mammary tumor xenografts. Cancer Lett. 2013;334(2):263–273. doi: 10.1016/j.canlet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 105.Zhang R.X., Cai P., Zhang T., et al. Polymer-lipid hybrid nanoparticles synchronize pharmacokinetics of co-encapsulated doxorubicin-mitomycin c and enable their spatiotemporal co-delivery and local bioavailability in breast tumor. Nanomedicine. 2016;12(5):1279–1290. doi: 10.1016/j.nano.2015.12.383. [DOI] [PubMed] [Google Scholar]
- 106.Hasenstein J.R., Shin H-C., Kasmerchak K., Buehler D., Kwon G.S., Kozak K.R. Anti-tumor activity of triolimus: a novel multi-drug loaded micelle containing paclitaxel, rapamycin and 17-Aag. Mol. Cancer Ther. 2012;11(10):2233–2242. doi: 10.1158/1535-7163.MCT-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu D., Li W., Zhang Y., Zhang B. Anti-tumor efficiency of paclitaxel and dna when co-delivered by ph responsive ligand modified nanocarriers for breast cancer treatment. Biomed. Pharmacother. 2016;83:1428–1435. doi: 10.1016/j.biopha.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 108.Rong L., Zhou S., Liu X., et al. Trastuzumab-modified dm1-loaded nanoparticles for HER2(+) breast cancer treatment: an in Vitro and in Vivo Study. Artif. Cells Nanomed. Biotechnol. 2017;46(8):1–11. doi: 10.1080/21691401.2017.1391821. [DOI] [PubMed] [Google Scholar]
- 109.Varshosaz J., Davoudi M.A., Rasoul-Amini S. Docetaxel-loaded nanostructured lipid carriers functionalized with trastuzumab (herceptin) for HER2-positive breast cancer cells. J. Liposome Res. 2017;28(4):1–11. doi: 10.1080/08982104.2017.1370471. [DOI] [PubMed] [Google Scholar]
- 110.Li L., Tong R., Li M., Kohane D.S. Self-assembled gemcitabine-gadolinium nanoparticles for magnetic resonance imaging and cancer therapy. Acta Biomater. 2016;33:34–39. doi: 10.1016/j.actbio.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Souza Albernaz M., Toma S.H., Clanton J., Araki K., Santos-Oliveira R. Decorated superparamagnetic iron oxide nanoparticles with monoclonal antibody and diethylene-triamine-pentaacetic acid labeled with thechnetium-99m and galium-68 for breast cancer imaging. Pharm. Res. 2018;35(1):24. doi: 10.1007/s11095-017-2320-2. [DOI] [PubMed] [Google Scholar]
- 112.Tezuka K., Takashima T., Kashiwagi S., et al. Phase I study of nanoparticle albumin-bound paclitaxel, carboplatin and trastuzumab in women with human epidermal growth factor receptor 2-overexpressing breast cancer. Mol. Clin. Oncol. 2017;6(4):534–538. doi: 10.3892/mco.2017.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee J.H., Nan A. Combination drug delivery approaches in metastatic breast cancer. J. Drug Deliv. 2012;2012:17. doi: 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu C-M.J., Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharmacol. 2012;83(8):1104–1111. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 115.Sparreboom A., Baker S.D., Verweij J. Paclitaxel in an albumin-stabilized nanoparticle: handy or just a dandy? J. Clin. Oncol. 2005;23(31):7765–7767. doi: 10.1200/JCO.2005.03.7135. [DOI] [PubMed] [Google Scholar]
- 116.Akala EO, Okunola O. 2013.
- 117.Adesina S.K., Wight S.A., Akala E.O. Optimization of the fabrication of novel stealth PLA-based nanoparticles by dispersion polymerization using D-optimal mixture design. Drug Dev Ind Pharm Drug Dev Ind Pharm. 2014;40(11):1547–1556. doi: 10.3109/03639045.2013.838578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adesina S.K., Holly A., Kramer-Marek G., Capala J., Akala E.O. Polylactide based paclitaxel loaded nanoparticles fabricated by dispersion polymerization: characterization, evaluation in cancer cell lines, and preliminary biodistribution studies. J. Pharm. Sci. 2014;103(8):2546–2555. doi: 10.1002/jps.24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ogunwuyi O., Adesina S.K., Akala E.O. D-optimal mixture experimental design for stealth biodegradable crosslinked docetaxel-loaded poly-E-caprolactone nanoparticles fabricated by dispersion polymerization. Pharmazie. 2015;70:165–176. [PubMed] [Google Scholar]
- 120.Oluwaseun O., Namita K., Kahli A.S., et al. Antiretroviral drugs-loaded nanoparticles fabricated by dispersion polymerization with potential for HIV/AIDS treatment. Infect Dis: Res Treat. 2016;9:21–32. doi: 10.4137/IDRT.S38108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puri R., Berhe S.A., Akala E.O. pH-sensitive polymeric nanoparticles fabricated by dispersion polymerization for the delivery of bioactive agents. Pharm. Nanotechnol. 2017;5:1–28. doi: 10.2174/2211738505666170110102320. [DOI] [PubMed] [Google Scholar]
- 122.Puri Reema, Adesina Simeon. 2018.
- 123.von Roemeling C., Jiang W., Chan C.K., Weissman I.L., Kim B.Y. Breaking down the barriers to precision cancer nanomedicine. Trends Biotechnol. 2017;35(2):159–171. doi: 10.1016/j.tibtech.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 124.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Emmanuel O.A., Simeon K.A. Fabrication of polymeric core-shell nanostructures. In: Grumezescu A.M., editor. Nanoscale fabrication, optimization, scale-up and biological aspects of pharmaceutical nanotechnology. Oxford: Elsevier; 2017. pp. 1–49. [Google Scholar]
- 126.Choi H.S., Ipe B.I., Misra P., Lee J.H., Bawendi M.G., Frangioni J.V. Tissue-and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009;9(6):2354–2359. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harris J.M., Chess R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 128.Schöttler S., Becker G., Winzen S., et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated Nanocarriers. Nat. Nanotechnol. 2016;11:372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 129.Carl D.W., Jonathan B.O., Hongbo G., Andrew E., Warren C.W.C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 130.Niewoehner J.B.B., Ludovic C., Eduard U., Hadassah S., Peter M., Petra R. 2 increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]


