Abstract
Background: Solubility is an important criterion for drug efficacy, independent of the route of administration. It also poses a major challenge for pharmaceutical indus-tries, which are developing new pharmaceutical products, since 40% of the active sub-stances being identified are either insoluble or poorly soluble in aqueous media.
Objective: The objective of this study was to develop nanoformulation of glipizide drug-loaded nanoparticles providing controlled release formulation.
Method: Nanoparticles were prepared by the solvent evaporation method. Eudragit RS100, a nonbiodegradable polymer with varying ratios was used for making the formula-tion. The effect of key formulation variables on the particle size and entrapment efficien-cy and drug loading of nanoparticles were studied by using factorial design.
Results: DSC thermograms indicate that glipizide was dispersed in an amorphous state in the polymer. TEM study indicates that the nanoparticles were in spherical shape. The mean diameter was dependent on the presence of the amount of Eudragit RS100 and vis-cosity of the organic phase. The in vitro study showed that the cumulative drug release was from 69.52-81.44% in 10 hrs at pH 6.8 in phosphate buffer respectively.
Conclusion: The developed NPs could reduce dose frequency, decrease side effects, and improve patient compliance. Using factorial design, maximum entrapment efficiency with minimum particle size could be achieved with a few experiments.
Keywords: Characterization, Eudragit RS-100, glipizide, optimization, solvent evaporation, nanoparticles
1. INTRODUCTION
Solubility is an important criterion for drug efficacy, independent of the route of administration. It also poses a major challenge for pharmaceutical industries, which are developing new pharmaceutical products, since 40% of the active substances being identified are either insoluble or poorly soluble in aqueous media. Over the last 10 years, nanoparticle (NP) engineering processes have been developed and reported for the enhancement of solubility of poorly aqueous soluble drugs [1].
Diabetes mellitus (DM) is one of the major incapacitating disorders spread worldwide, contributing to huge financial and health loses. Studies conducted in India highlighted that the prevalence of this metabolic dysfunction is high and increasing rapidly in the urban population due to sedentary lifestyle, aging, nutrition, stress, and obesity. The primary cause of Diabetes Mellitus Type 2 is that the pancreas may produce enough insulin to transport sugar into the cells, but the body may refuse to use the insulin [2]. Insulin helps unlock the cells of the body to allow the sugar to enter them so that glucose is transformed into energy. In Diabetes Type 2, the body refuses to use the insulin produced so insulin is unable to let the sugar enter the cells and transform into energy. The secondary reason is insufficient for the production of insulin by the pancreas. The second generation hypoglycemic agents are used for Non-Insulin Dependent Diabetes Mellitus (NIDDM) [3, 4].
glipizide is an oral hypoglycemic agent, which is a commonly prescribed drug for the treatment of patients with type II diabetes. It is used as an adjunct to diet to the management of type II (non-insulin dependent) diabetes mellitus in patients whose hyperglycemia cannot be controlled by diet and exercise alone. glipizide is a weak acid (pKa = 5.9) practically insoluble in water and acidic environment and highly permeable (class II) drugs according to the Biopharmaceutical Classification System (BCS) [5].
Biodegradable and non-biodegradable polymers are ideal carriers for the formulation of nanoparticles. Most biodegradable polymers consist of synthetic polyesters like polycyanoacrylate or ploy (D, L-lactide) and related polymers like poly (lactic acid) PLA or poly (lactide-co-glycolide) to give a few examples. The solvent evaporation method is one of the most preferred methods used for the preparation of nanoparticles [6, 7].
Eudragit polymers can be used to produce formulations which allow custom-tailored release profiles and are released over a specific period of time. Drug delivery can be controlled throughout the entire GI tract (GIT) to increase the therapeutic effect and patient compliance. Different polymer combinations of Eudragit RS 100 allow custom-tailored release profiles to achieve the desired drug delivery performance [8].
The aim of the present work is to formulate nanoparticles using Eudragit RS100 polymer using the solvent evaporation method and optimize and evaluate sustained release nanoparticles to improve its bioavailability [9, 10].
2. MATERIALS AND METHODS
2.1. Materials
Glipizide was purchased from Sigma-Aldrich. Eudragit RS100 polymer was a gift sample from Evonik Degussa India Private Limited, Mumbai. Dichloromethane, Polyvinyl alcohol and PLA polymer were also purchased from Sigma-Aldrich.
2.2. Factorial Design and Optimization
In this study, the first step was to screen using 32 factorial design. The second step was to optimize using 32 factorial designs to investigate the physicochemical properties of glipizide nanoparticles. Design-Expert (version 7.0.0; stat-Ease, Inc., Minneapolis, Minnesota, USA) was used for mathematical modeling and assessment of the response. A preliminary study was carried out based on prior knowledge from a literature survey [11, 12]. Based on the results obtained from preliminary experiments, the amount of Eudragit RS-100 (X1) and concentration of PVA (X2) was in the range of low, medium and high for independent variables which are presented in Table 1. Entrapment Efficiency (EE), Particle Size (nm) and drug loading were the response variables.
Table 1. Process variables and their levels for full factorial design.
| Independent Variables | Levels | ||
|---|---|---|---|
| Low (-1) | Medium (0) | High (+1) | |
| X1 | 50 | 100 | 150 |
| X2 | 0.15 | 0.30 | 0.45 |
In developing the regression equation, the test factors were coded according to equation 1.
= (Xi-)/ (1)
where xi is the coded value of ith independent variable, Xi is the natural value of the ith independent variable, is the natural value of the ith independent variable at the center points and Xi is the step change value. The multiple regressions according to the quadratic model were carried out using equation 2.
Y= b0 + + bij Xi Xj + (2)
where Y is the measured response, bo is the intercept term, bi, bij and bii are the measures of the variables Xi, XiXij and Xi.2 The variable XiXj represents the first-order interactions between Xi and Xj (i<j). Multiple regression was applied using Microsoft Excel in order to reduce the factors having a significant effect on the properties of formulations and then the best fitted mathematical model was selected on the basis of the results. The 3-D response surface plots and 2-D contour plots were studied using Design-Expert [11-13]. The results of full factorial design and observational data are given in Table 2.
Table 2. Results of 32 factorial design of glipizide loaded nanoparticles.
| Run |
Amount of Polymer
Eudragit RS-100 (mg) X1 |
Amount of Surfactant
PVA (% w/v) X2 |
Particle Size (nm)
Y1 |
Entrapment
Efficiency (%) Y2 |
% Drug Loading
Y3 |
|---|---|---|---|---|---|
| 1 | 150 | 0.3 | 857 | 62.56 | 16.87 |
| 2 | 100 | 0.3 | 423.8 | 77.97 | 37.94 |
| 3 | 100 | 0.3 | 465.3 | 77.29 | 38.12 |
| 4 | 50 | 0.15 | 377.8 | 64.49 | 32.56 |
| 5 | 100 | 0.3 | 510.6 | 77 | 38.51 |
| 6 | 100 | 0.45 | 257.7 | 81.23 | 40.12 |
| 7 | 100 | 0.3 | 354.8 | 77.81 | 37.12 |
| 8 | 100 | 0.15 | 532.5 | 75.12 | 36.1 |
| 9 | 150 | 0.45 | 720.9 | 65.18 | 18.23 |
| 10 | 100 | 0.3 | 585.5 | 69.75 | 33.12 |
| 11 | 50 | 0.45 | 243 | 77.81 | 37.12 |
| 12 | 50 | 0.3 | 336.2 | 71.73 | 35.54 |
| 13 | 150 | 0.15 | 864.5 | 58.43 | 15.43 |
2.3. Preparations of Glipizide Nanoparticles
Solvent evaporation method was used for the preparation of glipizide nanoparticles. Firstly, the emulsification of polymeric solution was done in an aqueous solution containing a surfactant. Then the evaporation of polymeric solution was done by precipitation of the polymer. In the solution of dichloromethane (DCM), acetone drug was dissolved. With constant stirring using a magnetic stirrer, the organic solution was added into an aqueous phase containing polyvinyl alcohol. The emulsion was sonicated using probe sonicator for 6 min to get nanosize of the emulsion. The organic solvent was then evaporated using constant stirring on a magnetic stirrer for about 4-5 hrs. After centrifugation (30 min, 10000 rpm), the nanoparticles were collected. The prepared emulsion was then kept for lyophilization for 48 hrs [14-16].
2.4. Size Measurement
Nanoparticles after freeze-drying were reconstituted in distilled water. Zeta potential of nanoparticles was determined by particle size analyzer (Zeta Sizer Ver System, UK). The measurement was done in triplicate [17, 18].
2.5. Drug Loading and Entrapment Efficiency
The entrapment efficiency and drug loading of nanoparticles containing glipizide were determined by spectrophotometric determination of nanoparticles at 276 nm in a supernatant centrifuged for 30 min at 10000 rpm [19, 20].
The entrapment efficiency and drug loading was determined from following equations:
Entrapment Efficiency
Drug Loading
2.6. Drug Release Studies
A modified dialysis method was used to evaluate the in vitro release of nanoparticles. 2 ml. of nanoparticles suspension was placed in a dialysis bag (cellophane membrane) which was tied and placed into 900 ml. of phosphate buffer with pH 6.8, at temperature 37°C and at 100rpm in USPXXV Type II (Paddle) dissolution apparatus [21]. At selected time intervals, 5 ml aliquots were withdrawn from the medium and replaced with the same amount of buffer. The data obtained from in vitro drug release were fitted to kinetic models to understand the mechanism of drug release from the nanoparticles. The study was performed in triplicate [22].
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR study was done using Fourier Transform Infrared spectrophotometer (Shimadzu, 8400S, Japan). The test samples were mixed with KBr, pressed into a disk and scanned from 400-4000 cm-1 [23].
2.8. Differential Scanning Calorimetry
The physical state characteristics of glipizide entrapped nanoparticles were characterized by Differential Scanning Calorimetry (DSC-60, Shimadzu, Japan). Each sample was selected in standard aluminum pans with lids and purged with air at a flow rate of 40 ml/min. The heat flows were recorded in the range of 30-300°C under inert nitrogen atmosphere [24].
2.9. Transmission Electron Microscopy
TEM analysis of the prepared nanoparticles was carried out to study the morphology of nanoparticles. For the study, a drop of nanoparticles suspension containing 0.01% of phosphotungstic acid was placed on a carbon film coated on a copper grid. The study was performed using Philips Technai-20, Japan. The copper grid was fixed into a sample holder and placed in a vacuum chamber of the transmission electron microscope and observed under low vacuum and the TEM images were recorded [25].
3. RESULTS AND DISCUSSION
3.1. Optimization and the Experiment
The 32 factorial design was used to study the responses for all formulations on the basis of variables. The responses observed for all formulations were Particle size (Y1), Entrapment Efficiency (Y2) and Drug Loading (Y3). Table 2 shows the experimental design of Eudragit RS-100 nanoparticles and the results of measured responses. The effect of the combination of polymer and surfactant (PVA) on entrapment efficiency and drug loading was studied using the response surface plot and the results of the response surface plot are given in Figs. (1-3).
Fig. (1).

Response surface plot showing combined effects of Eudragit rs-100 and pva on particle size of nanoparticles.
Fig. (3).

Response surface plot showing combined effect of Eudragit rs-100 and pva on percent drug loading of nanoparticles.
Based on the results obtained in preliminary experiments, the amount of Eudragit RS-100 (X1) and concentration of PVA (X2) were found to be major variables affecting the Particle size (Y1), Entrapment efficiency (Y2) and % Drug loading (Y3) of the nanoparticles. In case of particle size, the results showed that an increase in PS due to an increase in the polymer concentration and a decrease in the volume of organic phase. In case of drug entrapment efficiency, the results indicate that an increase in drug entrapment due to an increase in polymer concentration and a decrease in the solvent.
3.2. Response Surface Plots
Three-dimensional response surface plots generated by the Design Expert are presented in Fig. (1) for glipizide nanoparticles. Fig. (1) depicts the response surface plots for the particle size of glipizide nanoparticle which show an increase in PS due to an increase in the polymer concentration and a decrease in the volume of the organic phase [26-28]. Fig. (2) depicts the response surface plot for Drug entrapment efficiency which indicates an increase in drug entrapment due to an increase in polymer concentration and a decrease in the solvent. Fig. (3) shows the effect of concentration of polymer and PVA on drug loading.
Fig. (2).

Response surface plot showing combined effect of Eudragit rs-100 and pva on % entrapment efficiency of nanoparticles.
Quadratic model was found to be significant for particle size, entrapment efficiency and drug loading
variables. Table 3 shows the model summary statistics of responses.
Table 3. Model summary statistics of Eudragit rs100 nanoparticles formulation response to select suitable model.
| Response | Model | Adjusted R-Squared | Predicted R-Squared | Press | Significance |
|---|---|---|---|---|---|
| Y1 | Linear | 0.7812 | 0.6957 | 1.559E+005 | - |
| 2FI | 0.7569 | 0.5611 | 2.248E+005 | - | |
| Quadratic | 0.8743 | 0.7745 | 1.155E+005 | Suggested | |
| Y2 | Linear | 0.2623 | -0.2019 | 760.01 | - |
| 2FI | 0.2030 | -1.2967 | 1452.34 | - | |
| Quadratic | 0.8381 | 0.7427 | 162.70 | Suggested | |
| Y3 | Linear | 0.4518 | 0.1018 | 859.93 | - |
| 2FI | 0.3920 | -0.8333 | 1755.27 | - | |
| Quadratic | 0.9619 | 0.9570 | 41.14 | Suggested |
The relationship between the formulation variables and the responses in terms of coded factors is represented by the following equations [29, 30].
Particle Size (Y1) = +464.16+247.57* A -92.20* B -2.20* AB +142.04* A2 -59.46* B2
Entrapment Efficiency (Y2) = +76.36-4.64* A +4.36* B -1.64* AB -10.21* A2 +0.82* B2
Drug Loading (Y3) = +37.17-9.11* A +1.90* B -0.44* AB -11.49* A2 +0.42* B2
where
A = Amount of polymer
B = Amount of surfactant (PVA)
AB = Amount of polymer (Eudragit RS100) * surfactant (PVA)
3.3. Solution of Numerical Optimization
On the basis of desirability approach, the numerical optimization technique was used to develop a new formulation with the desired responses of minimum particle size, maximum entrapment efficiency and maximum drug loading [31, 32]. The desirability function combines all responses in one measurement, in order to achieve the desired goal. After obtaining the desired goal, the lower limit and upper limits were set for different points as shown in Table 4.
Table 4. Constraints of numerical optimization (Eudragit rs100 nanoparticles).
| Name | Goal |
Lower
Limit |
Upper
Limit |
Lower
Weight |
Upper
Weight |
Importance |
|---|---|---|---|---|---|---|
| A:Amount of polymer (Eudragit RS100) |
is in range | 50 | 150 | 1 | 1 | 3 |
| B:Surfactant | is in range | 0.15 | 0.45 | 1 | 1 | 3 |
| Particle Size (PS) | minimize | 243 | 864.5 | 1 | 1 | 4 |
| % Entrapment Efficiency (EE) | maximize | 58.43 | 81.23 | 1 | 1 | 3 |
| %Drug Loading (DL) | is in range | 15.43 | 40.12 | 1 | 1 | 4 |
By the numerical optimization and after confirmation of report, the formulation compositions are shown in the Table 5. The desirability approach provides a possibility to predict the optimum levels of the independent variables.
Table 5. Solution of numerical optimization (Eudragit RS100 Nanoparticles).
| Sr. No. |
Amount of Polymer
(EuRS100) |
Surfactant | Particle Size (PS) |
%Entrapment Efficiency
(EE) |
%Drug
Loading (DL) |
Desirability | Selection |
|---|---|---|---|---|---|---|---|
| 1 | 62.073 | 0.450 | 208.114 | 80.440 | 40.120 | 0.985 | Selected |
| 2 | 60.578 | 0.450 | 207.349 | 80.156 | 39.874 | 0.980 | - |
| 3 | 54.429 | 0.450 | 206.866 | 78.795 | 38.647 | 0.953 | - |
| 4 | 96.347 | 0.450 | 295.335 | 81.951 | 40.120 | 0.951 | - |
| 5 | 94.214 | 0.433 | 309.557 | 81.432 | 40.120 | 0.937 | - |
3.4. Determination of Particle Size and Percent Drug Entrapment
The freeze-dried nanoparticles were reconstituted in distilled water and their particle size was determined using a particle size analyzer (Zetasizer Ver System, UK). The particle size was increased by increasing the amount of polymer and this was due to the viscous nature of the polymer. The increase in the concentration of the surfactant led to a decrease in the particle size of nanoparticles (Fig. 4) [31, 32].
Fig. (4).

Particle size of glipizide loaded Eudragit rs100 nanoparticles of different formulations.
Entrapment efficiency and drug loading were increased with an increase in the polymer ratio (1:2). The decrease in entrapment efficiency and drug loading was studied after due to saturation capacity of polymer and formation of the more compact polymeric coat. However, drug loading and entrapment efficiency were increased with an increase in the amount of PVA. Due to large free surface, the smaller particle released the incorporated drug in faster rate which can lead to a faster
release of the drug incorporated. Nanoparticles had an average particle size of 257.7 ± 5.6 nm [33, 34].
3.5. Percentage Entrapment Efficiency and Drug Loading
The entrapment efficiency and drug loading were found to be in the range of 58.43% to 81.23% and 15.43% to 40.12% respectively. Entrapment efficiency and drug loading increased with an increase in the polymer ratio, up to a particular concentration (1:2). A decrease in entrapment efficiency and drug loading was observed after that due to the saturation capacity of the polymer and formation of the more compact polymeric coat which is shown in Figs. (5 and 6). However, the entrapment efficiency and drug loading were increased with increase in PVA concentration [35].
Fig. (5).

Entrapment efficiency of glipizide loaded Eudragit rs100 nanoparticles of different formulations.
Fig. (6).

Drug loading of glipizide loaded Eudragit rs100 nanoparticles different formulations.
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
The spectrum of glipizide and physical mixture of glipizide and Eudragit RS-100 is shown in Fig. (7). The FTIR spectra of Eudragit RS100 confirmed the peaks at 2981cm-1 because of CH aliphatic stretching and1723 cm-1 due to -CO stre-tching. Matching of FT-IR spectrum of glipizide and Eudragit was not involved in intermolecular interaction with the drug. The reduction in the intensity of peaks of nanoparticles formulation is probably due to weak electrostatic interaction between drug and ammonium group of polymer due to solvent evaporation. Results are shown in Table 6 [34, 35].
Fig. (7).
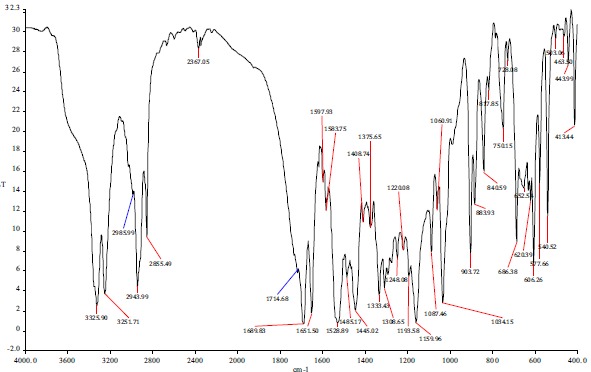
FTIR spectra of glipizide.
Table 6. FTIR interpretation of glipizide loaded rs100 optimized batch.
| Sr. No. | Interpretation | Peaks (cm-1) Observed |
|---|---|---|
| 1 | N-H bending | 1528.89 |
| 2 | S=O stretching | 1333.43 |
| 3 | N-H stretching | 3325.90, 3251.71 |
| 4 | C=O stretching | 1689.83 |
| 5 | CONH- stretching | 1651.50 |
| 6 | C=O stretching of ester group | 1714.68 |
3.7. Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) is one of the general methods to study the physicochemical interaction between the drug and excipients. DSC thermograph of Eudragit RS-100 is shown in Fig. (8).
Fig. (8).

DSC thermogram of glipizide loaded rs 100 optimized batch.
The optimized batch showed two endothermic peaks at 174.44°C due to the presence of Eudragit RS100 and 200.71°C due to the presence of glipizide. The endothermic peak of pure glipizide was found at 207.04°C [33, 34]. There was a slightl shift in the endothermic peak of glipizide in drug-loaded nanoparticles as compared to that of pure glipizide. This may be due to the drug being in an amorphous form rather than crystalline form. DSC studies show that there was compatibility between the drug and the polymers. The thermogram of optimized batch is shown in Fig. (8).
3.8. Dissolution Study
The drug release profile of the drug-loaded nanoparticles is shown in Figs. (9 and 10). Drug release studies of nanoparticles show the biphasic release profile. The release was associated with diffusion release with an initial fast release due to the smaller size of nanoparticles and the drug was able to reach the interface of dissolution medium easily [35, 36]. The release profiles showed the cumulative drug release from 69.52-81.44% in 10 hrs at pH 6.8. The increase in percent cumulative drug release with an increase in surfactant concentration could be attributed to a decrease in the particle size and increase in the surface area available for dissolution. The percent drug release was increased due an increase in the surfactant concentration and this can be attributed to a decrease in the particle size and an increase in the surface area. The release rate reduces as there is an increase in the molecular weight of polymers [37, 38].
Fig. (9).

Drug release profile of F1-F16.
Fig. (10).

Drug release profile of formulations (F7-F13).
3.9. Drug Release Kinetics
The drug release data of optimized batch of glipizide loaded Eudragit RS100 nanoparticles were fit to zero order, first order, and Higuchi and Korsmeyer-Peppas models. The value of correlation coefficient (R2) is shown in Table 7. The result of the highest R2 value of the optimized batch of Eudragit RS100 nanoparticles followed the Korsmeyer-Peppas model. Korsmeyer-Peppas model indicated that drug release followed a combination of diffusion as well as erosion mechanisms [36, 37].
Table 7. Values of correlation coefficients (R2) calculated after fitting the release profile of optimized batch obtained using different mathematical models.
| S. No. | Model | Value of R2 |
|---|---|---|
| 1 | Zero-order | 0.962 |
| 2 | First order | 0.987 |
| 3 | Higuchi order | 0.988 |
| 4 | Korsmeyer-Peppas model | 0.993 |
3.10. Transmission Electron Microscopy
The particle size of the optimized batch was determined by TEM analysis and the patterns were found to be uniform in size and spherical in shape. The image of particles is shown in Fig. (11).
Fig. (11).

TEM image of glipizide loaded rs 100 optimized batch.
CONCLUSION
Glipizide loaded Eudragit RS100 NPs were prepared using the solvent evaporation method. 32 factorial design was employed for the design of formulation. As indicated by the study formulation of glipizide, Eudragit RS100 has the ability to modify the physicochemical characteristics of the drug. Using factorial design maximum entrapment efficiency with minimum particle size could be achieved with few experiments. The response surface plots and contour plots were studied to confirm the same. In vitro release was found to follow the first order Fickian diffusion kinetics. There was an increase in drug encapsulation efficiency. The DSC confirms there is a decrease in crystallinity in the nanoparticles formulations. The intermolecular interaction between drug and Eudragit RS100 was detected in the FT-IR spectrum of the prepared formulation.
ACKNOWLEDGEMENTS
The author is highly thankful to the Chairman, Department of Pharmaceutical Sciences, for providing necessary facilities.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Dora C.P., Singh S.K., Kumar S., Datusalia A.K., Deep A. Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol Pharm Drug Res. 2010;67(3):283–290. [PubMed] [Google Scholar]
- 2.Baily C.J. Potential new treatments for type two diabetes. Trends Pharmacol. Sci. 2000;21:259–265. doi: 10.1016/s0165-6147(00)01506-6. [DOI] [PubMed] [Google Scholar]
- 3.Harlay C.R. Glipizide GRTS has advantages over other second generation sulfonylurea. Clin. Drug Investig. 2002;22:575–584. [Google Scholar]
- 4.Hesieh S.H., Lin J.D., Chang H.Y., Ho Ch., Liou M.J. Sustained release versus immediate release glipizide for the treatment of diabetes mellitus in Chinese patients: a randomized, double blind, double dummy, parallel group, 12 week clinical study. Clin. Ther. 2006;28:1318–1326. doi: 10.1016/j.clinthera.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Venkateswarlu K., Shanthi A. Formulation and evaluation of sustained release glipizide matrix. IOSR J Pharm Biol Sci. 2012;2(5):17–23. [Google Scholar]
- 6.Muthu M.S. Nanoparticles based on PLGA and its copolymer: an overview. Asian J Pharm. 2009;3:266–273. [Google Scholar]
- 7.Price J. Handbook of Pharmaceutical Excipients. London: The Pharmaceutical Press; 2003. pp. 1–917. [Google Scholar]
- 8.Singh S. Neelam, Arora S, Singla YP. An overview of multifaceted significance of eudragit polymers in drug delivery systems. Asian J Pharm Clin Res. 2015;8(5):1–6. [Google Scholar]
- 9.Chowdary K.P.R., Srinivasa Y.R. Design and in vitro evaluation of mucoadhesive controlled release oral tablet of glipizide. Indian J. Pharm. 2003;65:592–599. [Google Scholar]
- 10.Singh B., Bhatowa R., Tripathi C.B., Kapil R. Developing micro-/nanoparticulate drug delivery systems using “design of experiments”. Int. J. Pharm. Investig. 2011;1(2):75–81. doi: 10.4103/2230-973X.82395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma R.K., Garg S. Development and evaluation of osmotically controlled oral delivery system of glipizide. Eur. J. Pharm. Biopharm. 2004;57:513–525. doi: 10.1016/j.ejpb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Myers R.H., Montgomery D.C. Response surface methodology: process and product optimization using designed experiments. New York: John Wiley & Sons; 2002. [Google Scholar]
- 13.Thombre A.G., Denoto A.R., Gibbes D.C. Delivery of glipizide from asymmetric membrane capsules using encapsulated excepients. J. Control. Release. 1999;60:333–341. doi: 10.1016/s0168-3659(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 14.Florence A.T. Issues in oral nanoparticle drug carrier uptake and targeting. J. Drug Target. 2004;12:65–70. doi: 10.1080/10611860410001693706. [DOI] [PubMed] [Google Scholar]
- 15.Khafagy E.S., Morishita M., Onuki Y., Takayama K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv. Drug Deliv. Rev. 2007;59(15):1521–1546. doi: 10.1016/j.addr.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Patel J.K., Patel R.P., Amin A.F., Patel M.M. Formulation and evaluation of mucoadhesive glipizide microspheres. AAPS PharmSciTech. 2005;6:49–55. doi: 10.1208/pt060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harivardhan L.R., Murthy R.S.R. Influence of polymerization technique and experimental variables on the particle properties and release kinetics of methotrexate from poly (butylcyanoacrylate) nanoparticles. Acta Pharm. 2004;54:103–118. [PubMed] [Google Scholar]
- 18.Florence A., Hillery A., Hussain N., Jani P. Nanoparticles as carriers for oral peptide absorption: studies on particle uptake and fate. J. Control. Release. 1995;36(1-2):39–46. [Google Scholar]
- 19.Sonavane G.S., Devarajan P.V. Preparation of alginate nanoparticles using Eudragit E100 as a new complexing agent: development, in-vitro, and in-vivo evaluation. J. Biomed. Nanotechnol. 2007;3:160–169. [Google Scholar]
- 20.Zengshuan M.A., Yeoh H.H., Lim L.Y. Formulation pH modulates of insulin with chitosan nanoparticles. J. Pharm. Sci. 2002;91:1396–1404. doi: 10.1002/jps.10149. [DOI] [PubMed] [Google Scholar]
- 21.Crowley M.M., Schroeder B., Fredersdrof A., et al. Physicochemical properties and mechanism of drug release from ethylcellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int. J. Pharm. 2004;269:509–522. doi: 10.1016/j.ijpharm.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Ehtezazi T., Washington C., Melia C.D. First order release rate from porous PLA microspheres with limited exit on the exterior surface. J. Control. Release. 2000;66:27–38. doi: 10.1016/s0168-3659(99)00255-2. [DOI] [PubMed] [Google Scholar]
- 23.Akl M.A., Kartal-Hodzic A., Oksanen T., et al. Factorial design formulation optimization and in vitro characterization of curcumin-loaded PLGA nanoparticles for colon delivery. J. Drug Deliv. Sci. Technol. 2016;32:10–20. [Google Scholar]
- 24.Lokhande A., Mishra S., Kulkarni R., Naik J. Formulation and evaluation of glipizide loaded nanoparticles. J. Pharm. Pharm. Sci. 2013;5:147–151. [Google Scholar]
- 25.Nayak A.K., Pal D., Santra K. Ispaghula mucilage-gellan mucoadhesive beads of metformin HCl: development by response surface methodology. Carbohydr. Polym. 2014;107:41–50. doi: 10.1016/j.carbpol.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Malakar J., Nayak A.K., Goswami S. Use of response surface methodology in the formulation and optimization of bisoprolol fumarate matrix tablets for sustained drug release. ISRN Pharmacol. 2012;2012:1–10. doi: 10.5402/2012/730624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nayak A.K., Pal D., Santra K. Plantago ovata F. Mucilage-alginate mucoadhesive beads for controlled release of glibenclamide: development, optimization, and in vitro-in vivo evaluation. J. Pharm. (Cairo) 2013;2013:1–13. doi: 10.1155/2013/151035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shid R.L., Dhole S.N., Kulkarni N., Shid S.L. Formulation and evaluation of nanosuspension delivery system for simvastatin. Int J Pharm Sci Nanotechnol. 2014;7:2459–2476. [Google Scholar]
- 29.Naha P.C., Byrne H.J., Panda A.K. Role of polymeric excipients on controlled release profile of Glipizide from PLGA and Eudragit RS 100 Nanoparticles. J. Nanopharm. Drug Deliv. 2013;1(1):74–81. [Google Scholar]
- 30.Verma S., Singh S.K., Verma P.R. Fabrication of lipidic nanocarriers of loratadine for facilitated intestinal permeation using multivariate design approach. Drug Dev. Ind. Pharm. 2016;42(2):288–306. doi: 10.3109/03639045.2015.1052078. [DOI] [PubMed] [Google Scholar]
- 31.Bera K., Khanam J., Mohanraj K.P., Mazumder B. Design and evaluation of mucoadhesive beads of glipizide as a controlled release drug delivery system. J. Microencapsul. 2014;31(3):220–229. doi: 10.3109/02652048.2013.834989. [DOI] [PubMed] [Google Scholar]
- 32.Rao M.E., Swain S., Patra C.N., Mund S.P. Formulation Design, Optimization and Characterization of Eprosartan Mesylate Nanoparticles. Nanosci. Nanotechnol. Asia. 2018;8(1):130–143. [Google Scholar]
- 33.Petkar K.C., Chavhan S., Kunda N., et al. Development of novel octanoyl chitosan nanoparticles for improved rifampicin pulmonary delivery: optimization by factorial design. AAPS PharmSciTech. 2018;31:1–5. doi: 10.1208/s12249-018-0972-9. [DOI] [PubMed] [Google Scholar]
- 34.Verma U., Naik J.B., Deshmukh R., Mishra S. Development of biodegradable glimepiride loaded chitosan nano particles: a factorial design approach. Curr. Environ. Eng. 2018;5(1):68–77. [Google Scholar]
- 35.Jain S., Jain A., Bhargav S. Formulation and evaluation of embelin loaded pectin nanoparticles for the treatment of diabetes. Pancreatology. 2018;18(4):S39. [Google Scholar]
- 36.Reis C.P., Neufeld R.J., Veiga F. 2017. Preparation of drug-loaded polymeric nanoparticles. [DOI] [PubMed] [Google Scholar]
- 37.Landry F., Bazile D., Spenlehauer G., Veillard M., Kreuter J. Influence of the coating agents on the degradation of poly (D,L lactic acid) nanoparticles in model digestive fluids. S.T.P. Pharma Sci. 1996;6(3):195–202. [Google Scholar]
- 38.Parikh R., Parikh J., Dubey R., Soni H., Kapadia K. Poly (D,L-lactide-coglycolide) microspheres containing 5-fluorouracil: optimization of process parameters. AAPS PharmSciTech. 2003;4(2):E13. doi: 10.1208/pt040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this research are available within the article.


