Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease defined by the presence of upper and lower motor neuron dysfunction. Clinical presentation and disease progression are variable between patients with ALS, with psychosocial, neuropsychological, nutritional and genetic factors all related to ALS outcome. We present a detailed characterisation of a patient with familial ALS with a novel TBK1 mutation with coexistent brain atrophy, behavioural changes and metabolic dysfunction.
Methods
This male patient was first seen at age 62 years with a 3-month history of gait unsteadiness, weakness of the right arm and emotional lability. There was a history of depression. His father had died of ALS at age 67 years. At first assessment, there was weakness of the right upper limb, ALS Functional Rating Scale-Revised (ALSFRS-R) was 39 and his weight was 72 kg. After 6 months, there was onset of dysphagia. He had tongue fasciculations, brisk jaw jerk, weakness, wasting, brisk reflexes and fasciculations of upper and lower limbs. The ALSFRS-R was 34 and weight had further declined to 65 kg. Three months later, he had deteriorated further with severe weakness, nausea, anorexia and depression. ALSFRS-R was 25 and weight was 54 kg. His respiratory function declined from FVC of 4.57 L to 1.05 L over 6 months. He died ~16 months after the onset of symptoms.
The patient participated in a series of neuropsychological, imaging and metabolic studies of unselected consenting patients with ALS enrolled from our clinic.1 Neuropsychological assessment results for this patient are presented in online supplementary table 1. Comparison of his individual results with those of other patients with ALS is presented in online supplementary tables 2 to 4. Genetic assessment was done by massively parallel sequencing using a custom-designed neuromuscular gene capture panel that tests for 66 genes known to be associated with ALS (Nextera; Illumina). Genetic testing for the C9ORF72 repeat expansion was also performed, as described.1 Brain imaging was conducted using a whole-body 7 Tesla Siemens MR scanner (Magnetom 7T, Siemens Healthcare, Erlangen, Germany). For metabolic studies, resting energy expenditure was measured by indirect calorimetry (Quark, Cosmed) and body composition by air-displacement plethysmography (BodPod, Cosmed).
jnnp-2018-318823supp001.pdf (137.9KB, pdf)
Results
Genetic testing identified a heterozygous two base pair deletion in the TBK1 gene (NM_013254.3:c.2099_2100del; p.(Val700Glyfs*2); online supplementary figure 1). This variant is not present in the gnomAD population database. Truncating variants in this gene are known to be pathogenic (online supplementary table 5) and therefore was classified as likely pathogenic. No other causative genes were identified.
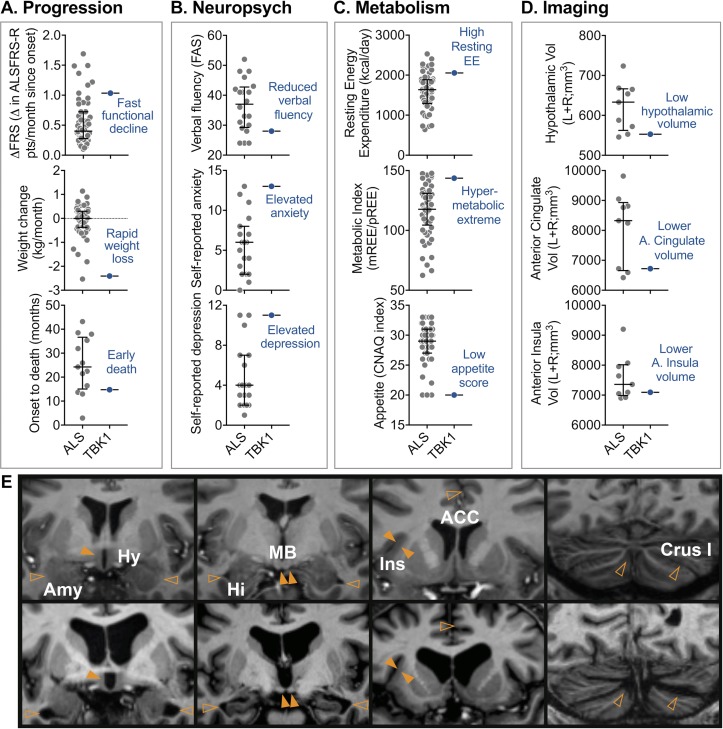
The patient had average age and a short diagnostic delay. Relative to other patients, he had faster disease progression, characterised by faster functional decline, rapid weight loss and earlier death (figure 1A). Neuropsychological assessment revealed a mild degree of cognitive dysfunction in the context of moderate mood and behavioural changes. Mild reductions were documented on executive function tasks that tap word fluency (figure 1B) and response inhibition (Stroop Test), except for severely impaired verbal inhibition (Hayling Test). In addition, mild dysfunction was observed in emotion recognition and auditory-verbal working memory. Self-reported measures of mood ascertained moderate levels of anxiety and depression; these scores were elevated compared with the other patients with ALS (figure 1B). Behavioural changes were evident in increased apathy and emotional lability. By contrast, general intelligence was within the average range, consistent with estimated premorbid levels, and performance was intact on tests of visual memory, auditory attention, visual perception and language. Further, spontaneous speech was fluent and well-articulated, with normal grammar and prosody, and visuomotor speed was in the high average range. This cognitive and behavioural profile reflects a degree of predominantly frontal lobe dysfunction and meets the recent Strong criteria for ALS with behaviour changes (ALSbi) but not ALS with cognitive impairment.2
Figure 1.
Assessment of a patient with ALS with a novel TBK1 mutation, demonstrating coexistent prognostic indicators of short survival. (A) Compared with an unselected population of patients with ALS, the patient with TBK1 had fast functional decline (ΔALSFRS-R), rapid weight loss and shorter survival. (B) Neuropsychological testing showed reduced verbal fluency, elevated self-reported anxiety and depression in the patient with TBK1. (C) Metabolic assessment revealed a high resting energy expenditure, contributing to hypermetabolism. The patient with TBK1 also presented with low appetite. These features have not been reported in patients with ALS with a TBK1 mutation. (E) Compared with a representative example of a patient with ALS (top panels), the patient with TBK1 (bottom panels) had marked brain atrophy and enlarged ventricles. Atrophy was pronounced for the hypothalamus and amygdala (left panels), hippocampus (second panel), anterior cingulate cortex and insula (second last panel) and the cerebellum (right panel). (D) Volume analysis confirmed reduced hypothalamic, anterior cingulate and anterior insula volumes. Data (ALS) presented as median with IQR. For the patient with TBK1, data points outside of the IQR suggest a peripheral phenotype. Brain volumes were corrected for age, sex and total intracranial volume (grey-matter+white-matter+CSF). Solid and hollow arrows signify regions with marked atrophy and enlarged cerebrospinal fluid (CSF) space, respectively. ACC, anterior cingulate cortex; ALS, amyotrophic lateral sclerosis; ALSFRS-R, ALS Functional Rating Scale-Revised; Amy, amygdala; Crus1, Cerebellum Crus 1; CNAQ, Council on Nutrition Appetite Questionnaire; ΔFRS, change in ALSFRS-R (total ALSFRS-R score minus 48 points divided by months since symptom onset); Hi, hippocampus; Hy, hypothalamus; Ins, anterior insula; kcal, kilocalories; MB, mammalian body; mREE, measured resting energy expenditure; pREE, predicted resting energy expenditure using the Nelson Prediction equation adjusting for fat free mass.
He had low fat mass and BMI (online supplementary table 3), increased resting energy expenditure and marked hypermetabolism (figure 1C). The patient presented with loss of appetite (figure 1C). He had marked brain atrophy and enlarged ventricles, compared with other patients with ALS (figure 1D and E). In particular, the volumes of the hypothalamus, anterior cingulate nucleus and anterior insula were among the lowest in patients with ALS (figure 1D; online supplementary table 4). Cortical thinning was also observed in both the cerebrum and cerebellum (figure 1E and online supplementary table 4).
Discussion
This patient had rapidly progressive ALS, with a novel frameshift mutation at codon 700 of the TBK1 gene in the optineurin binding site (online supplementary figure 1). Frameshift mutations in TBK1 are well-documented and generate premature termination codons (online supplementary table 5). Resulting mutant transcripts that contain a premature termination codon are degraded by a nonsense-mediated mRNA decay, resulting in a partial loss of protein.3
The broad phenotype of familial ALS with TBK1 mutations includes variable age and site of onset, survival, association with behavioural variant of FTD (online supplementary table 5) and widespread brain involvement on MRI.3 4 Our patient demonstrated short survival, neuropsychological findings of ALSbi and marked brain atrophy. He also had metabolic abnormalities with marked hypermetabolism, weight loss, reduced fat mass and reduced appetite. These metabolic features have not been previously described with TBK1 mutations (online supplementary table 5) but are important because metabolic abnormalities influence the outcome of disease.1 Metabolic observations therefore contribute to a comprehensive description of disease phenotype. Of interest, increased resting energy expenditure and weight loss in this individual occurred alongside hypothalamic atrophy. This is in line with previous observations in ALS where hypothalamic atrophy correlates with reductions in body mass index.5 The concurrent presentation of hypermetabolism, reduced appetite and hypothalamic atrophy could suggest that these phenomena are linked.
This is the first report to demonstrate metabolic abnormalities and hypothalamic atrophy in a patient with a TBK1 mutation. Given that these abnormalities are poor prognostic indicators and that some patients with TBK1 mutations are reported to have short survival, it is possible that these features will be found in other patients. Future cohort studies are needed to determine if coexistent hypermetabolism and hypothalamic atrophy contributes to disease heterogeneity in the ALS with TBK1 phenotype.
Acknowledgments
We thank all patients with ALS who participated in this study. We also acknowledge the facilities of the National Imaging Facility (NIF) at the Centre for Advanced Imaging, University of Queensland.
Footnotes
Contributors: Study concept and design: PAM, STN, CCG, FJS. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: PAM, STN, CCG, AC, GR, FJS. Critical revision of the manuscript for important intellectual content: PAM, STN, CCG, AC, GR, FJS. Administrative, technical or material support: PAM, STN, CCG, AC, GR, FJS. Supervision: CCG, GR, FJS.
Funding: This work was supported by funding from the Motor Neurone Disease Research Institute of Australia (Cunningham Collaboration MND Research Grant to PAM, RDH and STN; Cunningham Family MND Research Grant to FJS, STN, RDH and PAM), The University of Queensland (UQECR1719385 to STN), the CRC for Mental Health (OS), the QIMR Berghofer (Seed funding to CCG) and the Natural National Science Foundation of China (Project No. 61473234 to XH). The authors acknowledge valuable funding of Wesley Medical Research (Project No. 2016-32 to STN, FJS, RDH and PAM). STN is supported by the Scott Sullivan Fellowship (MND and Me Foundation, Royal Brisbane & Women’s Hospital Foundation and the Queensland Brain Institute) and the Australian Institute for Bioengineering and Nanotechnology at the University of Queensland. CCG is supported by a NHMRC Career Development Fellowship (APP1123674). SB is supported by an Australian Government Research Training Program Scholarship. MB is supported by an ARC Future Fellowship grant (FT140100865). GAR is supported by an Australian NHMRC Boosting Dementia Research Leadership Fellowship (APP1135769).
Competing interests: None declared.
Patient consent: Next of kin consent obtained.
Ethics approval: The Human Research Ethics Committee of the Royal Brisbane and Women's Hospital and the University of Queensland.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Steyn FJ, Ioannides ZA, van Eijk RPA, et al. . Hypermetabolism in ALS is associated with greater functional decline and shorter survival. J Neurol Neurosurg Psychiatry 2018;89:1016–23. 10.1136/jnnp-2017-317887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strong MJ, Abrahams S, Goldstein LH, et al. . Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017;18(3-4):153–74. 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Zee J, Gijselinck I, Van Mossevelde S, et al. . TBK1 mutation spectrum in an extended european patient cohort with frontotemporal dementia and amyotrophic lateral sclerosis. Hum Mutat 2017;38:297–309. 10.1002/humu.23161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Mossevelde S, van der Zee J, Belgian Neurology consortium. Clinical features of TBK1 carriers compared with C9orf72, GRN and non-mutation carriers in a Belgian cohort. Brain 2016;139(Pt 2):452–67. 10.1093/brain/awv358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorges M, Vercruysse P, Müller HP, et al. . Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2017;88:1033–41. 10.1136/jnnp-2017-315795 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2018-318823supp001.pdf (137.9KB, pdf)