Abstract
Objective
Evaluate the efficacy and safety of dual neutralisation of interleukin (IL)-17A and IL-17F with bimekizumab, a monoclonal IgG1 antibody, in addition to certolizumab pegol (CZP) in patients with rheumatoid arthritis (RA) and inadequate response (IR) to certolizumab pegol.
Methods
During this phase 2a, double-blind, proof-of-concept (PoC) study (NCT02430909), patients with moderate-to-severe RA received open-label CZP 400 mg at Weeks 0, 2 and 4, and 200 mg at Week 6. Patients with IR at Week 8 (Disease Activity Score 28-joint count C-reactive protein (DAS28(CRP))>3.2) were randomised 2:1 to CZP (200 mg every 2 weeks (Q2W)) plus bimekizumab (240 mg loading dose then 120 mg Q2W) or CZP plus placebo. The primary efficacy and safety variables were change in DAS28(CRP) between Weeks 8 and 20 and incidence of treatment-emergent adverse events (TEAEs).
Results
Of 159 patients enrolled, 79 had IR at Week 8 and were randomised to CZP plus bimekizumab (n=52) or CZP plus placebo (n=27). At Week 20, there was a greater reduction in DAS28(CRP) in the CZP-IR plus bimekizumab group compared with the CZP-IR plus placebo group (99.4% posterior probability). The most frequent TEAEs were infections and infestations (CZP plus bimekizumab, 50.0% (26/52); CZP plus placebo, 22.2% (6/27)).
Conclusions
PoC was confirmed based on the rapid decrease in disease activity achieved with 12 weeks of CZP plus bimekizumab. No unexpected or new safety signals were identified when neutralising IL-17A and IL-17F in patients with RA concomitantly treated with CZP, but the rate of TEAEs was higher with dual inhibition.
Keywords: anti-tnf, dmards (biologic), DAS28, rheumatoid arthritis, treatment
Key messages.
What is already known about this subject?
Significant increases in circulating T helper 17 cells and interleukin (IL)-17 production have been observed following inadequate response to tumour necrosis factor (TNF) inhibitors (anti-TNFs) in patients with rheumatoid arthritis.
It has been hypothesised that this compensatory amplification of IL-17 biology may contribute to the impaired response to anti-TNF treatment in some patients; however, clinical data substantiating this hypothesis are conflicting.
What does this study add?
We evaluated the efficacy and safety of dual neutralisation of IL-17A and IL-17F with bimekizumab, a monoclonal IgG1 antibody, in addition to certolizumab pegol in patients with rheumatoid arthritis and inadequate response to certolizumab pegol.
Proof-of-concept was confirmed based on the rapid decrease in disease activity achieved with 12 weeks of certolizumab pegol and bimekizumab treatment, with no unexpected or new safety findings identified.
How might this impact on clinical practice or future developments?
These findings support the potential to further explore concomitant neutralisation of multiple pathways in other patient populations where this treatment strategy may provide additional benefits.
Introduction
It is well documented that some patients with rheumatoid arthritis (RA), particularly those with poor prognostic factors, have an inadequate response (IR) to initial treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate (MTX). In these individuals, add-on treatment with tumour necrosis factor (TNF) inhibitors (anti-TNFs) is often considered.1 As a class, anti-TNFs (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab) comprise an effective treatment approach that has considerably improved the success of treatment for RA.2 3 However, sustained disease remission is only achieved by <10% of patients, and there remains a group of patients who fail to respond, or do not achieve an adequate response, even with anti-TNFs.4 Should patients fail to respond to one anti-TNF, they may be treated with another anti-TNF or a treatment with a different mode of action.1
Significant increases in circulating T helper 17 (Th17) cells and interleukin (IL)-17 production have been observed following IR to anti-TNFs in patients with RA.5–8 It has been hypothesised that this compensatory amplification of IL-17 biology may contribute to the impaired response to anti-TNF treatment in some patients; however, clinical data substantiating this hypothesis are conflicting. For example, phase 3 studies have shown that IL-17A blockade with secukinumab has minimal efficacy in patients with RA who have IR or intolerance to anti-TNFs,9–11 suggesting inhibition of IL-17A alone is insufficient to neutralise the inflammatory response in RA. Conversely, a phase 2 study demonstrated a modest but statistically greater American College of Rheumatology 20% improvement criteria (ACR20) response with ixekizumab, another higher affinity anti-IL-17A, compared with placebo after 12 weeks’ treatment in patients with RA with IR to anti-TNF therapy.12
In addition to IL-17A, Th17 cells secrete a number of pro-inflammatory cytokines including IL-22, IL-26, interferon (IFN)-γ, TNF, granulocyte-macrophage colony-stimulating factor, C-C motif chemokine ligand 20 and another member of the IL-17 family, IL-17F.13 Both IL-17A and IL-17F have been shown to independently co-operate with other cytokines to mediate chronic inflammation14; they share ~50% structural homology and overlapping but non-redundant biological functions,15–17 suggesting IL-17F may also play an important role in RA. Bimekizumab is a monoclonal immunoglobulin G1 antibody that potently and selectively neutralises the biological function of both IL-17A and IL-17F.18 In a proof-of-concept (PoC) study in patients with psoriatic arthritis, bimekizumab demonstrated rapid, profound responses in joint and skin, with no unexpected safety findings.18 In the phase 2b BE ABLE 1 study, rapid and substantial improvements were achieved with bimekizumab in patients with moderate-to-severe psoriasis.19 These data support the rationale for targeting both IL-17A and IL-17F in immune-mediated inflammatory disease. For those patients with RA and IR to anti-TNFs, neutralisation of both IL-17A and IL-17F in addition to TNF inhibition may reduce disease activity compared with inhibition of TNF alone. However, the potential safety effects of inhibiting these three cytokines together are not known.
Here, we report the efficacy and safety results of a phase 2a, randomised, double-blind, placebo-controlled PoC study (NCT02430909) evaluating certolizumab pegol, a Fc-free, PEGylated anti-TNF that provides rapid and sustained improvements to many patients with RA,20 21 in combination with bimekizumab in patients with moderate-to-severe RA who had an IR to certolizumab pegol.
Methods
Study design and treatment
This was a multicentre phase 2a, randomised, double-blind, placebo-controlled PoC study (NCT02430909) to assess the efficacy and safety of certolizumab pegol plus bimekizumab in patients with moderate-to-severe RA and IR to certolizumab pegol. This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice. Independent institutional review board approvals were obtained, and all patients provided written informed consent in accordance with local requirements.
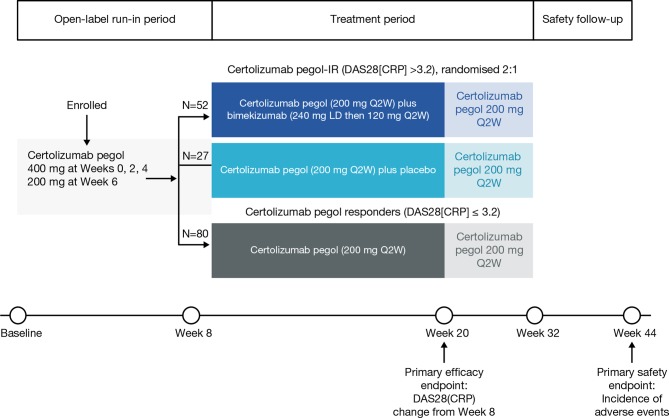
During an 8-week open-label, run-in period, patients received certolizumab pegol 400 mg at Weeks 0, 2 and 4, and then 200 mg at Week 6 (figure 1). Patients who responded to certolizumab pegol during the open-label run-in period remained on this treatment. Patients with IR to certolizumab pegol at Week 8, defined as Disease Activity Score 28-joint count C-reactive protein (DAS28(CRP)) >3.2, were randomised 2:1 to certolizumab pegol (200 mg every 2 weeks (Q2W)) plus bimekizumab (240 mg loading dose then 120 mg Q2W) or certolizumab pegol (200 mg Q2W) plus placebo. At Week 20, the add-on therapy (bimekizumab or placebo) was withdrawn; certolizumab pegol continued until Week 32, after which their treatment was determined by their clinician outside of the study protocol. There was a final follow-up visit at Week 44, 12 weeks after the end of study treatment.
Figure 1.
Study design. DAS28(CRP), Disease Activity Score 28-joint count (C-reactive protein); Q2W, once every 2 weeks.
Patients
Patients were aged 18–69 years with a diagnosis of adult-onset moderate-to-severe RA of ≥6 months’ duration as defined by ACR/European League Against Rheumatism 2010 classification criteria.22 Additional inclusion criteria were body mass index 18–35 kg/m2, with a body weight of ≥50 kg (men) or 45 kg (women); ≥6 tender joints (out of 68), ≥6 swollen joints (out of 66) and ≥10 mg/L CRP; and IR to ≥1 csDMARD. Patients with IR to csDMARDs could continue on stable doses of any permitted csDMARD; patients with a history of IR, but not currently receiving csDMARDS, were also eligible.
Key exclusion criteria were previous exposure to anti-TNFs, IL-17 inhibitors or bimekizumab; receipt of any investigational drug or experimental procedure within 90 days prior to baseline; and receipt of prohibited medications (online supplementary table S1). Patients with an active/high risk of infection, active or latent tuberculosis, known central nervous system demyelinating disorder or neoplastic disease within 5 years of study entry (with the exception of definitively treated basal or squamous carcinoma of the skin or carcinoma in situ) were excluded.
annrheumdis-2018-214943supp001.pdf (281.3KB, pdf)
Assessments
The primary efficacy endpoint was change in DAS28(CRP) between Weeks 8 and 20. Secondary efficacy endpoints were DAS28(CRP) remission (DAS28(CRP)<2.6) at Week 20, percentage of improvement in ACR criteria (ACRn), ACR20, ACR50 and ACR70 response between Weeks 8 and 20. DAS28(CRP) remission by visit was an exploratory endpoint. The primary safety endpoint was the incidence of adverse events (AEs) at follow-up (Week 44); results of clinical laboratory tests was an additional safety endpoint. Treatment-emergent AEs (TEAEs) that occurred during treatment with either bimekizumab or placebo were defined as any AE that started or worsened on or after the first dose of bimekizumab or placebo, up to 140 days after the last dose.
Statistical methods
The primary and secondary efficacy endpoints were analysed using a Bayesian approach. At the design and analysis stage, an informative prior23 (equivalent to approximately 13 patients) was assumed for the primary endpoint; this allowed for information borrowing from a previous study21 to augment the control data from the certolizumab pegol-IR plus placebo group (online supplementary methods). A Bayesian analysis of covariance was conducted with treatment as factor and Week 8 DAS28(CRP) as covariate. In addition, several sensitivity analyses were conducted for the primary efficacy endpoint (online supplementary methods). The change from Week 8 in the individual components of DAS28(CRP) at Week 20 was summarised for each treatment group using descriptive statistics. Additional analyses included Boolean, DAS28(CRP)[3] and Clinical Disease Activity Index (CDAI) remission (online supplementary methods). A Bayesian analysis using a logistic model with vague prior distributions was conducted for the secondary efficacy variables (DAS28(CRP) remission, ACRn, ACR20, ACR50 and ACR70 response). These endpoints were plotted over time by treatment group including the 95% confidence interval (CI) using a Wilson approximation.
Sample size calculations were based on a Bayesian analysis of the primary endpoint (online supplementary methods). A sample size of 60 patients across both treatment groups was deemed sufficient to determine the primary endpoint success criterion of the ≥97.5% probability that the change in DAS28(CRP) from Week 8 was greater for the certolizumab pegol-IR plus bimekizumab group than the certolizumab pegol-IR plus placebo group. The study had an 89% probability of detecting a difference of 0.7 in DAS28(CRP) change from Week 8 between the treatment groups at Week 20.
All analyses were performed using SAS V.9.3 or later, R V.2.10.1 or later, or WinBUGS V.1.4.
Results
Patients
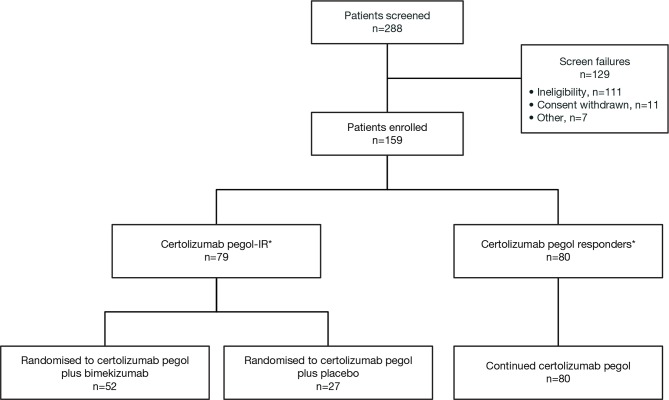
Of 159 patients enrolled, 79 had IR to certolizumab pegol at Week 8 and were randomised to certolizumab pegol plus bimekizumab (n=52) or certolizumab pegol plus placebo (n=27) (figure 2). Patients who achieved low disease activity (DAS28(CRP)≤3.2; n=80) at Week 8 continued to receive open-label certolizumab pegol.
Figure 2.
Patient disposition. *Inadequate response was defined as Disease Activity Score 28-joint count (C-reactive protein) >3.2. IR, inadequate response.
At baseline, demographics and disease characteristics were similar between the certolizumab pegol-IR plus bimekizumab group and the certolizumab pegol-IR plus placebo group (table 1). Disease characteristics at randomisation (Week 8; online supplementary table S2) were also similar between groups, although Patient’s Assessment of Arthritis Pain and Patient’s Global Assessment of Disease Activity (PtGADA) were numerically higher in the certolizumab pegol-IR plus bimekizumab group (53.4% and 53.7%, respectively) compared with the certolizumab pegol-IR plus placebo group (46% and 45.6%, respectively). Most patients received concomitant csDMARDs (80.8% in the certolizumab pegol-IR plus bimekizumab group and 96.3% in the certolizumab pegol-IR plus placebo group) (table 1). Demographics and characteristics for the certolizumab pegol responders group are also detailed in table 1.
Table 1.
Demographics and baseline disease characteristics
| Certolizumab pegol–IR plus bimekizumab (n=52) |
Certolizumab pegol–IR plus placebo (n=27) |
Certolizumab pegol responders (n=80) |
|
| Demographics, n (%) | |||
| Age, median (range), years | 53 (26–69) | 57 (30–67) | 58 (21–69) |
| Gender | |||
| Male | 7 (13.5) | 4 (14.8) | 13 (16.3) |
| Female | 45 (86.5) | 23 (85.2) | 67 (83.8) |
| Race | |||
| Caucasian | 52 (100) | 27 (100) | 80 (100) |
| Duration of RA | |||
| <2 years | 12 (23.1) | 8 (29.6) | 17 (21.3) |
| ≥2 years | 40 (76.9) | 19 (70.4) | 63 (78.8) |
| History of extra-articular features | 7 (13.5) | 1 (3.7) | 7 (8.8) |
| Anti-CCP positive | 39 (75) | 19 (70.4) | 66 (82.5) |
| Rheumatoid factor positive | 39 (75) | 22 (81.5) | 64 (80.0) |
| Prior csDMARDs* | 49 (94.2) | 27 (100) | 79 (98.8) |
| Methotrexate | 43 (82.7) | 22 (81.5) | 76 (95) |
| Methotrexate sodium | 6 (11.5) | 4 (14.8) | 5 (6.3) |
| Concomitant csDMARDs† | 42 (80.8) | 26 (96.3) | 71 (88.8) |
| Methotrexate | 29 (55.8) | 20 (74.1) | 63 (78.8) |
| Methotrexate sodium | 6 (11.5) | 3 (11.1) | 3 (3.8) |
| Disease characteristics, mean (SD) | |||
| SJC‡ | 13.6 (6.4) | 16.2 (7.9) | 11.1 (5.4) |
| TJC‡ | 20.6 (10.5) | 26.2 (12.3) | 20.3 (9.8) |
| PtAAP | 72.9 (15.5) | 73.1 (17.8) | 63.2 (21.4) |
| PtGADA | 74 (14.2) | 77.7 (17) | 64.3 (20.9) |
| HAQ-DI | 1.7 (0.6) | 1.9 (0.4) | 1.6 (0.6) |
| DAS28(CRP) | 6.1 (0.7) | 6.2 (0.8) | 5.7 (0.8) |
All patients received certolizumab pegol during the 8-week open-label run-in period.
*Prior medications include any medications that started prior to the start date of study medication.
†Concomitant medications are medications taken at least 1 day in common with the study medication, ie, whose start date is prior to the date of last study medication administration plus 14 days, and whose stop date is either missing, or on or after the date of first study medication administration.
‡SJC and TJC were based on 66 and 68 counts, respectively.
Anti-CCP, anti-cyclic citrullinated peptide;csDMARD, conventional synthetic disease-modifying antirheumatic drug; DAS28(CRP), Disease Activity Score 28-joint count (C-reactive protein);HAQ-DI, Health Assessment Questionnaire–Disability Index;PtAAP, Patient’s Assessment of Arthritis Pain;PtGADA, Patient’s Global Assessment of Disease Activity;RA, rheumatoid arthritis;SCJ, swollen joint count;TJC, tender/painful joint count.
Efficacy
PoC was confirmed based on the primary efficacy endpoint, with a greater reduction in DAS28(CRP) in the certolizumab pegol-IR plus bimekizumab group compared with the certolizumab pegol-IR plus placebo group from Week 8 to Week 20 (99.4% posterior probability by Bayesian analysis using an informative prior distribution). The estimated posterior group mean DAS28(CRP) change from Week 8 to Week 20 for the certolizumab pegol-IR plus bimekizumab group and the certolizumab pegol-IR plus placebo group were -1.41 (95% credible interval (CrI) -1.72, 1.09) and -0.82 (95% CrI -1.15, 0.49), respectively, with an estimated posterior mean treatment difference of 0.58 (95% CrI 0.13, 1.05). The results of sensitivity analyses of the primary efficacy variable were consistent with and supportive of the primary analysis (online supplementary results). The observed mean (SD) change from Week 8 to Week 20 in DAS28(CRP) was -1.40 (1.32) in the certolizumab pegol-IR plus bimekizumab group and -1.04 (0.90) in the certolizumab pegol-IR plus placebo group.
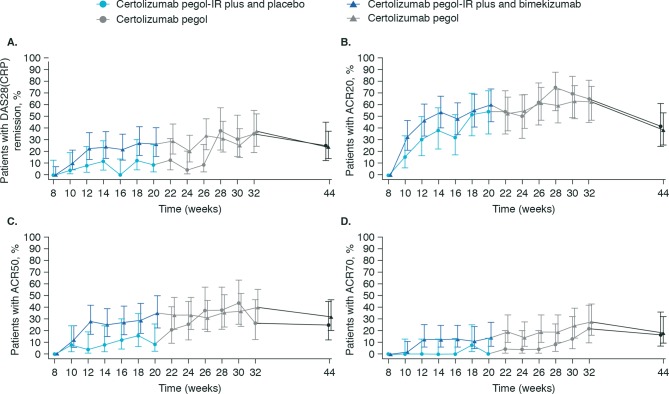
At Week 20, a greater percentage of patients in the certolizumab pegol-IR plus bimekizumab group achieved DAS28(CRP) remission compared with the certolizumab pegol-IR plus placebo group (97.6% posterior probability); the estimated posterior mean treatment difference in DAS28(CRP) remission was 17.8% (95% CrI 0.3, 33.8). The percentage of patients achieving DAS28(CRP) remission in the certolizumab pegol-IR plus bimekizumab group and the certolizumab-IR plus placebo group was similar during Weeks 2 to 8, and numerically higher during Weeks 10 to 26. By Week 32, after a further 12 weeks of certolizumab pegol treatment without the add-on therapy (bimekizumab or placebo), the percentage of patients achieving DAS28(CRP) remission was similar between the certolizumab pegol-IR plus bimekizumab group and certolizumab pegol-IR plus placebo group (figure 3A and online supplementary table S5).
Figure 3.
DAS28(CRP) remission by visit (A), percentage of ACR20 (B), ACR50 (C) and ACR70 (D) responders based on Week 8 in the certolizumab pegol-IR plus bimekizumab and certolizumab pegol-IR plus placebo groups. At Week 20, the add-on therapy (bimekizumab or placebo) was withdrawn; certolizumab pegol continued until Week 32. All patients continued certolizumab pegol therapy until Week 32, after which their treatment was determined by their clinician outside of the study protocol. There was a final follow-up visit at Week 44. See online supplementary table S5 for actual data. Error bars represent Wilson’s 95% CI. ACR20, ACR50, ACR70, American College of Rheumatology 20%, 50% and 70% improvement criteria; DAS28(CRP), Disease Activity Score 28-joint count (C-reactive protein).
Analysis of the DAS28(CRP) components showed numerically larger reductions in mean swollen joint count (SJC) and tender joint count (TJC) in the certolizumab pegol-IR plus placebo group from Week 8 to Weeks 20 and 32 compared with the certolizumab pegol-IR plus bimekizumab group; conversely, mean reductions in PtGADA were numerically greater in the certolizumab pegol-IR plus bimekizumab group (online supplementary table S3). Results of additional efficacy endpoints (including Boolean, DAS28(CRP)[3] and CDAI remission) are presented in online supplementary table S4.
The posterior probability of improvement in ACRn in the certolizumab pegol-IR plus bimekizumab group compared with the certolizumab pegol-IR plus placebo group at Week 20 was 70.6%; the estimated posterior mean treatment difference in ACRn was 5.38 (95% Crl -13.8, 25.3). Results of Bayesian analysis of ACR20, ACR50 and ACR70 response are detailed in online supplementary results; percentage of ACR20, ACR50 and ACR70 responders by visit is shown in figure 3, online supplementary table S5 and table 2.
Table 2.
DAS28(CRP) and ACR response rates at weeks 20 and 32
| Certolizumab pegol–IR plus bimekizumab (n=52) |
Certolizumab pegol–IR plus placebo (n=27) |
|
| DAS28(CRP) <3.2, n (%) | ||
| Week 20 | 21 (45.7) | 7 (29.2) |
| Week 32 | 26 (60.5) | 11 (47.8) |
| ACR20, n (%) | ||
| Week 20 | 26 (60.5) | 13 (54.2) |
| Week 32 | 25 (62.5) | 15 (65.2) |
| ACR50, n (%) | ||
| Week 20 | 15 (34.9) | 2 (8.3) |
| Week 32 | 16 (40.0) | 6 (26.1) |
| ACR70, n (%) | ||
| Week 20 | 6 (14.0) | 0 |
| Week 32 | 11 (27.5) | 5 (21.7) |
ACR20, ACR50, ACR70, American College of Rheumatology 20%, 50% and 70% improvement criteria.
DAS28(CRP), Disease Activity Score 28-joint count (C-reactive protein).
Safety
Mean durations of exposure for the certolizumab pegol-IR plus bimekizumab group and the certolizumab pegol-IR plus placebo group were similar (80.1 days vs 78.3 days, respectively). Across all parts of the study, the mean duration of exposure for certolizumab pegol was 205.8 days. A greater percentage of patients experienced TEAEs with certolizumab pegol plus bimekizumab compared with certolizumab pegol plus placebo (78.8% (41/52) vs 59.3% (16/27)) (table 3). Severe TEAEs were reported for one patient in each treatment group (one patient in the certolizumab pegol-IR plus bimekizumab group experienced haematoma and psoas abscess; one patient in the certolizumab pegol-IR plus placebo group experienced meningitis). There were no deaths in the certolizumab pegol-IR plus bimekizumab group; one patient in the certolizumab pegol-IR plus placebo group experienced a TEAE resulting in death (meningitis).
Table 3.
TEAEs during certolizumab pegol plus bimekizumab and certolizumab pegol plus placebo treatment
| Variable, n (%)* | Certolizumab pegol–IR plus bimekizumab (n=52) |
Certolizumab pegol–IR plus placebo (n=27) |
| Any TEAE | 41 (78.8) | 16 (59.3) |
| Serious TEAEs | 2 (3.8) | 3 (11.1) |
| Discontinuation due to TEAEs | 4 (7.7) | 3 (11.1) |
| Severe TEAEs | 1 (1.9) | 1 (3.7) |
| Deaths | 0 | 1 (3.7) |
| Most common TEAEs by SOC and PT (reported in ≥5% of patients) | ||
| Infections and infestations | 26 (50.0) | 6 (22.2) |
| Nasopharyngitis | 4 (7.7) | 2 (7.4) |
| Upper respiratory tract infection | 3 (5.8) | 1 (3.7) |
| Pharyngitis | 3 (5.8) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | 5 (9.6) | 7 (25.9) |
| Rheumatoid arthritis | 3 (5.8) | 4 (14.8) |
| Skin and subcutaneous disorders | 8 (15.4) | 1 (3.7) |
| Dermatitis allergic | 3 (5.8) | 0 (0.0) |
| Gastrointestinal disorders | 6 (11.5) | 1 (3.7) |
| Stomatitis | 3 (5.8) | 0 (0.0) |
| General disorders and administration site reactions | 4 (7.7) | 1 (3.7) |
| Investigations | 4 (7.7) | 1 (3.7) |
| Vascular disorders | 2 (3.8) | 3 (11.1) |
| Hypertension | 0 (0.0) | 3 (11.1) |
| Blood and lymphatic system disorders | 3 (5.8) | 0 (0.0) |
| Injury, poisoning and procedural complications | 1 (1.9) | 2 (7.4) |
TEAEs during treatment were defined as an adverse event that started or worsened on or after the first dose of bimekizumab or placebo up to 140 days after the final dose. TEAEs were coded using MedDRA V.19.0.
*n=number of patients reporting at least one TEAE within the SOC/PT.
csDMARD, conventional synthetic disease-modifying antirheumatic drug; PT, preferred term;SOC, system organ class;TEAE, treatment-emergent adverse event.
The most frequent TEAEs were infections and infestations, with a higher incidence seen with certolizumab pegol plus bimekizumab (50.0% (26/52)) compared with certolizumab pegol plus placebo (22.2% (6/27)). The most common non-serious infections reported by ≥5% of patients in either group were nasopharyngitis, upper respiratory tract infection and pharyngitis (table 3). Skin and subcutaneous disorders and gastrointestinal disorders also occurred with a higher incidence in the certolizumab pegol plus bimekizumab group compared with certolizumab pegol plus placebo. Eight patients in the certolizumab pegol plus bimekizumab group reported nine skin and subcutaneous TEAEs, including three of allergic dermatitis, two of rash (both reported by the same patient) and one each of photosensitivity reaction, dermatitis, atopic dermatitis and skin lesion. Six patients reported eight gastrointestinal disorder TEAEs with certolizumab pegol plus bimekizumab, which included three TEAEs of stomatitis, two of dry mouth and one each of diarrhoea, dysphagia and tongue geographic.
Serious infections were experienced by one patient in the certolizumab pegol-IR plus bimekizumab group (psoas abscess 98 days after final bimekizumab dose) and one patient in the certolizumab pegol-IR plus placebo group (meningitis leading to death, 89 days after last placebo dose); both were considered unrelated to study treatment by the investigator.
Treatment-emergent grade 3 low neutrophil values were experienced by three patients (5.8%) in the certolizumab pegol-IR plus bimekizumab group and one patient (3.7%) in the certolizumab pegol-IR plus placebo. In most patients, this was a transient event that resolved and patients remained on treatment; one patient in the certolizumab pegol-IR plus bimekizumab group withdrew from the study. No additional patterns of change in any laboratory parameters were identified.
Overall, the safety profile for certolizumab pegol plus bimekizumab was consistent with previous findings for bimekizumab. The safety profile in the certolizumab pegol responders group (online supplementary results, online supplementary table S6) was also consistent with the known safety profile of certolizumab pegol.24
Discussion
This study tested the principle of enhancing an IR to an anti-TNF therapy, in this case certolizumab pegol, by the addition of another treatment, in this case bimekizumab. The primary endpoint for the study was met, with a greater reduction in DAS28(CRP) in the certolizumab pegol-IR plus bimekizumab group at Week 20, compared with the certolizumab pegol-IR plus placebo group. Treatment with certolizumab pegol plus bimekizumab was also associated with a greater percentage of patients achieving DAS28(CRP) remission at Week 20 compared with certolizumab pegol plus placebo. Secondary efficacy outcomes, such as ACR50 and ACR70 at Week 20, also showed evidence for greater benefit with certolizumab pegol plus bimekizumab compared with certolizumab pegol plus placebo.
DAS28(CRP) response in the certolizumab pegol plus bimekizumab group was maintained for an additional 24 weeks of treatment with certolizumab pegol. Clinical response to certolizumab pegol is usually achieved within 12 weeks of treatment24; however, the certolizumab pegol-IR plus placebo group showed continued gradual improvement, such that by Week 32 the improvements reached were similar to the certolizumab pegol-IR plus bimekizumab group. Further work is required to determine whether treatment with certolizumab pegol plus bimekizumab for 12 weeks was sufficient to downregulate the inflammatory response to a level that could be maintained once treatment with bimekizumab was withdrawn. In addition, changes to individual components of the DAS28(CRP) suggested that improvements in the certolizumab pegol-IR plus bimekizumab group may have been mostly attributable to changes in PtGADA, with little impact of the combined treatment on SJC or TJC. It is notable that this group had a markedly higher baseline PtGADA score at randomisation.
In contrast to our findings, a recent study found no difference in efficacy with dual inhibition of TNF and IL-17A with ABT-122 compared with inhibition of TNF alone with adalimumab.25 However, patients in this study were anti-TNF naïve whereas patients in our study demonstrated IR to anti-TNF treatment. The difference in efficacy may also be attributable to the difference in IL-17 inhibition: ABT-122 inhibits IL-17A but bimekizumab inhibits both IL-17A and IL-17F. Any potential gains in efficacy as a result of targeting two inflammatory pathways must be considered in balance with associated risks. Indeed, previous attempts to combine anti-TNF therapy with other modulators of the immune response, for example, etanercept plus abatacept (CTLA-4Ig) and etanercept plus anakinra (IL-1R antagonist), have resulted in increased safety risks, including increased rates of infection in patients with RA.26 27 The safety profile of certolizumab pegol plus bimekizumab in this study was consistent with that expected in patients receiving certolizumab pegol for RA21 and patients with psoriasis or psoriatic arthritis receiving bimekizumab.18 19 There were no unexpected safety findings specifically associated with the combination of certolizumab pegol and bimekizumab. Of note, the incidence of infections and infestations in the certolizumab pegol-IR plus bimekizumab group was over double that of the certolizumab pegol-IR plus placebo group (50% vs 22.2%). This was not due to an increased rate of any particular AE; nasopharyngitis and upper respiratory tract were the most common infections, with similar incidence in both treatment groups.
There is evidence to suggest that a higher baseline frequency of Th17 cells is associated with poor response to anti-TNF therapy.5–8 The biology underlying the observed rises in Th17 numbers and IL-17A production after anti-TNF treatment in patients with RA is not fully understood. As noted by Hull et al, patients with higher baseline frequencies of circulating Th17 cells may have more IL-17-predominant disease and could therefore obtain greater benefit from the combined inhibition of IL-17A, IL-17F and TNF.7 However, the study population was not large enough to identify any subpopulations that may have achieved particular benefit from the combination of certolizumab pegol plus bimekizumab. A further limitation of this study was that the effect of bimekizumab treatment alone was not assessed, including the effect of IL-17 inhibition on CRP, independent of clinical activity. In addition, evaluation of response to certolizumab pegol after 8 weeks may have resulted in initiation of additional treatment with bimekizumab in patients who would have met response criteria given additional time. The bimekizumab regimen in this study was significantly less than the maximum human exposure observed at the time of study initiation in patients with mild psoriasis (640 mg).28 This was to allow for the different study population and any possible drug−drug interactions that might have increased exposure to bimekizumab; the dose regimen used for certolizumab pegol is an approved dose regimen in patients with RA.24
A rapid increase in response was achieved with 12 weeks of certolizumab pegol plus bimekizumab in patients with IR to certolizumab pegol. Neutralising both IL-17A and IL-17F in patients with moderate-to-severe RA treated with certolizumab pegol and background csDMARDs did not give rise to unexpected or new safety signals, although the rate of TEAEs was higher with dual inhibition. Overall, these findings support the potential to further explore concomitant neutralisation of multiple pathways in other patient populations where this treatment strategy may provide additional benefits.
Acknowledgments
The authors thank the patients and their caregivers, in addition to the investigators, their teams, and the UCB Pharma RA0123 clinical team, who contributed to this study. The study (NCT02430909) was funded by UCB Pharma. The authors acknowledge Alvaro Arjona, PhD, of UCB Pharma for publication and editorial support; Chetan Mistry, MSc, of Veramed for additional statistical support; and Olivier Harari, FRCP, PhD, formerly of UCB Pharma for support and technical expertise in the development of study design. The authors acknowledge Rosalie Richards, PhD, of iMed Comms, Macclesfield, UK, an Ashfield Company, part of UDG Healthcare plc, for medical writing support that was funded by UCB Pharma in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). PCT would like to acknowledge personal support from the National Institute for Health Research, Oxford Biomedical Research Centre and Arthritis Research UK.
Footnotes
Handling editor: Josef S Smolen
Contributors: PCT was the co-ordinating investigator. SG, PCT, IBM, GS, RL and MILW contributed to study conduct and/or data collection. All authors analysed and/or interpreted the data. All authors collaborated in the drafting and critical revision of the manuscript, with the support of a professional medical writer funded by UCB Pharma. All authors approved the final version of the manuscript and vouch for the accuracy of the analyses and the fidelity of the study to the protocol.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SG is an employee at UCB Pharma and reports a patent pending. PCT reports grants and consultancy for UCB Pharma and Janssen, grants from Lilly and Celgene, and consultancy for AbbVie, Biogen and Novartis. IBM reports grants from AstraZeneca, Compugen and Roche, consultancy and grants from UCB Pharma, Novartis and Celgene, honoraria from UCB Pharma, grants and honoraria from BMS and Janssen, and consultancy from AbbVie, Galvani, Lilly and Pfizer. GS reports personal fees from AbbVie and Pfizer, grants and consultancy for UCB Pharma, and grants and personal fees from Celgene, Novartis, Lilly, BMS and Chugai. RL reports honoraria from UCB Pharma, Novartis, AbbVie and Pfizer, and grants and honoraria from Lilly, Janssen and BMS. DB is an employee at UCB Pharma. LI is an employee at UCB Pharma. FS is an employee at UCB Pharma and has a patent pending. MILW is an employee at UCB Pharma. SS is an employee at UCB Pharma.
Patient consent for publication: Obtained.
Ethics approval: Independent institutional review board approvals were obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised IPD and redacted study documents which may include raw datasets, analysis-ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.clinicalstudydatarequest.com and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password-protected portal.
References
- 1. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 2. Aaltonen KJ, Virkki LM, Malmivaara A, et al. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS ONE 2012;7:e30275 10.1371/journal.pone.0030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma X, Xu S. TNF inhibitor therapy for rheumatoid arthritis. Biomedical Reports 2013;1:177–84. 10.3892/br.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furst DE, Pangan AL, Harrold LR, et al. Greater likelihood of remission in rheumatoid arthritis patients treated earlier in the disease course: results from the Consortium of Rheumatology Researchers of North America registry. Arthritis Care Res 2011;63:856–64. 10.1002/acr.20452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen D-Y, Chen Y-M, Chen H-H, et al. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res Ther 2011;13 10.1186/ar3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alzabin S, Abraham SM, Taher TE, et al. Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann Rheum Dis 2012;71:1741–8. 10.1136/annrheumdis-2011-201024 [DOI] [PubMed] [Google Scholar]
- 7. Hull DN, Cooksley H, Chokshi S, et al. Increase in circulating Th17 cells during anti-TNF therapy is associated with ultrasonographic improvement of synovitis in rheumatoid arthritis. Arthritis Res Ther 2016;18 10.1186/s13075-016-1197-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hull DN, Williams RO, Pathan E, et al. Anti-tumour necrosis factor treatment increases circulating T helper type 17 cells similarly in different types of inflammatory arthritis. Clin Exp Immunol 2015;181:401–6. 10.1111/cei.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dokoupilová E, Aelion J, Takeuchi T, et al. Secukinumab after anti-tumour necrosis factor-α therapy: a phase III study in active rheumatoid arthritis. Scand J Rheumatol 2018;47:276–81. 10.1080/03009742.2017.1390605 [DOI] [PubMed] [Google Scholar]
- 10. Tahir H, Deodhar A, Genovese M, et al. Secukinumab in active rheumatoid arthritis after anti-TNFα therapy: a randomized, double-blind placebo-controlled phase 3 study. Rheumatol Ther 2017;4:475–88. 10.1007/s40744-017-0086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blanco FJ, Möricke R, Dokoupilova E, et al. Secukinumab in active rheumatoid arthritis: a phase III randomized, double‐blind, active comparator- and placebo‐controlled study. Arthritis Rheum 2017;69:1144–53. 10.1002/art.40070 [DOI] [PubMed] [Google Scholar]
- 12. Genovese MC, Greenwald M, Cho C-S, et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheum 2014;66:1693–704. 10.1002/art.38617 [DOI] [PubMed] [Google Scholar]
- 13. van Hamburg JP, Tas SW. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J Autoimmun 2018;87:69–81. 10.1016/j.jaut.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 14. Maroof A, Smallie T, Archer S, et al. 699 dual IL-17A and IL-17F inhibition with bimekizumab provides evidence for IL-17F contribution to immune-mediated inflammatory skin response. J Invest Dermatol 2017;137 10.1016/j.jid.2017.02.722 [DOI] [Google Scholar]
- 15. Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med 2008;205:1063–75. 10.1084/jem.20071978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hymowitz SG, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. Embo J 2001;20:5332–41. 10.1093/emboj/20.19.5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wright JF, Bennett F, Li B, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 2008;181:2799–805. 10.4049/jimmunol.181.4.2799 [DOI] [PubMed] [Google Scholar]
- 18. Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018;77:523–32. 10.1136/annrheumdis-2017-212127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both IL-17A and IL-17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded placebo-controlled phase 2B trial. J Am Acad Dermatol 2018. [DOI] [PubMed] [Google Scholar]
- 20. Keystone E, Landewé R, van Vollenhoven R, et al. Long-term safety and efficacy of certolizumab pegol in combination with methotrexate in the treatment of rheumatoid arthritis: 5-year results from the RAPID 1 trial and open-label extension. Ann Rheum Dis 2014;73:2094–100. 10.1136/annrheumdis-2013-203695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keystone E, Heijde Dvander, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319–29. 10.1002/art.23964 [DOI] [PubMed] [Google Scholar]
- 22. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 23. Spiegelhalter DJ, Best NG. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost-effectiveness modelling. Stat Med 2003;22:3687–709. 10.1002/sim.1586 [DOI] [PubMed] [Google Scholar]
- 24. EMA Cimzia summary of product characteristics, 2018. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001037/WC500069763.pdf [Accessed 24 Apr 2018].
- 25. Genovese MC, Weinblatt ME, Aelion JA, et al. ABT-122, a bispecific dual variable domain immunoglobulin targeting tumor necrosis factor and interleukin-17A, in patients with rheumatoid arthritis with an inadequate response to methotrexate. Arthritis Rheum 2018;70:1710–20. 10.1002/art.40580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weinblatt M, Schiff M, Goldman A, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis 2007;66:228–34. 10.1136/ard.2006.055111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004;50:1412–9. 10.1002/art.20221 [DOI] [PubMed] [Google Scholar]
- 28. Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol 2017;83:991–1001. 10.1111/bcp.13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2018-214943supp001.pdf (281.3KB, pdf)