Abstract
The cornea was the first human solid tissue to be transplanted successfully, and is now a common procedure in ophthalmic surgery. The grafts come from deceased donors. Corneal therapies are now being developed that rely on tissue from living-related donors. This presents new ethical challenges for ophthalmic surgeons, who have hitherto been somewhat insulated from debates in transplantation and donation ethics. This paper provides the first overview of the ethical considerations generated by ocular tissue donation from living donors and suggests how these might be addressed in practice. These are discussed in the context of a novel treatment for corneal limbal stem cell deficiency. This involves limbal cell grafts which are transplanted, either directly or after ex vivo expansion, onto recipient stem cell-deficient eyes. Where only one eye is diseased, the unaffected eye can be used as a source of graft tissue. Bilateral disease requires an allogenic donation, preferably from a genetically related living donor. While numerous papers have dealt with the theory, surgical approaches and clinical outcomes of limbal stem cell therapies, none has addressed the ethical dimensions of this form of tissue donation.
Keywords: clinical ethics, donation/procurement of organs/tissues, transplantation
Introduction
The first successful corneal transplant was performed in 1905 by Eduard Zirm, using the cornea of a boy aged 11 years, who was due to have an evisceration. The cornea was grafted to a farm labourer who had been blinded by severe alkali burns.1 This serendipitous living donation paved the way for the field of corneal transplantation, which now relies on deceased donation. Due to the immune privilege of the eye, corneas are not routinely human leucocyte antigen (HLA)-matched, resulting in more potential donors. In addition, corneas do not need to be retrieved from donors immediately on death, as is the case with other solid organs, and can be stored for several days after procurement.2 There is no upper age limit3 and donations can be retrieved from non-hospital environments such as hospices, nursing home and funeral parlours. Despite this, there is still a severe shortage of donor corneas relative to demand worldwide, with only 1 cornea being available for every 70 needed.4 Living-related donors are not considered viable sources of corneas, as donation would result in a blind eye. Recent advances in corneal treatments, such as limbal stem cell transplantation for limbal stem cell deficiency (LSCD), do, however, depend on living donors as we shall now explain.
In healthy people, limbal stem cells regenerate the corneal epithelium and prevent conjunctival cells from migrating over the corneal surface. After severe injury, typically chemical burns, chronic inflammation and certain genetic diseases, the limbal stem cells may be lost and the cornea becomes vascularised and opaque, leading to blindness5 6 (figure 1). In such cases, standard corneal transplants fail because of the inability to maintain a healthy epithelium. Limbal stem cell transplantation is designed to address this problem by replacing the damaged or lost limbal stem cells (LSC) and restoring the ocular surface, which in turn increases the success rates of subsequent sight-restoring corneal transplants.7 8 Limbal stem cell donations only entail the removal of small piece of tissue from the donor’s eye. This is placed on the recipient’s injured eye either immediately or after ex vivo expansion (figure 2). When the disease is unilateral, the patient can donate stem cells from their own healthy eye but in bilateral cases the options are limited to allogeneic cell sources. Either deceased or living-related donors’ tissue may be used, but the latter is preferred as it has the advantage of providing a higher degree of histocompatibility.
Figure 1.
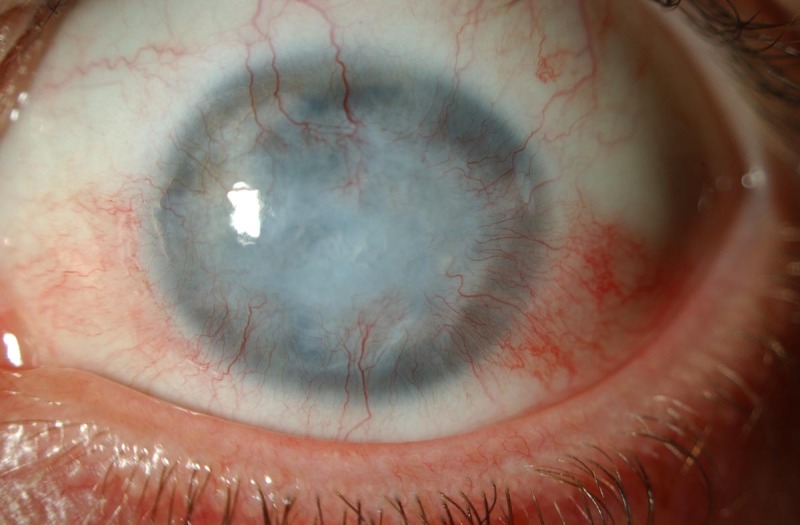
Example of total limbal stem cell deficiency following a chemical burn. Note the opacification of the cornea and the presence of neovessels over 360°.
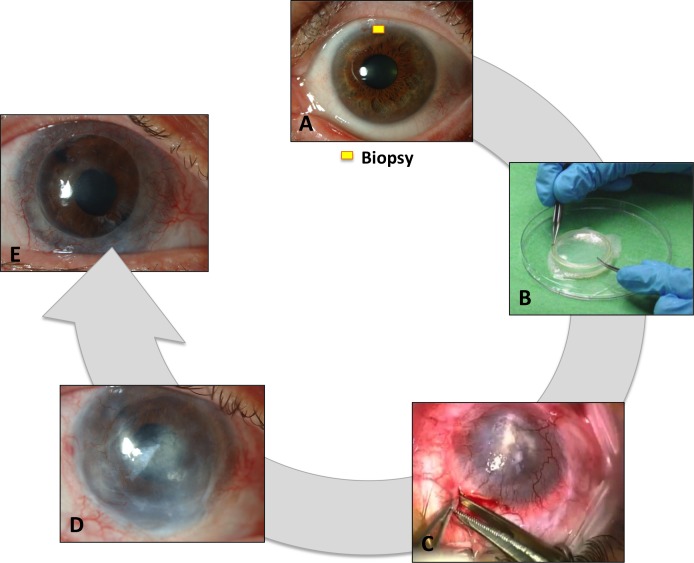
Figure 2.
Different steps of a cultivated limbal stem cell transplantation: (A) limbal biopsy harvest of donor eye; (B) ex vivo cultivation of the cells; (C) transplantation of the compound graft onto the recipient eye; (D) result post cultivated limbal epithelial transplantation (CLET); (E) result following sight restoring penetrating keratoplasty.
These advances mean that corneal surgeons, for the first time, have to consider the ethical issues surrounding living donation that are familiar to other transplant specialists. Given the traditional dependence on deceased donation and potential for corneas to be retrieved many hours after death and preserved in banks, corneal surgeons have been largely insulated from transplant ethics controversies. They may not, therefore, be accustomed to considering what the ethical norms for donation are or should be. They are also unlikely to be engaged with current ethical controversies in living donation. As the technology improves, corneal surgeons will have to apply, and adhere to, the ethical principles and practices that have guided other transplant disciplines for decades. While there is literature to support and promote good practices in relation to living donation of lung and liver lobes, kidney, bone marrow, umbilical cord blood and uterus, we have found no extant work that identifies and addresses ethical issues in living corneal donation. This paper aims to address this deficit.
Harms and benefits
In living donation, the donor bears the risks without direct clinical benefit. This is unlike therapeutic procedures, where known risks are outweighed by anticipated benefits. As such, one could argue that all living donation contravenes the most basic principle of medical ethics: first do no harm. Arguments countering this approach point to non-clinical or ‘indirect’ benefits that family members get from helping each other. After all, their interests are often intertwined, particularly in the case of parents and children. In the context of life-threatening conditions like liver failure, for example, a family member may feel that preventing the death of a loved one, by donating a liver lobe, is of direct benefit to them—it avoids (or postpones) the pain of bereavement.
It might, therefore, be argued that what counts from the living donors’ perspective is that, in their autonomous judgement, the harms and risks to themselves are outweighed by the benefit to the recipient, and what counts from the perspective of the surgeon retrieving the tissue is that the benefit to the recipient should outweigh the harms and risks to the donor.9 Before proceeding to any kind of surgery, a surgeon must be satisfied that there is a favourable balance of harms and benefits. This is no less true in case of corneal surgery. The potential harm of frustrating the autonomous wishes of the donor, as well as concerns about the potential harms risked by the donation, should be taken into account.
More widely, ethical features include the burdens and costs of not treating potential recipients. Visual impairment and blindness cause considerable economic burden for both affected persons, their caregivers and society. These costs increase with the degree of visual impairment, with the mean annual expenses per patient estimated at US$ purchasing power parities (PPP) 14 822– 24 180 in case of blindness, which is almost twice the cost for non-blind patients.10
A classic deceased-donor corneal transplant could be performed in an LSC-deficient eye but the prognosis is poor without LSCs. It may not, therefore, be a fair and prudent use of corneal tissue that could be used more effectively in an alternate recipient. Limbal stem cell transplantation aims to restore the limbal microenvironment and the anterior cornea: it is not a life-saving procedure for the recipient. It may, however, significantly improve their quality of life by improving vision and alleviate symptoms, such as pain or photophobia. Living-related donors of stem cells are helping their family members and are helping to maximise the absolute number of donated corneas available, by reducing the demand for them.
The consent process
The value of respecting the donor’s autonomous judgement of the risks and potential benefits of donation is weight-bearing in arguments in favour of living donation. A robust consent process is one way to safeguard autonomy. Corneal surgeons are experienced in gaining consent prior to conducting surgery on their patients. In living corneal cell donation, gaining consent for eye surgery entails agreement to surgery, but without the therapeutic purpose. There is no direct clinical benefit for the donor; instead, the clinical benefits—if successful—will accrue to the recipient. Its success will, however, bring other sorts of benefits to the donor, such as the family member’s visual rehabilitation.
This process also needs to reflect the fact that successful living donation requires a tandem consent process: both donor and recipient need to agree. In the case of directed living donation, where tissue passes between prespecified individuals, the identity of those individuals is a key factor. So, for example, one might imagine a parent who is willing to receive limbal tissue, but not from their own adult child because they are unwilling to agree to their offspring taking any risk to his/her eyesight, however small, for their benefit. Equally, one can imagine a donor who might be willing to provide limbal tissue for some family members, but not others.
Capacity
Capacity may fluctuate and individuals who have the capacity to make more simple decisions might not necessarily be able to make more complex ones. To have capacity, an individual must be able understand the information necessary to make a decision, retain this information, be able to deliberate and be able to communicate the outcome of his/her deliberations. There is often a legal presumption in favour of capacity in the case of adults/individuals of legal age to consent to or refuse medical interventions. This presumption does not, however, permit practitioners to abrogate responsibility for assessing capacity of individuals who have reached the age of majority (or other relevant legal threshold). If there is uncertainty about capacity, this may indicate a need to involve other professionals or to slow down the process and greatly simplify the information provided. The presumption in favour of capacity serves as a reminder to practitioners that an individual can be rendered incompetent by poor communications skills, such as the use of technical terms, visible impatience and inflexibility in their approach to how complex information is transmitted. Capacity is clearly interconnected, therefore, with information: both recipients and donors need adequate knowledge and comprehension of the risks, benefits, alternatives and outcomes to provide adequate informed consent.
Information
As a minimum, the information provided to both donor and recipient should include a description of, and discussion about, the diagnosis and prognosis, the procedure, overview of the purpose and effectiveness of the intervention, any contraindications, medical uncertainties, side effects and risks, necessary aftercare, potential financial and other social consequences and similar, relevantly detailed information about other available alternatives, including doing nothing.
In the case of limbal tissue donation specifically, the aim is to restore the ocular surface and eventually reverse the recipient’s corneal blindness by using the donated limbal tissue. The donation or ‘biopsy procedure’ itself only takes a few minutes and requires minimal aftercare. That being said, patients often feel more anxious about eye surgery specifically than other surgical interventions. Indeed, sight has been considered the most important of the five senses.11 The risks related to tissue removal should be discussed with the potential donor. While these are less significant than solid organ donation, the special nature of the eye should still be taken into account. Surgical risks in conjunctival limbal donation include the development of LSCD in the donor eye, pseudopterygium, filamentary keratitis and microperforation during surgery.12–14 Modern stem cells techniques were specifically developed to use smaller biopsies, which has significantly reduced the risks to the donor eye. It also offers the possibility of taking a second biopsy, if needed; however, in this case the process of gaining consent would need to be repeated. The consequences of a relative deciding not to donate are not life-threatening for the potential recipient, but they may have to wait longer for a suitable deceased donation, or ultimately their LSCD may not be cured, both of which can have an impact on their quality of life.
For the potential recipient, the surgical risks related to receiving a stem cell donation are less than those for routine corneal transplants. The culture protocols of the CLET technique have, however, introduced new issues. There is the potential for contamination with infectious agents related to the use of animal and/or human tissue.15 The use of allogeneic donor cells includes the risk of rejection, although HLA-compatible grafts may improve survival. Because this is a tandem process, the donor needs to be provided with clear information about the risks and consequences of transplant failure for the recipient, and the recipient needs to understand the risks and consequences for the potential donor. Information provision for each party needs to be equally rigorous.
Voluntariness
The potential for coercion to be brought to bear on donors outside of the clinic environment is a common feature of all types of living tissue donation. Obviously, a decision is not an autonomous one if it is made as a result of coercion, and the line between coercion and reasonable persuasion is a fine one.16 Family pressures, emotions or other external influences may play a role in manipulating the donor’s voluntary choices or might otherwise cause unnecessary anxiety or distress. Deciding against donation may provoke family hostility or generate feelings of guilt.17 Undue family influence may not be immediately obvious. However, ophthalmologists tasked with screening potential donors have an obligation to engage in detailed, private conversations to rule out the possibility of any coercion.18
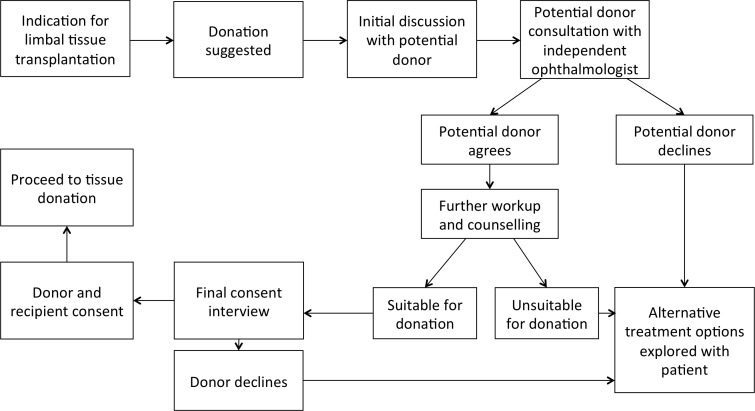
In the case of living kidney donation, it is common—after an initial willingness to donate has been established—for the care of donors, including that related to consent, to be passed to a team that is independent of the potential recipient. This avoids the possibility of coercion in the clinic, or any potential appearance of coercion. The living donation process is new in corneal transplant surgery and affects relatively few patients. It may not be economically viable for clinics to establish parallel recipient/donor teams until numbers increase substantially. It is also likely that in smaller centres only one corneal surgeon will be involved, which will also make it logistically and economically difficult to establish a dedicated, independent team of practitioners (including a dedicated coordinator and counsellor) to serve living donors. In large centres this may be feasible and should therefore be considered. All centres, however small, which recruit living donors must have a transparent and robust consent process. Most centres in high-income countries should be able to provide an independent (of the recipient) ophthalmic specialist to discuss the process with the donor, including any fears, concerns or reservations. Figure 3 illustrates this suggested consent process. Additional training and support in the ethical dimensions of providing care to living donors should be included as part of any training packages devised for new corneal surgery that depends on them, and additional training should also be provided to any non-participating, independent ophthalmic specialists who will be used to support the donors.
Figure 3.
Flow diagram of the suggested consent process.
All processes should comply with existing legal framework on tissue donation, such as the Tissues and Cell Directive (2004/23/EC) for Europe, which defines safety and quality standards for tissues and cell donation.19
Payment for tissues donation is not permitted in the European Union, and other jurisdictions partly because it is regarded as a potentially coercive inducement. The Declaration of Istanbul also prohibits all forms of transplant tourism and the trafficking of donors or their tissue across jurisdictional borders.20 This Declaration is endorsed by WHO and is implemented as one of the 11 Guiding Principles on Human Cell, Tissue and Organ Transplantation.21
Decision
Both the donor and the recipient should be given time to consider the information provided, including seeking the advice and counsel of others they trust. This is both an ethical requirement and a pragmatic one. It is sometimes assumed that people will feel rushed into consenting, but other people, if rushed, may be more inclined to refuse since this maintains the status quo. Although it is not often conceived of this way, such a rushed refusal would also be less than voluntary and therefore potentially as unethical as a rushed consent. People who agree in haste may also be more inclined to change their minds, or not to have grasped the gravity of the commitments involved. A change of mind may create disappointment and resentment within the family and also waste valuable clinical time and money with unnecessary workup procedures. Failure to fully understand what is required may result in less than optimal self-care following the procedure. The expectations of the donor and recipient, as well as their continued agreement, should, however, always be reviewed by the person carrying out the intervention immediately prior to its being performed.
Donor unwillingness to consent
A donor who expresses reluctance in a private conversation needs to be managed sensitively, including ensuring that the response from the healthcare practitioners is not itself coercive (eg, expressing disapproval or disappointment verbally or non-verbally). Moreover, donors are as entitled to enjoy a confidential relationship with their doctor as recipients. As we are recommending that the donor is seen by an independent ophthalmologist, this specialist will need to discuss with the potential donor whether the reasons for their reluctance can be shared with the recipient team and ultimately the recipient. If they are not willing to discuss their reluctance with the potential recipient and are unwilling to give the practitioners permission to disclose the information, this presents a difficulty. The potential recipient may expect to be given a reason as to why the donation cannot go ahead.
One solution is for staff to follow George Canguilhem’s distinction between ‘truth’ as ‘quality by which things appear such as they are’, versus, ‘true’ (vrai), as used in Latin in the sense of ‘real’ and ‘regular’ or ‘correct'.22 Here, a distinction is being drawn between disclosure of the whole truth and being generally honest, while disclosing less detail. A donor could, for instance, be declared as ‘unsuitable’ since being reluctant to consent would render them ineligible to proceed. The decision about how to proceed in circumstances such as these will depend on available alternatives (eg, other living-related donors or deceased donation). On the whole, however, it might be best to counsel openness and transparency wherever possible, especially given the importance of maintaining relationships of trust and confidence in dealings with these two individuals and between the professional as a whole and the public. These sorts of issues are not unusual in circumstances where practitioners have dealings with different family members, such as genetics and general practice.
Donor screening and dealing with unanticipated, adverse results
Infectious diseases such syphilis, hepatitis and HIV must be ruled out to avoid transmitting these to the recipient. HLA-matching may also be necessary to reduce the risk of rejection. Information about such tests should be clearly explained to the donor prior to screening, including the right to receive the confirmed results. Ethical issues arise when positive test results (eg, positive serology for HIV) are unexpected or where previously undisclosed non-paternity is revealed. Either may render the donor unsuitable. We might suppose that someone who has agreed to a test has agreed to receive the results. Although the term ‘screening’ is used in this context, it is not quite the same as its use in relation to population screening. The donor is not being tested as an asymptomatic member of an ‘at-risk’ population, where the burdens and benefits have been carefully calculated as part of a conventional screening programme, and where treatment has to be available for any conditions detected.23 24 Nor are they being tested because they have symptoms that need investigation and treatment, conducted within a regular healthcare context. In these latter contexts, those conducting the tests will have knowledge and expertise in relation to the conditions for which donors are being tested. This may not be the case among corneal surgeons, and protocols will need to be developed within clinics to ensure the compassionate management and referral on for treatment of those who are given adverse, potentially stigmatising results.
Ethical issues in relation to the obligations to disclose serious infectious disease to others who may be at risk of infection could be passed on with a referral, leaving it to those with more expertise on the receiving team to offer advice and counselling. This solution assumes, however, that the potential donor will agree to a referral, so plans need to be placed for how to manage anyone who refuses. In addition, certain infectious diseases are notifiable to the relevant authorities for disease control and surveillance. Information about these legal obligations needs to be provided as part of the consent process.
The issue of how to respond to suspected misattributed paternity is widely discussed in the transplant, genetics and in other bioethics literature.25–27 Clinics that offer donation schemes need to counsel individuals ahead of testing about this possibility and include information about any policies in place regarding (non)disclosure. In some cases, it may be appropriate to offer the donor the option of not receiving results. This is consistent with how other potential, unexpected results are managed where no treatment is available and/or there is no risk to others. The choice to opt-out of receiving results in other circumstances will inevitably generate difficult ethical issues and is best avoided. If necessary, an alternative donor could be found or the plans for transplant in the case of this potential recipient changed.
Infection risk is usually initially assessed by administering a questionnaire to the potential donor, covering ‘Creutzfeldt-Jakob Disease’ and rabies among others. If the donor knows that they are HIV positive, for example, they can then be excluded straight away. Potential donors should not be asked to complete such questionnaires, either verbally or in writing, while they are in a public area, given that these checklists include sensitive information. A copy of the form should be provided to them during the initial consultation to be completed in private and the potential recipient should also not be present. These measures protect the privacy of the donor and may encourage greater honesty in the questionnaire’s completion.
Conclusion
It is imperative that all clinicians involved with any form of (living-related) tissue donation engage with the related ethical issues. For corneal surgeons, this field is relatively new and there are currently no specific professional guidelines dealing with these practices. In this paper, we have set out some of the important ethical considerations and considered how these might be managed in practice, with the aim of provoking discussion and improving provision of local and specialty policies and guidelines to promote good practice in this emerging area of living donation.
Acknowledgments
The authors acknowledge the advice andhelpful comments from Gerda Van Beeumen, Carina Koppen, Sean O Dubhghaill and the anonymous reviewers, which have helped to improve this paper.
Footnotes
Contributors: JB, SND, HD contributed in medical conception, writing, reviewing.
Funding: The work is supported by the EU Horizon2020 project ARREST BLINDNESS (grant agreement number 667400).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Crawford AZ, Patel D V, McGhee CN. A brief history of corneal transplantation: From ancient to modern. Oman J Ophthalmol 2013;6:12–17. 10.4103/0974-620X.122289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pels E, Beele H, Claerhout I. Eye bank issues: II. Preservation techniques: warm versus cold storage. Int Ophthalmol 2008;28:155–63. 10.1007/s10792-007-9086-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borderie VM. Donor selection, retrieval and preparation of donor tissue: Donor selection : Bredehorn-Mayr T, Duncker GIW, Armitage WJ, Eye Banking. Developments in Ophthalmology. 43 Basel: Karger, 2009:22–30. 10.1159/000223836 [DOI] [PubMed] [Google Scholar]
- 4. Gain P, Jullienne R, He Z, et al. . Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 2016;134:167–73. 10.1001/jamaophthalmol.2015.4776 [DOI] [PubMed] [Google Scholar]
- 5. Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med 2012;1:110–115. 10.5966/sctm.2011-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Q, Deng SX, Xu J. In vivo confocal microscopy of congenital aniridia-associated keratopathy. Eye 2013;27:763–6. 10.1038/eye.2013.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rama P, Matuska S, Paganoni G, et al. . Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010;363:147–55. 10.1056/NEJMoa0905955 [DOI] [PubMed] [Google Scholar]
- 8. Sepsakos L, Cheung AY, Holland EJ. Outcomes of keratoplasty after ocular surface stem cell transplantation. Cornea 2017;36:1025–30. 10.1097/ICO.0000000000001267 [DOI] [PubMed] [Google Scholar]
- 9. Abecassis M, Adams M, Adams P, et al. . Live Organ Donor Consensus Group. Consensus statement on the live organ donor. JAMA 2000;284:2919–26. [DOI] [PubMed] [Google Scholar]
- 10. Köberlein J, Beifus K, Schaffert C, et al. . The economic burden of visual impairment and blindness: a systematic review. BMJ Open 2013;3:e003471 10.1136/bmjopen-2013-003471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Leo D, Hickey PA, Meneghel G, et al. . Blindness, fear of sight loss, and suicide. Psychosomatics 1999;40:339–44. 10.1016/S0033-3182(99)71229-6 [DOI] [PubMed] [Google Scholar]
- 12. Gris O, Güell JL, del Campo Z. Limbal-conjunctival autograft transplantation for the treatment of recurrent pterygium. Ophthalmology 2000;107:270–3. 10.1016/S0161-6420(99)00041-X [DOI] [PubMed] [Google Scholar]
- 13. Dua HS, Azuara-Blanco A. Allo-limbal transplantation in patients with limbal stem cell deficiency. Br J Ophthalmol 1999;83:414–9. 10.1136/bjo.83.4.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morgan S, Murray A. Limbal autotransplantation in the acute and chronic phases of severe chemical injuries. Eye 1996;10:349–54. 10.1038/eye.1996.72 [DOI] [PubMed] [Google Scholar]
- 15. Behaegel J, Ní Dhubhghaill S, Koppen C, et al. . Safety of Cultivated Limbal Epithelial Stem Cell Transplantation for Human Corneal Regeneration. Stem Cells Int 2017;2017:1–11. 10.1155/2017/6978253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powers P. Persuasion and coercion: a critical review of philosophical and empirical approaches. HEC Forum 2007;19:125–43. 10.1007/s10730-007-9035-4 [DOI] [PubMed] [Google Scholar]
- 17. Dunstan GR. The ethics of organ donation. Br Med Bull 1997;53:921–39. 10.1093/oxfordjournals.bmb.a011659 [DOI] [PubMed] [Google Scholar]
- 18. Truog RD. The ethics of organ donation by living donors. N Engl J Med 2005;353:444–6. 10.1056/NEJMp058155 [DOI] [PubMed] [Google Scholar]
- 19. Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Off J Eur Union 2004;L102:48–58. [Google Scholar]
- 20. Steering Committee of the Istanbul Summit. Organ trafficking and transplant tourism and commercialism: the Declaration of Istanbul. Lancet 2008;372:5–6. 10.1016/S0140-6736(08)60967-8 [DOI] [PubMed] [Google Scholar]
- 21. Sixty-Third World Health Assembly, World Health Organization. WHO Guiding Principles on Human Cell, Tissue and Organ Transplantation. Cell Tissue Bank 2010;11:413–9. 10.1007/s10561-010-9226-0 [DOI] [PubMed] [Google Scholar]
- 22. Canguilhem G. Writings on medicine. New York: Fordham University Press, 2012. [Google Scholar]
- 23. Wilson JM, Jungner YG. [Principles and practice of mass screening for disease]. Bol Oficina Sanit Panam 1968;65:281–393. [PubMed] [Google Scholar]
- 24. Andermann A, Blancquaert I, Beauchamp S, et al. . Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ 2008;86:317–9. 10.2471/BLT.07.050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ross LF. Good ethics requires good science: why transplant programs should not disclose misattributed parentage. Am J Transplant 2010;10:742–6. 10.1111/j.1600-6143.2009.03011.x [DOI] [PubMed] [Google Scholar]
- 26. Davis DS. The changing face of "misidentified paternity". J Med Philos 2007;32:359–73. 10.1080/03605310701515294 [DOI] [PubMed] [Google Scholar]
- 27. Mandava A, Millum J, Berkman BE. When should genome researchers disclose misattributed parentage? Hastings Cent Rep 2015;45:28–36. 10.1002/hast.452 [DOI] [PMC free article] [PubMed] [Google Scholar]