Abstract
Background:
A variety of measures have been developed to screen for hazardous or harmful drinking. The Alcohol Use Disorders Identification Test—Consumption (AUDIT-C) is one of the screening measures recommended by the U.S. Preventive Services Task Force. Annual administration of the AUDIT-C to all primary care patients is required by the U.S. Veterans Affairs Health System. The availability of data from the repeated administration of this instrument over time in a large patient population provides an opportunity to evaluate the utility of the AUDIT-C for identifying distinct drinking groups.
Methods:
Using data from the Million Veteran Program cohort, we modeled group-based drinking trajectories using 2,833,189 AUDIT-C scores from 495,178 Veterans across an average 6-year time period. We also calculated patients’ age-adjusted mean AUDIT-C scores to compare to the drinking trajectories. Finally, we extracted data on selected clinical diagnoses from the EHR and assessed their associations with the drinking trajectories.
Results:
Of the trajectory models, the four-group model demonstrated the best fit to the data. AUDIT-C trajectories were highly correlated with the age-adjusted mean AUDIT-C scores (rs=0.94). Those with an alcohol use disorder diagnosis had 10 times the odds of being in the highest trajectory group (consistently hazardous/harmful) compared to the lowest drinking trajectory group (infrequent). Those with hepatitis C, PTSD, liver cirrhosis, and delirium had 10%, 7%, 21%, and 34%, respectively, higher odds of being classified in the highest drinking trajectory group vs. the lowest drinking trajectory group.
Conclusion:
Trajectories and age-adjusted mean scores are potentially useful approaches to optimize the information provided by the AUDIT-C. In contrast to trajectories, age-adjusted mean AUDIT-C scores also have clinical relevance for real-time identification of individuals for whom an intervention may be warranted.
Keywords: alcohol use disorder, hazardous drinking, group-based trajectory modeling, electronic health record data
Introduction
Hazardous drinking, defined as alcohol consumption that increases the risk of physical or psychological harm (Saunders et al., 1993), is highly prevalent among Veterans, with possible estimates for Afghanistan (OEF) and Iraq (OIF) Veterans ranging from 27% – 40% (Calhoun et al., 2016). It is known that drinking patterns vary over the life course and to characterize drinking more accurately studies have examined longitudinal measures of alcohol use rather than a single, cross-sectional measure. Several recent studies have used latent growth mixture modeling to identify trajectories of alcohol use from repeated measures of the Alcohol Use Disorders Identification Test–Consumption (AUDIT-C) questionnaire, a widely used, valid, and reliable alcohol screening instrument for the identification of hazardous and harmful drinking (Jacob et al., 2013, Marshall et al., 2015, Justice et al., 2017). The 3-item AUDIT-C measures alcohol consumption (Bush et al., 1998, Bradley et al., 2007) and was derived from the 10-item AUDIT (Saunders et al., 1993). The AUDIT-C was recommended by the US Preventive Services Task Force for the identification of hazardous drinking (Moyer, 2013) and its annual administration to all Veterans in primary care is required by the U.S. Veterans Affairs Health System. We use data from the Million Veteran Program, a large sample of volunteers who gave access to their electronic health records, to evaluate different approaches to the use of repeated measurement of the AUDIT-C to identify clinically informative phenotypic groups.
Previous studies that have employed trajectory modeling in the Veteran population have consistently identified four distinct classes of alcohol use (Jacob et al., 2013, Jacob et al., 2005, Jacob et al., 2009, Jacob et al., 2010, Jacob et al., 2012, Marshall et al., 2015, Justice et al., 2017, Marshall et al., 2017, Fuehrlein et al., 2018). However, the detection of longitudinal patterns can be sensitive to both the composition of the sample and its size. Indeed, the largest of these studies consisted of less than 4,000 Veterans, likely limiting the ability to examine more than four drinking groups due to unstable estimates. Further, previous analyses have been restricted to Veterans of a narrow age range or who served in a particular conflict (e.g., Vietnam, OEF/OIF) and it is unclear whether those findings generalize to the Veteran population as a whole.
We conducted the largest analysis of longitudinal drinking patterns among US Veterans to date, using data from the multi-generational Million Veteran Program (MVP), which currently includes more than 700,000 participants (Gaziano et al., 2016). Herein, we utilized group-based trajectory modeling and proposed a new longitudinal measure, an age-adjusted mean AUDIT-C score. To characterize the individuals in the alcohol risk trajectory groups, we evaluated differences in patient demographics and known correlates of alcohol use and/or alcohol use disorder (AUD) across the trajectory groups. The resultant findings may yield a more generalizable characterization of lifetime drinking patterns among US Veterans compared to previous studies.
Materials and Methods
Sample
This study was conducted with the MVP cohort that was available as of August 2017. Gaziano et al. (2016) has described the MVP study procedures and sampling methods. Briefly, any Veteran able to sign informed consent was eligible for enrollment in MVP. After giving consent, Veterans were asked to complete a self-report questionnaire, provide a blood sample for genetic analysis, and allow access to their electronic health record (EHR). Enrollment for MVP began in early 2011 at the Boston, Massachusetts and West Haven, Connecticut VA facilities. At the time of analysis, the MVP cohort had enrolled 510,358 Veterans from VA Medical Centers across the United States; that number has now grown to more than 700,000.
Within the MVP cohort there were 496,035 individuals with AUDIT-C data in their EHR. Because the AUDIT-C was not a required annual assessment in primary care until the 2008 fiscal year, we excluded participants whose only AUDIT-C score was prior to October 1, 2007 and any individual AUDIT-C scores prior to that date for included Veterans. We also excluded individuals less than 18 or greater than 90 years of age. This yielded an analytic sample of 495,178 Veterans with a total of 2,833,189 AUDIT-C scores.
Measures
The AUDIT-C consists of the first three items of the 10-item AUDIT, developed by the World Health Organization (Saunders et al., 1993). The three questions, which cover the past year, are: (1) How often do you have a drink containing alcohol? (Response options: never, monthly or less, 2–4 times a month, 2–3 times a week, 4 or more times a week); (2) How many standard drinks containing alcohol do you have on a typical day? (Response options: 1 or 2, 3 or 4, 5 or 6, 7 to 9, 10 or more); and (3) How often do you have six or more drinks on one occasion? (Response options: never, less than monthly, monthly, weekly, daily or almost daily). The responses to each question are coded from 0 to 4. Total AUDIT-C scores range from 0 to 12 with a higher score indicating greater risk of hazardous/harmful drinking. Positive screening thresholds for AUDIT-C are ≥3 in women and ≥4 in men (Bradley et al., 2007).
Diagnoses associated with hazardous/harmful drinking were ascertained based on the presence or absence of ICD-9-CM codes available in the EHR and included: post-traumatic stress disorder (PTSD), bipolar disorder (BPD), liver cirrhosis, alcohol use disorder (AUD), delirium, and hepatitis C infection (HCV). The specific ICD-9 codes used to indicate the presence of these diagnoses are available upon request. Demographic characteristics (sex, age, race, and ethnicity) were self-reported and identified in the MVP questionnaire completed by participants at enrollment, or were obtained from the EHR.
Data Analysis
Trajectory analysis has been shown to be a useful strategy to examine trends in drinking over time (Justice et al., 2017, Marshall et al., 2015, Jacob et al., 2013). Semi-parametric group-based trajectory analyses identify distinct groups of individual trajectories on an outcome over time and allow the profiling of individuals’ characteristics within the groups. Groups of individuals with similar trajectories are identified based on maximum likelihood estimates, with the posterior probability of membership in each group calculated for all individuals. The trajectory for which a person has the highest posterior probability of membership is the group to which they are assigned. We used the SAS procedure, Proc Traj (Jones et al., 2001), with a zero-inflated Poisson distribution to estimate trajectories of AUDIT-C scores over 9 years in the MVP cohort, with age as the time scale to account for decreased drinking with increasing age (Grant et al., 2017). A previous study using these methods in a smaller Veteran cohort yielded 4 groups, but estimates for a larger number of trajectories were unstable because of the limited sample size (Justice et al., 2017). In the present study, using the much larger MVP sample, we were able to evaluate trajectory stratification for up to 6 groups. To determine the optimal number of trajectories, we used the absolute value of the Bayesian Information Criterion (BIC), a standard measure to compare trajectory models, where a BIC closer to zero is indicative of better model fit. Finally, we computed entropy, a measure of classification uncertainty, with values closer to 1 indicating greater certainty in discrimination (Celeux and Soromenho, 1996) and also examined the proportion of individuals assigned to the smallest group using 1% as a minimum acceptable threshold (Klijn et al., 2017). The starting polynomial order was set at quartic (the highest allowed in Proc Traj) a priori since we had an average of 7 time points per person. Once we selected the optimal number of classes, we used backward elimination to determine the final polynomial order; all terms were significant in the 4-group quartic model, thus none were removed.
We also calculated an overall mean AUDIT-C score for each individual, and an age-adjusted mean AUDIT-C score, and evaluated their association with the trajectories by computing Spearman’s correlation coefficients. To compute the age-adjusted mean, we used age 50 as the reference point and created weights to down-weight AUDIT-C scores for individuals younger than 50 and up-weight AUDIT C scores for individuals older than 50. To create the weights, we first calculated the mean AUDIT-C score for each individual age and divided the mean at age 50 by each age-specific mean. This produced weights ranging from 0.56 to 1.69. Then each AUDIT-C score was multiplied by the appropriate weight (i.e., an AUDIT-C score for an individual at age 30 was multiplied by the corresponding weight for age 30). The weighted AUDIT-C scores for an individual were added and then divided by the sum of the weights used for that individual.
Lastly, to characterize the trajectories, we described patient characteristics across drinking trajectory groups using means, standard deviations, and percentages. We evaluated associations between patient characteristics (i.e., demographics and clinical diagnoses) and trajectory group using unadjusted generalized logit regression models with the trajectories as the dependent variable and the “infrequent” group as the base (comparison) outcome. We also evaluated the association between age-adjusted mean AUDIT-C scores and AUD using a Wilcoxon rank-sum test. All analyses were conducted using SAS 9.2 (Cary, North Carolina) and an alpha of 0.05 denoted statistical significance.
Results
On average, Veterans included in the analysis had 7 AUDIT-C assessments (sd=3.2, median=7.0, interquartile range [IQR]=5, 8) over 6 years (sd=2.3, median=6.0, mode=8.0, minimum=1). Nearly half (49%) of the 2.8 million AUDIT-C scores used in the analysis had a value of 0 (25th percentile=0, 75th percentile=2). Most of the sample was white (74.1%), non-Hispanic (92.3%), and male (91.4%), with an average age of 62 years (sd=13.8; Table 1).
Table 1.
Demographic and clinical characteristics of the total sample and stratified by alcohol use trajectory group.1
| Total (n=495,178) |
Infrequent (n=172,870) |
Lower risk (n=161,110) |
Potentially hazardous/ harmful (n=127,772) |
Consistently hazardous/ harmful (n=33,426) |
|
|---|---|---|---|---|---|
| Female2 | 8.6 (42,562) | 10.2 (17,635) | 11.4 (18,303) | 4.7 (5,952) | 2.0 (672) |
| Age - mean (SD) | 61.7 (13.8) | 62.8 (13.3) | 60.0 (14.2) | 62.1 (13.8) | 62.6 (13.9) |
| Race3 | |||||
| AA | 19.6 (96,947) | 20.6 (35,563) | 20.0 (32,168) | 17.1 (21,892) | 14.3 (4,763) |
| EA | 74.1 (366,937) | 70.3 (121,522) | 70.6 (113,807) | 75.4 (96,324) | 78.2 (26,141) |
| Other/Mixed race4 | 6.3 (31,293) | 9.1 (15,785) | 9.4 (15,135) | 7.5 (9,556) | 7.6 (2,522) |
| Ethnicity | |||||
| Hispanic/Latino | 6.6 (32,644) | 6.1 (10,618) | 7.4 (11,898) | 6.2 (7888) | 6.7 (2240) |
| Not Hispanic/Latino | 92.0 (455,690) | 92.4 (159,798) | 91.3 (147,012) | 92.5 (118,147) | 91.9 (30,733) |
| Unknown | 1.4 (6,844) | 1.4 (2,454) | 1.4 (2200) | 1.4 (1737) | 1.4 (453) |
| Hepatitis C infection | 6.4 (31,878) | 7.4 (12,759) | 5.8 (9,308) | 5.6 (7,125) | 8.0 (2,686) |
| PTSD | 25.1 (124,142) | 26.0 (44,952) | 25.4 (40,942) | 22.8 (29,110) | 27.3 (9,138) |
| Bipolar disorder | 12.7 (62,772) | 14.0 (24,277) | 12.9 (20,773) | 10.3 (13,163) | 13.6 (4,559) |
| Liver cirrhosis | 3.0 (15,021) | 3.8 (6,617) | 2.4 (3,894) | 2.3 (2,979) | 4.6 (1,531) |
| AUD | 19.9 (98,574) | 12.5 (21,636) | 14.4 (23,240) | 26.4 (33,746) | 59.7 (19,952) |
| Delirium | 4.3 (21,074) | 4.9 (8,546) | 3.8 (6,120) | 3.3 (4,225) | 6.5 (2,183) |
SD=standard deviation; AA=African American; EA=European American; PTSD=posttraumatic stress disorder; AUD=alcohol use disorder.
All values are % (n) unless otherwise indicated.
Sex is missing for 6 individuals
Race is missing for 1 individual
Other race includes: American Indian, Alaska Native, Asian, Native Hawaiian, Other Pacific Islander, Other, and Unknown.
One-, two-, three-, four-, five-, and six-group trajectories were modeled to determine the best fit for the data. Table 2 shows the change in fit statistics moving from a one-group model to a six-group model. There was significant improvement in BIC going from a one- to three-group model, a moderate improvement from a three- to a four-group model, and only modest incremental improvement between the four-, five-, and six-group models. At the tails of the distribution of age, where the sample size was relatively small (e.g., there were 46 individuals who were 22 years old in the highest trajectory), the six-group trajectory model produced unstable estimates. Thus, only the four- and five-group models were considered further. We also examined the median probability of group assignment within the four- and five-group models. In the four-group model, the median probability of membership in each group was greater than 90%; 0.2% or less in each group had a random (i.e., 50%) chance of group assignment and 80% or more in each group had a greater than 70% probability of assignment. In the five-group model, the median probability of group membership ranged from 79% to 96%, up to 1.5% in each group had a random chance of group assignment, and assignment greater than 70% ranged from 65% to 89% of members across groups. Because of the consistently high probability of group assignment for all groups in the four-group model and the small (potentially unstable) class size of the five-group model, we chose the four-group model.
Table 2.
Comparison of trajectory models by AIC, BIC, entropy, posterior probabilities, and sample size per class.
| Number of classes |
AIC | BIC | Entropy | Median Posterior Probability per Class |
Sample Size per Class (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| 1 | −5601101 | −5601129 | -- | 1.00 | 100 | -- | -- | -- | -- | -- |
| 2* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 3 | −3986857 | −3986951 | 0.841 | 1.00/0.98/1.00 | 38.4 | 39.6 | 22.1 | -- | -- | -- |
| 4 | −3928041 | −3928169 | 0.803 | 0.99/0.93/0.92/0.96 | 34.4 | 33.1 | 25.8 | 6.8 | -- | -- |
| 5 | −3910603 | −3910765 | 0.746 | 0.96/0.81/0.79/0.88/0.96 | 31.0 | 23.9 | 24.2 | 18.0 | 3.0 | -- |
| 6 | −3904701 | −3904895 | 0.689 | 0.70/0.86/0.74/0.74/0.81/0.94 | 27.0 | 14.0 | 25.4 | 19.3 | 12.2 | 2.1 |
AIC=Akaike information criterion; BIC=Bayesian information criterion.
Did not converge.
Similar to what was previously reported by Marshall et al. (2015) and Justice et al. (2017), the drinking trajectories (based on AUDIT-C scores) in our four-group model could be described as “infrequent,” “lower risk,” “potentially hazardous/harmful,” and “consistently hazardous/harmful” alcohol use. The majority of the sample fell into the infrequent (34%) and lower risk (33%) groups, with 26% categorized as potentially hazardous/harmful and 7% as consistently hazardous/harmful. Figure 1 shows the trajectories across increasing age. The prevalence of women and African Americans (AAs) decreased across trajectory severity (Table 1). While the prevalence of AUD increased with heavier drinking trajectories, the prevalence of other clinical diagnoses associated with alcohol use tended to decrease from the infrequent use to the moderate use trajectories (i.e., lower risk and potentially hazardous/harmful groups). The consistently hazardous/harmful group had the highest prevalence of all available medical and psychiatric diagnoses except bipolar disorder.
Figure 1.
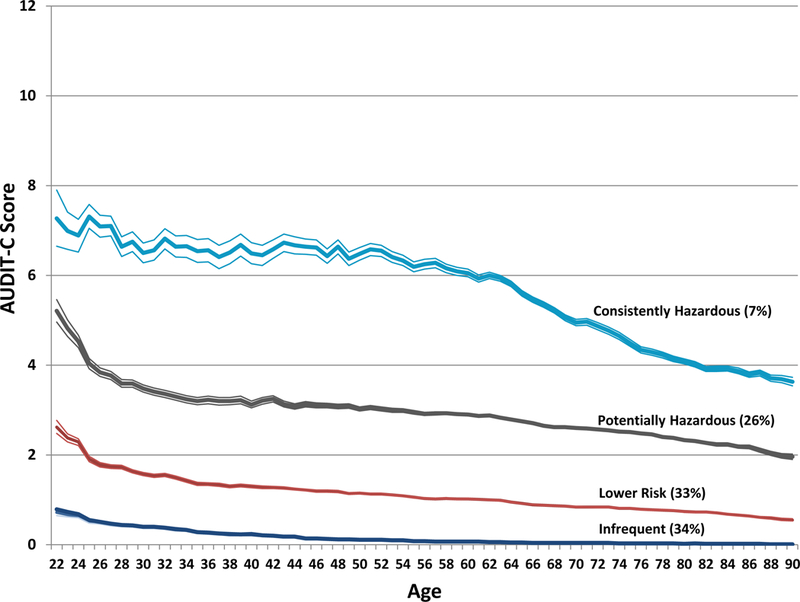
Estimated mean AUDIT-C score across age by alcohol use trajectory group.
The AUDIT-C trajectories were highly correlated with patients’ overall mean AUDIT-C score (rs=0.94, p<.0001) and age-adjusted mean AUDIT-C score (rs=0.94, p<.0001). The distribution of the age-adjusted mean AUDIT-C score by trajectory group can be seen in Figure 2. The overwhelming majority (97%) of the infrequent drinking trajectory (group 1) had an age-adjusted mean AUDIT-C score of 0. Within the lower risk trajectory (group 2), 69% had an age-adjusted mean AUDIT-C score of 1. The highest trajectory groups, 3 and 4, had a wider range of age-adjusted mean AUDIT-C scores. The potentially hazardous/harmful trajectory (group 3) included age-adjusted mean scores from 1 to 8 and the consistently hazardous/harmful trajectory (group 4) included scores from 2 to 12. Within the potentially hazardous/harmful drinking group, the most common AUDIT-C age-adjusted mean scores were 2 (37%) and 3 (39%), whereas in the consistently hazardous/harmful group, the bulk of the distribution of scores (70%) was between 4 and 6.
Figure 2.
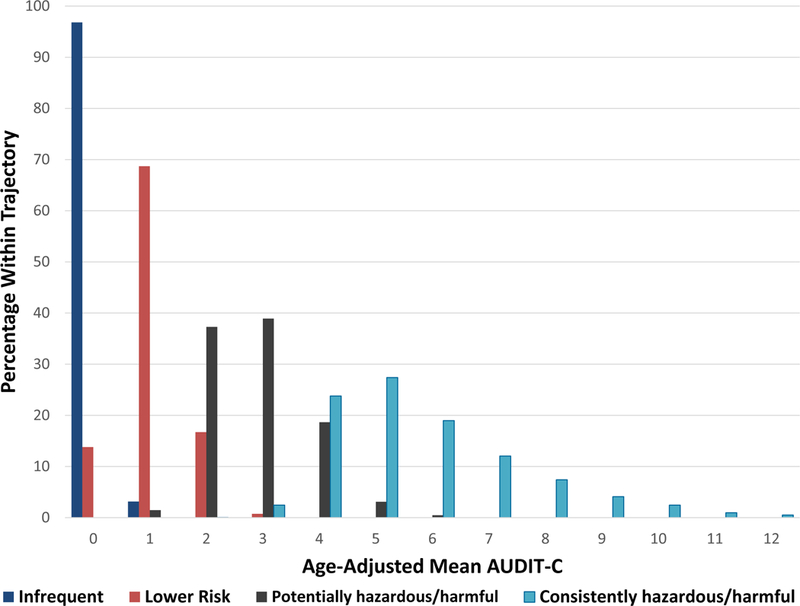
Distribution of age-adjusted mean AUDIT-C scores by alcohol use trajectory group.
As can be seen in Table 3, the generalized logit models showed that women were substantially and significantly less likely than men and AAs were significantly less likely than European Americans (EAs) to be categorized as potentially hazardous/harmful or consistently hazardous/harmful drinkers than infrequent drinkers (potentially hazardous/harmful: odds ratio [OR]=0.43, 95% confidence interval [CI]=0.42, 0.44; consistently hazardous/harmful: OR=0.18, 95% CI=0.17, 0.20). AAs had 38% lower odds of being in the consistently hazardous/harmful trajectory vs. the infrequent trajectory (OR=0.62, 95% CI=0.60, 0.64) and 22% lower odds of being in the potentially hazardous/harmful trajectory vs. the infrequent trajectory (OR=0.78, 95% CI=0.76, 0.79) compared to EAs. Patients with documented HCV infection had 10% greater odds of being in the consistently hazardous/harmful trajectory vs. the infrequent trajectory compared to patients without documented HCV infection (OR=1.10, 95% CI=1.05, 1.15) and patients with PTSD had 7% higher odds of being in the consistently hazardous/harmful trajectory vs. the infrequent trajectory compared to those without PTSD (OR=1.07, 95% CI=1.04, 1.10). Lastly, individuals with cirrhosis had 21% greater odds (OR=1.21, 95% CI= 1.14, 1.28) and those with delirium 34% greater odds (OR=1.34, 95% CI=1.28, 1.41) of being consistently hazardous/harmful drinkers vs. infrequent drinkers compared to those without cirrhosis or delirium, respectively.
Table 3.
Unadjusted generalized logits models of demographic and clinical correlates and alcohol use trajectory group.
| Infrequent (n=172,870) |
Lower risk (n=161,110) |
Potentially hazardous/harmful (n=127,772) |
Consistently hazardous/harmful (n=33,426) |
|
|---|---|---|---|---|
| Female | REF | 1.13 (1.10, 1.15) | 0.43 (0.42, 0.44) | 0.18 (0.17, 0.20) |
| Age (10 year increments) | REF | 0.86 (0.86–0.87) | 0.96 (0.96–0.97) | 0.99 (0.98–1.00) |
| Race (ref=white) | ||||
| Black/AA | REF | 0.97 (0.95–0.98) | 0.78 (0.76–0.79) | 0.62 (0.60–0.64) |
| Other/Mixed race | REF | 1.02 (1.00–1.05) | 0.76 (0.74–0.78) | 0.74 (0.71–0.78) |
| Ethnicity (ref=not Hispanic/Latino) | ||||
| Hispanic/Latino | REF | 1.22 (1.19–1.25) | 1.01 (0.98–1.04) | 1.10 (1.05–1.15) |
| Unknown | REF | 0.97 (0.92–1.03) | 0.96 (0.90–1.02) | 0.96 (0.87–1.06) |
| Hepatitis C | REF | 0.77 (0.75, 0.79) | 0.74 (0.72, 0.76) | 1.10 (1.05, 1.15) |
| PTSD | REF | 0.97 (0.96, 0.99) | 0.84 (0.83, 0.85) | 1.07 (1.04, 1.10) |
| Bipolar disorder | REF | 0.91 (0.89, 0.92) | 0.70 (0.69, 0.72) | 0.97 (0.93, 1.00) |
| Liver cirrhosis | REF | 0.62 (0.60, 0.65) | 0.60 (0.57, 0.63) | 1.21 (1.14, 1.28) |
| AUD | REF | 1.18 (1.16, 1.20) | 2.51 (2.46, 2.56) | 10.35 (10.08, 10.62) |
| Delirium | REF | 0.76 (0.73, 0.79) | 0.66 (0.63, 0.68) | 1.34 (1.28, 1.41) |
All values are odds ratio (95% confidence interval); REF=reference group; AA=African American; PTSD=posttraumatic stress disorder; AUD=alcohol use disorder.
There was a positive, dose-response relationship between drinking trajectory and the presence of an AUD diagnosis – as the trajectory group increased in the amount of drinking, the odds of an AUD diagnosis increased. Specifically, individuals with an AUD diagnosis had 18% greater odds of being classified as lower risk drinkers vs. infrequent drinkers (OR=1.18, 95% CI=1.16, 1.20), 2.5 times greater odds of being potentially hazardous/harmful drinkers vs. infrequent drinkers (OR=2.51, 95% CI=2.46, 2.56), and more than 10 times greater odds of being classified as consistently hazardous/harmful drinkers vs. infrequent drinkers (OR=10.35, 95% CI=10.08, 10.62) compared to those without an AUD diagnosis. We also examined the relationship between age-adjusted mean AUDIT-C and AUD; the median age-adjusted mean AUDIT-C score for those with AUD (median=2.21, mean=2.66) was significantly higher than that of those without AUD (median=0.80, mean=1.21; p<.0001).
Conclusions
We found that a 4-level-trajectory model best characterized longitudinal drinking patterns in the MVP sample. Although many times larger than the Veterans Aging Cohort sample (Justice et al. 2017), the increased statistical power that made it feasible to examine a greater number of groups in trajectory analysis did not lead us to a more complex pattern of trajectories. The finding that trajectories were highly correlated with patients’ overall age-adjusted mean AUDIT-C scores suggests that the age-adjusted mean may be preferable, as it is a simpler means to characterize hazardous/harmful drinking patterns.
Women were significantly less likely to be in the highest drinking trajectories than men consistent with the observation that, overall, women drink less than men (Grant et al., 2017). Interestingly, sex differences in drinking patterns are diminishing and women’s alcohol use is beginning to look more like men’s (White et al., 2015). Though outside the scope of this study, the AUDIT-C data available in the VA EHR could be useful for examining such temporal trends. We also found that AAs were less likely to be classified as being at-risk for harmful drinking than EAs. Recent epidemiological findings show that whites are more likely than other racial or ethnic groups to report current alcohol use, and their prevalence of binge and heavy alcohol use was among the highest of all racial/ethnic groups (U.S. Department of Health and Human Services, 2016, Grant et al., 2017).
An important criterion validation of both the trajectories and age-adjusted mean AUDIT C was the strong association with AUD diagnoses. Similarly, a cross-sectional analysis of VA EHR data showed that the prevalence of documented AUD or substance use disorder (SUD) increased with increasing AUDIT-C-based risk (Williams et al., 2014). Although there is a strong relationship evident between the recorded diagnosis of AUD and AUDIT-C score, it is important to recognize that the two measures do not completely map onto one another. This is particularly apparent for the group characterized as having potentially hazardous/harmful alcohol use, where only 26% had a clinical diagnosis of AUD. Boscarino and colleagues (2017) also found a modest correlation between ICD-9 codes and positive AUDIT-C screening in their study of chronic hepatitis C patients. Therefore, using both AUD and AUDIT-C trajectories may provide greater information than either alone, a finding that is consistent with that reported by Justice et al. (2018) using a genetic criterion measure.
In addition, HCV infection was associated with greater odds of consistently hazardous/harmful drinking than infrequent drinking. This is particularly important because of the interaction of heavy alcohol consumption and HCV in causing liver disease (Fuster et al., 2016, Fuster and Samet, 2018). Heavy alcohol consumption can potentiate HCV-related cytotoxicity and oxidative stress (Fuster et al., 2016), increase the rate of HCV-induced fibrosis (Fuster and Samet, 2018) and the risk of liver cirrhosis and hepatocellular carcinoma (Fuster et al., 2016), and reduce the effect of HCV treatment (Fuster and Samet, 2018). Individuals with an HCV diagnosis were significantly less likely to be categorized into the lower risk and potentially hazardous/harmful trajectories vs. the infrequent drinking trajectory compared to those without HCV. A recent study conducted in a Veteran sample, which largely overlaps with the cohort analyzed herein, found that individuals infected with HCV were more likely to underreport their alcohol consumption (Eyawo et al., 2018), which could explain this unexpected result.
The presence of PTSD, liver cirrhosis, and delirium (common comorbid conditions with AUD) were all associated with reduced odds of being classified in the lower risk and potentially hazardous/harmful drinking trajectories than the infrequent drinking trajectory. Although there is substantial evidence that light and moderate alcohol use is associated with lower alcohol-related morbidity and mortality risk than abstinence (e.g., Kinder et al., 2009, Baumeister et al., 2006, Bradley et al., 2011b), a recent, large meta-analysis of the effects of alcohol use showed that risk of all-cause mortality rises with increasing levels of consumption, and there is no safe drinking level (GBD 2016 Alcohol Collaborators, 2018). One potential explanation for the observed associations in these two trajectory groups is that the clinical diagnoses in the EHR are not sensitive measures, as they depend upon clinician judgment and reporting, which are often unreliable (Kim et al., 2012, Samuel et al., 2015). Thus, their prevalence in the two intermediate trajectory groups may be underestimates. Another possible explanation is that there are individuals in the infrequent alcohol use group who, despite being classified as infrequent drinkers were not always so, having quit heavy drinking after they experienced adverse medical outcomes (Kerr and Ye, 2010, Liang and Chikritzhs, 2011). Yet another explanation could be that social support and more active physical and social lives, both of which may be conferred by moderate alcohol use, improve health functioning (Gros et al., 2016).
This study has several limitations. First, AUDIT-C scores were based on self-report and though the instrument has been found to be a valid and reliable measure of risk of harmful drinking (Bradley et al., 2007), individuals may underreport their drinking behavior. Indeed, as described by Bradley et al. (2011), there is evidence of underreporting in self-report in VA clinical settings compared to anonymous surveys. Second, the clinical correlates that we examined were binary variables that identified the presence of a diagnostic code in the EHR, which does not take into account diagnoses that may have been given to the participants in settings outside the VA. Thus, they likely are an underestimate of the true prevalence of these clinical phenomena. Third, because this was an exclusively Veteran sample, conclusions based on these data may not generalize to the population at large. Fourth, the modeling procedure used herein constrains the variance of AUDIT-C to be zero within each trajectory across time (Jung and Wickrama, 2008) and, consequently, any variation in AUDIT-C is explained by trajectory group membership, which can lead to over-extraction, that is, overestimation of the number of classes. Additionally, individual assignment to a trajectory group was based on the highest posterior probability of membership, which could result in misclassification of individuals. However, we conducted a sensitivity analysis removing all individuals assigned to a group with probability less than 80% and did not observe a substantive change in our findings. This, as well as high entropy (80%) and high median posterior probability of assignment for each group (>90%), partially mitigates the concern for impact of over-extraction and misclassification.
Despite these limitations, we obtained findings that are consistent with a prior analysis that used repeated AUDIT-C scores collected over a period of six years to measure harmful drinking (Justice et al., 2017). We conclude that age-adjusted mean AUDIT-C has implications for the identification of individuals for whom an intervention may be warranted, as these values could be updated in real time, providing an opportunity for early intervention. In contrast, trajectories can only be calculated after the fact, once all the data are available.
Acknowledgments
Financial Support and Acknowledgments:
This research is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration, and was supported by award #VHA-ORD i01 BX003341. This publication does not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Disclosure: Dr. Kranzler has been an advisory board member, consultant, or CME speaker for Alkermes, Indivior and Lundbeck. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the last three years by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor, and Amygdala Neurosciences. He is also named as an inventor on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018. The other authors have no disclosures to make.
References
- BAUMEISTER SE, SCHUMANN A, NAKAZONO TT, ALTE D, FRIEDRICH N, JOHN U & VOLZKE H 2006. Alcohol consumption and out-patient services utilization by abstainers and drinkers. Addiction, 101, 1285–91. [DOI] [PubMed] [Google Scholar]
- BRADLEY KA, DEBENEDETTI AF, VOLK RJ, WILLIAMS EC, FRANK D & KIVLAHAN DR 2007. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res, 31, 1208–17. [DOI] [PubMed] [Google Scholar]
- BRADLEY KA, LAPHAM GT, HAWKINS EJ, ACHTMEYER CE, WILLIAMS EC, THOMAS RM & KIVLAHAN DR 2011a. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med, 26, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY KA, RUBINSKY AD, SUN H, BRYSON CL, BISHOP MJ, BLOUGH DK, HENDERSON WG, MAYNARD C, HAWN MT, TONNESEN H, HUGHES G, BESTE LA, HARRIS AH, HAWKINS EJ, HOUSTON TK & KIVLAHAN DR 2011b. Alcohol screening and risk of postoperative complications in male VA patients undergoing major non-cardiac surgery. J Gen Intern Med, 26, 162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSH K, KIVLAHAN DR, MCDONELL MB, FIHN SD & BRADLEY KA 1998. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med, 158, 1789–95. [DOI] [PubMed] [Google Scholar]
- CALHOUN PS, SCHRY AR, WAGNER HR, KIMBREL NA, DENNIS P, MCDONALD SD, BECKHAM JC, DEDERT EA, KUDLER H & STRAITS-TROSTER K 2016. The prevalence of binge drinking and receipt of provider drinking advice among US veterans with military service in Iraq or Afghanistan. Am J Drug Alcohol Abuse, 42, 269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CELEUX G & SOROMENHO G 1996. An entropy criterion for assessing the number of clusters in a mixture model. Journal of classification, 13, 195–212. [Google Scholar]
- EYAWO O, MCGINNIS KA, JUSTICE AC, FIELLIN DA, HAHN JA, WILLIAMS EC, GORDON AJ, MARSHALL BDL, KRAEMER KL, CRYSTAL S, GAITHER JR, EDELMAN EJ, BRYANT KJ & TATE JP 2018. Alcohol and Mortality: Combining Self-Reported (AUDIT-C) and Biomarker Detected (PEth) Alcohol Measures Among HIV Infected and Uninfected. J Acquir Immune Defic Syndr, 77, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUEHRLEIN BS, KACHADOURIAN LK, DEVYLDER EK, TREVISAN LA, POTENZA MN, KRYSTAL JH, SOUTHWICK SM & PIETRZAK RH 2018. Trajectories of alcohol consumption in U.S. military veterans: Results from the National Health and Resilience in Veterans Study. Am J Addict. [DOI] [PubMed] [Google Scholar]
- FUSTER D & SAMET JH 2018. Alcohol Use in Patients with Chronic Liver Disease. N Engl J Med, 379, 1251–1261. [DOI] [PubMed] [Google Scholar]
- FUSTER D, SANVISENS A, BOLAO F, RIVAS I, TOR J & MUGA R 2016. Alcohol use disorder and its impact on chronic hepatitis C virus and human immunodeficiency virus infections. World J Hepatol, 8, 1295–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAZIANO JM, CONCATO J, BROPHY M, FIORE L, PYARAJAN S, BREELING J, WHITBOURNE S, DEEN J, SHANNON C & HUMPHRIES D 2016. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. Journal of clinical epidemiology, 70, 214–223. [DOI] [PubMed] [Google Scholar]
- GBD 2016 ALCOHOL COLLABORATORS 2018. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet, 392, 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANT BF, CHOU SP, SAHA TD, PICKERING RP, KERRIDGE BT, RUAN WJ, HUANG B, JUNG J, ZHANG H, FAN A & HASIN DS 2017. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74, 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROS DF, FLANAGAN JC, KORTE KJ, MILLS AC, BRADY KT & BACK SE 2016. Relations between Social Support, PTSD Symptoms, and Substance Use in Veterans. Psychol Addict Behav, 30, 764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB T, BLONIGEN DM, HUBEL K, WOOD PK & HABER JR 2012. Drinking course through midlife based on diagnostic versus quantity-frequency indices. Alcohol Clin Exp Res, 36, 477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB T, BLONIGEN DM, KOENIG LB, WACHSMUTH W & PRICE RK 2010. Course of alcohol dependence among Vietnam combat veterans and nonveteran controls. J Stud Alcohol Drugs, 71, 629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB T, BLONIGEN DM, UPAH R & JUSTICE A 2013. Lifetime drinking trajectories among veterans in treatment for HIV. Alcohol Clin Exp Res, 37, 1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB T, BUCHOLZ KK, SARTOR CE, HOWELL DN & WOOD PK 2005. Drinking trajectories from adolescence to the mid-forties among alcohol dependent males. J Stud Alcohol, 66, 745–55. [DOI] [PubMed] [Google Scholar]
- JACOB T, KOENIG LB, HOWELL DN, WOOD PK & HABER JR 2009. Drinking trajectories from adolescence to the fifties among alcohol-dependent men. J Stud Alcohol Drugs, 70, 859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES BL, NAGIN DS & ROEDER K 2001. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociological Methods & Research, 29, 374–393. [Google Scholar]
- JUNG T & WICKRAMA KAS 2008. An Introduction to Latent Class Growth Analysis and Growth Mixture Modeling. Social and Personality Psychology Compass, 2, 302–317. [Google Scholar]
- JUSTICE AC, MCGINNIS KA, TATE JP, XU K, BECKER WC, ZHAO H, GELERNTER J & KRANZLER HR 2017. Validating Harmful Alcohol Use as a Phenotype for Genetic Discovery Using Phosphatidylethanol and a Polymorphism in ADH1B. Alcoholism: Clinical and Experimental Research, 41, 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR WC & YE Y 2010. Relationship of Life-Course Drinking Patterns to Diabetes, Heart Problems, and Hypertension Among Those 40 and Older in the 2005 U.S. National Alcohol Survey. J Stud Alcohol Drugs, 71, 515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM HM, SMITH EG, STANO CM, GANOCZY D, ZIVIN K, WALTERS H & VALENSTEIN M 2012. Validation of key behaviourally based mental health diagnoses in administrative data: suicide attempt, alcohol abuse, illicit drug abuse and tobacco use. BMC Health Serv Res, 12, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINDER LS, BRYSON CL, SUN H, WILLIAMS EC & BRADLEY KA 2009. Alcohol screening scores and all-cause mortality in male Veterans Affairs patients. J Stud Alcohol Drugs, 70, 253–60. [DOI] [PubMed] [Google Scholar]
- KLIJN SL, WEIJENBERG MP, LEMMENS P, VAN DEN BRANDT PA & LIMA PASSOS V 2017. Introducing the fit-criteria assessment plot - A visualisation tool to assist class enumeration in group-based trajectory modelling. Stat Methods Med Res, 26, 2424–2436. [DOI] [PubMed] [Google Scholar]
- LIANG W & CHIKRITZHS T 2011. Reduction in alcohol consumption and health status. Addiction, 106, 75–81. [DOI] [PubMed] [Google Scholar]
- MARSHALL BD, OPERARIO D, BRYANT KJ, COOK RL, EDELMAN EJ, GAITHER JR, GORDON AJ, KAHLER CW, MAISTO SA & MCGINNIS KA 2015. Drinking trajectories among HIV-infected men who have sex with men: a cohort study of United States veterans. Drug and alcohol dependence, 148, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL BDL, TATE JP, MCGINNIS KA, BRYANT KJ, COOK RL, EDELMAN EJ, GAITHER JR, KAHLER CW, OPERARIO D, FIELLIN DA & JUSTICE AC 2017. Long-term alcohol use patterns and HIV disease severity. Aids, 31, 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYER VA 2013. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med, 159, 210–8. [DOI] [PubMed] [Google Scholar]
- SAMUEL AM, LUKASIEWICZ AM, WEBB ML, BOHL DD, BASQUES BA, DAVIS KA & GRAUER JN 2015. ICD-9 diagnosis codes have poor sensitivity for identification of preexisting comorbidities in traumatic fracture patients: A study of the National Trauma Data Bank. J Trauma Acute Care Surg, 79, 622–30. [DOI] [PubMed] [Google Scholar]
- SAUNDERS JB, AASLAND OG, BABOR TF, DE LA FUENTE JR & GRANT M 1993. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, S. A. A. M. H. S. A., CENTER FOR BEHAVIORAL HEALTH STATISTICS AND QUALITY 2016. National Survey on Drug Use and Health.
- WHITE A, CASTLE IJP, CHEN CM, SHIRLEY M, ROACH D & HINGSON R 2015. Converging patterns of alcohol use and related outcomes among females and males in the United States, 2002 to 2012. Alcoholism: clinical and experimental research, 39, 1712–1726. [DOI] [PubMed] [Google Scholar]
- WILLIAMS EC, RUBINSKY AD, LAPHAM GT, CHAVEZ LJ, RITTMUELLER SE, HAWKINS EJ, GROSSBARD JR, KIVLAHAN DR & BRADLEY KA 2014. Prevalence of clinically recognized alcohol and other substance use disorders among VA outpatients with unhealthy alcohol use identified by routine alcohol screening. Drug Alcohol Depend, 135, 95–103. [DOI] [PubMed] [Google Scholar]


