Atherosclerosis is a multifactorial pathological process, during which the physiological composition of arterial walls is structurally and functionally altered. The atheroma growth - accumulation of fibro-adipose and degenerative material in the artery walls – leads to the stiffening of vessels and the narrowing of the lumen, limiting the blood flow. Such reduction of blood flow represents only the initial complication of the formation of the atherosclerotic plaque whereas the persistence of the insult, with the hemodynamic instability that characterizes the atherosclerotic artery, can induce evolution of the plaque versus an instable phenotype with major vulnerability and probability of rupture [1]. The atherosclerotic plaque rupture leads to the exposure of the highly thrombogenic necrotic core material, with subsequent platelet activation and formation of thrombi that can block blood flow in loco, or break away and enter in the bloodstream, obstructing other vessels with smaller diameter. Therefore, the atherosclerotic process can cause coronary artery disease (CAD), ischemic stroke, and peripheral artery disease, representing a major life-threatening disorder.
The main risk factors underlying the development of the atherosclerotic plaque are:
Elevated blood levels of lipids; essentially cholesterol in LDL form, which can deposit along arterial wall and, in combination with oxidative stress, can cause endothelial leak, with incorporation of the lipids inside the intima of the vessel.
High blood pressure; the exposition of endothelium to constant high pressure can trigger atheroma neo-formation; moreover, the hemodynamic instability derived from suddenly high pick of blood pressure, can also represent an insult for pre-existent plaque, causing its rupture.
Tobacco smoking; both above risk factors are exacerbated by smoke through its ability to potentiate hypertension, and to affect lipid metabolism increasing LDL levels. Cigarette smoke represents an independent risk factor for atherosclerosis, since chemical constituents of smoke have high oxidant and inflammatory power that can directly induce endothelial damage and potentiate inflammatory response.
1. Smoking and atherosclerosis: direct proportionality?
Smoke has a profound effect on vessel homeostasis through the activity of single smoking compounds (e.g. nicotine, carbonyl compounds, acrolein, methylvinylketone) and/or their combinatory action; it can affect each stage of the atherosclerotic process, from development until degeneration and rupture of the atherosclerotic plaque with consequent thrombotic events. Cigarette smoking is an aerosol of more than 4000 different compounds, constituted by ~92% of gaseous phase, including carbon monoxide/dioxide and ammonia, and ~8% of particulate phase, characterized by phenol, naphthalene, nicotine [2]. Smoking is considered to be one of the primary causes of vascular injury and atherosclerosis [3], and different studies have verified this effect; in particular for in vivo studies, the ApoE−/− mouse model represents a relevant tool for assessing the contribute of smoking on atherosclerotic process [4]. Using this model, smoke has been shown to increase the plaque size and the severity of atherosclerosis [5]. In the paper published by Cheezum and colleagues in this issue of Atherosclerosis, the direct proportionality in the relationship of smoking and atherosclerosis is examined in detail, in a population of 1798 subjects referred for coronary computed tomographic angiography [6]. The Authors provide clinical evidence that smoking exposure has a dose dependent association with the presence of extensive and calcified atherosclerotic plaques. Importantly, they also show that smoking cessation is associated with reduced CAD severity: in particular, subjects, who quit more than twelve years before the angiography, have a significantly lower probability of obstructive CAD compared with current smokers [6]. However, the molecular mechanisms and cellular responses underlying the relationship between smoke and atherosclerosis are not fully understood, also because in this complex process different cell types are involved, with different responses to various compounds of smoke.
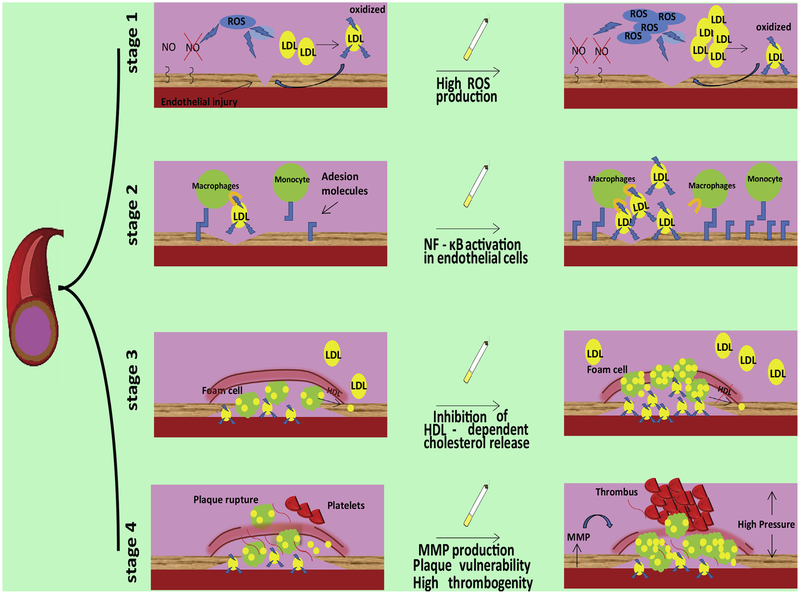
Nevertheless, smoking can affect each single stage of the atherosclerotic disease (Fig. 1): (1) endothelial damage/activation; (2) endothelial interaction with monocyte and their recruitment; (3) formation of foam cells with genesis of the lipid core, and fibrous cap of the plaque; (4) plaque vulnerability and eventual destabilization and rupture.
Fig. 1.
Effects of smoke on the four stages of the atherosclerotic process.
2. Atherosclerotic stage 1: oxidant power and metabolic effects of smoking
The atherosclerotic process begins when an endothelial injury occurs; damaged endothelial cells assume an activated phenotype that is able to induce an inflammatory response, which plays a pivotal role in the regulation of successive phases. Oxidative stress is one of the main mechanisms underlying endothelial injury, eventually leading to plasmamembrane damages, and influencing the activity of numerous enzymes indispensable for endothelial function (e.g. endothelial nitric oxide synthase, eNOS) [7]. Being a main source of reactive oxygen species (ROS), the exposition to smoke can severely affect this stage: the superoxide anion, contained in the gas phase of smoke, reacts with NO, forming peroxynitrite that induces protein nitration causing decreased activity of eNOS. Notably, the reduced bioavailability of NO is one of the main factors altering endothelial homeostasis. Moreover, aldeydes in the smoke can activate NADPH–oxidases, increasing ROS levels in endothelial cells [8]. Equally important, the carbonyl compounds present in the smoke, given their extremely hydrophilic nature, pass through the airway epithelial barrier affecting systemic oxidative status and causing carbonylation, an irreversible modification of different proteins, thereby deeply altering intracellular signaling pathways. The pro-oxidative effects of smoke have been demonstrated also by in vivo studies in ApoE−/− mice, showing an increase of markers of oxidative stress, oxidized LDL, cholesterol esters and ceramide [9]. Oxidized LDL represents the key substrate for the formation of the lipidic core inside the plaque. Also for this aspect, the exposition to smoke plays a key role in atherogenesis, inducing an increase of LDL and reduction of HDL in the blood and promoting the oxidation of LDL. Consequently, smoke is able to act on main mechanisms implicated in the initial triggering of atherosclerosis process: primary endothelial injury through oxidative stress, LDL increase through metabolic alterations, and induction of LDL oxidation due to direct oxidant capacity of smoke components. All of these effects strongly predispose to successive phases of atherosclerosis.
3. Atherosclerotic stage 2: pro-inflammatory effects of smoking
The endothelial injury induces an inflammatory response characterized by changes in pattern of membrane protein expression, production of cytokines and chemokines facilitating the interaction between monocytes and endothelial cells, eventually leading to the recruitment of inflammatory cells from circulation. Smoking has a relevant role in this phase. Indeed, smoke exposition induces NF-κB activation in endothelial cells, increasing the expression of adhesion molecules on the plasmamembrane [10]. The up-regulation of inflammatory genes, including Interleukin-1 and COX-2, after smoke exposure, has been demonstrated in ApoE−/− mice, with proatherogenic effects [11]. Furthermore, nicotine has been shown to trigger the secretion of pro-inflammatory adipokines from the perivascular adipose tissue [12]. Mounting evidence supports the view of a chronic inflammatory state that is common in smokers, characterized by depression of suppressor T-cell function. Thus, smoke affects most of the inflammatory events implicated in atherosclerotic development.
4. Atherosclerotic stage 3: smoke and vascular remodeling
After their interaction with endothelial cells, monocytes infiltrate the intima of the vessel; then, the differentiated macrophages interact and incorporate oxidized LDL. Macrophages with high content of cholesterol, known as foam cells, and extracellular triglycerides and oxidized LDL, accumulate inside the intima, forming the lipid core of atheroma. In this stage, tobacco smoke has been shown to impair HDL-mediated cholesterol efflux from macrophages, increasing cellular accumulation of lipid [4]. Moreover, smoking increases the expression of molecules, including interferon beta and PDGF, that trigger proliferation and migration of vascular smooth muscle cells and the switch from contractile to secretory phenotype [13,14]; in this form, vascular smooth muscle cells are able to release pro-inflammatory factors and extracellular matrix components. This evidence demonstrates the effect of cigarette smoke in inducing and/or accelerating changes in cellular pathological phenotypes, regulating vessel remodeling during the formation of the atherosclerotic plaque.
5. Atherosclerotic stage 4: smoke and plaque destabilization
The clinical outcome of atherosclerosis is essentially determined by plaque destabilization and rupture, leading to exposition of sub-endothelial matter to platelets, ensuing thrombotic events. Many intrinsic factors over time can affect plaque instability; among these, the most relevant are the degradation of extracellular matrix proteins and their reduced synthesis, the increased infiltration of inflammatory cells, and intra-plaque hemorrhage. All of these phenomena are affected by smoke. Indeed, atherosclerotic plaques of smokers are characterized by a predominance of lipid core, and the fibrotic cap is thinner than in non-smokers [3]. This configuration is partly due to augmented metalloproteinase activity in the plaque of smokers, in particular matrix metallo-proteinases (MMPs) are up-regulated in endothelial cells through a mechanism dependent by Tumor Necrosis Factor-α (TNF-α); moreover, in smokers the production of MMPs by macrophages is significantly increased whereas the activity of collagene-forming enzymes is reduced [15,16]. Smoking also triggers the infiltration and activation of macrophages inside the lesion, and their conversion in foam cells, contributing to the growth of lipid core. Intra-plaques hemorrhage due to the formation of neo-vessels in the intima, plays a crucial role in determining plaque instability, and the genesis of immature and leaky vessels is more accentuated in smokers than non-smokers [3]. Smoking is also essential in the process of platelet activation and adhesion to endothelium, as demonstrated by experiments in which the serum of smokers was able to activate platelets of non-smokers [17]. In addition to these effects, tobacco smoke influences plaque instability also through means of indirect events, for instance via the activation of the sympathetic system, which can cause an increase in blood pressure, determinant for plaque damage and instability. This evidence shows that smoking can also affect the degenerative stage of atherosclerotic process, fostering thrombotic events, thereby influencing the clinical outcome of atherosclerosis.
6. Actual benefits of smoking cessation: “QUIT SMOKING. NOW.”
Smoking causes approximately 6 million of deaths per year worldwide, representing a field of urgent intervention to safeguard public health. Many research groups are committed to found molecules that can limit the damage of smoke, principally on vessels and heart. Given the strong pro-oxidant effect of smoke, many of these molecules are antioxidants. For instance, vitamin E administration to ApoE−/− mice reduces the increase of oxidized LDL and markers of oxidative stress induced by smoke exposure [18]; similarly, pomegranate juice reduces lipid accumulation in macrophages [4]. However, the main significant results in reducing risk factors for cardiovascular disease associated to smoking derived from actual cessation of smoke exposure: the healing process begins just 20 minutes after the last cigarette; after one year, a reduction of 50% of coronary artery disease occurs, and between 5 and 15 years from smoking cessation, acute coronary syndrome and stroke are reduced to values similar to non-smokers [19]. Further studies are necessary to completely understand the exact mechanisms underlying the vascular damage induced by smoking, and even more the mechanisms by which smoking cessation has a so powerful effect in reverting the phenotype. As mentioned above, atherosclerosis represents a multifactorial process and the genetic background plays a relevant role during atherogenesis in addition to environmental factors: hence, different genetic configurations and their combinations might explain the heterogeneity of smoking susceptibility [1,3]. Intriguingly, smoking cessation positively affects metabolism, with a regularization of lipid profile: in ApoE−/− mice, the smoke-dependent increase of lipid levels in the blood is reversed after 3 months of smoking cessation [20]. Some beneficial effects of smoking cessation are not immediate, and some of them respect a kinetic that also includes phases of worsening. For instance, biomarkers of atherosclerosis, such as α1-antitrypsin LDL and serum amyloid complex, increase during the first 3 months following smoking cessation and decrease between 3 and 12 months after cessation [21]. This phenomenon of initial worsening is attributable, at least in part, to the association of these two markers also with obesity, so the increase observed in the first months is due to the increase of body weight that characterizes the first period of smoking cessation. Furthermore, the adipose tissue is an important source of inflammatory cytokines that can concur to atherosclerotic plaque development, somehow explaining the strong association of atherosclerosis with obesity. In this context, the adipose tissue has been shown to contain higher levels of the pro-inflammatory cytokines TNF-α and Interleukin-6 (IL-6) in smokers than in non-smokers, and these cytokines after smoking cessation return to levels comparable to non-smokers [22]. The anti-atherosclerotic effects of smoking cessation are further validated by a significant reduction of platelet volume and platelet aggregability [23], thus affecting also the stage 4 of the atherosclerotic process, characterized by thrombotic events. Crucially, a recent study conducted on 290,215 subjects provided evidence that also individuals who smoke fewer than 1 cigarette per day have higher mortality risk than never smokers and would benefit from cessation [24]. Therefore, it is never too late to quit!
Financial support
Dr. Gaetano Santulli is supported by the National Institutes of Health (Grant K99/R00 DK107895).
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Contributor Information
Jessica Gambardella, Columbia University Medical Center, New York, NY, United States; Department of Medicine, Surgery, and Dentistry, University of Salerno, Salerno, Italy.
Celestino Sardu, Inselspital Universitätsspitals,Bern, Switzerland; “John Paul II” Research and Care Foundation, Campobasso, Italy; Department of Medical, Surgical, Neurological, Metabolic, and Geriatric Sciences, Second University of Naples, Naples, Italy.
Cosimo Sacra, “John Paul II” Research and Care Foundation, Campobasso, Italy.
Carmine Del Giudice, Department of Advanced Biomedical Sciences, University of Naples “Federico II”, Naples, Italy.
Gaetano Santulli, Columbia University Medical Center, New York, NY, United States; Department of Advanced Biomedical Sciences, University of Naples “Federico II”, Naples, Italy.
References
- [1].Novak J, Olejnickova V, Tkacova N, et al. , Mechanistic role of MicroRNAs in coupling lipid metabolism and atherosclerosis, Adv. Exp. Med. Biol 887 (2015) 79–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ambrose JA, Barua RS, The pathophysiology of cigarette smoking and cardiovascular disease: an update, J. Am. Coll. Cardiol 43 (2004) 1731–1737. [DOI] [PubMed] [Google Scholar]
- [3].Csordas A, Bernhard D, The biology behind the atherothrombotic effects of cigarette smoke, Nat. Rev. Cardiol 10 (2013) 219–230. [DOI] [PubMed] [Google Scholar]
- [4].Lo Sasso G, Schlage WK, Boue S, et al. , The Apoe(−/−) mouse model: a suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction, J. Transl. Med 14 (2016) 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].von Holt K, Lebrun S, Stinn W, et al. , Progression of atherosclerosis in the Apo E−/− model: 12–month exposure to cigarette mainstream smoke combined with high–cholesterol/fat diet, Atherosclerosis 205 (2009) 135–143. [DOI] [PubMed] [Google Scholar]
- [6].Cheezum MK, et al. , Association of tobacco use and cessation with coronary atherosclerosis, Atherosclerosis (2016). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tollefson AK, Oberley–Deegan RE, Butterfield KT, et al. , Endogenous enzymes (NOX and ECSOD) regulate smoke–induced oxidative stress, Free Radic. Biol. Med 49 (2010) 1937–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rahman MM, Laher I, Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms, Curr. Vasc. Pharmacol 5 (2007) 276–292. [DOI] [PubMed] [Google Scholar]
- [9].Boue S, Tarasov K, Janis M, et al. , Modulation of atherogenic lipidome by cigarette smoke in apolipoprotein E–deficient mice, Atherosclerosis 225 (2012) 328–334. [DOI] [PubMed] [Google Scholar]
- [10].Collins T, Endothelial nuclear factor–kappa B and the initiation of the atherosclerotic lesion, Lab. Invest 68 (1993) 499–508. [PubMed] [Google Scholar]
- [11].Barbieri SS, Weksler BB, Tobacco smoke cooperates with interleukine1beta to alter beta–catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenasee2 expression in vitro and in vivo, FASEB J 21 (2007) 1831–1843. [DOI] [PubMed] [Google Scholar]
- [12].Wang CN, Yang GH, Wang ZQ, et al. , Role of perivascular adipose tissue in nicotineinduced endothelial cell inflammatory responses, Mol. Med. Rep 14 (2016) 5713–5718. [DOI] [PubMed] [Google Scholar]
- [13].Demirjian L, Abboud RT, Li H, et al. , Acute effect of cigarette smoke on TNF–alpha release by macrophages mediated through the erk1/2 pathway, Biochim. Biophys. Acta 1762 (2006) 592–597. [DOI] [PubMed] [Google Scholar]
- [14].Santulli G, Wronska A, Uryu K, et al. , A selective microRNA–based strategy inhibits restenosis while preserving endothelial function, J. Clin. Invest 124 (2014) 4102–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Raveendran M, Senthil D, Utama B, et al. , Cigarette suppresses the expression of P4Halpha and vascular collagen production, Biochem. Biophys. Res. Commun 323 (2004) 592–598. [DOI] [PubMed] [Google Scholar]
- [16].O’Toole TE, Zheng YT, Hellmann J, et al. , Acrolein activates matrix metallo-proteinases by increasing reactive oxygen species in macrophages, Toxicol. Appl. Pharmacol 236 (2009) 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ljungberg LU, Persson K, Eriksson AC, et al. , Effects of nicotine, its metabolites and tobacco extracts on human platelet function in vitro, Toxicol Vitro 27 (2013) 932–938. [DOI] [PubMed] [Google Scholar]
- [18].Kunitomo M, Yamaguchi Y, Kagota S, et al. , Biochemical evidence of atherosclerosis progression mediated by increased oxidative stress in apolipoprotein E–deficient spontaneously hyperlipidemic mice exposed to chronic cigarette smoke, J. Pharmacol. Sci 110 (2009) 354–361. [DOI] [PubMed] [Google Scholar]
- [19].Frey P, Waters DD, DeMicco DA, et al. , Impact of smoking on cardiovascular events in patients with coronary disease receiving contemporary medical therapy (from the Treating to New Targets [TNT] and the Incremental Decrease in End Points through Aggressive Lipid Lowering [IDEAL] trials), Am. J. Cardiol 107 (2011) 145–150. [DOI] [PubMed] [Google Scholar]
- [20].Lietz M, Berges A, Lebrun S, et al. , Cigarette–smoke–induced atherogenic lipid profiles in plasma and vascular tissue of apolipoprotein E–deficient mice are attenuated by smoking cessation, Atherosclerosis 229 (2013) 86–93. [DOI] [PubMed] [Google Scholar]
- [21].van Lammeren GW, den Ruijter HM, Vrijenhoek JE, et al. , Time℃dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery, Circulation 129 (2014) 2269–2276. [DOI] [PubMed] [Google Scholar]
- [22].Mach L, Bedanova H, Soucek M, et al. , Tobacco smoking and cytokine levels in human epicardial adipose tissue: Impact of smoking cessation, Atherosclerosis 255 (2016) 37–42. [DOI] [PubMed] [Google Scholar]
- [23].Inoue T, Hayashi M, Uchida T, et al. , Significance of platelet aggregability immediately after blood sampling and effect of cigarette smoking, Platelets 12 (2001) 415–418. [DOI] [PubMed] [Google Scholar]
- [24].Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, et al. , Association of Long-term, Low-Intensity Smoking With All-Cause and Cause-Specific Mortality in the National Institutes of Health-AARP Diet and Health Study, JAMA Intern. Med 177 (1) (2017) 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]