Abstract
The small conducting airways are the major site of obstruction in COPD. This report examines small airway pathology using a novel combination of multi-detector row computed tomography (MDCT), microCT and histology.
Airway branches visible on specimen MDCT were counted and the dimensions of the 3rd–5th generation airways were computed, whereas the terminal (TB), preterminal (TB-1) and pre-preterminal (TB-2) bronchioles were examined with microCT and histology in 8 explanted lungs with end-stage COPD and 7 unused donor lungs that served as controls.
On MDCT, COPD lungs showed a decrease in the number of 2–2.5mm diameter airways and the lumen area of 5th generation airways while on microCT there was a reduction in the number of TBs as well as a decrease in the luminal areas, wall volumes and alveolar attachments to the walls of TB, TB-1, and TB-2. The combination of microCT and histology showed increased B cell infiltration into the walls of TB-1 and TB-2 and this change was correlated with a reduced number of alveolar attachments in COPD.
Small airways disease extends from 2mm diameter airways to the terminal bronchioles in COPD. Destruction of alveolar attachments may be driven by a B cell-mediated immune response in the preterminal bronchioles.
Introduction
A series of reports from the 1960s [1–4] established that the small conducting airways < 2mm in diameter offer < 10% of the total resistance to airflow in health but become the major site of increased resistance in chronic obstructive pulmonary disease (COPD). Based on these and other data, Mead postulated that the small conducting airways < 2mm in diameter are a “quiet” zone within normal lungs where disease can accumulate over many years without being noticed [5]. Since then, numerous reports have shown that the disease in the small conducting airways in COPD is characterized by chronic inflammation, lumen narrowing, wall thickening, and loss of alveolar attachment to the outer walls of these airways [6–12]. In addition, the introduction of multi-detector row computed tomography (MDCT) in the 1990s made it possible to show that abnormalities in the 2–3mm in diameter airways visible on MDCT is closely associated with airflow obstruction in COPD [13]. More recent reports using MDCT and microCT have shown that the terminal bronchioles (TB) are narrowed and extensively destroyed in lungs from patients with end-stage COPD treated by lung transplantation [14]. Furthermore, microCT studies have shown lumen narrowing, wall thickening, and loss of alveolar attachments in preterminal bronchioles (TB-1) in the centrilobular emphysema phenotype of COPD [15].
The purpose of this report is to use a very recently developed approach capable of linking MDCT and microCT scans to histology in exactly the same samples of tissue [16] in order to determine if small airways disease extends from the smaller bronchi and bronchioles (2–2.5mm in diameter) that define the start of the lung’s quiet zone to the preterminal (TB-1 and TB-2) and terminal bronchioles (TB) that are the last purely conducting airways in the lung’s quiet zone.
Methods
Informed consent
Informed consent was obtained directly from persons with very severe COPD waiting for lung transplantation and from the donor’s next of kin under conditions that allows organs considered unsuitable for transplantation to be released for research.
MDCT and MicroCT scans
In this study, all specimens were right explanted lungs. The procedures used to prepare the lung tissue for MDCT and microCT imaging followed by histology have been described detail elsewhere [14–16]. Briefly, 8 lungs affected by COPD were donated by subjects undergoing lung transplantation for COPD; all had GOLD 4 disease. Seven unused donor lungs (3 never and 4 former smokers) served as controls. The lungs were fully inflated with air to a positive pressure of 30 cm H2O, deflated and held at constant pressure (10 cm H2O) while the lungs were surrounded by liquid nitrogen vapor and frozen solid. The inflated lung specimen was kept frozen while MDCT scans were obtained using either a Siemens Sensation 16 (120 kVp, 250 mA, pitch: 1.25, 1mm contiguous images) or a GE Discovery CT750 HD (120 kVp, 250 mA, pitch: 1.375, 0.625mm contiguous images) CT scanner and reconstructed using a high spatial frequency reconstruction algorithm (Siemens: B60f, GE: Bone). The frozen specimen was then cut into 2cm-thick transverse slices from lung apex to base and regions of mild to moderate emphysema were specifically identified by visually inspecting photographs of the slices from the COPD lungs. Frozen cores of tissue 14mm in diameter and 2cm in length were removed from the selected lung regions of COPD lungs as well as from randomly selected regions of control lungs. The cores were imaged on a Nikon HMX 225ST micro CT scanner with 11μm isotropic voxel resolution at −30C° using a technique recently described in detail by Vasilescu et al [16].
MDCT analysis
The Apollo 2.0 software (VIDA diagnostic Inc, Iowa, USA) was used to analyze the tracheobronchial tree in the MDCT specimen scans. The airway tree was automatically segmented based on the region growing method. A new seed point was manually set to facilitate further region growing when necessary based on a recently reported method [17]. The numbers of branches in each generation of airway was automatically counted using the segmented airway trees. The luminal and wall areas were measured for the 3rd, 4th and 5th generation airways of the RB1 and RB10 paths, and averaged for each of the generations. The percentage of low attenuation lung tissue < −950 Hounsfield units (LAA%) [18, 19] was also calculated to estimate the severity of the emphysema in the entire right lungs.
MicroCT analysis
The mean linear intercept (Lm) and the numbers of terminal bronchioles/ ml lung were measured in each tissue core as previously described [14]. One terminal bronchiole (TB), defined as the last purely conducting airway, was randomly selected in each tissue core, and then preterminal bronchioles (TB-1) [15] and pre-preterminal bronchiole (TB-2) were identified by tracking back from the selected TBs [14]. Cross-sectional micro CT images of each of these airways perpendicular to the centerline of their lumens were reconstructed, and the bronchiolar length (distance between two branch points), luminal and wall areas, wall thickness and volume, internal diameter, number of alveolar attachments to their wall, and number of alveolar attachments adjusted by the outer perimeter were obtained using previously reported methods [15].
Histology
Portions of the frozen cores of tissue examined by microCT were fixed overnight in alcohol-based formalin kept at −20C°, warmed up to room temperature, processed into paraffin, and cut into 4μm-thick sections [16]. The examination of hematoxylin and eosin stained sections using a light microscope made it possible to register the histological sections to microCT scans to identify TB, TB-1, and TB-2. For immunohistochemistry, the sections were incubated with the primary antibodies to identify specific cell types (see supplementary Table E1) followed by secondary antibodies attached to the alkaline phosphatase system that amplified signals. The volume fractions of the airway walls occupied by immune cell sub-types were calculated for all identifiable TB, TB-1, and TB-2 on histological sections. In addition, the perimeter of the basement membrane (Pbm), lumen and wall areas of relatively circular airways (long axis / short axis < 3) were measured and histological wall thickness was calculated by dividing wall areas by Pbm according to a previously reported manual tracing technique [10]. Since basement membrane length is not affected by inflation level or extent of smooth muscle contraction [10, 20], the area enclosed by the basement membrane in a hypothetical maximally dilated circular airway (Amax) can be calculated as; Amax = Pbm2 / (4*Pi), The degree of luminal narrowing can then be calculated as 1-(measured luminal area/Amax) as reported by Bosken et al [11].
Statistical analysis
Data are expressed as mean ± SD. Either t-tests or Wilcoxon rank sum tests were used for comparison between control and COPD groups. Tukey’s adjustment was used for multiple comparisons. Pearson correlation tests were performed after log-transformation of the volume fraction of immune cells. Statistical analysis were performed with the R program [21].
Results
Table 1 shows that the 7 control subjects and 8 subjects with COPD were well matched for age, sex, and height while the controls were heavier. All subjects with COPD were former smokers, whereas 3 controls were never smokers.
Table1.
Demographics of subjects
| Control | COPD | P value | |
|---|---|---|---|
| No. subjects | 7 | 8 | |
| Age | 59 ± 11 | 56 ± 6 | 0.51 |
| Sex (Male : Female) | 4 : 3 | 3 : 5 | 0.62 |
| Height | 176 ± 5 | 171 ± 9 | 0.21 |
| Weight | 85 ± 18 | 65 ± 13 | 0.03 |
| Smoking history (Never: Former) | 3 : 4 | 0 : 8 | |
| FEV1 (%predicted) | NA | 25 ± 6 |
Data are expressed as mean ± SD per sample. FEV1 = forced expiratory volume in 1 second, Never = never smoker, Former = former smoker, NA = not available.
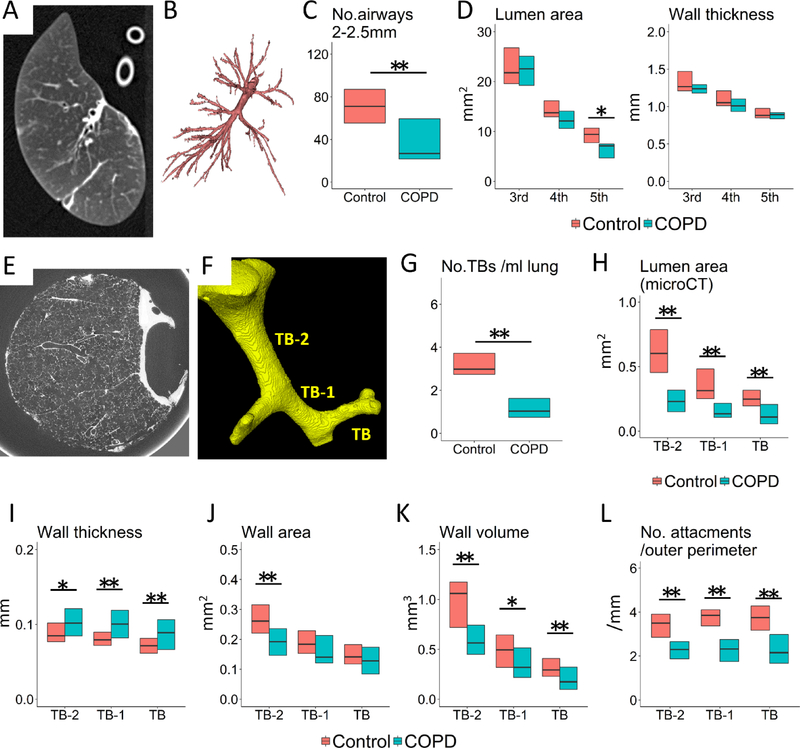
Figure 1 A and B show a MDCT specimen scan used to reconstruct the airway tree using the Apollo software. Table 2 shows that total lung volume and emphysematous change on specimen MDCT scans were substantially greater in COPD than in controls. There was a downward trend in total airway number, wall and lumen area in lungs affected by COPD compared to controls, which became statistically significant for the number of airway branches 2–2.5mm in diameter (Figure 1 C) and luminal areas of the 5th generation airways (Figure 1 D). Figure 1D also shows no difference in the wall thickness of the 3rd, 4th, and 5th generation airways between COPD and controls.
Figure 1. Specimen MDCT and microCT analysis of airways.
(A) shows a MDCT scan, from which the airway tree is segmented (B). MDCT measurements show the number of airway branches 2 to 2.5 mm in diameter (C) and luminal area of the 5th generation airways (sub-subsegmental) are decreased in COPD compared to control lungs while wall thickness does not differ (D). (E) shows a microCT scan, from which the lumen of terminal (TB), preterminal (TB-1), and pre-preterminal (TB-2) bronchioles were segmented (F). MicroCT measurements show the number of TBs (G) and the luminal areas of TB, TB-1, and TB-2 (H) are decreased in COPD compared to control lungs. These measurements also show increases in the wall thickness of TB, TB-1, and TB-2 (I) and the wall area of TB-2 (J) and decreases in the wall volume (K) and the number of alveolar attachments per outer perimeter (L) of TB, TB-1, and TB-2 in COPD. * p<0.05 and ** p<0.01.
Table2.
Quantitative measurements of explanted lungs with specimen MDCT scans
| Control | COPD | P value | |
|---|---|---|---|
| Total lung volume (L) | 2661 ± 691 | 4244 ± 662 | 0.0006 |
| LAA% (%) | 13.7 ± 8.6 | 51.3 ± 6.3 | <0.0001 |
| Airway count | |||
| >3 mm in diameter | 127 ± 52 | 117 ± 56 | 0.71 |
| 2.5 – 3.0 mm in diameter | 59 ± 15 | 48 ± 26 | 0.35 |
| 2 – 2.5 mm in diameter | 71 ± 20 | 38 ± 22 | 0.007 |
| Luminal area (mm2) | |||
| 3rd generation | 23.3 ± 5.2 | 23.3 ± 7.5 | 1.00 |
| 4th generation | 15.0 ± 3.3 | 12.7 ± 4.9 | 0.31 |
| 5th generation | 9.2 ± 2.0 | 6.4 ± 2.1 | 0.02 |
| Wall area (mm2) | |||
| 3rd generation | 30.5 ± 6.1 | 26.7 ± 4.2 | 0.20 |
| 4th generation | 20.3 ± 4.2 | 17.0 ± 3.8 | 0.13 |
| 5th generation | 13.5 ± 2.7 | 10.8 ± 2.3 | 0.06 |
Data are expressed as mean ± SD. LAA% =low attenuation area percent of the lung with attenuation values below −950 HU.
Figure 1E and F show how the microCT scans were used to identify TB and to trace back from TB to TB-1 and TB-2. Table 3 compares microCT data from 38 samples from 7 control lungs to 40 samples from 8 explanted COPD lungs, and supplementary Figure E1 shows that the distribution of the positions of these samples within the lungs was not different between control and COPD. The number of TBs per ml lung and luminal areas and alveolar attachments of TB, TB-1, and TB-2 were reduced in COPD (Table 3, Figure 1G and H). The wall thickness of TB-2, TB-1 and TB (Figure 1I) was increased without an increase in wall area (Figure 1J) in these airways, whereas the wall volumes (Figure 1K) and the number of alveolar attachments adjusted by the outer perimeter (Figure 1L) were decreased in COPD.
Table3.
MicroCT findings of sample cores
| Control (N=7) | COPD (N=8) | P value | |
|---|---|---|---|
| No. sample cores | 38 | 40 | |
| Lm (μm) | 390 ± 73 | 1198 ± 491 | <0.0001 |
| No. terminal bronchioles (/ml) | 3.3 ± 1.2 | 1.2 ± 0.7 | <0.0001 |
| Internal diameter of lumen (mm) | |||
| TB | 0.58 ± 0.12 | 0.40 ± 0.22 | <0.0001 |
| TB-1 | 0.68 ± 0.17 | 0.44 ± 0.18 | <0.0001 |
| TB-2 | 0.88 ± 0.20 | 0.58 ± 0.12 | <0.0001 |
| Bronchiolar length (mm) | |||
| TB | 2.10 ± 0.56 | 1.50 ± 0.58 | <0.0001 |
| TB-1 | 2.76 ± 0.89 | 2.29 ± 0.89 | 0.03 |
| TB-2 | 3.64 ± 0.94 | 3.15 ± 0.95 | 0.09 |
| No. alveolar attachments | |||
| TB | 9.7 ± 1.8 | 4.8 ± 1.4 | <0.0001 |
| TB-1 | 11.1 ± 2.1 | 5.5 ± 1.3 | <0.0001 |
| TB-2 | 12.6 ± 2.0 | 6.4 ± 2.0 | <0.0001 |
Data are expressed as mean ± SD per sample. In COPD, 39 terminal (TB), 32 preterminal (TB-1), and 29 pre-preterminal (TB-2) bronchioles were used to measure internal diameter, bronchiolar lumen, and the number of alveolar attachments to the outer wall, while 38 TB, 36 TB-1, and 29 TB-2 were used in controls. When there were multiple bronchioles at the same generations, one bronchiole was randomly chosen.
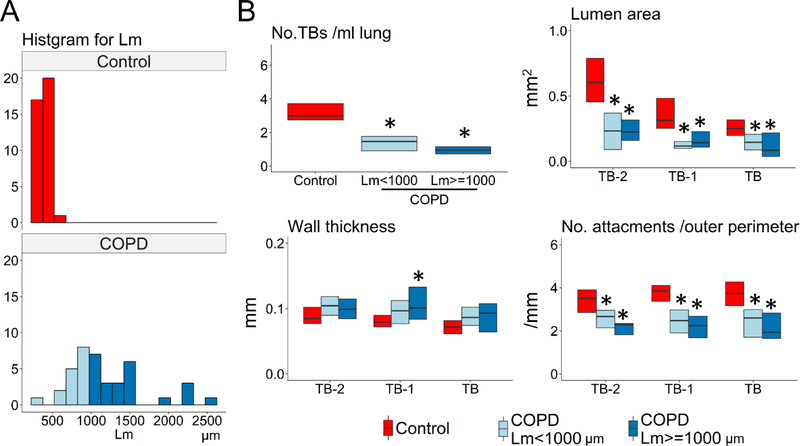
Figure 2A shows the distributions of Lm for samples from controls and COPD subjects. Figure 2B shows that the number of terminal bronchioles, lumen area, and number of alveolar attachments to TB-2, TB-1, and TB were decreased in samples with mild emphysema (Lm < 1000μm) in the COPD lungs compared to controls. Supplementary Figure E2 shows no differences in LAA% (MDCT) or Lm, the number of terminal bronchioles, lumen areas, and the number of alveolar attachments (microCT) between controls with and without a smoking history.
Figure 2. MDCT and microCT comparisons between control and COPD samples with different severity of emphysema.
(A) A histogram for mean linear intercept (Lm) in control and COPD. In order to compare mild emphysematous regions of the COPD lungs with controls, COPD samples were divided into those with Lm <1000 μm (n=16) and Lm ≥1000 μm (n=24). (B) The number of terminal bronchioles (TBs) per ml lung, the mean lumen area, and the number of alveolar attachments per length of outer perimeter of TB, TB-1, and TB-2 were decreased in both groups of COPD samples compared to controls. * p<0.05 vs control.
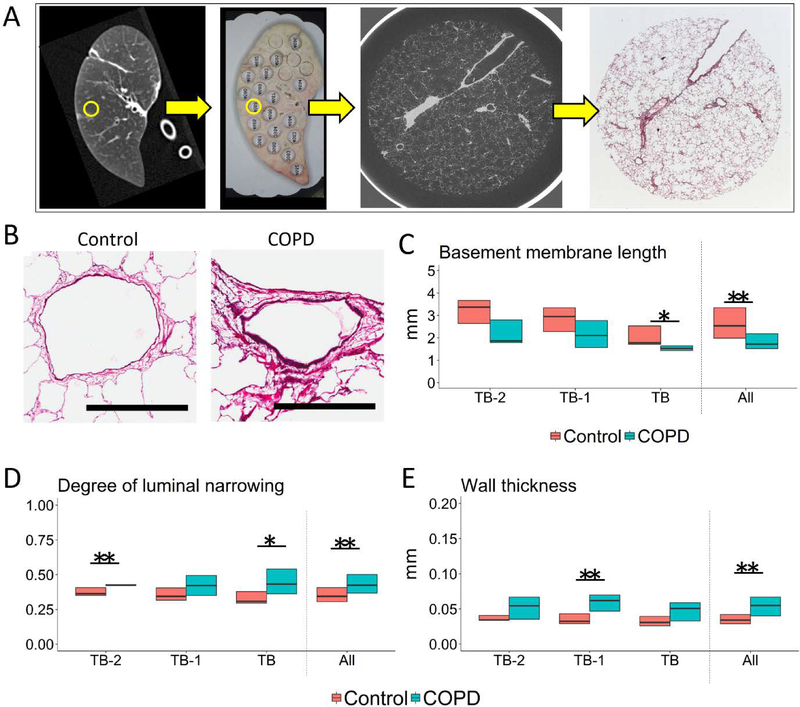
Figure 3A shows a specimen MDCT scan that was matched with a photograph of the corresponding lung slice where the circled area indicates a region sampled for microCT and histology. Figure 3B shows representative cross-sections of TB-1 in control and COPD. Histological analysis of airways that were cut circularly, showed a downward trend in the perimeter of basement membrane in COPD compared to control, which became statistical significant for TB (Figure 3C). In contrast, there was trend toward greater luminal narrowing (Figure 3D) and wall thickness (Figure 3E) in COPD, which became statistical significant for TB-1.
Figure 3. Structural evaluation of small airways on histology combined with microCT scan.
(A) An example of a MDCT scan of a specimen (left panel) matched with a photograph of the corresponding lung slice on which the circled area indicates the region that was subsequently used for microCT and histology. This allowed for comparison of the microCT to the histology of the same airway. By matching a microCT image to a histological section, this technique enabled identification of terminal (TB), preterminal (TB-1), and pre-preterminal (TB-2) bronchioles on histology. (B) Representative cross-sections of TB-1 in control and COPD. Scale bar indicates 0.5mm. (C, D & E) show histological measurements of relatively circular bronchioles (9 TB, 11 TB-1, and 5 TB-2 in control and 8 TB, 11 TB-1, and 5 TB-2 in COPD) (C) Basement membrane length, (D) Degree of airway narrowing (E) and Wall thickness. The degree of airway narrowing was calculated as; 1 – (measured luminal area) / (calculated area enclosed by the basement membrane in hypothetical maximally dilated airways). “All” on the x-axis indicates the data when combining all circular bronchioles including TB-2, TB-1, and TB (n=25 in control and n=24 in COPD). *p<0.05 and **p<0.01.
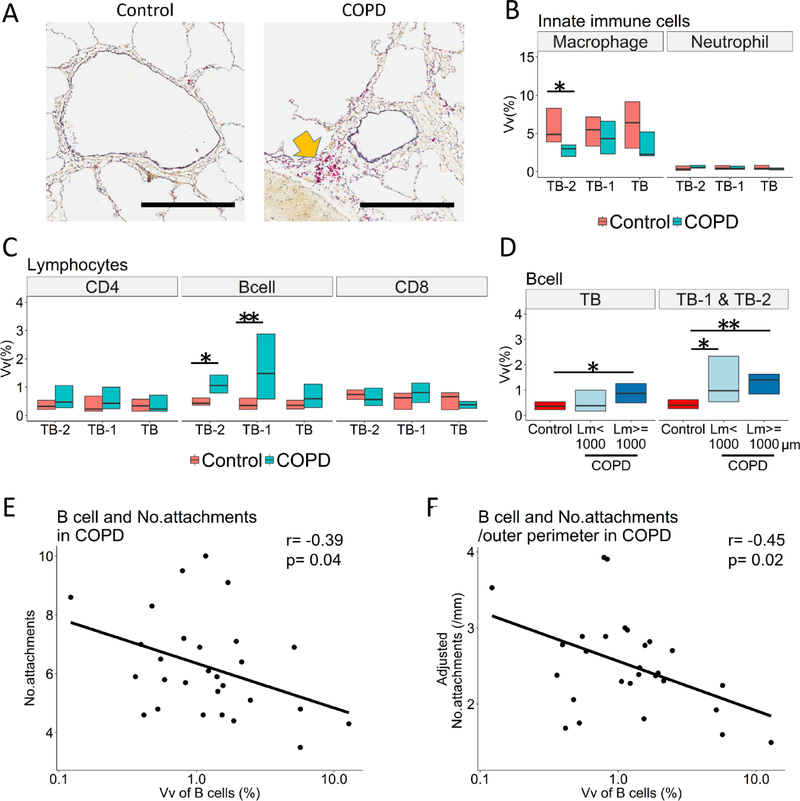
Figure 4A shows representative CD79a staining of TB-1 in control and COPD. The data from immunochemical staining of all circular and non-circular airways show that in COPD the macrophages stained with a CD68 antibody were decreased in TB-2 (Figure 4B) and the B cells stained with a CD79a antibody were increased in TB-1 and TB-2 (Figure 4A and C). Figure 4D shows an increase in B cells in TB-1 and TB-2 for samples with Lm < 1000μm and Lm ≥ 1000μm in the COPD lungs. Moreover, the increased B cell infiltration into TB-1 and TB-2 in COPD was associated with a reduced number of alveolar attachments/airway (Figure 4E) and with the number of alveolar attachments adjusted by the outer perimeter (Figure 4F) of the same airways, but not with the wall thickness or luminal area (Supplementary Table E2).
Figure 4. A combined analysis of immunohistochemistry and microCT images to assess infiltration of immune cells and structural changes in small airways.
(A) Representative immunohistochemistry with CD79a antibody (B cell marker, indicated by a yellow arrow) in preterminal bronchiole (TB-1) of control and COPD. Scale bar indicates 0.5mm. TB and TB-2 indicate terminal and pre-preterminal bronchioles. (B and C) Volume fractions (Vv) of the airway wall occupied by immune cells using all circular and non-circular bronchioles (15–16 TB, 15–17 TB-1, and 9–10 TB-2 in control and 17–18 TB, 15–16 TB-1, and 13 TB-2 in COPD). (D) A significantly greater B cell infiltration into the walls of TB1 and TB-2 in COPD samples with mean liner intercept (Lm) <1000 μm (n=14) and those with Lm ≥1000 μm (n=15) compared to controls (n=24). (E) and (F) show the relationships of Vv of B cells in the wall of TB-1 and TB-2 with the number of alveolar attachments per airway (r=−0.39, p=0.04) and with the number of alveolar attachments per mm of outer perimeter (r=−0.45, p=0.02), respectively. *p<0.05 and **p<0.01.
Discussion
In this study, the combined use of MDCT and microCT provides new information about the lung’s “quiet” zone in COPD by showing that both the numbers of airways 2–2.5mm in diameter identifiable on MDCT scans and terminal bronchioles (TB) on microCT scans are decreased, and the lumens of the 5th generation airways and the preterminal and terminal bronchioles (TB-2, TB-1, and TB) are narrowed in COPD. These data suggest that the disease in the lung’s “quiet zone” extends from airways 2–2.5mm in diameter to the terminal bronchioles. Furthermore, the combination of microCT and histology allowed us, for the first time, to show that increased B cell infiltration into the walls of TB-1 and TB-2 in COPD is associated with destruction of alveolar attachments to these airways.
A major advantage of the present report is that it is based on advances in technology [16] that allows the bronchioles to be accurately located using microCT and then processed for histology. This makes it possible to study tissue remodeling and destruction in relation to inflammatory-immune cell infiltration into the terminal, and preterminal bronchioles (i.e.TB-2, TB-1, and TB) defined by microCT. Consequently, the present data extend numerous histological reports on the small airways disease in COPD [10–12, 14] by showing the wall thickening and luminal narrowing of TB-1 and the increased B cell infiltration into TB-1 and TB-2 that are associated with loss of alveolar attachments.
Increased B cell infiltration into small airways and parenchyma has been recognized as a major histological feature of COPD [10, 22–24], and gene expression analyses have suggested an association between emphysematous destruction and B cell related immune pathways [25, 26]. However, despite the fact that small airways, generally defined as bronchioles < 2mm in diameter, include relatively larger bronchioles (1–2mm in diameter) as well as preterminal and terminal bronchioles (0.5–0.7mm in diameter), no report has examined B cell infiltration for specific generations of small airways. Moreover, although abnormalities in the terminal and preterminal bronchioles are important pathological lesions of COPD [14, 15], the mechanism by which an increase in B cell infiltration contributes to structural changes in these airways is not understood. Thus, the present results showing the association between increased B cells and decreased alveolar attachments in TB-1 and TB-2 of the COPD lungs substantially extends the previous findings, and suggests that persistent B cell mediated immune response causes destruction of alveolar attachments, which eventually promotes the progression of emphysema.
It should be also noted that the reduction in TB number, luminal narrowing, reduced alveolar attachments, and increased B cell infiltration in COPD lungs were present even in samples with mild emphysema (Lm < 1000μm). This suggests that these pathological changes in small airways may develop prior to the establishment of severe emphysematous destruction.
The microCT data show reduced bronchiolar lengths in TB and TB-1 but not TB-2 We postulate that this shortening of airways is the consequence of axial retraction of the airway tree induced by breaks in the elastic fibers that run longitudinally along its entire length from the trachea to the most peripheral bronchioles and alveoli [27, 28]. The results also show an increase in wall thickness of TB-2, TB-1 and TB without an increase in wall area and volume. This discrepancy between wall thickness and area is consistent with our recent report on the structure of preterminal bronchioles [15], and can be explained by the greater reduction in luminal areas than in total areas (i.e. wall +lumen area). In addition, the observation that wall volumes and number of alveolar attachments of all these generations of small airways are reduced in COPD supports the hypothesis that the destruction of terminal and preterminal bronchioles as well as surrounding alveolar tissue is a major pathological feature of COPD [8, 10, 11, 14, 15].
The perimeter of the basement membrane or of the internal luminal has been used to estimate airway size because this length is unlikely to be influenced by tissue processing (such as the degree of lung inflation) or the extent of smooth muscle contraction [10, 20]. In the present study, we found that the length of basement membrane of TB is shorter in COPD than control. This is consistent with a previous report by Bosken et al [11], who observed a reduction in the internal perimeter of membranous bronchioles in COPD. Based on the result showing the degree of luminal narrowing in the COPD is greater than controls, we speculate that in addition to the shortening of the basement membrane, excessive folding of the airways due to the formation and contraction of scar tissues during an abnormal repair process accounts for the reduction in luminal area in COPD.
The MDCT measurements confirm the reduced numbers of 2–2.5mm diameter airways while the wall areas of the 5th generation airways tended to be less in the COPD lungs (p= 0.06). These reductions in airway count and wall area in COPD are consistent with recent MDCT reports on total airway counts [17] and airway dimensions [29, 30]. Because the airway tree was segmented based on the region growing method, the segmentation process would be terminated at a narrow point even when the lumen peripheral to this narrow point remained open [17]. The present finding of luminal narrowing of the 5th generation airways without wall thickening, leads us to speculate that the reduced number of 2–2.5 mm diameter airways reflects luminal narrowing of these airways in COPD.
Explanted lungs were inflated with a positive pressure of 30cm H2O to achieve full inflation, then deflated and held at constant pressure (10cm H2O) while frozen solid. We chose 10 cm H2O as the static pressure since 30cm H2O pressure may cause over-inflation and air leaking into the interstitium and because there is only a relatively small (~ 10%) reduction of lung volume in going from 30 to 10 cm H2O on the deflation limb of the pressure volume curve [31, 32].
Some limitations in this study need to be mentioned. The present study provides information on airways only from subjects with end-stage COPD treated by lung transplantation. It is possible that small airways pathology in mild and moderate COPD is different from end-stage of COPD. Since potential therapeutic interventions are mainly aimed at subjects with mild to moderate COPD, the airways’ pathology of this population using their lung tissues obtained at surgery should be investigated in the future. The second limitation is that although the present study examined two generations proximal to terminal bronchioles (0.88 ± 0.20mm in diameter in controls), more proximal bronchioles (1–2mm in diameter) could not be identified because the size of the sample cores is limited. Finally, although LAA% was measured using the MDCT scans of the explanted frozen lungs, an influence of the process of explanting and freezing lungs on CT density is not clear. Since the pulmonary blood volume was lost during the explanation process, this might have contributed to a reduction of lung density and an increased LAA%.
In conclusion, this study shows that small airways disease extends from 2mm diameter airways to the terminal bronchioles in COPD. Further, the combination of microCT with histology provides important new information associating the infiltration of B cells with the destruction of alveolar attachments to the outer wall of the preterminal bronchioles, which we postulate represents the site of the spread of this destructive process from the airway lumen and wall into the alveolar tissue.
Supplementary Material
Acknowledgments
Funding sources:
US National Institute of Health (R01 HL122438), Canadian Institutes of Health Research (Thoracic imaging network of Canada), Investigator initiated contracts from the Grifols and Respivert corporations.
Footnotes
”Take home” message:
B cell infiltration into the preterminal bronchioles is associated with destruction of alveolar attachments in COPD.
This article has an online data supplement
References
- 1.Weibel ER. Morphometry of the Human Lung. Springer Verlag and Academic Press, Heidelberg-New York: 1963. [Google Scholar]
- 2.Green M How big are the bronchioles?. St Thomas Hospital gazette 1965: 63: 136–139. [Google Scholar]
- 3.Macklem PT, Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol 1967: 22(3): 395–401. [DOI] [PubMed] [Google Scholar]
- 4.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968: 278(25): 1355–1360. [DOI] [PubMed] [Google Scholar]
- 5.Mead J The lung’s “quiet zone”. N Engl J Med 1970: 282(23): 1318–1319. [DOI] [PubMed] [Google Scholar]
- 6.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978: 298(23): 1277–1281. [DOI] [PubMed] [Google Scholar]
- 7.Hale KA, Ewing SL, Gosnell BA, Niewoehner DE. Lung disease in long-term cigarette smokers with and without chronic air-flow obstruction. Am Rev Respir Dis 1984: 130(5): 716–721. [DOI] [PubMed] [Google Scholar]
- 8.Saetta M, Ghezzo H, Kim WD, King M, Angus GE, Wang NS, Cosio MG. Loss of alveolar attachments in smokers. A morphometric correlate of lung function impairment. Am Rev Respir Dis 1985: 132(4): 894–900. [DOI] [PubMed] [Google Scholar]
- 9.Verbeken EK, Cauberghs M, Lauweryns JM, van de Woestijne KP. Anatomy of membranous bronchioles in normal, senile and emphysematous human lungs. J Appl Physiol (1985) 1994: 77(4): 1875–1884. [DOI] [PubMed] [Google Scholar]
- 10.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004: 350(26): 2645–2653. [DOI] [PubMed] [Google Scholar]
- 11.Bosken CH, Wiggs BR, Pare PD, Hogg JC. Small airway dimensions in smokers with obstruction to airflow. Am Rev Respir Dis 1990: 142(3): 563–570. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein R, Ma HD, Ghezzo H, Whittaker K, Fraser RS, Cosio MG. Morphometry of small airways in smokers and its relationship to emphysema type and hyperresponsiveness. Am J Respir Crit Care Med 1995: 152(1): 267–276. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Ito Y, Betsuyaku T, Nishimura M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006: 173(12): 1309–1315. [DOI] [PubMed] [Google Scholar]
- 14.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Pare PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011: 365(17): 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanabe N, Vasilescu DM, McDonough JE, Kinose D, Suzuki M, Cooper JD, Pare PD, Hogg JC. Micro-Computed Tomography Comparison of Preterminal Bronchioles in Centrilobular and Panlobular Emphysema. Am J Respir Crit Care Med 2017: 195(5): 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasilescu DM, Phillion AB, Tanabe N, Kinose D, Paige DF, Kantrowitz JJ, Liu G, Liu H, Fishbane N, Verleden SE, Vanaudenaerde BM, Lenburg ME, Stevenson CS, Spira A, Cooper JD, Hackett TL, Hogg JC. Non-destructive cryo micro CT imaging enables structural and molecular analysis of human lung tissue. J Appl Physiol (1985) 2016: jap 00838 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirby M, Tanabe N, Tan WC, Zhou G, Obeidat M, Hague CJ, Leipsic J, Bourbeau J, Sin DD, Hogg JC, Coxson HO. Total Airway Count on Computed Tomography and the Risk of COPD Progression: Findings from a Population-based Study. Am J Respir Crit Care Med 2017. [DOI] [PubMed] [Google Scholar]
- 18.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1996: 154(1): 187–192. [DOI] [PubMed] [Google Scholar]
- 19.Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988: 94(4): 782–787. [DOI] [PubMed] [Google Scholar]
- 20.James AL, Hogg JC, Dunn LA, Pare PD. The use of the internal perimeter to compare airway size and to calculate smooth muscle shortening. Am Rev Respir Dis 1988: 138(1): 136–139. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. URL http://www.R-project.org/. 2015.
- 22.Baraldo S, Turato G, Lunardi F, Bazzan E, Schiavon M, Ferrarotti I, Molena B, Cazzuffi R, Damin M, Balestro E, Luisetti M, Rea F, Calabrese F, Cosio MG, Saetta M. Immune activation in alpha1-antitrypsin-deficiency emphysema. Beyond the protease-antiprotease paradigm. Am J Respir Crit Care Med 2015: 191(4): 402–409. [DOI] [PubMed] [Google Scholar]
- 23.Polverino F, Baraldo S, Bazzan E, Agostini S, Turato G, Lunardi F, Balestro E, Damin M, Papi A, Maestrelli P, Calabrese F, Saetta M. A novel insight into adaptive immunity in chronic obstructive pulmonary disease: B cell activating factor belonging to the tumor necrosis factor family. Am J Respir Crit Care Med 2010: 182(8): 1011–1019. [DOI] [PubMed] [Google Scholar]
- 24.Polverino F, Seys LJ, Bracke KR, Owen CA. B cells in chronic obstructive pulmonary disease: moving to center stage. Am J Physiol Lung Cell Mol Physiol 2016: 311(4): L687–L695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell JD, McDonough JE, Zeskind JE, Hackett TL, Pechkovsky DV, Brandsma CA, Suzuki M, Gosselink JV, Liu G, Alekseyev YO, Xiao J, Zhang X, Hayashi S, Cooper JD, Timens W, Postma DS, Knight DA, Lenburg ME, Hogg JC, Spira A. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med 2012: 4(8): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faner R, Cruz T, Casserras T, Lopez-Giraldo A, Noell G, Coca I, Tal-Singer R, Miller B, Rodriguez-Roisin R, Spira A, Kalko SG, Agusti A. Network Analysis of Lung Transcriptomics Reveals a Distinct B-Cell Signature in Emphysema. Am J Respir Crit Care Med 2016: 193(11): 1242–1253. [DOI] [PubMed] [Google Scholar]
- 27.Mitzner W Emphysema--a disease of small airways or lung parenchyma? N Engl J Med 2011: 365(17): 1637–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macklin CC. A note on the elastic membrane of the bronchial tree of mammals, with an interpretation of its functional significance. Anat Rec 1922: 24(3): 119–+. [Google Scholar]
- 29.Washko GR, Diaz AA, Kim V, Barr RG, Dransfield MT, Schroeder J, Reilly JJ, Ramsdell JW, McKenzie A, Van Beek EJ, Lynch DA, Butler JP, Han MK. Computed tomographic measures of airway morphology in smokers and never-smoking normals. J Appl Physiol (1985) 2014: 116(6): 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith BM, Hoffman EA, Rabinowitz D, Bleecker E, Christenson S, Couper D, Donohue KM, Han MK, Hansel NN, Kanner RE, Kleerup E, Rennard S, Barr RG. Comparison of spatially matched airways reveals thinner airway walls in COPD. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 2014: 69(11): 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berend N, Skoog C, Thurlbeck WM. Pressure-volume characteristics of excised human lungs: effects of sex, age, and emphysema. Journal of applied physiology: respiratory, environmental and exercise physiology 1980: 49(4): 558–565. [DOI] [PubMed] [Google Scholar]
- 32.Salmon RB, Primiano FP Jr., Saidel GM, Niewoehner DE. Human lung pressure-volume relationships: alveolar collapse and airway closure. Journal of applied physiology: respiratory, environmental and exercise physiology 1981: 51(2): 353–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.