Abstract
Neonicotinoid insecticides are widely used replacements for organophosphate and carbamate insecticides, but the extent of human exposure is largely unknown. On the other hand, based on urinary concentrations of DEET metabolites, human exposure to N,N-diethyl-m-toluamide (DEET) appears to be widespread. We developed a fast online solid-phase extraction high-performance liquid chromatography-isotope dilution tandem mass spectrometry (HPLC-MS/MS) method to measure in 200 μL of human urine the concentrations of six neonicotinoid biomarkers (acetamiprid, N-desmethyl-acetamiprid, clothianidin, imidacloprid, 5-hydroxy-imidacloprid, thiacloprid), and two DEET biomarkers (3-diethyl-carbamoyl benzoic acid, 3-ethyl-carbamoyl benzoic acid). Limits of detection ranged from 0.01 to 0.1 μg/L, depending on the biomarker. Accuracy ranged from 91 to 116% and precision ranged from 3.7 to 10 %RSD. The presented method can be used to increase our understanding of exposure to neonicotinoid insecticides and DEET, and to evaluate the potential health effects from such exposures.
Keywords: DEET; N,N-Diethyl-m-toluamide; Neonicotinoids; Biomarkers; HPLC-MS/MS; Online SPE
Introduction
Pesticides include a wide variety of products such as fungicides, herbicides, insecticides, and insect repellents. Neonicotinoid insecticides are used as agricultural insecticides [1–4] and are also quite effective for flea control in cats and dogs [5]. In 2014, neonicotinoids dominated more than 25% of the insecticide market [6], although geographic differences may exist. For example, in 2012, sales in Latin America, Asia, and North America accounted for 75% of the global market, while sales in Europe only accounted for 11% [6]. Neonicotinoids have chemical structures similar to nicotine and target the insect’s nicotinic acetylcholine receptors (nAChRs) exciting the nerve cells, causing trembling and shaking, paralysis [7], and even death [2, 8]. The neonicotinoid family includes acetamiprid, clothianidin, imidacloprid, nitenpyram, nithiazine, thiacloprid, and thiamethoxam [9]; in 2009, imidacloprid was the world’s top selling insecticide [3].
Neonicotinoids are persistent in the environment and have been detected in food, streams, and other environmental matrices [10, 11]. Neonicotinoids are used for growing genetically modified corn, soybeans, cotton, sunflowers, and canola, as well as various other genetically modified and non-genetically modified vegetables and fruits [12]. Approximately 90% of the corn and 50% of the soybeans planted in the USA have been treated with neonicotinoids [1]. Neonicotinoids are systemic in nature: their relatively high water solubility readily facilitates absorption through roots and leaves and distribution to all plant tissues, meaning neonicotinoids are not easily washed off food [5, 10]. Imidacloprid was detected in about 70% of fruits and vegetables procured in 2012 from Boston neighborhood grocery stores; several other neonicotinoids were also detected in 72% of fruits and 45% of vegetables [10].
Active ingredients of neonicotinoids have been alleged as one of the factors that lead to the development of the honeybee colony collapse disorder syndrome [13, 14] and the decline of insectivorous birds [15]. Furthermore, although neonicotinoids have relatively low toxicity to mammals and humans compared with organophosphate and carbamate insecticides, in vitro and in vivo data suggest potential toxic effects of neonicotinoids on mammals, and even humans, including reproductive toxicology, neurotoxicity, immunotoxicity, hepatotoxicity/hepatocarcinogenicity, and genetic toxicity [16]. Long-term impacts of neonicotinoids on the environment are currently unknown and information on exposure to neonicotinoids in the US general population is not readily available even though human exposure may be on the rise because of increased use of these insecticides.
Neonicotinoids can be metabolized in mammals by phase I enzymes [17, 18]. Human cytochrome P450 recombinant enzymes convert thiamethoxam to clothianidin, clothianidin to desmethyl-clothianidin, and thiamethoxam to desmethyl-thiamethoxam [18]. Imidacloprid produces several in vitro metabolites [17]; 5-hydroxy-imidacloprid is one of the principal in vitro products [19]. Some of these phase I neonicotinoid metabolites can undergo phase II reactions such as glutathione conjugation, glycine conjugation, acetylation, and glucuronidation [20, 21] to facilitate elimination. There are currently no human in vivo metabolism studies; however, several neonicotinoids and some of their metabolites were identified in the urine of patients suspected of neonicotinoid pesticide poisoning [22, 23]. These included acetamiprid; N-desmethyl-acetamiprid; three imidacloprid metabolites: 5-hydroxy-imidacloprid, 4,5-dehydro-imidacloprid, and 4,5-dihydroxy-imidacloprid; two clothianidin metabolites: N-desmethyl-clothianidin, N-(2-(methylsulfinyl) thiazole-5-carboxyl)-glycine; and a common metabolite of acetemiprid and imidacloprid (N-(6-chloronicotinoyl)-glycine 4) [22, 23]. Some of these metabolites are potential biomarkers of human exposure.
Analytical methods using mass spectrometry for identifying and quantifying neonicotinoids and their metabolites in human urine are described in the literature and include GC-MS [24], GC-MS/MS [25], LC-TOFMS [23], LC-MS/MS-ESI [26–30], and UHPLC-Orbitrap MS [31].
N,N-Diethyl-m-toluamide, commonly known as DEET, is the principal ingredient in many personal insect repellents worldwide and is highly effective against potential disease vectors such as mosquitoes, biting flies, and ticks [32]. Every year, approximately one third of the US population uses DEET-containing insect repellents [33] and more than 500 products are currently registered with the U.S. Environmental Protection Agency in a variety of liquids, lotions, gels, sprays, sticks, and impregnated materials with DEET concentrations ranging from 5 to 99% [34]. In the US general population, exposure to DEET is widespread [35]. Identifying adequate metabolites as DEET exposure biomarkers is important because relying on DEET itself can lead to exposure misclassification [35, 36].
Reliable and accurate methods to obtain population exposure data for DEET and neonicotinoid insecticides are needed to better understand prevalence of exposure, and to evaluate whether exposure to these compounds may have any adverse effects on human health. In this paper, we describe an online solid-phase extraction high-performance liquid chromatography-isotope dilution tandem mass spectrometry (HPLC-MS/MS) method to quantify in 200 μL of urine the concentrations of six neonicotinoid biomarkers: acetamiprid, clothianidin, imidacloprid, thiacloprid, acetamiprid-N-desmethyl, and 5-hydroxy-imidacloprid, and two DEET biomarkers: 3-diethyl-carbamoyl benzoic acid (DCBA) and 3-ethyl-carbamoyl benzoic acid (ECBA).
Materials and methods
Reagents and chemicals
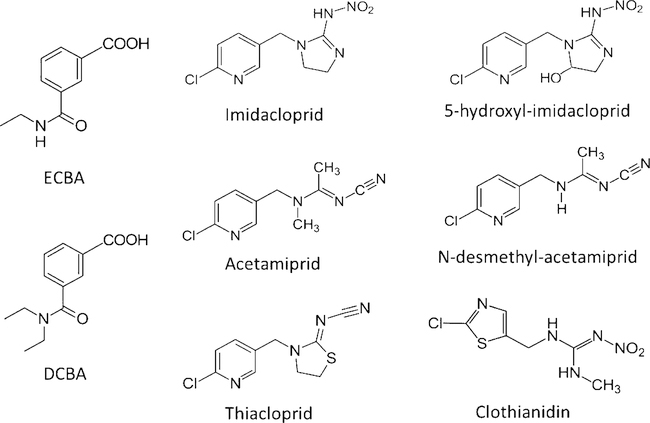
HPLC grade methanol, acetonitrile, and water were purchased from Fisher Scientific (Pittsburg, PA). Formic acid, acetamiprid, N-desmethyl-acetamiprid, thiacloprid, and thiacloprid-d4 were purchased from Fluka (Seelze, Germany). 4-Methylumbelliferone-13C4 was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). Phosphoric acid, β-glucuronidase (E. coli), imidacloprid, clothianidin-d3, imidacloprid-d4, and 4-methylumbelliferyl-β-D-glucuronide hydrate were purchased from Sigma-Aldrich (St. Louis, MO). ECBA, DCBA, and acetamiprid-d5 were purchased from Cerilliant (Round Rock, TX). ECBA-d5, DCBA-d10, and N-desmethyl-acetamiprid-(2H, 13C, 15N2) were purchased from CanSyn Chemical Corporation (Toronto, Canada). Potassium phosphate dibasic trihydrate was purchased from MP Biomedicals (Santa Ana, CA). Clothianidin was purchased from Chemservice (West Chester, PA). 5-Hydro-imidacloprid-d4 was purchased from ClearSynth (Mississauga, ON, Canada). 5-Hydroxy-imidacloprid was a gift from Dr. Heiko Käfferlein of the Institute for Prevention and Occupational Medicine of the German Social Accident Insurance (Institute of the Ruhr-University, Bochum, Germany). Reagents, solvents, and standard materials were used without additional purification. The chemical structures of the target analytes are shown in Fig. 1.
Fig. 1.
Chemical structures of two oxidative metabolites of DEET, 3-diethyl-carbamoyl benzoic acid (DCBA) and 3-ethyl-carbamoyl benzoic acid (ECBA); four neonicotinoid insecticides: acetamiprid, clothianidin, imidacloprid, and thiacloprid; and two neonicotinoid metabolites: N-desmethyl-acetamiprid and 5-hydroxy-imidacloprid
Human urine collection for method development and validation
Individual urine samples for method development and validation were collected in March of 2017 in Atlanta, GA, from anonymous male and female donors with no documented exposure to DEET or neonicotinoids. A Human Subjects Institutional Review Board at The Centers for Disease and Control Prevention (CDC) reviewed and approved the collection protocol. A waiver of informed consent was requested under 45 CFR 16.116(d). Ten additional urine samples were purchased in May 2017 from BioIVT, Inc and 50 more in April 2018 to test the suitability of the method. The company had IRB approval to collect urine and obtained informed consent from donors. CDC’s use of the commercial urine was consistent with the IRB approval and donor consent. No personal identifiers were provided to CDC.
Preparation of native standard solutions, calibrators, internal standard spiking solution, and enzyme spiking solution
Stock solutions for native acetamiprid, N-desmethyl-acetamiprid, clothianidin, imidacloprid, and thiacloprid were purchased as certified solutions at 1 mg/mL in acetonitrile, except imidacloprid which was in acetone. Solutions for native DCBA, ECBA, and 5-hydroxy-imidacloprid were made from powder dissolved in acetonitrile at 1 mg/mL, except ECBA, which was prepared at 0.1 mg/mL because of its inability to solubilize at higher concentrations. Individual stock solutions were combined in various proportions to prepare 12 individual calibrator spiking solutions such that a 50-μL spike into 200 μL of deionized water resulted in concentrations ranging from 0.025 to 50.0 μg/L, except DCBA which had concentrations ranging from 0.12 to 250 μg/L.
Stable isotopically labeled analogs of all target analytes, except for 5-hydroxy-imidacloprid which used a surrogate for its internal standard (ISTD), were prepared at 1 mg/mL in acetonitrile. The stocks were combined and diluted with water resulting in an ISTD spiking solution such that a 100-μL spike into 200 μL of urine resulted in concentrations ranging from 12 to 50 μg/L, depending on the analyte. This ISTD spiking solution also contained 4-methylumbelliferyl-β-D-glucuronide hydrate and 4-methylumbelliferone-13C4 at concentrations that resulted in 1 and 0.35 mg/L urine, respectively. For each sample, we monitored the deconjugated 4-methylumbelliferone/4-methylumbelliferone-13C4 peak area ratio to confirm completion of the enzymatic deconjugation reaction (ratio between 1.8 and 2 based on spiked concentrations was considered acceptable).
An enzyme spiking solution was prepared weekly by gently dissolving β-glucuronidase in 0.2 M potassium phosphate buffer (pH = 6.8 ± 0.1) such that a 300-μL spike in 200 μL of urine contained 1 Fishman unit per microliter of urine. The 0.2 M buffer solution was prepared in 1 L volumes and kept refrigerated for up to 2 months.
Sample preparation
Samples were prepared for analysis in 96-well plates using a Tomtec Quadra 3 liquid handler (Hamden, CT, USA). To each well, 100 μL of ISTD spiking solution, 200 μL of urine, 300 μL of enzyme spiking solution, and 650 μL of HPLC grade water were added. Standard calibrators (S1-S12) had an additional 50 μL of standard spiking solution added. Plate contents were gently mixed on the Tomtec and incubated for 17 h at 37 °C. After incubation, 100 μL of 10% formic acid was added to each well. Following an additional mix on the Tomtec, plates were covered with pre-slit sealing mats and loaded into a 10 °C refrigerated autosampler for injection.
Online solid-phase extraction and analytical separation
We used a Thermo Scientific™ Dionex UltiMate™ 3000 Rapid Separation Dual HPLC system (Sunnyvale, CA, USA) consisting of (a) a DGP-3600RS module with dual independent operating Ternary Rapid Separation pumps, (b) an SRD-3600 Integrated Solvent and Degasser rack, (c) a WPS-3000TRS temperature-controlled autosampler, and (d) a TCC-3000RS column thermostat compartment equipped with one six-port switching valve. The first pump was dedicated to online solid-phase extraction (SPE) and we refer to it as the SPE pump. The second pump was used for analytical separation and we refer to it here as the analytical pump. We used a Chromolith Flash RP-18e monolithic 25 × 4.6 mm (EMD Chemicals, Gibbstown, NJ) as the SPE column and a Thermo Scientific™ Hypersil Gold aQ column 150 × 4.6 mm, 3 μm particle size, with guard column of the same packing, as the analytical column. The SPE pump mobile phase A was 5% methanol in 0.1% aqueous formic acid, and mobile phase B was 100% methanol. The analytical pump mobile phase A was 0.1% aqueous formic acid, and mobile phase B was 100% acetonitrile.
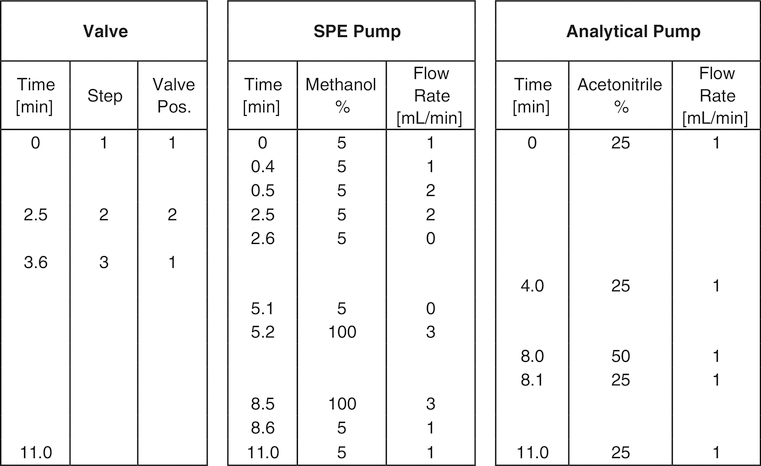
To isolate the target compounds, the online SPE method used a three-step process: SPE loading/washing, SPE eluting, and analytical chromatographic separation. Flow rates and valve switching times are shown in Fig. 2. A diagram is shown in Electronic Supplementary Material (ESM, Fig. S1). Briefly, in step 1 (SPE loading), 500 μL is injected into the SPE column using the SPE pump. The SPE pump washes the SPE column with 100% A at 1 mL/min for 0.4 min then immediately increases the flow to 2 mL/min for two additional minutes and then flow is stopped. At 2.5 min, the switching valve is activated initiating step 2 (SPE eluting). Switching of the valve reroutes the analytical pump’s flow (25% B at 1 mL/min) through the SPE column in a reverse direction and in line with the analytical column. After 1 min, all target analytes completely elute onto the analytical column and the switching valve returns to its initial load/wash position. In step 3 (analytical chromatographic separation), the analytical pump begins a gradient at 4 min from 25% B to 50% B ending at 8 min and then back to 25% B at 8.1 min. All target analytes elute from the analytical column between 6 and 9 min. From 5.2 to 8.5 min, the SPE pump independently washes the SPE cartridge with 100% B at 3 mL/min and then equilibrates the SPE column with 100% A from 8.6–11 min at 1 mL/min, making it ready for the next injection.
Fig. 2.
Valve positions, SPE and analytical pumps timing chart, and solvent conditions
Mass spectrometer settings
We used a Thermo Scientific Vantage triple quadrupole (TSQ) mass spectrometer (San Jose, CA, USA) equipped with a Heated Electrospray Ionization (HESI) probe. The HESI probe used the following settings: spray voltage (3200 V), vaporizer temperature (500 °C), sheath gas flow rate (50 arbitrary units [AU]), sweep gas flow rate (1.0 AU), auxiliary gas flow rate (5 AU), and capillary temperature (400 °C). Nitrogen was used as sheath, sweep, and auxiliary gas. Argon was used as the collision gas at 1.5 mTorr. The mass spectrometer operated in positive polarity selected reaction monitoring (SRM) mode, with a scan time of 0.06 s for each ion transition. Table 1 shows the mass transitions and collision energies for each compound of interest.
Table 1.
Tandem mass spectrometer parameters
| Analyte | Ion type | Precursor ion (m/z) | Product ion (m/z) | Collision energy (V) |
|---|---|---|---|---|
| ECBA | Quantitation | 194 | 149 | 15 |
| Confirmation | 194 | 121 | 23 | |
| Internal standard | 199 | 149 | 17 | |
| DCBA | Quantitation | 222 | 149 | 12 |
| Confirmation | 222 | 121 | 27 | |
| Internal standard | 232 | 149 | 19 | |
| Acetamiprid | Quantitation | 223 | 126 | 19 |
| Confirmation | 223 | 90 | 33 | |
| Internal standard | 226 | 126 | 21 | |
| N-Desmethyl-acetamiprid | Quantitation | 209 | 126 | 17 |
| Confirmation | 209 | 90 | 31 | |
| Internal standard | 215 | 126 | 17 | |
| Clothianidin | Quantitation | 250 | 132 | 15 |
| Confirmation | 250 | 113 | 29 | |
| Internal standard | 253 | 172 | 13 | |
| Imidacloprid | Quantitation | 256 | 209 | 14 |
| Confirmation | 256 | 175 | 16 | |
| Internal standard | 260 | 213 | 17 | |
| 5-Hydroxy-imidacloprid | Quantitation | 272 | 225 | 16 |
| Confirmation | 272 | 191 | 19 | |
| Internal standard | 276 | 229 | 17 | |
| Thiacloprid | Quantitation | 253 | 126 | 19 |
| Confirmation | 253 | 90 | 34 | |
| Internal standard | 257 | 126 | 21 |
Daily operation and quality control procedures
Quality control (QC) materials were prepared from a urine pool obtained from multiple anonymous donors. The pool was divided into two sub pools that were enriched with native target analytes to create low-concentration (QCL, 4–10 μg/L) and high-concentration (QCH, 16–90 μg/L) QC materials. The two pools were mixed overnight at 5 °C, dispensed in 2-mL portions in polypropylene vials, and stored long term at − 70 °C and up to 12 weeks at − 20 °C. Each QC material was characterized by repeated measurements to define mean concentrations and 95% and 99% control limits of each target analyte.
An analytical run included 12 calibration standards, two reagent blanks, four QC materials (2 QCL and 2 QCH), and up to 72 study samples. A standard check was analyzed at the beginning of each analytical run, to confirm acceptable chromatographic retention time and shape, and mass spectrometry sensitivity. Raw data from the analytical run were integrated using Xcalibur 2.2 software (Thermo Scientific, San Jose, CA) and transferred to a database where data were checked against previously established values and flagged, if not acceptable, for example, LOD values, standard curve y-intercept values, quantification and confirmation concentration difference, internal standard area count, and relative retention time. Samples outside acceptable ranges were re-extracted and reanalyzed. Samples with results above the calibration curve were re-extracted with a smaller sample volume. QCL and QCH data were statistically analyzed applying modified Westgard QC rules [37] using SAS (SAS Institute Inc., Cary, NC) to ensure statistical control of the analytical run.
Method validation
Precision was determined by measuring two replicates of the two QC urine pools (low and high concentrations). Pools were analyzed in 25 analytical runs over 2 months using two identical analytical systems and two different analysts. Precision was expressed as percent relative standard deviation (%RSD). Mean and standard deviations were used to determine quality control limits used in analyzing 60 test samples.
Because certified reference materials were not available for the target analytes, we determined accuracy and selectivity by spiked recovery (measured concentration / known concentration × 100). We spiked 20 individual urine samples with known concentrations (low, medium, and high) of native analytes. Measured concentrations were corrected for any endogenous amount of the target compounds, if applicable, by subtracting the blank measured concentration. The accuracy was expressed as the average of the 20 spiked recovery values. The acceptable range for accuracy was 85 to 115% [38]. Selectivity was indirectly evaluated using the spiked recovery data. The reasoning was that if the results were accurate, the method used must also be selective [39].
The limit of detection (LOD) was determined using 25 repeat measurements of low-concentration standards spiked in urine. For each analyte, from a plot of the standard deviation of the 25 measured values at each concentration versus the spiked concentrations, we determined the y-intercept (S0, standard deviation at zero concentration) using linear regression analysis. We calculated LODs as 3 times S0 [40]. Linearity was evaluated by performing both linear and quadratic regressions over several standard curves and by analyzing residual plots.
Stability of the target analytes was evaluated in both water and urine. Stability in water was determined by aliquotting 400 μL of a 25 μg/L solution of the combined native compounds into clear silanized 2-mL screw-capped vials and storing them at − 70, − 20, 5, 25, and 37 °C. The ISTD spiking solution was stored at − 70 °C. Aliquots stored at 25 °C were also dispensed into amber vials to test for potential degradation from ultraviolet light. The vials stored at 25 °C remained on a lab benchtop under room light for the duration of the testing period. Periodically, over 120 days, a vial from each temperature location was removed along with a vial of ISTD and brought to room temperature. One hundred microliters of the native solution and 50 μL of ISTD were added to silanized vials in triplicate, vortex mixed, and analyzed. Plots of the responses (native/ISTD) versus time were used to evaluate possible degradation. Because the ISTD stored at − 70 °C was assumed to be stable, a negative slope was interpreted as possible degradation of the native.
Stability of response ratios (native/ISTD) was also tested in water at 37 °C by adding target compounds and their respective ISTDs into water. Twenty-one samples were prepared in silanized vials. Three vials were analyzed immediately (time zero). The remaining 18 samples were placed in a 37 °C oven. Periodically, throughout a 34-h time period, three vials were removed and analyzed. Regression analysis was applied to plots of response ratios versus time spent at 37 °C. From the linear regression equations, a percent gain or loss in response factors over a 17-h period was calculated. A zero slope indicated that native and ISTD had the same thermal stability characteristics.
Stability of the target analytes in urine was determined using two urine pools, spiked at low and high concentrations. Pools were analyzed in triplicate before and after (a) three freeze-thaw cycles, (b) being kept on the lab bench for 24 h, and (c) prepared urine samples were left in the autosampler kept at 10 °C for 24 h. Percent differences of the before and after measured values were determined, with ± 15% being acceptable [38].
Relative matrix effects were evaluated by measuring the variability of standard curve slopes prepared in five individual urine specimens as recommended by Matuszewski [41]. For each individual urine, a standard curve was prepared and analyzed every day for 5 days, the resulting five slopes from each individual urine were averaged, and the percent RSD of the averaged slopes was calculated. Values < 3–4% indicated that the internal standard was effective at correcting for relative matrix effects [41].
Results and discussion
SPE/analytical columns selection
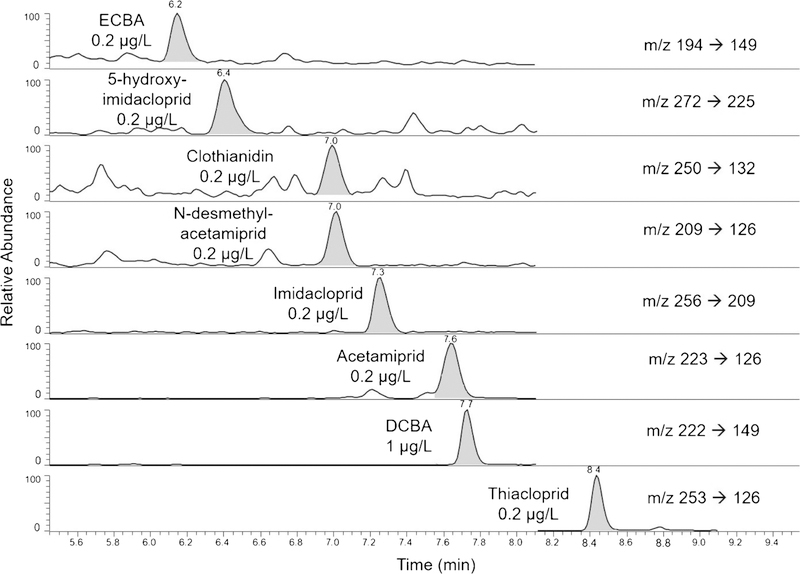
We evaluated several online SPE columns including Sunfire® C8 5 μm, Sunfire® C18 5 μm, Waters XSelect™ HHS PFP 5 μm, Waters XSelect® CSH™ Fluoro-Phenyl 5 μm, Waters Oasis® HLB 5 μm, and Chromolith Flash RP-18e monolithic 25 × 4.6 mm. The Waters Oasis® HLB performed adequately and has recently been used for the quantification of several neonicotinoid biomarkers [31]. Nevertheless, we chose the Chromolith Flash RP-18e because, under our experimental conditions, it provided the cleanest background and fewest chromatographic interferences. The target compounds eluted from the SPE column in less than 1 min with 25% acetonitrile, and effectively refocused on a ThermoScientific Hypersyl GOLD™ aQ polar encapped analytical column. We chose the analytical column primarily because it produced the sharpest and narrowest peaks of all columns tested (analytical peak widths were approximately 15 s, Fig. 3). All target compounds eluted between 6 and 9 min.
Fig. 3.
Extracted ion chromatogram of an analytical standard in urine diluted 1:1 with HPLC grade water. Concentrations are given for each analyte
Method validation
Precision, accuracy (spike recovery), and LOD parameters are summarized in Table 3. Precision (%RSD) ranged from 3.7 to 10.2%. Precision was < 8% for all analytes except 5-hydroxy-imidacloprid (9.2% [QCL], 10.2% [QCH]). Accuracy ranged from 91.2 to 107% at the low concentration, from 93.2 to 116% at the medium concentration, and from 94.8 to 114% at the high concentration (Table 3), all within the 85 to 115% acceptable range [38], except for 5-hydroxy-imidacloprid at the medium concentration level. The acceptable accuracy data also support the selectivity of the method [38, 39]. Compared to the other analytes, we attribute the lower accuracy of 5-hydroxy-imidacloprid (107, 116, and 114% at the low, medium, and high concentrations, respectively) and precision (~ 10%) to not having an exact isotopically labeled analog, which was not commercially available at the time. Therefore, we used a surrogate ISTD which had the OH functional group on the 5 position of the 6-chloro-3-pyridinyl moiety instead of the 5 position of the 1H-imidazol moiety. This surrogate ISTD eluted about 20 s earlier than the native 5-hydroxy-imidacloprid, which likely further contributed to reducing its effectiveness to compensate for matrix effects and to a concomitant loss in accuracy and precision.
Table 3.
Detection frequency, median, and concentration range for DEET and neonicotinoid biomarkers measured in 60 anonymous adult volunteers
| Analyte | Detection frequency (%) | Median (μg/L) | Range (μg/L) |
|---|---|---|---|
| Acetamiprid | 2 | <LOD | <LOD–0.14 |
| N-Desmethyl-acetamiprid | 90 | 0.17 | <LOD–2.2 |
| Clothianidin | 37 | <LOD | <LOD–2.2 |
| DCBA | 95 | 4.0 | <LOD–433 |
| Imidacloprid | 30 | <LOD | <LOD–0.98 |
| ECBA | 83 | 0.92 | <LOD–142 |
| 5-Hydroxy-imidacloprid | 42 | <LOD | <LOD–9.2 |
| Thiacloprid | 0 | NA | NA |
Limit of detection (LOD) in μg/L: acetamiprid and clothianidin = 0.1; N-desmethyl-acetamiprid and 5-hydroxyimidacloprid= 0.03; imidacloprid and ECBA = 0.05; DCBA = 0.06; and thiacloprid = 0.01 NA, not applicable
The LODs ranged from 0.01 to 0.1 μg/L (Table 2). These values are similar to those reported before [26–29, 31, 42, 43] and adequate for quantification of these biomarkers at trace levels.
Table 2.
Method validation data for DEET and neonicotinoid biomarkers
| Analyte | Accuracya (%RSD)b |
Precisionc |
LODd (μg/L) | |||
|---|---|---|---|---|---|---|
| 1.6 μg/L | 6.3 μg/L | 25 μg/L | QCL (4 μg/L) | QCH (16 μg/L) | ||
| Acetamiprid | 100 (2.4) | 104 (2.6) | 104 (4.1) | 3.7 | 3.9 | 0.1 |
| N-Desmethyl-acetamiprid | 97.9 (3.7) | 99.4 (3.4) | 99.4 (4.2) | 5.0 | 4.4 | 0.03 |
| Clothianidin | 91.2 (12.6) | 93.2 (5.1) | 94.8 (6.1) | 6.2 | 5.1 | 0.1 |
| DCBA | 96.6 (5.6) | 98.8 (5.2) | 97.1 (6.6) | 6.3 | 6.1 | 0.06 |
| Imidacloprid | 96.6 (6.4) | 102 (3.9) | 102 (4.4) | 4.2 | 4.6 | 0.05 |
| ECBA | 100 (6.6) | 102 (3.9) | 101 (3.9) | 7.2 | 6.9 | 0.05 |
| 5-Hydroxy-imidacloprid | 107 (15.8) | 116 (14.5) | 114 (12.5) | 9.2 | 10.2 | 0.03 |
| Thiacloprid | 95.7 (5.4) | 99.3 (5.3) | 99.5 (5.9) | 6.8 | 6.3 | 0.01 |
Accuracy calculated by averaged spike recovery, n = 20 individual urine samples
%RSD of individual spiked recovery values from n = 20 individual urine samples
Total precision expressed as %RSD, n = 25 runs using pooled urine, two instruments, and two analysts over 10 weeks
Limit of detection was determined using 25 repeat measurements of low-concentration standards spiked in urine and calculated as 3 × S0 where S0 is considered the standard deviation of measured values at zero concentration
Quadratic regressions showed no statistical significance (p > 0.05) for the squared term. Linear regressions of standard curves from 15 runs on 15 different days for all analytes produced R-squared values > 0.98 (range, 0.9837 to 0.9976) and were linear over 2.5 orders of magnitude. Residual plots for all analytes were acceptable.
The stability of the DEET and neonicotinoid biomarkers in human urine and water was assessed under several storage/ handling conditions. At 25 °C, there were no differences in the degradation curves associated with storing the analytes in amber versus clear vials. In deionized water, we observed degradation of 5-hydroxy-imidacloprid at 25 °C and 37 °C, and, to a lesser degree, of acetamiprid, but only at 37 °C. We estimate that native 5-hydroxy-imidacloprid and acetamiprid concentrations decreased about 9% and 6%, respectively, after 17 h at 37 °C. However, the method was still accurate for two main reasons. First, each analytical run included a standard curve which underwent the exact same treatment as the samples being analyzed. Any bias caused by degradation of the native or ISTD in the samples being analyzed was offset by the same thermal degradation occurring in the native or ISTD of the standard calibrators. Second, with the exception of 5-hydroxy-imidacloprid, the method used stable isotopically labeled analogs as ISTDs which theoretically behaved as their unlabeled counterparts. The response ratios for each analyte in the test samples stored for 17 h at 37 °C varied less than 2% for all analytes except for 5-hydroxy-imidacloprid, which decreased about 7%.
Stability of target analytes in urine showed percent differences in measured amounts below ± 15% for all analytes at both low and high levels except one value for 5-hydroxyimidacloprid which had a percent difference of − 16% for the high pool. The larger variability for 5-hydroxyimidacloprid was likely caused by the lack of an exact stable isotopically labeled analog.
Relative matrix effects were negligible for most analytes as indicated by the %RSD of the averaged slopes (0.9 to 3.3%), suggesting no negative impact on method performance [41]. The higher %RSD for 5-hydroxy-imidacloprid (10.5%) was likely the result of the unavailability of a stable isotopically labeled analog. The low RSD values for the other seven analytes stress the importance and effectiveness of using stable isotopically labeled analogs as ISTDs to greatly reduce or eliminate relative matrix effects [41].
Method application
We detected the DEET metabolites in most of the 60 commercial samples analyzed (DCBA, 95%; ECBA, 83%) (Table 3). Furthermore, concentrations of DCBA and ECBA strongly correlated (R2 = 0.983). These results suggest widespread exposure to DEET in these convenience samples and are in agreement with data from the 2007–2014 National Health and Nutrition Examination Survey (NHANES) showing that DCBA was detected in most US residents over the 8-year timespan [44]. Furthermore, because DCBA is a known biomarker of exposure to DEET [35], the strong correlation between DCBA and ECBA suggests that ECBA is also an acceptable urinary biomarker of DEET. The most commonly detected neonicotinoid biomarkers were N-desmethyl-acetamiprid (90%), 5-hydroxy-imidacloprid (42%), clothianidin (37%), and imidacloprid (30%). Acetamiprid was detected in 2% of the samples, and thiacloprid was not detected in any. These data suggest that neonicotinoid metabolites are better biomarkers than the parent compounds themselves.
Conclusions
We present a sensitive, accurate, and precise high-throughput method for quantifying trace concentrations of DEET and neonicotinoid biomarkers in human urine using online SPE and isotope dilution HPLC-MS/MS. Our results confirm the applicability of the method for biomonitoring purposes to evaluate human exposure to DEET and neonicotinoids and suggest that neonicotinoid and neonicotinoid metabolites can serve as biomarkers of exposure. We plan to use the method to obtain reference ranges for these pesticides biomarkers in large-scale epidemiological studies such as NHANES.
Supplementary Material
Acknowledgements
We thank Charlie Chambers for technical assistance and Dr. Peter Kuklenyik for the diagram in the supplemental information.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare they have no competing interests.
Disclaimer The use of trade names is for identification purposes only and does not constitute any endorsement by the US Department of Health and Human Services or the Centers for Disease Control and Prevention. The conclusions and related findings presented in this report are those of the authors and do not necessarily represent an official position of the US Centers for Disease Control and Prevention.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00216-018-1481-0) contains supplementary material, which is available to authorized users.
References
- 1.Douglas MR, Tooker JF. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest min U.S. field crops. Environ Sci Technol. 2015;49(8):5088–97. 10.1021/es506141g. [DOI] [PubMed] [Google Scholar]
- 2.Goulson D REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol. 2013;50(4): 977–87. 10.1111/1365-2664.12111. [DOI] [Google Scholar]
- 3.Jeschke P, Nauen R, Schindler M, Elbert A. Overview of the status and global strategy for neonicotinoids. J Agric Food Chem. 2011;59(7):2897–908. 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- 4.Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, et al. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int. 2015;22(1):5–34. 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45(1):247–68. 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 6.Bass C, Denholm I, Williamson MS, Nauen R. The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol. 2015;121:78–87. 10.1016/j.pestbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci. 2001;22(11):573–80. 10.1016/S0165-6147(00)01820-4. [DOI] [PubMed] [Google Scholar]
- 8.Kasiotis KM, Machera K. Neonicotinoids and their metabolites in human biomonitoring: a review. Hellenic Plant Protect J. 2015;8(2): 33–45. 10.1515/hppj-2015-0006. [DOI] [Google Scholar]
- 9.Casida JE. Neonicotinoid metabolism: compounds, substituents, pathways, enzymes, organisms, and relevance. J Agric Food Chem. 2011;59(7):2923–31. 10.1021/jf102438c. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Tao L, McLean J, Lu C. Quantitative analysis of neonicotinoid insecticide residues in foods: implication for dietary exposures. J Agric Food Chem. 2014;62(26):6082–90. 10.1021/jf501397m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hladik ML, Main AR, Goulson D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ Sci Technol. 2018. 10.1021/acs.est.7b06388. [DOI] [PubMed] [Google Scholar]
- 12.Cimino AM, Boyles AL, Thayer KA, Perry MJ. Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environ Health Perspect. 2017;125(2):155–62. 10.1289/ehp515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.vanEngelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, Nguyen BK, et al. Colony collapse disorder: a descriptive study. PLoS One. 2009;4(8):e6481 10.1371/journal.pone.0006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hladik ML, Kolpin DW, Kuivila KM. Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ Pollut. 2014;193:189–96. 10.1016/j.envpol.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Hallmann CA, Foppen RPB, van Turnhout CAM, de Kroon H, Jongejans E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature. 2014;511:341 10.1038/nature13531. Accessed 13 Aug 2013 [DOI] [PubMed] [Google Scholar]
- 16.Han W, Tian Y, Shen X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: an overview. Chemosphere. 2018;192:59–65. 10.1016/j.chemosphere.2017.10.149. [DOI] [PubMed] [Google Scholar]
- 17.Dick RA, Kanne DB, Casida JE. Substrate specificity of rabbit aldehyde oxidase for nitroguanidine and nitromethylene neonicotinoid insecticides. Chem Res Toxicol. 2006;19(1):38–43. 10.1021/tx050230x. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Dick RA, Ford KA, Casida JE. Enzymes and inhibitors in neonicotinoid insecticide metabolism. J Agric Food Chem. 2009;57(11):4861–6. 10.1021/jf900250f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz-Jander DA, Casida JE. Imidacloprid insecticide metabolism: human cytochrome P450 isozymes differ in selectivity for imidazolidine oxidation versus nitroimine reduction. Toxicol Lett. 2002;132(1):65–70. 10.1016/S0378-4274(02)00068-1. [DOI] [PubMed] [Google Scholar]
- 20.Ford KA, Casida JE. Chloropyridinyl neonicotinoid insecticides: diverse molecular substituents contribute to facile metabolism in mice. Chem Res Toxicol. 2006;19(7):944–51. 10.1021/tx0600696. [DOI] [PubMed] [Google Scholar]
- 21.Ford KA, Casida JE. Unique and common metabolites of thiamethoxam, clothianidin, and dinotefuran in mice. Chem Res Toxicol. 2006;19(11):1549–56. 10.1021/tx0601859. [DOI] [PubMed] [Google Scholar]
- 22.Marfo JT, Fujioka K, Ikenaka Y, Nakayama SM, Mizukawa H, Aoyama Y, et al. Relationship between urinary N-desmethyl-acetamiprid and typical symptoms including neurological findings: a prevalence case-control study. PLoS One. 2015;10(11):e0142172 10.1371/journal.pone.0142172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taira K, Fujioka K, Aoyama Y. Qualitative profiling and quantification of neonicotinoid metabolites in human urine by liquid chromatography coupled with mass spectrometry. PLoS One. 2013;8(11):e80332 10.1371/journal.pone.0080332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomura H, Ueyama J, Kondo T, Saito I, Murata K, Iwata T, et al. Quantitation of neonicotinoid metabolites in human urine using GC-MS. J Chromatogr B. 2013;941:109–15. 10.1016/j.jchromb.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Uroz FJ, Arrebola FJ, Egea-Gonzalez FJ, Martinez-Vidal JL. Monitoring of 6-chloronicotinic acid in human urine by gas chromatography-tandem mass spectrometry as indicator of exposure to the pesticide imidacloprid. Analyst. 2001;126(8):1355–8. [DOI] [PubMed] [Google Scholar]
- 26.Harada KH, Tanaka K, Sakamoto H, Imanaka M, Niisoe T, Hitomi T, et al. Biological monitoring of human exposure to neonicotinoids using urine samples, and neonicotinoid excretion kinetics. PLoS One. 2016;11(1):e0146335 10.1371/journal.pone.0146335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueyama J, Nomura H, Kondo T, Saito I, Ito Y, Osaka A, et al. Biological monitoring method for urinary neonicotinoid insecticides using LC-MS/MS and its application to Japanese adults. J Occup Health. 2014;56(6):461–8. 10.1539/joh.140077-OA. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Liu T, Liu F, Zhang J, Wu Y, Sun H. Occurrence and profile characteristics of the pesticide imidacloprid, preservative parabens, and their metabolites in human urine from rural and urban China. Environ Sci Technol. 2015;49(24):14633–40. 10.1021/acs.est.5b04037. [DOI] [PubMed] [Google Scholar]
- 29.Yamamuro T, Ohta H, Aoyama M, Watanabe D. Simultaneous determination of neonicotinoid insecticides in human serum and urine using diatomaceous earth-assisted extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr B. 2014;969:85–94. 10.1016/j.jchromb.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Li Z, Chang CH, Lou JL, Zhao MR, Lu C. Potential human exposures to neonicotinoid insecticides: a review. Environ Pollut. 2018;236:71–81. 10.1016/j.envpol.2017.12.101. [DOI] [PubMed] [Google Scholar]
- 31.López-García M, Romero-González R, Lacasaña M, Garrido Frenich A. Semiautomated determination of neonicotinoids and characteristic metabolite in urine samples using TurboFlow™ coupled to ultra high performance liquid chromatography coupled to Orbitrap analyzer. J Pharm Biomed Anal. 2017;146:378–86. 10.1016/j.jpba.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 32.CDC (2017) Protection against mosquitoes, ticks, & other Arthropods, Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases; https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travelconsultation/protection-against-mosquitoes-ticks-other-arthropods. Accessed 5 July 2018. [Google Scholar]
- 33.EPA (2017) DEET. https://www.epa.gov/insect-repellents/deet. Accessed 5 July 2018.
- 34.EPA (2017) Find the insect repellent that is right for you. http://www.epa.gov/insect-repellents/find-insect-repellent-right-you. Accessed 5 July 2018.
- 35.Calafat AM, Baker SE, Wong LY, Bishop AM, Morales AP, Valentin-Blasini L. Novel exposure biomarkers of N,N-diethyl-mtoluamide (DEET): data from the 2007–2010 National Health and Nutrition Examination Survey. Environ Int. 2016;92–93:398–404. 10.1016/j.envint.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ATSDR (2017) Toxicological profile for DEET (N,N-diethyl-meta-toluamide). U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry; https://www.atsdr.cdc.gov/ToxProfiles/tp185.pdf. Accessed 13 Aug 2018 [PubMed] [Google Scholar]
- 37.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27(20):4094–106. 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 38.FDA (2018) Bionalytical method validation-guidance for industry. May 2018 edn. Food and Drug Administration Center for Drug Evaluation and Research, [Google Scholar]
- 39.Bader MBD, Gæen T, Schaller KH, Scherer G, Angerer J. Reliability criteria for analytical methods [Biomonitoring Methods, 2010]. In: GmbH&Co W-VV, editor. The MAK-Collection for occupational health and safety, vol 12, Biomonitoring Methods., vol Part IV; 2010. 10.1002/3527600418.bireliabe0012. [DOI] [Google Scholar]
- 40.Taylor J Quality assurance of chemical measurements. 1st ed: CRC Press; 1987. [Google Scholar]
- 41.Matuszewski BK. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC-MS bioanalysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830(2):293–300. 10.1016/j.jchromb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Kuklenyik P, Baker SE, Bishop AM, Morales AP, Calafat AM. Online solid phase extraction-high performance liquid chromatography-isotope dilution-tandem mass spectrometry approach to quantify N,N-diethyl-m-toluamide and oxidative metabolites in urine. Anal Chim Acta. 2013;787:267–73. 10.1016/j.aca.2013.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osaka A, Ueyama J, Kondo T, Nomura H, Sugiura Y, Saito I, et al. Exposure characterization of three major insecticide lines in urine of young children in Japan—neonicotinoids, organophosphates, and pyrethroids. Environ Res. 2016;147:89–96. 10.1016/j.envres.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 44.CDC (2018) Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2018 Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences; https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Mar2018.pdf. Accessed July 5, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.