TO THE EDITOR:
Congenital diarrhea is rare with only a few well-described etiologies. Congenital tufting enteropathy (CTE) represents one of such rare entities. CTE typically presents within the first few months of life with failure to thrive, chronic diarrhea, and occasionally, persistent emesis. Unlike the more common causes of diarrhea, CTE is not associated with inflammation or infection. It is characterized by crypt hyperplasia and near total or total villous atrophy involving the epithelium of the small intestine [Sherman et al., 2004]. Isolated epithelial tufts are usually present in the duodenum and jejunum that comprised enterocytes with surrounding disturbance of the plasma membrane resulting in a teardrop shape [Reifen et al., 1994; Beck et al., 2001]. Some patients may have associated defects such as choanal and esophageal atresia, punctated keratitis, skeletal dysplasia, and polymalformation with syndromic appearance [Abely et al., 1998; Bird et al., 2007; El-Matary et al., 2007; Goulet et al., 2007].
CTE was first described in 1994 [Reifen et al., 1994], with an annual incidence estimated at ∼1/100,000 live births in Western Europe [Goulet et al., 2007]. As a result of its rarity, the genetic basis of CTE was not uncovered until recently. Last year, mutations in the gene for epithelial cell adhesion molecule (EpCAM) were identified in CTE by studying only two affected patients from the same family of Mexican-American origin [Sivagnanam et al., 2008]. Additional mutations were also found in two other patients of Native-Canadian and Russian ethnicity, respectively. No EpCAM mutation was identified in a patient of English-Italian descent with a mild form of the disease, suggesting the possibility of heterogeneity or sequence variation within promoter and intronic regions of EpCAM. In this study we describe a consanguineous family of Pakistani origin with a child affected by CTE in whom a novel homozygous nonsense mutation was identified in EpCAM, while the parents carried the same mutation in a heterozygous form. Our observations further the evidence for EpCAM as the gene responsible for CTE.
The female infant was born at 38 weeks gestation after an uneventful pregnancy and delivery to a G1P1 mother with Pakistani descent. Both the parents and maternal grandparents are first cousins. At birth, the baby weighed 2,640 g (25th centile), length was 48.5 cm (25th centile), and head circumference was 34 cm (25th centile). She presented at 1 month of age with a 1-week history of persistent watery diarrhea and frequent emesis resulting in failure to thrive. At this time, her weight was 2,350 g (<3rd centile), length 49 cm (3rd centile), and head circumference 34 cm (3rd centile). She was hospitalized and total parenteral nutrition was initiated since her diarrhea, emesis, poor feeding, and failure to thrive persisted. An extensive inpatient evaluation included a metabolic and infectious work-up that was unrevealing, in addition to upper endoscopy and colonoscopy. Pathology results indicated crypt hyperplasia and villous atrophy on four sections of small intestine. Of particular importance, semi-thin sections stained with toluidine blue displayed epithelial tufting in which enterocytes extended from the surface in a characteristic tear dropped shape. A repeat endoscopy 2 months later confirmed the above results and the diagnosis of CTE.
Informed consent of the subjects was obtained according to the Institutional Review Board guidelines at UCSD. Patient and parents were recruited, blood samples were collected, and genomic DNA was extracted. In addition, available endoscopic duodenal tissue from the affected subject and age-matched normal control was obtained.
Primers were designed to amplify the coding exons of EpCAM including intron/exon boundaries as described by Sivagnanam et al. [2008]. Direct sequencing of PCR amplified products of DNA was performed using a 3730xl DNA analyzer (Applied Biosystems Inc., Foster City, CA). Immunofluorescent staining of formaldehyde fixed, paraffin-embedded duodenal biopsy tissue was performed using techniques previously described [Sivagnanam et al., 2008]. One sample from the affected patient and one sample from the control patient were stained using mouse monoclonal anti-EpCAM antibody (clone 323/A3, Abcam, Cambridge, MA) and a fluorescent secondary antibody (goat polyclonal to mouse IgG FITC) at a dilution of 1/1,000. Isotype controls lacking primary antibody were also performed.
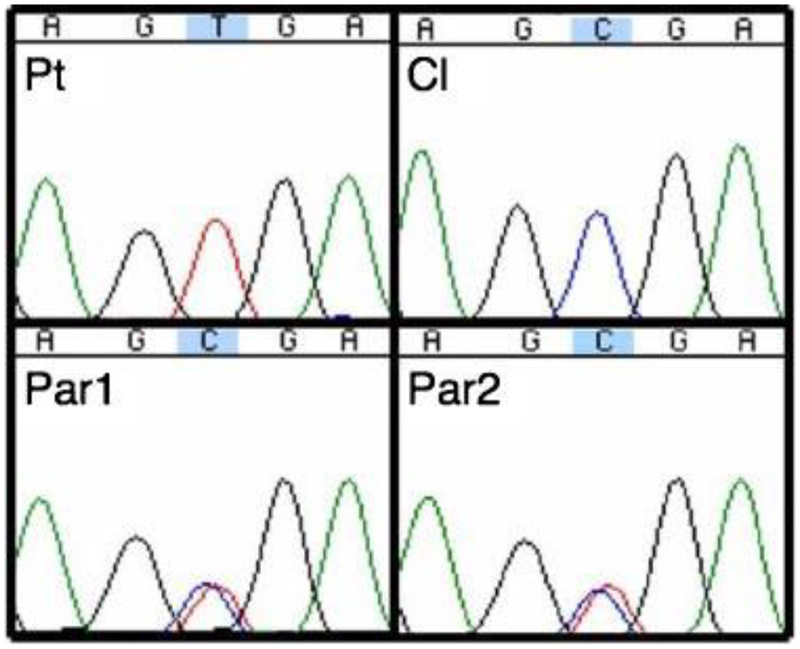
Direct sequencing of EpCAM revealed a novel mutation in the affected patient. A homozygous C>T substitution was identified in the affected patient at c.412 of exon 3 of EpCAM (Fig. 1) resulting in amino acid change from arginine (CGA) to a stop codon (UGA). The parents were found to be heterozygous for this variant, consistent with autosomal recessive inheritance.
FIG. 1.
Sequence chromatogram of the novel EpCAM exon 3 mutation. Nucleotides across x-axis with c.412 C>T mutation in blue/gray highlight. Homozygous mutant affected patient (Pt), heterozygous mother (Par1) and father (Par2), normal control (Cl). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
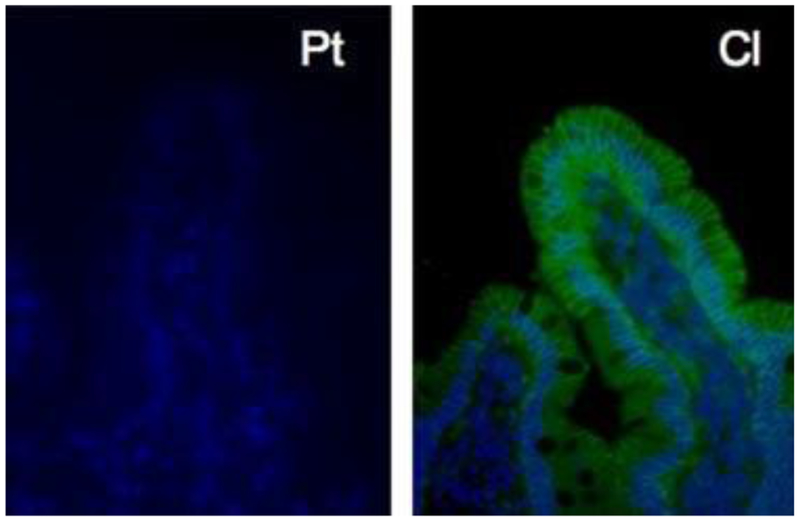
To assess protein expression in the intestine, fluorescent immunohistochemical (IHC) staining was performed on duodenal biopsy tissue demonstrating markedly decreased EpCAM staining throughout the tissue sample from the affected subject compared to control tissue (Fig. 2).
FIG. 2.
Fluorescent immunohistochemistry of duodenal tissue demonstrating lack of EpCAM staining (green/gray) in the affected patient (Pt) as compared with control patient tissue (Cl). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In this study we describe a novel EpCAM mutation associated with CTE in a consanguineous family. This is the first report of a nonsense mutation in EpCAM, which is consistent with absent EpCAM protein expression observed by IHC in the intestinal tissue from the affected patient and a clinically severe presentation. This finding strengthens the pathogenic role of EpCAM in CTE and supports its autosomal recessive inheritance pattern. Identification of additional EpCAM mutations will be necessary before genotype–phenotype correlations can be established. Nevertheless, it should be emphasized that none of the CTE patients with definite EpCAM mutations have had associated congenital malformations thus far suggesting that this genetic defect may cause isolated intestinal tufting and congenital diarrhea. The majority of reported CTE cases indeed had no associated malformations. Additionally, choanal atresia, corneal erosions, and polymalformation are frequent features of SPINT2-related syndromic congenital sodium diarrhea [Heinz-Erian et al., 2009]. Similar somatic defects have been attributed to associate with CTE in the isolated syndromic cases (see end of first paragraph). Therefore, it is plausible that syndromic CTE results from mutations in other genes than EpCAM and the epithelial tufting in these patients may be a common intestinal phenotype of converging molecular pathways. Thus, genetic analyses of EpCAM, SPINT2, and potential other genes identified in the future will help distinguish between such phenotypically overlapping entities. While normal EpCAM expression appears to be required for appropriate gut development and function, its specific role in the intestine is still unclear.
ACKNOWLEDGMENTS
We would like to acknowledge Hal Hoffman, James Mueller, Richard Kolodner, and Ludwig Institute of Cancer Research for support and advice. M.S. was supported by generous funding from the American College of Gastroenterology and the North American Society of Pediatric Gastroenterology Hepatology and Nutrition/Children’s Digestive Health, and Nutrition Foundation. R.K. was supported by funding from the Broad Medical Research Program, the Broad Foundation (IBD-0252) and a young investigator joint award from the Crohn’s and Colitis Foundation of America-Children’s Digestive Health and Nutrition Foundation/North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (CCFA #2426).
Grant sponsor: American College of Gastroenterology; Grant sponsor: North American Society of Pediatric Gastroenterology Hepatology, Nutrition/Children’s Digestive Health and Nutrition Foundation, and Crohn’s and Colitis Foundation Broad Foundation.
REFERENCES
- Abely M, Hankard GF, Hugot JP, Cezard JP, Peuchmaur M, Navarro J. 1998. Intractable infant diarrhea with epithelial dysplasia associated with polymalformation. J Pediatr Gastroenterol Nutr 27:348–352. [DOI] [PubMed] [Google Scholar]
- Beck NS, Kang IS, Suh YL. 2001. Protracted diarrhea: Results of the five-year survey in a tertiary hospital in Korea. J Korean Med Sci 16:736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird LM, Sivagnanam M, Taylor S, Newbury RO. 2007. A new syndrome of tufting enteropathy and choanal atresia, with ophthalmo-logic, hematologic and hair abnormalities. Clin Dysmorphol 16:211–221. [DOI] [PubMed] [Google Scholar]
- El-Matary W, Dalzell AM, Kokai G, Davidson JE. 2007. Tufting enteropathy and skeletal dysplasia: Is there a link? Eur J Pediatr 166:265–268. [DOI] [PubMed] [Google Scholar]
- Goulet O, Salomon J, Ruemmele F, de Serres NP, Brousse N. 2007. Intestinal epithelial dysplasia (tufting enteropathy). Orphanet J Rare Dis 2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz-Erian P, Muller T, Krabichler B, Schranz M, Becker C, Ruschendorf F, Nurnberg P, Rossier B, Vujic M, Booth IW, Holmberg C, Wijmenga C, Grigelioniene G, Kneepkens CM, Rosipal S, Mistrik M, Kappler M, Michaud L, Do’czy LC, Siu VM, Krantz M, Zoller H, Utermann G, Janecke AR. 2009. Mutations in SPINT2 cause a syndromic form of congenital sodium diarrhea. Am J Hum Genet 84:188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifen RM, Cutz E, Griffiths AM, Ngan BY, Sherman PM. 1994. Tufting enteropathy: A newly recognized clinicopathological entity associated with refractory diarrhea in infants. J Pediatr Gastroenterol Nutr 18:379–385. [PubMed] [Google Scholar]
- Sherman PM, Mitchell DJ, Cutz E. 2004. Neonatal enteropathies: Defining the causes of protracted diarrhea of infancy. J Pediatr Gastroenterol Nutr 38:16–26. [DOI] [PubMed] [Google Scholar]
- Sivagnanam M, Mueller JL, Lee H, Chen Z, Nelson SF, Turner D, Zlotkin SH, Pencharz PB, Ngan BY, Libiger O, Schork NJ, Lavine JE, Taylor S, Newbury RO, Kolodner RD, Hoffman HM. 2008. Identification of EpCAM as the gene for congenital tufting enteropathy. Gastroenterology 135:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]