Abstract
Objective:
To assess ischemic stroke patients regarding the relationship between lesion locations, swallowing impairment, medical and demographic factors and 1) oral intake improvement and 2) feeding tube dependency at discharge from their acute hospital stay.
Methods:
We conducted an exploratory, retrospective observational longitudinal cohort study of acute, first-ever, ischemic stroke patients. Patients who had an initial non-oral feeding recommendation from a speech and language pathologist and who underwent a modified barium swallow study within their hospital stay were included. Oral intake status was measured with the Functional Oral Intake Scale (FOIS) as the change in FOIS during the hospital stay and as feeding tube dependency at hospital discharge. Associations were assessed with multiple linear regression modelling controlling for age, comorbidities and hospital length of stay.
Results:
We included 44 stroke patients. At hospital discharge, 93% of patients had oral intake restrictions and 30% were feeding tube dependent. Following multiple linear regression modeling, age, damage to the left superior frontal gyrus, dorsal anterior cingulate gyrus, hypothalamus, and nucleus accumbens were significant predictors for FOIS change. Feeding tube dependency showed no significant associations with any prognostic variables when controlling for confounders.
Conclusions:
The vast majority of patients with an initial non-oral feeding recommendation are discharged with oral intake restrictions indicating a continued need for swallowing assessments and treatment after discharge. Lesion locations associated with motivation, reward and drive to consume food as well as swallowing impairment, higher age and more comorbidities were related to less oral intake improvement.
Keywords: dysphagia, acute stroke, deglutition, deglutition disorders, gastric feeding tube
Introduction
Swallowing disorders, dysphagia, are a common and disabling condition after stroke affecting the majority of acute stroke patients [1, 2]. Dysphagia is associated with stroke mortality [3], serious short- and long-term complications, such as pneumonia [1, 4], malnutrition, and dehydration [5], and has a negative impact on patients’ self-esteem, enjoyment of life and socialization [6].
While some stroke patients with dysphagia recover spontaneously, some patients require therapy to improve, and, unfortunately, some patients with dysphagia never recover, even with therapy [7–10]. Early predictions of who will recover are essential to streamline and individualize patient care, facilitate recovery, make informed decisions for rehabilitation and counsel patients and families. For example, if clinicians can better predict severe and long-lasting oral intake impairment versus early recovery, they can allocate resources such as feeding tubes and intensive rehabilitation. However, it is not well understood which factors contribute to dysphagia recovery offering motivation for the current study.
Likely, several factors impact post-stroke dysphagia recovery, including intrasubject factors and treatment specific factors. One intrasubject factor could be lesion location(s), as it has been shown that lesion locations are associated with stroke recovery in general [11, 12]. However, whether lesion locations are associated with the nature and extent of dysphagia recovery remains speculative [13]. For example, previous research suggests that dysphagia recovery may depend on whether lesions occur bilaterally or unilaterally [14], or whether the dominant or non-dominant swallowing hemisphere is lesioned [15, 16]. Moreover, different lesion locations or combinations of lesion locations might be associated with the extent of recovery at different time points after stroke [17, 18].
Other than lesion locations, intrasubject factors such as genetic polymorphisms [19], stroke type [20], degree of leukoaraiosis [20], previous cognitive impairment [20], baseline National Institute of Health Stroke Scale (NIHSS) score [14, 20], intubation [14], and identified aspiration [14] have been linked to post-stroke dysphagia recovery. Further, treatment specific factors such as specificity, intensity, and repetition [21], and the onset time of treatment after stroke [22] have also been associated with post-stroke dysphagia recovery.
The available evidence, however, has limitations that may have influenced study results. For example, many studies did not use instrumental assessments to determine dysphagia occurrence, did not use instrumental assessments for all patients, did not control for the initial dysphagia severity, or applied lesion location analyses lacking precision by using categorical variables and choosing large ROIs (e.g. the entire brainstem instead of dividing the brainstem into its different components).
The objective of our study was to assess factors including precise stroke lesion locations, swallowing physiology, medical and demographic characteristics and their relationship with the acute recovery of post-stroke dysphagia. Our primary hypothesis was that swallowing recovery is associated with distinct lesion locations independent of confounding factors such as age, lesion volume and length of hospital stay (LOS). Because the relationship between lesion locations and swallow recovery is understudied and poorly understood, we defined our study as exploratory. We measured recovery during the acute hospital stay using the surrogate of 1) improvement in oral intake and 2) absence of feeding tube dependency. Oral intake status is a functional and highly relevant patient-centered outcome. To limit confounding factors for recovery, we only included first-ever ischemic stroke patients with an initial non-oral intake recommendation (non per os = NPO) who all received a Modified Barium Swallow Study (MBSS) during their hospital stay.
Methods
Patients
We conducted a retrospective observational longitudinal cohort study of acute stroke patients admitted between 2008 and 2017 to the Medical University of South Carolina (USA). We selected patients from our larger study of 68 ischemic stroke patients on the influence of stroke lesion location on swallowing physiology [23]. These 68 patients of the larger study were a convenience sample identified based on retrospective chart reviews. Patients were included if they presented with radiographically confirmed acute, ischemic, first-ever stroke, underwent diffusion weighted MRI (DW-MRI) scanning and a MBSS during their acute hospital stay. Patients were excluded if they had a history or neuroimaging (radiographical) evidence of previous strokes or diseases commonly affecting swallowing (e.g. head and neck cancer, Parkinson’s, dementia), or were younger than 21 years. For the study presented here, we selected patients from our larger study, if the speech and language pathologist (SLP) recommended NPO intake at the very first SLP and patient encounter and they were diagnosed as dysphagic by an SLP. Demographic and medical information were extracted from medical charts.
Dependent variables
Change in oral intake from the first to last SLP encounter was used as a surrogate for swallow recovery. We used the Functional Oral Intake Scale (FOIS) [24] to measure oral intake status. The FOIS is a validated and reliable 7-point ordinal scale ranging from a score of “1” denoting “nothing by mouth” to a score of “7” denoting “total oral diet with no restrictions”. The primary outcome was overall change in oral intake from the first to last SLP encounter. The secondary outcome was feeding tube dependency (FOIS score 1–3) at the last SLP encounter. Since we only included patients who had a recommendation of NPO (FOIS score 1) at their first SLP encounter, all patients had the same initial FOIS level.
It is standard of care, at the Medical University of South Carolina (USA) and elsewhere, that SLPs assess stroke patients with dysphagia and provide recommendations regarding their oral intake based on their clinical and/or instrumental swallowing assessment. SLPs recommend whether patients with dysphagia can safely eat and/or drink and if they need modifications of food and liquid consistencies. For patients who have signs/symptoms or confirmed dysphagia and who are not safe to eat or drink anything, SLPs will recommend NPO. MBSSs assist in diagnosing the underlying swallowing impairment and inform treatment decisions. The medical team, consisting of physicians, SLPs, dietitians and nurses, will discuss a treatment plan and recommend a form of alternative feeding (e.g. nasogastric tube feeding or intravenous fluids). At every encounter, the SLP re-evaluates the dysphagia status and updates the oral intake recommendations if changes have occurred. The oral intake recommendation at the last SLP encounter before hospital discharge was used to determine the primary and secondary outcome measures.
Independent variables
We investigated the relationship between the following independent variables with the dependent variables oral intake improvement, and feeding tube dependency: stroke lesion locations, lesion volume (in ml), physiological swallowing impairment, modified Rankin Scale (mRS), National Institute of Health Stroke Scale (NIHSS), Charlson Comorbidity Index (CCI), number of days between MRI and MBSS, LOS (in days), and age (in years), sex, race. All variables except lesion location and volume, and physiological swallowing impairment were extracted from chart review.
Lesion locations and volume from DW-MRI
Neuroimaging data was obtained from conventional standard of care brain MRIs that all patients underwent for stroke diagnosis. All patients received whole brain DW-MRI, with voxel-wise resolution ranging from 0.9375×0.9375×3.0000mm to 1.4458×1.4458×6.0000mm. Since the patient recruitment period ranged from 2008 to 2017, MRI sequence details varied as a result of improvements in diagnostic radiology technology during this time. Using the software dcm2niix, we converted MR DICOM images into NIfTI files [25]. Using the software MRIcron (www.mricron.com), one rater (JW) manually drew the acute stroke lesions in the DW-MRIs. A neurologist (LB) reviewed all lesion drawings for accuracy and precision. At the time of lesion drawings, both raters (JW, LB) were blinded to the FOIS results. Stroke lesions were spatially normalized into standard space to allow the comparison of lesion locations across individuals by using SPM12 and open source Matlab scripts developed in-house [26]. We first smoothed the lesion maps by removing uneven edges using a 3mm full-width half maximum Gaussian kernel; second, we used SPM12’s unified segmentation-normalization to apply an enantiomorphic approach [27] to normalize the DW-MRI onto the standard space (1×1×1mm chimeric T1-weighted image, with the corresponding to the stroke lesion being replaced by the mirrored equivalent region in the intact hemisphere). We determined lesion locations by using the Johns Hopkins University (JHU) neuroanatomical atlas that segments the brain into 189 regions [28]. Lesion status was measured on a continuous scale as percentage lesion of each region of interest (ROI) of the JHU atlas. We determined total lesion volume in cubic mm based on the number of voxels being lesioned.
Region of interest selection
We selected ROIs based on previous published literature (original research articles, reviews, meta-analyses, case reports) that identified ROIs associated with swallowing, dysphagia occurrence, dysphagia recovery, or food consumption. These a-priori ROIs are listed in Table 1.
Table 1:
Selected regions of interest (ROIs) for the lesion location analyses.
| Brain region | Reference |
|---|---|
| Primary motor and sensory cortex | [35, 45–48] |
| Supplementary motor area | [45–47, 49] |
| Superior frontal gyrus | [50, 51] |
| Middle frontal gyrus | [48] |
| Inferior frontal gyrus | [17, 18, 48, 52–54] |
| Orbitofrontal cortex | [34, 45] |
| Operculum | [34, 47, 48, 55] |
| Parietal lobe | [35, 46, 53] |
| Angular gyrus | [34] |
| Supramarginal gyrus | [34, 55] |
| Superior temporal gyrus | [34, 48] |
| Insula | [46–48, 56] |
| Cingulate gyrus | [35, 46, 47] |
| Basal ganglia, putamen, caudate | [45] |
| Thalamus | [35, 57] |
| Hypothalamus | [58–60] |
| Amygdala | [50, 61] |
| Nucleus accumbens | [58, 62] |
| Corona radiata | [18, 63] |
| Internal capsule | [17, 45] |
| External capsule | [18, 63] |
| Superior longitudinal fasciculus | [34, 63] |
| Periventricular white matter | [64, 65] |
| Cerebellum | [35, 50, 66] |
| Pons | [67, 68] |
| Medulla | [67, 69–71] |
Swallowing physiology impairment
Swallow physiology measures were derived from MBSSs that all followed the standardized and validated Modified Barium Swallow Impairment Profile (MBSImP™©) [29]. The MBSImP assesses in total 17 swallow physiology components including 6 oral, 10 pharyngeal and 1 esophageal component. Further, entrance of bolus material in the upper airway was measured with the validated and standardized Penetration-Aspiration Scale (PAS) [30] as the worst score across swallows. One rater (JW) scored the MBSImP and PAS for all MBSSs and scored 20% of the same MBSS again with at least 2 weeks in between to establish intra-rater reliability. A second rater (JC) scored 20% of all MBSSs to establish inter-rater reliability. Intra- and inter-rater reliability were good to excellent for the MBSImP and PAS.
Statistical analyses
Using Spearman correlation analyses, we assessed associations between the independent and dependent variables. We interpreted the size of correlations with |r| < 0.3 as weak, 0.3 ≤ |r| < 0.5 as moderate, and |r| ≥ 0.5 as strong [31]. Linear and logistic multivariable regression modelling was used to assess the strength of the relationship between two variables while controlling for other variables. P values < 0.05 were considered statistically significant.
The script NiiStat (version 9, released October 2016, Neuroimaging Informatics Tools and Resources Clearinghouse) running on Matlab (version R2016b) and SPM (version 12) was used to determine percentage lesion in the JHU ROIs. SAS statistical software (version 9.4, released 2016, SAS Institute, Inc., Cary, N.C., USA) or IBM SPSS Statistics for Windows (version 24, released 2016, IBM Corp., Armonk, N.Y., USA) were used for any other statistical analyses. The study was approved by our Institutional Review Board.
Results
Patient characteristics
In total, 44 patients were included in the study. Demographic and medical information are shown in Table 2. On average, patients were 67 years old and presented at admission with moderate stroke severity based on the NIHSS. About half of all patients received tissue plasminogen activator (tPA) and the majority were discharged to rehabilitation facilities. Further, on average, 1.55 days (SD 2.24) after hospital admission, patients received a DW-MRI and on average 3.59 days (SD 4.33) after the initial DW-MRI, patients underwent a MBSS. The first SLP encounter was on average 1 day (SD 1, range 0–7) after admission, and the last SLP encounter was on average 1 day before discharge (SD 1, range 0–4).
Table 2:
Demographic and medical information.
| Demographical Information | ||
|---|---|---|
| Age, in years, mean (SD; range) | 67.48 (15.89; 28–90) | |
| Gender, N (%) | Female | 23 (52%) |
| Male | 21 (48%) | |
| Race, N (%) | White or Caucasian | 29 (66%) |
| Black or African-American | 15 (34%) | |
| Asian | 0 (0%) | |
| Other/Unknown | 0 (0%) | |
| Ethnicity, N (%) | Not Hispanic or Latino | 44 (100%) |
| Hispanic or Latino | 0 (0%) | |
| Status at hospital admission and stroke characteristics | ||
| National institute of health stroke scale, N, mean (SD; range) | N=42, 14.31 (5.94; 2–26) | |
| Modified rankin scale, N, median (range) | N=37, 0 (0–4) | |
| Charlson comorbidity index, mean (SD; range) | 0.86 (1.34; 0–6) | |
| Lesion volume (in ml / cc), mean (SD; range) | 112.28 (97.95; 0.43–360.72) | |
| Hospital course | ||
| Length of hospital stay (in days), mean (SD; range) | 12.82 (14.29; 2–90) | |
| Tissue plasminogen activator, N (%) | 21 (48%) | |
| Thrombectomy, N (%) | 11 (25%) | |
| Intubation, N (%) | 6 (14%) | |
| Tracheotomy, N (%) | 2 (5%) | |
| Hospital discharge | ||
| Percutaneous endoscopic gastrostomy, N (%) | 10 (23%) | |
| Nasogastric feeding tube | 3 (7%) | |
| Discharge destination, N (%) | Rehabilitation facility | 28 (63.6%) |
| Home / home health | 6 (13.6%) | |
| (Skilled) nursing facility | 2 (4.55%) | |
| Long term care hospital | 0 (0.0%) | |
| Transitional care unit | 1 (2.3%) | |
| Other hospital | 1 (2.3%) | |
| Hospice / home hospice | 2 (4.5%) | |
| Deceased | 1 (2.3%) | |
| Unknown | 3 (6.8%) | |
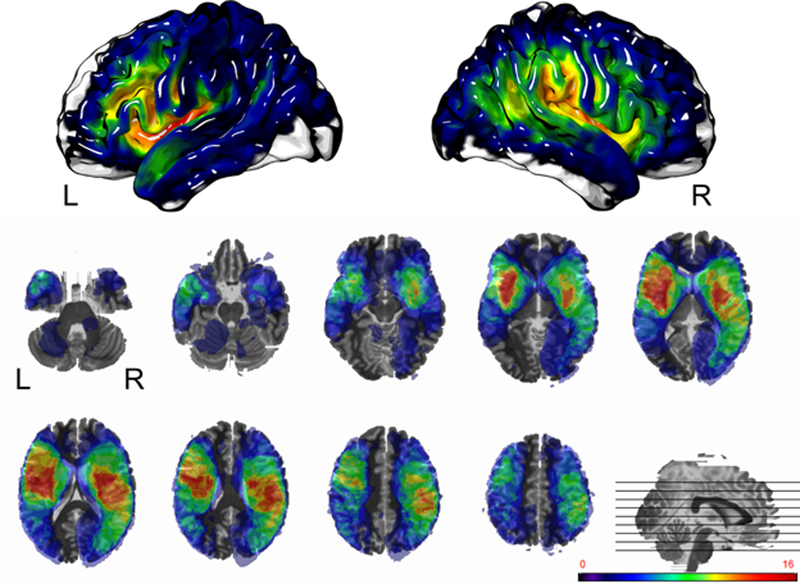
The distribution and locations of the stroke lesions of all 44 patients is shown in Fig. 1 by illustrating which brain regions were lesioned in how many patients. Middle cerebral artery territories in both hemispheres were the most commonly lesioned brain regions. The highest number of patients showed lesions in the left posterior insula and left external capsule, thus, the highest relative power was in these regions. Further, Fig. 1 illustrates that some brain regions (e.g. pons) were not lesioned in any of the 44 patients, and thus, were not included in the statistical analyses.
Fig. 1:
Lesion overlap of all included stroke patients (N=44). The first row shows the cortical surfaces of the left and right hemispheres, the second and third rows show axial brain slices. Different colors represent different numbers of patients with lesions in that area. As denoted by the color bar, red highlighted areas were lesioned in the highest number of patients (>10 patients), whereas purple highlighted regions were lesioned in very few patients, and not highlighted areas were not lesioned in any patient. L = left hemisphere; R = right hemisphere.
Table 3 gives an overview of the distribution of PAS and MBSImP component and total scores. Components 13 (pharyngeal contraction) and 17 (esophageal clearance) had substantially missing data (39% and 66% of patients had no score, respectively). Missing data is common in patients with neurological disorders because they are often unable to reposition to the anterior-posterior position required to accurately detail the integrity of these two components. For all other PAS, component, and total scores the group of 44 patients had a broad data distribution with representations of almost all possible scores.
Table 3:
Distribution of Modified Barium Swallow Impairment Profile (MBSImP) and Penetration-Aspiration Scale (PAS) scores across all included patients (N=44) (higher values indicate more severe impairment).
| Variable (possible score range) | Median | Range | Missing data |
|---|---|---|---|
| MBSImP components | |||
| C1- Lip closure (0–4) | 2 | 0–4 | 0 |
| C2 - Tongue control (0–3) | 1 | 0–3 | 0 |
| C3 - Bolus preparation (0–3) | 0 | 0–3 | 7 |
| C4 - Bolus transport (0–4) | 2 | 0–3 | 0 |
| C5 - Oral residue (0–4) | 2 | 1–4 | 0 |
| C6 - Initiation of pharyngeal swallow (0–4) | 3 | 1–3 | 0 |
| C7 - Soft palate elevation (0–4) | 0 | 0–2 | 0 |
| C8 - Laryngeal elevation (0–3) | 1 | 0–2 | 0 |
| C9 - Anterior hyoid excursion (0–2) | 1 | 0–2 | 0 |
| C10 - Epiglottic movement (0–2)t | 1 | 0–2 | 0 |
| C11 - Laryngeal vestibular closure (0–2) | 1 | 0–2 | 0 |
| C12 - Pharyngeal stripping wave (0–2) | 1 | 0–2 | 0 |
| C13 - Pharyngeal contraction (0–3) | 0 | 0–2 | 17 |
| C14 - PES opening (0–3) | 1 | 0–2 | 0 |
| C15 - Tongue base retraction (0–4) | 2 | 1–3 | 0 |
| C16 - Pharyngeal residue (0–4) | 2 | 1–3 | 0 |
| C17 - Esophageal clearance (0–4) | 1 | 0–2 | 29 |
| MBSImP total scores | |||
| Oral total (0–22) | 11.5 | 0–18 | 0 |
| Pharyngeal total (0–29) | 8 | 0–19 | 0 |
| PAS (1–8) | 3 | 1–8 | 0 |
C=components; MBSImP=Modified Barium Swallow Impairment Profile; PAS= Penetration-Aspiration Scale
FOIS score distribution
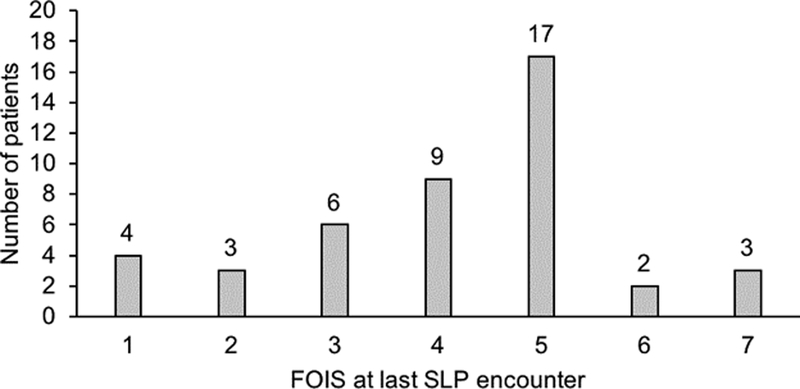
Fig. 2 shows the distribution of FOIS scores at the last SLP encounter. The median FOIS score at hospital discharge (last SLP encounter) was 4.5 (range 1–7). At time of hospital discharge, 13 patients (29.5%) were completely or in part feeding tube dependent (FOIS 1–3). Thirty-one of 44 patients (70.5%) who were initially non-per-os (NPO) were discharged on an oral diet without feeding tube dependency (FOIS 4–7). Three of the 31 patients (6.8%) returned to a normal oral diet (FOIS 7).
Fig. 2:
Distribution of FOIS scores at last speech and language pathologist’s (SLP) encounter.
Change in oral diet: FOIS change from first to last SLP encounter
Lesion location and volume
Four JHU ROIs showed significant correlations (with correlations coefficients between r=−0.3 and r=−0.4) with more damage in the ROI being associated with less improvement on the FOIS. The significant ROIs were: left superior frontal gyrus (frontal pole), left dorsal anterior cingulate gyrus, left hypothalamus, and left nucleus accumbens. These associations all remained statistical significant after controlling for age, CCI and LOS in multivariable linear regression modelling. Total lesion volume did not correlate with FOIS change and was not a significant factor after controlling for confounders.
Physiological swallowing impairment
FOIS change had a significant negative correlation with MBSImP components 4 (bolus transport) (r=−0.15, p=0.02), 10 (epiglottic movement) (r=−0.37, p=0.01), 14 (pharyngoesophageal segment opening) (r=−0.30, p=0.05), and PAS (r=−0.38, p=0.01). After controlling for age, CCI and LOS, the MBSImP components 4 (bolus transport) (ß=−0.40, p=0.08), and 10 (epiglottic movement) (ß=−0.73, p=0.07), and PAS (ß=−0.28, p=0.09) showed a trend towards significance.
Other demographic and medical variables
FOIS change had a significant negative correlation with age (r=−0.39, p=0.01) and CCI (r=−0.35, p=0.02). Age remained significant after controlling for CCI and LOS (ß=−0.03, p=0.04). No other correlations or multivariable regression models were statistical significant.
Feeding tube dependency
At discharge, 13 (29.55%) patients were feeding tube dependent (10 patients had a percutaneous endoscopic gastrostomy (PEG) tube, 3 patients opted out of a PEG tube placement and kept a nasogastric tube). No statistically significant differences were detected between feeding tube dependent and non-dependent patients in terms of age, gender, race, ethnicity, NIHSS, mRS, CCI, lesion volume, and LOS.
Lesion location and volume
Three JHU regions showed significant correlations (range r= −0.38 - −0.30, p<0.05) with more damage in the ROI being associated with feeding tube dependency at the last SLP encounter: right superior parietal gyrus, right angular gyrus, and left cingulate gyrus. When controlling for age, CCI and LOS in a multivariable logistic regression model, none of the regions remained statistically significant. Total lesion volume did not correlate with feeding tube dependency and was not a significant factor after controlling for confounders.
Physiological swallowing impairment
The MBSImP component 6 (initiation of the pharyngeal swallow) significantly correlated with feeding tube dependency at discharge (r=−0.29, p=0.05). After controlling for age, CCI and LOS, component 6 (initiation of the pharyngeal swallow) showed a trend towards significance (p=0.09).
Other demographic and medical variables
CCI significantly correlated (r=−0.29, p=0.05) with feeding tube dependency at discharge, but did not remain significant in adjusted analyses. No other variables were statistically significantly associated with feeding tube dependency.
Discussion
The goal of our exploratory study was to assess factors associated with recovery of swallowing impairment after stroke. We measured recovery of swallowing impairment as the change in oral intake during the hospital stay and feeding tube dependency at hospital discharge. We only included patients with a recommendation of NPO at the first SLP encounter who received a MBSS during their hospital stay to ensure the best possible comparability between patients and their potential for recovery. The majority of patients in our study showed substantial improvement during their hospital stay: 70% of all 44 included patients progressed to an oral diet without feeding tube dependency while 30% remained feeding tube dependent at discharge. However, more than 90% of patients were on a restricted diet (ranging from tube fed to oral diet with restrictions) at discharge, emphasizing the importance of post-discharge follow up.
Change in oral diet: FOIS change from first to last SLP encounter
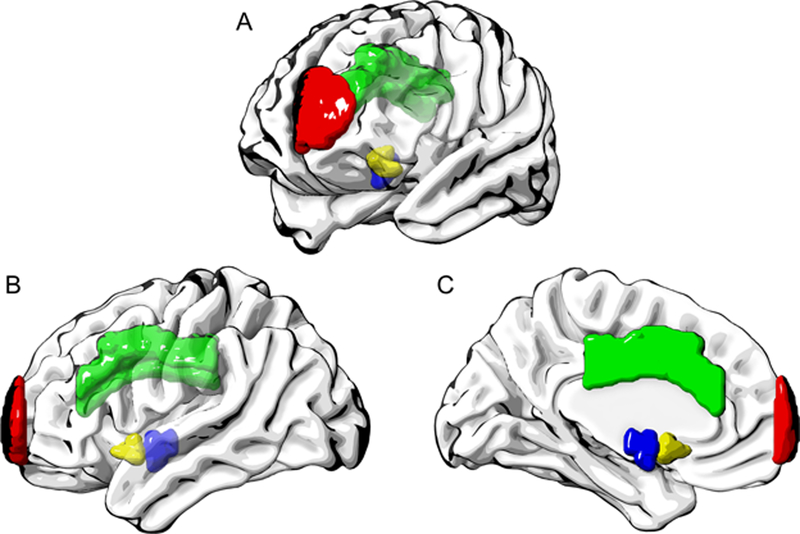
We found that while controlling for confounders, regional lesion burden in the left superior frontal gyrus (frontal pole), left dorsal anterior cingulate gyrus, left hypothalamus, and left nucleus accumbens were all significantly associated with less improvement in oral intake. Fig. 3 visualizes the location of these ROIs in a standard brain from different angles. These ROIs mainly comprise structures of the limbic system (cingulate gyrus, hypothalamus) or are connected to the limbic system (nucleus accumbens). These structures are known to be involved in emotions, motivation and reward functions, as well as hunger and thirst regulation. Further, all significant ROIs were located in the left hemisphere. This is in contrast to results from previous studies where we found heavy right hemisphere associations for distinct physiological swallowing impairments [32, 33]. These findings implicate that regions controlling food motivation and consumption might be more important for improvement in oral intake than regions involved in the sensorimotor control of swallowing. This suggests that more attention should be given to the factors of motivation and interest in food consumption during clinical assessments and treatments.
Fig. 3:
Region of interests associated with less oral intake improvement after controlling for age, CCI and LOS in multivariable linear regression modelling. A = oblique view; B = left hemisphere, lateral; C = left hemisphere, medial; red = left superior frontal gyrus (frontal pole); green = left dorsal anterior cingulate gyrus; blue = left hypothalamus; yellow = left nucleus accumbens.
Moreover, neither total lesion volume, nor any of the ROIs with the on average highest lesion load (percent damage across patients) were significantly associated with FOIS change. Thus, improvement in oral intake during the hospital stay is not a simple function of how much of a patient’s brain was damaged. Older age, higher comorbidity score, and higher (worse) scores on PAS and three MBSImP components – 4 (bolus transport), 10 (epiglottic movement), 14 (pharyngoesophageal segment opening) – were significantly, but weakly correlated with less improvement in oral intake during the hospital stay. Only age remained an independent predictor after controlling for confounders. The weak associations between swallowing components and oral intake change might be a result of the latency between the MBSS and the oral intake status at discharge as the MBSSs were not necessarily performed close to discharge. Future studies with larger sample sizes that include a MBSS near discharge are warranted to investigate this relationship in more detail.
Feeding tube dependency
We did not find any independent predictors for feeding tube dependency at hospital discharge after controlling for confounders. Reasons why we were not able to reveal independent predictors for feeding tube dependency are speculative. One possible reason is insufficient statistical power. Compared to FOIS change, where we revealed several independent predictors, feeding tube dependency was a binary outcome, and only 30% of patients (13 patients) had a feeding tube at time of discharge.
Further, feeding tube dependency might be related to other existing factors that go beyond stroke characteristics and a patient’s health status. Previous evidence suggests that patient-centered factors are not the only drivers of the decision to place a gastrostomy tube in a stroke patient. Instead, hospital factors, such as stroke volume or for-profit status [38], and/or next level care institutions’ preference for admittance of patients with PEG tubes instead of nasogastric tubes may influence gastrostomy tube placement during the acute hospital stay [39, 40].
Other factors influencing post-stroke swallow recovery
Stroke recovery depends on multiple factors including age, genetic factors [19], complications following the stroke (e.g. hemorrhagic transformation) [41], patient health status, and rehabilitation therapy [42, 43]. In turn, the success of rehabilitation therapy also depends on a variety of factors, for example the treatment’s specificity, intensity, and repetition [21, 42], the patient’s lifestyle and time after stroke [43], or the preservation of structural brain networks [44]. In the study presented here, we investigated a few select demographic and medical factors, but were not able to account for other potentially important factors, such as genetic factors and rehabilitation therapy.
Moreover, we used oral intake (FOIS) as a surrogate for recovery of swallow impairment. Oral intake status is a functional and highly relevant patient-centered outcome; however, oral intake status does not allow for conclusions about the presence, degree and type of dysphagia. Oral intake recommendations should be based on a patient’s swallowing ability, but are also based on other factors, such as agitation, limited alertness, and the amount of food and liquid consumed to meet nutrition and hydration needs. Further, oral intake recommendations might also reflect clinicians’ confidence or comfort in what they believe the patient can tolerate. By only including patients who had a MBSS, information on swallow physiology was available for all patients in our study, and we attempted to control for variation related to the available information on swallow physiology. Nevertheless, future studies are needed that investigate dysphagia recovery by assessing swallowing impairment with MBSSs at different time points post stroke. Results of changes in swallowing impairment could then be correlated to other swallowing outcomes, such as medical complications, oral intake or self-perceived quality of life.
Limitations
Our study has other limitations in addition to factors influencing stroke recovery that we were not able to include. For example, the lesion location analyses were mainly restricted to supratentorial lesion locations, because only very few patients had infratentorial lesions. Thus, we were not able to investigate relationships between lesions in the brainstem or cerebellum and oral intake improvement. We carefully selected patients to control for heterogeneities in patients’ diagnoses and dysphagia management. However, this selection bias limits the generalizability of our results to patients who underwent a MBSS during their acute hospital stay. Further, some of our statistical analyses may have been underpowered, and thus, limited the detection of additional factors associated with oral intake improvement and feeding tube dependency.
Conclusions
In this study of 44 stroke patients, 90% of those with an initial NPO recommendation did not progress to a normal oral diet (FOIS 1–6) and 30% remained feeding tube dependent (FOIS 1–3). These findings of persistent dysphagia indicate a continued need for swallowing re-evaluation and treatment after discharge from the acute hospital stay. A novel finding was, lesion locations associated with motivation, reward and drive to consume food were related to less oral intake improvement at discharge. In patients who demonstrated oral intake improvement, multiple factors were related to the magnitude of improvement including severity of swallowing impairment, age and severity of comorbidities albeit with weak associations.
Compliance with ethical standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Because the study was retrospective, informed consent was waived and not obtained from the participants included in this study.
Acknowledgements:
We would like to thank the Center for Biomedical Research Excellence (COBRE) in Stroke Recovery at the Medical University of South Carolina (MUSC) in Charleston, South Carolina who provided resources for patient identification and recruitment (Grant Number 5P20GM109040).
Funding:
The project described was supported in part by the NIH National Center for Advancing Translational Sciences (NCATS) through Grant Number UL1 TR001450, SCTR Pilot Project 17254.
References
- [1].Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63. [DOI] [PubMed] [Google Scholar]
- [2].Daniels SK, Foundas AL. Lesion localization in acute stroke patients with risk of aspiration. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 1999;9(2):91–8. [DOI] [PubMed] [Google Scholar]
- [3].Sharma JC, Fletcher S, Vassallo M, Ross I. What influences outcome of stroke--pyrexia or dysphagia? Int J Clin Pract. 2001;55:17–20. [PubMed] [Google Scholar]
- [4].Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124:328–36. [DOI] [PubMed] [Google Scholar]
- [5].Crary MA, Humphrey JL, Carnaby-Mann G, Sambandam R, Miller L, Silliman S. Dysphagia, nutrition, and hydration in ischemic stroke patients at admission and discharge from acute care. Dysphagia. 2013;28:69–76. [DOI] [PubMed] [Google Scholar]
- [6].Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17:139–46. [DOI] [PubMed] [Google Scholar]
- [7].Langdon PC, Lee AH, Binns CW. Dysphagia in acute ischaemic stroke: severity, recovery and relationship to stroke subtype. J Clin Neurosci. 2007;14:630–4. [DOI] [PubMed] [Google Scholar]
- [8].Mann G, Hankey GJ, Cameron D. Swallowing Function After Stroke : Prognosis and Prognostic Factors at 6 Months. Stroke. 1999;30:744–8. [DOI] [PubMed] [Google Scholar]
- [9].Smithard DG, O’Neill PA, England RE, Park CL, Wyatt R, Martin DF, et al. The natural history of dysphagia following a stroke. Dysphagia. 1997;12:188–93. [DOI] [PubMed] [Google Scholar]
- [10].Broadley S Predictors of prolonged dysphagia following acute stroke. J Clin Neurosci. 2003;10(3):300–5. [DOI] [PubMed] [Google Scholar]
- [11].Hope TM, Seghier ML, Leff AP, Price CJ. Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage Clinical. 2013;2:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Plowman E, Hentz B, Ellis C Jr., Post-stroke aphasia prognosis: a review of patient-related and stroke-related factors. J Eval Clin Pract. 2012;18:689–94. [DOI] [PubMed] [Google Scholar]
- [13].Lowell SY, Reynolds RC, Chen G, Horwitz B, Ludlow CL. Functional connectivity and laterality of the motor and sensory components in the volitional swallowing network. Experimental brain research. 2012;219:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kumar S, Doughty C, Doros G, Selim M, Lahoti S, Gokhale S, et al. Recovery of swallowing after dysphagic stroke: an analysis of prognostic factors. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;23:56–62. [DOI] [PubMed] [Google Scholar]
- [15].Verin E, Michou E, Leroi AM, Hamdy S, Marie JP. “Virtual” lesioning of the human oropharyngeal motor cortex: a videofluoroscopic study. Archives of physical medicine and rehabilitation. 2012;93:1987–90. [DOI] [PubMed] [Google Scholar]
- [16].Mistry S, Verin E, Singh S, Jefferson S, Rothwell JC, Thompson DG, et al. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J Physiol. 2007;585:525–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Galovic M, Leisi N, Muller M, Weber J, Abela E, Kagi G, et al. Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke. 2013;44:2760–7. [DOI] [PubMed] [Google Scholar]
- [18].Galovic M, Leisi N, Pastore-Wapp M, Zbinden M, Vos SB, Mueller M, et al. Diverging lesion and connectivity patterns influence early and late swallowing recovery after hemispheric stroke. Human brain mapping. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jayasekeran V, Pendleton N, Holland G, Payton A, Jefferson S, Michou E, et al. Val66Met in brain-derived neurotrophic factor affects stimulus-induced plasticity in the human pharyngeal motor cortex. Gastroenterology. 2011;141:827–36 e1–3. [DOI] [PubMed] [Google Scholar]
- [20].Toscano M, Cecconi E, Capiluppi E, Vigano A, Bertora P, Campiglio L, et al. Neuroanatomical, Clinical and Cognitive Correlates of Post-Stroke Dysphagia. Eur Neurol. 2015;74:171–7. [DOI] [PubMed] [Google Scholar]
- [21].Robbins J, Butler SG, Daniels SK, Diez Gross R, Langmore S, Lazarus CL, et al. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. Journal of speech, language, and hearing research : JSLHR. 2008;51:S276–300. [DOI] [PubMed] [Google Scholar]
- [22].Bakhtiyari J, Sarraf P, Nakhostin-Ansari N, Tafakhori A, Logemann J, Faghihzadeh S, et al. Effects of early intervention of swallowing therapy on recovery from dysphagia following stroke. Iran J Neurol. 2015;14:119–24. [PMC free article] [PubMed] [Google Scholar]
- [23].Wilmskoetter J, Martin-Harris B, Bonilha L, Elm J, Cucciare J, Bonilha H. Mapping acute lesion locations to physiological swallow impairments after stroke. 26th Annual Meeting Dysphagia Research Society. Baltimore (MD) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Archives of physical medicine and rehabilitation. 2005;86:1516–20. [DOI] [PubMed] [Google Scholar]
- [25].Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods. 2016;264:47–56. [DOI] [PubMed] [Google Scholar]
- [26].Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nachev P, Coulthard E, Jager HR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. Neuroimage. 2008;39:1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Faria AV, Joel SE, Zhang Y, Oishi K, van Zjil PC, Miller MI, et al. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies. Neuroimage. 2012;61:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, et al. MBS measurement tool for swallow impairment--MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
- [31].Cohen J Statistical power analysis for the behavioral sciences 2nd ed. ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- [32].Wilmskoetter J, Martin-Harris B, Pearson WG Jr., , Bonilha L, Elm JJ, Horn J, et al. Differences in swallow physiology in patients with left and right hemispheric strokes. Physiology & behavior. 2018;194:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wilmskoetter J, Bonilha L, Martin-Harris B, Elm JJ, Horn J, Bonilha HS. Mapping acute lesion locations to physiological swallow impairments after stroke. NeuroImage: Clinical. 2019:101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Suntrup-Krueger S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, et al. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 2: Oropharyngeal residue, swallow and cough response, and pneumonia. Eur J Neurol. 2017;24:867–74. [DOI] [PubMed] [Google Scholar]
- [35].Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Human brain mapping. 2009;30:3209–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wilmskoetter J, Simpson AN, Simpson KN, Bonilha HS. Practice Patterns of Percutaneous Endoscopic Gastrostomy Tube Placement in Acute Stroke: Are the Guidelines Achievable? Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2016;25:2694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wilmskoetter J, Simpson AN, Logan SL, Simpson KA, Bonilha H. Impact of Gastrostomy Feeding Tube Placement on the 1-Year Trajectory of Care in Patients After Stroke. Nutrition in Clinical Practice. 2018. [DOI] [PubMed] [Google Scholar]
- [38].George BP, Kelly AG, Schneider EB, Holloway RG. Current practices in feeding tube placement for US acute ischemic stroke inpatients. Neurology. 2014;83:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jauch EC, Saver JL, Adams HP Jr., , Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- [40].Plonk WM Jr. To PEG or not to PEG. Practical Gastroenterology. 2005;29:16+9–31. [Google Scholar]
- [41].Bustamante A, Garcia-Berrocoso T, Rodriguez N, Llombart V, Ribo M, Molina C, et al. Ischemic stroke outcome: A review of the influence of post-stroke complications within the different scenarios of stroke care. Eur J Intern Med. 2015. [DOI] [PubMed] [Google Scholar]
- [42].Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. Journal of speech, language, and hearing research : JSLHR. 2008;51:S225–39. [DOI] [PubMed] [Google Scholar]
- [43].Warraich Z, Kleim JA. Neural plasticity: the biological substrate for neurorehabilitation. PM R. 2010;2:S208–19. [DOI] [PubMed] [Google Scholar]
- [44].Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Success of Anomia Treatment in Aphasia Is Associated With Preserved Architecture of Global and Left Temporal Lobe Structural Networks. Neurorehabilitation and neural repair. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gonzalez-Fernandez M, Kleinman JT, Ky PK, Palmer JB, Hillis AE. Supratentorial regions of acute ischemia associated with clinically important swallowing disorders: a pilot study. Stroke. 2008;39:3022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia. 2007;22:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–71. [DOI] [PubMed] [Google Scholar]
- [48].Martin R Cerebral cortical representation of automatic and volitional swallowing in humans. Journal of neurophysiology. 2001;85(2):938–50. [DOI] [PubMed] [Google Scholar]
- [49].Huckabee ML, Deecke L, Cannito MP, Gould HJ, Mayr W. Cortical control mechanisms in volitional swallowing: the Bereitschaftspotential. Brain Topogr. 2003;16:3–17. [DOI] [PubMed] [Google Scholar]
- [50].Hamdy S Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. Journal of neurophysiology. 1999;81(4):1917–26. [DOI] [PubMed] [Google Scholar]
- [51].Martin R, Barr A, MacIntosh B, Smith R, Stevens T, Taves D, et al. Cerebral cortical processing of swallowing in older adults. Experimental brain research. 2007;176:12–22. [DOI] [PubMed] [Google Scholar]
- [52].Dziewas R Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. NeuroImage. 2003;20(1):135–44. [DOI] [PubMed] [Google Scholar]
- [53].Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, et al. Neurophysiology of swallowing: effects of age and bolus type. Neuroimage. 2009;44:982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Experimental brain research. 2001;140:280–9. [DOI] [PubMed] [Google Scholar]
- [55].Suntrup S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, et al. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: Dysphagia incidence, severity and aspiration. European journal of neurology. 2015. [DOI] [PubMed] [Google Scholar]
- [56].Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia. 1997;12(3):146–56. [DOI] [PubMed] [Google Scholar]
- [57].Mosier KM. Lateralization of cortical function in swallowing: a functional MR imaging study. Am J Neuroradiol. 1999;20(8):1520–6. [PMC free article] [PubMed] [Google Scholar]
- [58].Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Frontiers in systems neuroscience. 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Smeets PA, Charbonnier L, van Meer F, van der Laan LN, Spetter MS. Food-induced brain responses and eating behaviour. Proc Nutr Soc. 2012;71:511–20. [DOI] [PubMed] [Google Scholar]
- [60].Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–72. [DOI] [PubMed] [Google Scholar]
- [61].Saker P, Farrell MJ, Egan GF, McKinley MJ, Denton DA. Overdrinking, swallowing inhibition, and regional brain responses prior to swallowing. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:12274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63:415–22. [DOI] [PubMed] [Google Scholar]
- [63].Galovic M, Leisi N, Muller M, Weber J, Tettenborn B, Brugger F, et al. Neuroanatomical correlates of tube dependency and impaired oral intake after hemispheric stroke. Eur J Neurol. 2016. [DOI] [PubMed] [Google Scholar]
- [64].Moon HI, Nam JS, Leem MJ, Kim KH. Periventricular White Matter Lesions as a Prognostic Factor of Swallowing Function in Older Patients with Mild Stroke. Dysphagia. 2017. [DOI] [PubMed] [Google Scholar]
- [65].Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41:482–6. [DOI] [PubMed] [Google Scholar]
- [66].Rangarathnam B, Kamarunas E, McCullough GH. Role of cerebellum in deglutition and deglutition disorders. Cerebellum. 2014;13:767–76. [DOI] [PubMed] [Google Scholar]
- [67].Flowers HL, AlHarbi MA, Mikulis D, Silver FL, Rochon E, Streiner D, et al. MRI-Based Neuroanatomical Predictors of Dysphagia, Dysarthria, and Aphasia in Patients with First Acute Ischemic Stroke. Cerebrovasc Dis Extra. 2017;7:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schmahmann JD, Ko R, MacMore J. The human basis pontis: motor syndromes and topographic organization. Brain : a journal of neurology. 2004;127:1269–91. [DOI] [PubMed] [Google Scholar]
- [69].Steinhagen V, Grossmann A, Benecke R, Walter U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke. 2009;40:1903–6. [DOI] [PubMed] [Google Scholar]
- [70].Moon HI, Pyun SB, Kwon HK. Correlation between Location of Brain Lesion and Cognitive Function and Findings of Videofluoroscopic Swallowing Study. Ann Rehabil Med. 2012;36:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chun MH, Kim D, Chang MC. Comparison of Dysphagia Outcomes Between Rostral and Caudal Lateral Medullary Infarct Patients. Int J Neurosci. 2017:1–24. [DOI] [PubMed] [Google Scholar]